Introduction
Salicylate is a widely prescribed compound known for
its anti-pyretic, anti-inflammatory and analgesic properties
(1). However, salicylate has been
reported to have numerous undesirable side effects, including
gastrointestinal irritation and bleeding, tinnitus,
hypersensitivity as well as central nervous system (CNS) symptoms
(2). It has been suggested that
salicylate affects neuronal function via interactions with specific
membrane channels/receptors (3);
however, the majority of studies to date have focused on its
effects on the auditory system (4–6).
Salicylate treatment was reported to increase spontaneous neuronal
activity and decrease the efficacy of inhibitory neurotransmission
in the inferior colliculus and auditory cortex (7,8).
However, the effect of salicylate on neural plasticity in the
hippocampus, a key structure for numerous complex brain functions,
including exploration, cognition, and memory, remains to be
elucidated (9).
Tinnitus is the perception of sound in the absence
of an external acoustic stimulus. It is not clear whether chronic
tinnitus can be solely attributed to damage to the auditory system;
of note, an association between tinnitus and emotional state has
been reported (10–13), indicating that tinnitus may also
result from the dysfunction of non-auditory brain regions such as
the hippocampus (14). The
hippocampus receives either direct or indirect input from the
central auditory system (15),
which in turn receives projections from limbic regions that
modulate neuronal activity and plasticity (16).
Long-term plasticity is dependent on rapid, de
novo protein synthesis. Immediate-early genes (IEGs), including
activity-regulated cytoskeleton-associated protein (Arc/Arg) 3.1
and early growth response gene 1 (Egr-1), were reported to have
major roles in transcription-dependent plasticity (17). The aim of the present study was to
evaluate the expression of these genes, as well as that of
N-methyl D-aspartate (NMDA) receptor subunit 2B (NR2B), in
order to measure the neuronal activity and morphology of neurons in
the hippocampal CA1 area of rats following chronic exposure to
salicylate.
Materials and methods
Animals
Experimental procedures were approved by the Animal
Care and Use Committee of the Nantong University School of Medicine
(Jiangsu, China). A total of 54 male Sprague-Dawley rats (Shanghai
Super-B&K Laboratory Animal Corp. Ltd., Shanghai, China), aged
2–3 months (weight, 250–350 g) were divided into five groups:
(1) Control group (n=12);
(2) acute treatment group
administered a single salicylate injection (n=6); (3) chronic treatment group (S10)
administered daily injections of salicylate for 10 days (n=12);
(4) recovery group (S10+R14) with
a 14-day recovery period following chronic salicylate treatment
(n=6); and (5) recovery group
(S10+R28) with a 28-day recovery period following chronic
salicylate treatment (n=9).
Experimental design and salicylate
administration
Sodium salicylate (Sigma-Aldrich, Shanghai, China)
was dissolved in normal saline [9% (w/v) NaCl] for a final
concentration of 100 mg/ml. Rats in the acute treatment group
received a single intraperitoneal (i.p.) injection of salicylate
(300 mg/kg). Rats were then anesthetized using sodium pentobarbital
(40 mg/kg, i.p.; P3761, Sigma-Aldrich, St. Louis, MO, USA) and
sacrificed 2 h post-treatment. Animals in the chronic treatment
groups were administered i.p. injections of salicylate daily at
08:00 h for 10 consecutive days. Rats in the S10 group were
sacrificed at 08:00 h on day 11, while those in S10+R14 and S10+R28
groups were sacrificed at 08:00 h on days 25 and 39, respectively.
Animals in the control group were administered an i.p. injection of
saline at 08:00 h for 10 consecutive days.
Reverse transcription quantitative PCR
(RT-qPCR)
Rats were sacrificed by decapitation following an
i.p. injection of sodium pentobarbital (40 mg/kg body weight). The
CA1 region of the hippocampus was immediately dissected and total
RNA was extracted using TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer’s
instructions. RNA was quantified by measuring absorbance at 260/280
nm (TENOVO International Co., Ltd., Beijing, China) and
complementary DNA (cDNA) was obtained using a Reverse Transcription
kit (DRR036A; Takara Bio Inc., Otsu, Japan). Primers for Arg3.1,
Egr-1, NR2B and GAPDH were obtained from Shanghai Sangon Biological
Engineering Technology and Services Co., Ltd. (Shanghai, China).
