Introduction
Gastric cancer (GC) remains a significant threat to
human life, although significant progress in the diagnosis and
treatment of this disease has been achieved (1). As with all solid tumors, GC is
thought to initiate and progress through a series of genetic
alterations. Recently, increasing attention has been focused on
gene therapy.
Inhibitor of apoptosis-stimulating protein of p53
(iASPP) acts as a negative regulator of p53 function, inhibiting
p53 by directly binding to its DNA-binding domains (2,3). The
p53-binding region of iASPP has a certain similarity with that of
its other family members, p63 and p73 (4). Previous studies have revealed that
iASPP may also interact with p63 and p73 and affect their function
(5). Furthermore, iASPP, also
known as RelA-associated inhibitor, is able to regulate the
function of nuclear factor-κB (NF-κB) (6,7).
iASPP, the only homologue of the ASPP family, was
identified as an oncogene by detection of abnormal overexpression
of iASPP in several types of human cancer, including breast
carcinomas (8,9), acute leukemia (10), lung cancer (11) and hepatocellular carcinoma
(12). These data indicated that
iASPP may be important in the development of tumors in humans.
However, only a few studies have investigated the role of iASPP in
human GC (13). In the present
study, the expression of iASPP in GC tissues and GC cell lines was
analyzed and then the potential role of iASPP in the GC cell lines
was examined in vivo and in vitro.
Materials and methods
Tissue samples and
immunohistochemistry
GC tissue (46 samples) and the adjacent normal
gastric mucosal tissue (30 samples) were collected from patients
who underwent surgery at the Second Affiliated Hospital of
Chongqing Medical University (Chongqing, China) between September
2012 and March 2014. All GC tissues were confirmed by pathological
examination, and the adjacent normal gastric tissues were obtained
from 5 cm away from the GC tissues. Informed consent was obtained
from the patients and the present study received approval from the
Institutional Review Board of the Second Affiliated Hospital of
Chongqing Medical University. The present study was conducted in
accordance with the ‘Biomedical Research Involving Human Ethics
Review (Tentative)’ regulation of the Ministry of Health and the
Declaration of Helsinki on Ethical Principles for Medical Research
Involving Human Subjects.
The expression of iASPP protein in the samples was
detected using immunohistochemistry. The GC tissue and adjacent
normal gastric mucosal tissue samples were paraffin-embedded
(Sigma-Aldrich, St. Louis, MO, USA) and 4-µm sections were
prepared. The sections were incubated with 3%
H2O2 (Sigma-Aldrich) for 10 min at room
temperature to eliminate endogenous peroxidase activity.
Subsequently, the sections were incubated with monoclonal mouse
iASPP antibody (ab49805; 1/1,000; Abcam, Cambridge, UK) for 90 min
at 37°C and with a peroxidase-conjugated goat anti-mouse
immunoglobulin IgG (SC-2005; 1:500; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) for 20 min at room temperature.
3,3′-diaminobenzidine reagent (Sigma-Aldrich) was added onto each
section, and subsequently, counter-staining was performed with
hematoxylin (Sigma-Aldrich). The sections were then dehydrated in
graded alcohol (50, 70, 85, 95 and 100%) and xylene
(Sigma-Aldrich), cleared in distilled water and mounted with
neutral gum (Bioworld Technology, Inc., St. Louis Park,. MN,
USA).
Cell lines
The GC cell lines (MKN45, BGC-823 and SGC-7901) were
purchased from the American Type Culture Collection (Manassas, VA,
USA). The GES-1 cell line was obtained from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
The GC cell lines were routinely maintained in RPMI 1640 medium
supplemented with 10% fetal bovine serum (Gibco Life Technologies,
Carlsbad, CA, USA) without antibiotics in a humidified atmosphere
of 5% CO2 at 37°C. The GES-1 cell line was cultured in
Dulbecco’s modified Eagle’s medium containing 10% fotal bovine
serum (Gibco Life Technologies) and 10 mg/ml vancomycin (Santa Cruz
Biotechnology, Inc.).