Primer sequences were as follows: Arc forward (F),
5′-CTGCCACAGAAGCAGGGTGA-3′ and Arc reverse (R),
5′-AGGGTGCCCACCACATACTGA-3′; Egr-1-F, 5′-GAACAACCCTACGAGCACCTG-3′
and Egr-1-R, 5′-GCCACAAAGTGTTGCCACTG-3′; NR2B-F,
5′-TGGCTATCCTGCAGCTGTTTG-3′ and NR2B-R,
5′-TGGCTGCTCATCACCTCATTC-3′; and GAPDH-F,
5′-GGCACAGTCAAGGCTGAGAATG-3′ and GAPDH-R,
5′-ATGGTGGTGAAGACGCCAGTA-3′. SYBR Premix Ex Taq (DRR420A; Takara
Bio, Inc., Otsu, Japan) was used as the reaction mixture.
The cycling program was as follows: 95°C for 30 sec;
40 cycles of 95°C for 5 sec, 60°C for 34 sec; and a final
dissociation stage using the ABI 7500 real-time PCR system (Applied
Biosystems, Foster City, CA, USA). The amplification efficiency of
the target and reference (GAPDH) were assumed to be equal. Relative
quantification and calculations were performed using the
comparative threshold (Ct) cycle method (2−ΔΔCt)
(18).
Western blot analysis
Total protein was extracted from tissue samples and
the concentration was determined using an ultraviolet
spectrophotometer (DR/4000UV-VIS; Hach, Loveland, CO, USA).
SDS-PAGE was performed using a 12% polyacrylamide gel to resolve
Arg3.1 and Egr-1, while 8% polyacrylamide was used for NR2B.
Proteins were transferred to polyvinylidene difluoride membranes,
which were incubated in blocking buffer (Tris-buffered saline,
containing 0.1% Tween-20 and 5% skimmed milk powder), and then
incubated with primary antibodies diluted in the same buffer
overnight at 4°C. Following washes in Tris-buffered saline with
0.1% Tween-20, membranes were incubated with secondary antibodies
in blocking buffer for 2 h at room temperature. The primary
antibodies used were rabbit polyclonal anti-Arc (1:1,000, ab23382;
Abcam, Cambridge, MA, USA), rabbit anti-Egr-1 (1:1000, 4153S; Cell
Signaling Technology, Danvers, MA, USA) and rabbit anti-NR2B
(1:1,000, 4212S; Cell Signaling Technology); followed by the
secondary antibody goat anti-rabbit immunoglobulin G horseradish
peroxidase (1:5,000; Jackson ImmunoResearch, Inc., West Grove, PA,
USA). Immunoreactivity was visualized using the SuperSignal West
Pico Chemiluminescent Substrate system (Pierce Biotechnology, Inc.,
Rockford, IL, USA). Band intensity was quantified using Image Lab
software version 3.0 (Bio-Rad, Hercules, CA, USA), and values for
Arc, Egr-1 and NR2B are expressed relative to GAPDH.
Immunohistochemistry
Rats were anesthetized using 2% sodium pentobarbital
and perfused through the ascending aorta with normal saline
followed by 4% (v/v) paraformaldehyde. The brain was removed and
the hippocampal CA1 region was dissected, then embedded in
paraffin. Paraffin blocks were sectioned at 10 µm, sections
were then collected on slides and deparaffinized using xylene prior
to rehydration through a graded alcohol series. Sections were
washed in phosphate-buffered saline (PBS; pH 7.4) with 0.05%
Tween-20 (PBST) and then blocked with 1% normal goat serum in PBS
for 30 min at 37°C. Consecutive sections were incubated with one of
the following primary antibodies: rabbit anti-Arc (1:50, ab23382;
Abcam), rabbit anti-Egr-1 (1:50, 4153S; Cell Signaling Technology)
or rabbit anti-NR2B (1:50, 4212S; Cell Signaling Technology).
Sections were rinsed with PBST and incubated with horseradish
peroxidise-conjugated secondary antibody (goat anti-rabbit IgG,
1:2,000; Sigma-Aldrich, Gillingham, UK) for 1 h at room
temperature. Immunoreactivity was visualized by treating the
sections with 0.015% (v/v) H2O2 in
3,3′-diaminobenzidinetetrahydrochloride/Tris-buffered saline for 10
min at room temperature.
Coronal sections of the hippocampal CA1 region were
visualized using an Axioplan 2 imaging microscope (Carl Zeiss
Microimaging Inc., Thornwood, NY, USA) and analyzed using ImageJ
software version 1.48 (National Institutes of Health, Bethesda, MD,
USA) to quantify the density of immunoreactive neurons as the
number of positive neurons/section. Hippocampal neurons from the
same side of the brain were counted for each sample.
Transmission electron microscopy
(TEM)
In order to determine whether changes in IEG
expression were due to, or produced by, alterations in synaptic
properties, hippocampal CA1 neurons of nine animals (three each
from the control, S10 and S10+R28 groups) were examined using TEM.
Anesthetized rats were perfused via the ascending aorta with 2%
(v/v) glutaraldehyde (Sigma-Aldrich) in saline. Following
perfusion, brains were removed and the hippocampus was dissected,
then washed in 0.1 M phosphate buffer. Tissue samples were immersed
in 2% glutaraldehyde and 1% osmium tetroxide (Sigma-Aldrich) for 2
h at 4°C, then dehydrated in a graded ethanol series. Following
displacement of ethanol with propylene oxide (Sigma-Aldrich), the
tissue was embedded in Epon (Sigma-Aldrich) and sectioned along the
coronal plane with a diamond knife (FernAnclez-hIorln 1953; Ivan
Sorvall, Inc., New York, NY, USA) at a thickness of 70 µm.
The sections were stained with lead citrate and observed using a
CM-120 electron microscope (Philips, Eindhoven, Netherlands).
ImageJ software was used for quantitative analysis of three
sections in the superficial layers of the hippocampal CA1 region,
performed separately for each hemisphere (19). The number of synaptic vesicles,
thickness of the postsynaptic density (PSD), width of the synaptic
cleft and curvature of the synaptic interface were measured
(20).
Statistical analysis
Statistical analyses were performed using SPSS
version 19 (SPSS, Inc., Chicago, IL, USA) and all data are
presented as the mean ± standard deviation. Based on the
distribution of data and homogeneity of variance, the unpaired,
two-tailed Student’s t-test and one-way analysis of variance
followed by Dunnett’s post-hoc tests were used to compare the
results from each group. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
Expression of IEGs in the hippocampal CA1
region
RT-qPCR and western blot analysis were used to
evaluate the expression of IEGs in the hippocampal CA1 region
following treatment with salicylate. mRNA and protein expression of
Arg 3.1 were upregulated in rats subjected to acute and chronic
(S10) salicylate exposure (P<0.05) (Fig. 1). By contrast, levels in the
S10+R14 and S10+R28 groups demonstrated no significant difference
from those of controls. For Egr-1 and NR2B, mRNA and protein levels
were upregulated in the S10 group; however, the two recovery groups
showed no significant difference compared to those of the control
animals (P<0.05). These results therefore indicated that gene
expression returned to baseline levels following cessation of
salicylate treatment.
 | Figure 1Effect of salicylate administration on
the expression of Arg3.1, Egr-1, and NR2B mRNA and protein in the
hippocampal CA1 area. mRNA expression levels for (A) Arg3.1, (B)
Erg-1 and (C) NR2B were quantified using reverse transcription
quantitative polymerase chain reaction. Transcript levels were
normalized to GAPDH protein expression levels of (D) Arg3.1, (E)
Erg-1 and (F) NR2B were quantified using western blot analysis.