Lentivirus transfection
Downregulation of iASPP was achieved by infecting
the cells with the iASPP-small interfering (si)RNA lentivirus
(Genepharma Co., Ltd., Shanghai, China). Target cells were plated
in six-well plates at 20–30% confluence and incubated for 12 h
prior to the infections with iASPP-small interfering (si)RNA- or
scrambled control-siRNA-expressing lentiviruses (Genepharma Co.,
Ltd.). When the infections were performed, the culture medium was
replaced with a supernatant fluid, which contained an appropriate
viral titer (1 ml/well). After incubating at 37°C for 12 h, the
viral supernatant was replaced with fresh media. The infected cells
were selected using puromycin (2 mg/ml; Santa Cruz Biotechnology,
Inc.) following incubation for 48 h. Successful infection was
confirmed via expression of green fluorescent protein as confirmed
using an inverted fluorescence microscope (Leica DMI4000 B; Leica
Microsystems GmbH, Wetzlar, Germany). The knockdown efficiency was
determined using western blot analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the GES-1, MKN-45,
SGC-7901 and BGC-823 cells using the RNAiso reagent (Takara Bio,
Inc., Otsu, Japan) according to the manufacturer’s instructions.
The RNA was reverse-transcribed into cDNA using the PrimeScript II
First Strand cDNA synthesis kit (Takara Bio, Inc.). RT-qPCR was
performed using LightCycler real-time PCR with the SYBR Premix Ex
Taq™ kit for Perfect Real-Time (Takara Bio, Inc.). Primers were
purchased from Takara Bio., Inc., and the sequences for PCR
amplification of the iASPP gene were as follows: Forward,
5′-GCGGTGAAGGAGATGAACGA-3′ and reverse,
5′-TGATGAGGAAATCCACGATAGAGTAG-3′. The primer sequences for the
internal control β-actin were as follows: Forward,
5′-CCACGAAACTACCTTCAACTCC-3′ and reverse,
5′-GTGATCTCCTTCTGCATCCTGT-3′. The PCR cycling conditions were as
follows: 94°C for 60 sec, followed by 40 cycles of 94°C for 40 sec,
60°C for 40 sec and 6 min extension at 72°C. The relative gene
expression levels were calculated using the 2−ΔΔCT
method.
Protein preparation and western
blotting
The target cells were washed twice with
phosphate-buffered saline (PBS), harvested in
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China) and flash-frozen on dry ice.
Following allowing the cells to thaw, the lysates were collected
with a rubber scraper, sonicated and centrifuged at 12,000 × g (4°C
for 20 min). The total protein concentration was measured using a
Pierce bicinchoninic acid protein assay kit (Pierce Biotechnology,
Inc., Rockford, IL, USA). To perform the western blot analysis,
proteins were resolved using 10% SDS-PAGE (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and transferred onto a polyvinylidene
difluoride membrane (Merck Millipore, Darmstadt, Germany).
Subsequently, the membranes were blocked for 1 h with 5% non-fat
milk at room temperature and then incubated overnight at 4°C with
the iASPP and β-actin primary antibodies. The secondary antibody
was goat anti-mouse IgG conjugated to horseradish peroxidase
(1:3,000). The signal was detected using an enhanced
chemiluminescence reagent (EMD Millipore, Billerica, MA, USA). To
analyze the iASPP protein levels, monoclonal mouse antibodies
against the iASPP protein (828 amino acids, 92 kDa, 1:5,000, Abcam)
were used. For the loading control, a monoclonal mouse β-actin
antibody (A5441; 42 kDa, 1:5,000, Sigma-Aldrich) was used.
Cell viability and colony formation
assays
The effect of iASPP-siRNA on cell proliferation was
detected using an MTT assay. The target cells were seeded into
96-well plates at a density of 1×104 cells/well. An MTT
solution (5 mg/ml MTT, 20 ml; Sigma-Aldrich) was added to the
cultures (total volume of 200 ml) and incubated for 4 h at 37°C.
Following removal of the culture medium, the remaining crystals
were dissolved in dimethyl sulfoxide (Sigma-Aldrich) and the
absorbance at 560 nm was measured using a Multiskan MK3 microplate
reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
The effect of iASPP-siRNA on the colony forming
ability of the target cells was detected using a colony formation
assay. To perform this assay, the target cells were seeded in
six-well plates at a low density (1,000 cells/plate) and cultured
until visible colonies appeared. The colonies were then stained
with Giemsa stain (Santa Cruz Biotechnology, Inc.) and were
counted.
Detection of apoptosis
To further elucidate the association between iASPP
and GC cells, the rate of cell apoptosis was determined using flow
cytometry. The target cells were collected and washed twice with
ice-cold PBS buffer. The apoptosis rate of cells was detected with
an Annexin V-FITC Apoptosis Detection kit (eBioscience, Inc., San
Diego, CA, USA) and propidium iodide (Sigma-Aldrich) double
staining according to the manufacturer’s instructions. Flow
cytometric analysis was performed using the BD LSRI flow cytometer
(BD Biosciences, Franklin Lakes, NJ, USA) and data were analyzed
using the CellQuest 5.1 software (BD Biosciences).
Xenograft experiment
Male athymic nude mice (6–8 weeks old), were
obtained from the Animal Experimental Centre of Chongqing Medical
University. To establish the GC model, equal numbers of MKN-45
cells (1×106) infected with iASPP-siRNA or control-siRNA
lentivirus were injected subcutaneously into the right rear flank
of each mouse (four mice per group). Tumor growth was observed
daily in each group. The tumor volume was calculated as
(LxS2)/2 where L is the longest tumor axis and S is the
shortest tumor axis. At four weeks following injection, all mice
were sacrificed via anesthesia using sodium pentobarbital
(Sigma-Aldrich), then subjected to cervical dislocation, the
xenografts were then resected from the mice and flash frozen in
liquid nitrogen for further analysis. The present study was
conducted in strict accordance with the recommendations of the
Guide for the Care and Use of Laboratory Animals of Chongqing
Medical University. The protocol was approved by the Committee on
the Ethics of Animal Experiments of Chongqing Medical University.
All surgical procedures were performed under sodium pentobarbital
(Sigma-Aldrich) anesthesia and all efforts were made to minimize
suffering.
Statistical analysis
All experiments were repeated three times. Data were
analyzed using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA).
Values are expressed as the mean ± standard deviation. The
statistical significance of the differences among the groups was
evaluated using a t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
iASPP is upregulated in GC tissues and
cell lines
According to the results of the immunohistochemical
analysis, the expression of iASPP in GC samples was significantly
upregulated in GC tissues compared with that in their adjacent
normal tissues (cells with brown staining in the cytoplasm or
nucleus were regarded as iASPP-positive cells) (Fig. 1). According to the RT-qPCR and
western blot analyses, the expression levels of iASPP were higher
in the MKN-45, BGC-823 and SGC-7901 cell lines compared with those
in the GES-1 cell line, illustrating that iASPP may be associated
with the development of GC (Fig.
1). The expression of iASPP was higher in the MKN-45 and
SGC-7901 cell lines compared with that in the other cell lines;
therefore, these two cell lines were selected as the target cells
for subsequent experiments. The MKN-45 cell line contains the
wild-type p53 gene (14), whereas
the SGC-7901 cell line carries a mutated p53 gene (15).