GAPDH was used as a loading control. Arg3.1 expression was
upregulated in acute and S10 groups compared to that of the control
groups. Similarly, increased expression of Egr-1 and NR2B was
observed in animals in the S10 group. *P<0.05
compared to controls. Arg3.1, activity-regulated
cytoskeleton-associated protein; Egr-1, early growth response gene
1; NR2B, N-methyl D-aspartate (NMDA) receptor subunit 2B;
CA1, Cornu Ammonis 1; mRNA, messenger RNA; IEG,
immediate-early genes; S10, chronic salicylate treatment group; R#,
number of recovery days. |
In addition, immunohistochemical examination of IEG
expression in the hippocampal CA1 region revealed an increased
number of Arg3.1-, Egr-1- and NR2B-positive neurons in the S10
group compared with that of the control group (Fig. 2).
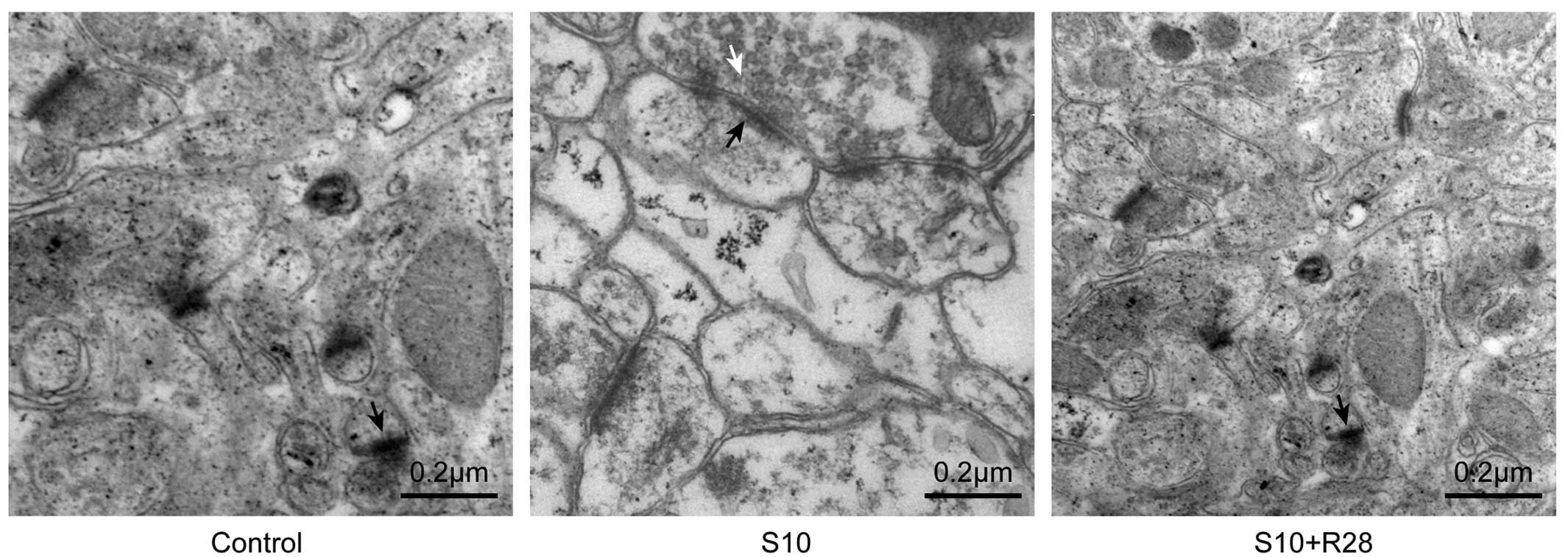
Ultrastructural alterations of
synapses
Examination of synaptic ultrastructure of
hippocampal CA1 neurons revealed an increased number of synaptic
vesicles (P<0.05), thicker PSD (P<0.05) and increased
synaptic interface curvature (P<0.05) in the S10 group compared
to those of the control animals (Fig.
3 and Table I).
 | Table ISynaptic parameters for the different
experimental groups. |
Table I
Synaptic parameters for the different
experimental groups.
| Synaptic
parameters | Controls | S10 | S10+R28 |
|---|
| Vesicles
(number/µm2) | 5±3 | 73±24b | 6±3 |
| Cleft width
(µm) | 0.0083±0.0023 | 0.0088±0.0023 | 0.0082±0.0016 |
| Postsynaptic density
thickness (µm) | 0.035±0.008 | 0.048±0.009a | 0.038±0.011 |
| Curvature | 0.63±0.22 | 0.95±0.21a | 0.68±0.26 |
In conclusion, these results demonstrated that
salicylate induced the upregulation of IEG expression and resulted
in physical alterations to the synaptic structure of hippocampal
CA1 neurons that persisted for the duration of salicylate
exposure.
Discussion
As one of the most commonly used non-prescription
drugs on the market, salicylate and its derivative aspirin, are
able to penetrate the blood-brain barrier; with cerebrospinal
concentration of salicylate reaching several millimolars in an
animal model (21). The results of
the present study demonstrated that long-term administration of
salicylate within this concentration range induces the expression
of Arg3.1, Egr-1, and NR2B. Given the roles of the NMDA receptor in
synaptic plasticity and the role of Arg3.1 in cytoskeletal
remodeling (22), the observed
ultrastructural changes at hippocampal CA1 neuronal synapses in the
salicylate treatment group indicated that these morphological
changes may be a direct consequence of IEG upregulation.
High-intensity noise was reported to induce
hippocampal plasticity and the symptoms of tinnitus by altering the
response of place cells (23).
However, whether salicylate induces plasticity in the hippocampus
remains to be elucidated, although this effect has been observed in
the peripheral and central auditory systems (24). Upregulation of NR2B in the
forebrain of transgenic mice was reported to be associated with the
activation of NMDA receptors, which are critical for regulating the
age-dependent thresholds of plasticity and memory formation
(25). Egr-1 was found to be
essential for the persistence of late-phase long-term potentiation
in the hippo-campus and the consolidation of several forms of
long-term memory (26); Arg3.1 was
reported to have comparable roles in long-term memory consolidation
and synaptic plasticity (27).
Arg3.1 acts as an effector protein at synapses; Arg3.1 mRNA is
trafficked to dendrites and accumulates at sites of synaptic
activity, where it is locally translated and induces homeostatic
scaling of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
receptors and structural modifications (28). Therefore, the results of the
present study, which demonstrated the upregulation of Arg3.1, Egr-1
and NR2B in the hippocampus following chronic salicylate
administration, reflect, in part, the establishment of long-term
plastic changes.
Salicylate was reported to inhibit cochlear
cyclooxygenase and stimulate arachidonic acid production, which
facilitated the NMDA receptor response to glutamate released
spontaneously by inner hair cells (29). In a fear memory consolidation
paradigm, NMDA receptor-mediated alterations in extracellular
signal-regulated kinase/mitogen-activated protein kinase signaling
promoted the transcription and translation of the IEG Egr-1 in
neurons of the lateral nucleus of the amygdale (30). Egr family members regulate Arg3.1
transcription, and Egr-1 activation modulate later phases of
activity-dependent Arg3.1 transcription in the dentate gyrus and
CA1 area of the hippocampus (31).
This therefore indicated that the observed ultrastructural changes
resulting from salicylate treatment were due to NMDA
receptor-mediated activation of Egr-1, followed by upregulation of
Arg3.1 expression and remodeling of cytoskeletal components.
To date, few studies have investigated the time
course of changes occurring in the hippocampus following salicylate
exposure. The present observation that Arg3.1 levels significantly
increased in the acute as well as the chronic treatment groups
suggested that Arg3.1 acts immediately to fine-tune the neuronal
response to activity. However, the transcript and protein levels of
NR2B, Egr-1 and Arg3.1 returned to normal 14 days post-cessation of
salicylate treatment, consistent with the reversible increases in
cochleoneural activity (32),
distortion product otoacoustic emissions (33), and cochlear prestin expression
(34) in the auditory system
reported by previous studies. These fluctuations indicated a
homeostatic mechanism through which the nervous system adapts to
novel stimulation, providing a basis for long-term plasticity.
Salicylate-treated rats had a greater number of
presynaptic vesicles, thicker PSDs and increased synaptic interface
curvature. This suggested that synapses are primed for increased
neurotransmitter release and synaptic transmission, which may
results in greater synaptic efficacy. The observations were
comparable with ultrastructural changes reported by a previous
study, which found large, lucent pleomorphic vesicles in the
synapses of the dorsal cochlear nucleus of the chinchilla following
acoustic trauma (35). Of note,
images from the TEM analysis showed that there were fewer
microtubules and neurofilaments in the synapses of
salicylate-treated animals, which may possibly lead to reduced
vesicular and protein transport to the synapse. Further studies are
required to determine whether this was a compensatory reduction in
response to salicylate-induced hyperactivity.
In conclusion, the results of the present study
demonstrated that chronically administered salicylate produced
transient changes in the expression of IEGs as well as the synaptic
ultrastructure in hippocampal CA1 neurons. Future studies are
required in order to investigate whether these changes are
associated with tinnitus in these animals by employing the
gap-startle paradigm, which could ultimately provide insight into
the role of the hippocampus in the development of tinnitus in
humans.
Acknowledgments
This study was supported by grants from the
Affiliated Hospital of Nantong University Postdoctoral Foundation
(no. 128385) and the Postdoctoral Foundation of Jiangsu Province
(no. 1302083C).
References
|
1
|
Stolzberg D, Salvi RJ and Allman BL:
Salicylate toxicity model of tinnitus. Front Syst Neurosci.
6:282012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gong N, Zhang M, Zhang XB, Chen L, Sun GC
and Xu TL: The aspirin metabolite salicylate enhances neuronal
excitation in rat hippocampal CA1 area through reducing GABAergic
inhibition. Neuropharmacology. 54:454–463. 2008. View Article : Google Scholar
|
|
3
|
Liu Y, Li X, Ma C, Liu J and Lu H:
Salicylate blocks L-type calcium channels in rat inferior
colliculus neurons. Hear Res. 205:271–276. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu SS, Mei L, Chen JY, Huang ZW and Wu H:
Effects of salicylate on the inflammatory genes expression and
synaptic ultrastructure in the cochlear nucleus of rats.
Inflammation. 37:365–373. 2014. View Article : Google Scholar
|
|
5
|
Chen G, Feng L, Liu Z, Sun Y, Chang H and
Cui P: Both central and peripheral auditory systems are involved in
salicylate-induced tinnitus in rats: a behavioral study. PLoS One.
9:e1086592014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen GD, Stolzberg D, Lobarinas E, Sun W,
Ding D and Salvi R: Salicylate-induced cochlear impairments,
cortical hyperactivity and re-tuning, and tinnitus. Hear Res.
295:100–113. 2013. View Article : Google Scholar
|
|
7
|
Basta D and Ernst A: Effects of salicylate
on spontaneous activity in inferior colliculus brain slices.
Neurosci Res. 50:237–243. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang HT, Luo B, Zhou KQ, Xu TL and Chen L:
Sodium salicylate reduces inhibitory postsynaptic currents in
neurons of rat auditory cortex. Hear Res. 215:77–83. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sadegh M, Fathollahi Y and Semnanian S:
The chronic treatment in vivo of salicylate or morphine alters
excitatory effects of subsequent salicylate or morphine tests in
vitro in hippocampus area CA1. Eur J Pharmacol. 721:103–108. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dobie RA: Depression and tinnitus.
Otolaryngol Clin North Am. 36:383–388. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Falkenberg ES and Wie OB: Anxiety and
depression in tinnitus patients: 5-year follow-up assessment after
completion of habituation therapy. Int J Otolaryngol.
2012:3754602012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Langguth B, Landgrebe M, Kleinjung T, Sand
GP and Hajak G: Tinnitus and depression. World J Biol Psychiatry.
12:489–500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zielińska-Bliźniewska H and Olszewski J:
Tinnitus and depression. Otolaryngol Pol. 63:20–23. 2009.In Polish.
View Article : Google Scholar
|
|
14
|
Landgrebe M, Langguth B, Rosengarth K, et
al: Structural brain changes in tinnitus: grey matter decrease in
auditory and non-auditory brain areas. Neuroimage. 46:213–218.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Munoz-Lopez MM, Mohedano-Moriano A and
Insausti R: Anatomical pathways for auditory memory in primates.
Front Neuroanat. 4:1292010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weinberger NM: Associative
representational plasticity in the auditory cortex: a synthesis of
two disciplines. Learn Mem. 14:1–16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng F, Yao H, Bai X, et al:
Platelet-derived growth factor-mediated induction of the synaptic
plasticity gene Arc/Arg3.1. J Biol Chem. 285:21615–21624. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2[-Delta Delta C(T)] Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Juiz JM, Luján R, Domínguez del Toro E,
Fuentes V, Ballesta JJ and Criado M: Subcellular
compartmentalization of a potassium channel (Kv1.4): preferential
distribution in dendrites and dendritic spines of neurons in the
dorsal cochlear nucleus. Eur J Neurosci. 12:4345–4356.
2000.PubMed/NCBI
|
|
20
|
Güldner FH and Ingham CA: Increase in
postsynaptic density material in optic target neurons of the rat
suprachiasmatic nucleus after bilateral enucleation. Neurosci Lett.
17:27–31. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jastreboff PJ, Hansen R, Sasaki PG and
Sasaki CT: Differential uptake of salicylate in serum,
cerebrospinal fluid, and perilymph. Arch Otolaryngol Head Neck
Surg. 112:1050–1053. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maddox SA and Schafe GE: The
activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is
required for reconsolidation of a Pavlovian fear memory. J
Neurosci. 31:7073–7082. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goble TJ, Møller AR and Thompson LT: Acute
high-intensity sound exposure alters responses of place cells in
hippocampus. Hear Res. 253:52–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Panford-Walsh R, Singer W, Rüttiger L, et
al: Midazolam reverses salicylate-induced changes in brain-derived
neurotrophic factor and arg3.1 expression: implications for
tinnitus perception and auditory plasticity. Mol Pharmacol.
74:595–604. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang YP, Shimizu E, Dube GR, et al:
Genetic enhancement of learning and memory in mice. Nature.
401:63–69. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Davis S, Renaudineau S, Poirier R, Poucet
B, Save E and Laroche S: The formation and stability of recognition
memory: what happens upon recall? Front Behav Neurosci. 4:1772010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Plath N, Ohana O, Dammermann B, et al:
Arc/Arg3.1 is essential for the consolidation of synaptic
plasticity and memories. Neuron. 52:437–444. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shepherd JD, Rumbaugh G, Wu J, et al:
Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors.
Neuron. 52:475–484. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ruel J, Chabbert C, Nouvian R, et al:
Salicylate enables cochlear arachidonic-acid-sensitive NMDA
receptor responses. J Neurosci. 28:7313–7323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ota KT, Monsey MS, Wu MS, Young GJ and
Schafe GE: Synaptic plasticity and NO-cGMP-PKG signaling
coordinately regulate ERK-driven gene expression in the lateral
amygdala and in the auditory thalamus following Pavlovian fear
conditioning. Learn Mem. 17:221–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Penke Z, Chagneau C and Laroche S:
Contribution of Egr1/zif268 to activity-dependent Arc/Arg3.1
transcription in the dentate gyrus and area CA1 of the hippocampus.
Front Behav Neurosci. 5:482011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cazals Y, Horner KC and Huang ZW:
Alterations in average spectrum of cochleoneural activity by
long-term salicylate treatment in the guinea pig: a plausible index
of tinnitus. J Neurophysiol. 80:2113–2120. 1998.PubMed/NCBI
|
|
33
|
Huang ZW, Luo Y, Wu Z, Tao Z, Jones RO and
Zhao HB: Paradoxical enhancement of active cochlear mechanics in
long-term administration of salicylate. J Neurophysiol.
93:2053–2061. 2005. View Article : Google Scholar
|
|
34
|
Yang K, Huang ZW, Liu ZQ, Xiao BK and Peng
JH: Long-term administration of salicylate enhances prestin
expression in rat cochlea. Int J Audiol. 48:18–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Du X, Chen K, Choi CH, et al: Selective
degeneration of synapses in the dorsal cochlear nucleus of
chinchilla following acoustic trauma and effects of antioxidant
treatment. Hear Res. 283:1–13. 2012. View Article : Google Scholar
|