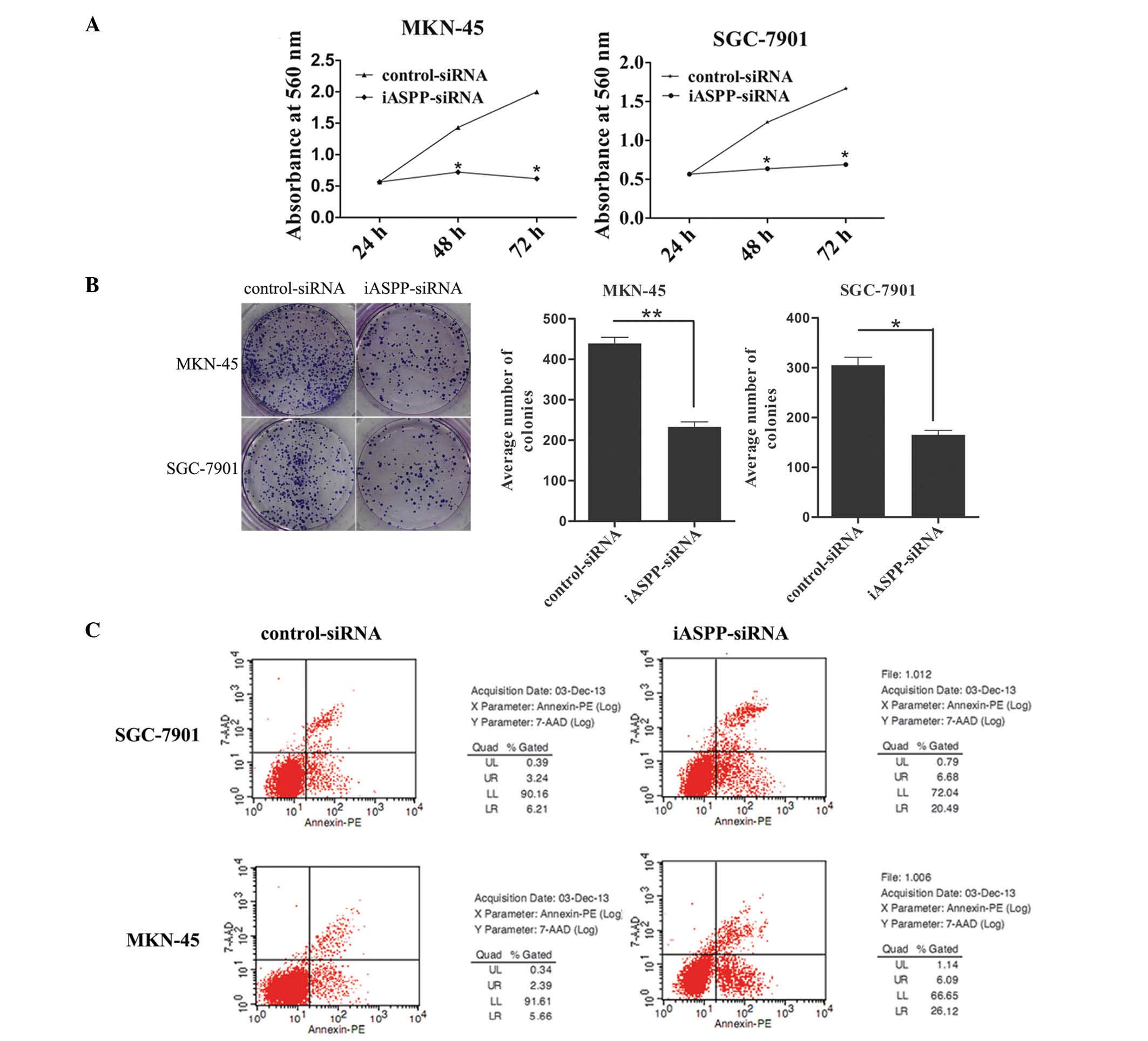
Inhibition of iASPP expression inhibits
proliferation and colony forming ability and promotes apoptosis in
GC cells
To examine the functional significance of iASPP in
GC, GC cell lines (MKN-45 and SGC-7901) were infected with
lentivirus containing iASPP-siRNA or scrambled control siRNA.
Western blotting was performed to assess iASPP protein levels.
Infection of cells with lentivirus containing iASPP-siRNA
significantly reduced iASPP protein expression levels in the MKN-45
and SGC-7901 cells (Fig. 2). By
contrast, the control siRNA had no effect on iASPP protein levels.
As shown in Fig. 3, the decreased
expression of iASPP reduced the proliferation and colony forming
ability of cells. The iASPP-siRNA lentivirus-infected cells formed
fewer colonies compared with the control-siRNA lentivirus-infected
cells. Flow cytometric analysis revealed that decreased expression
of iASPP enhanced the levels of cell apoptosis.
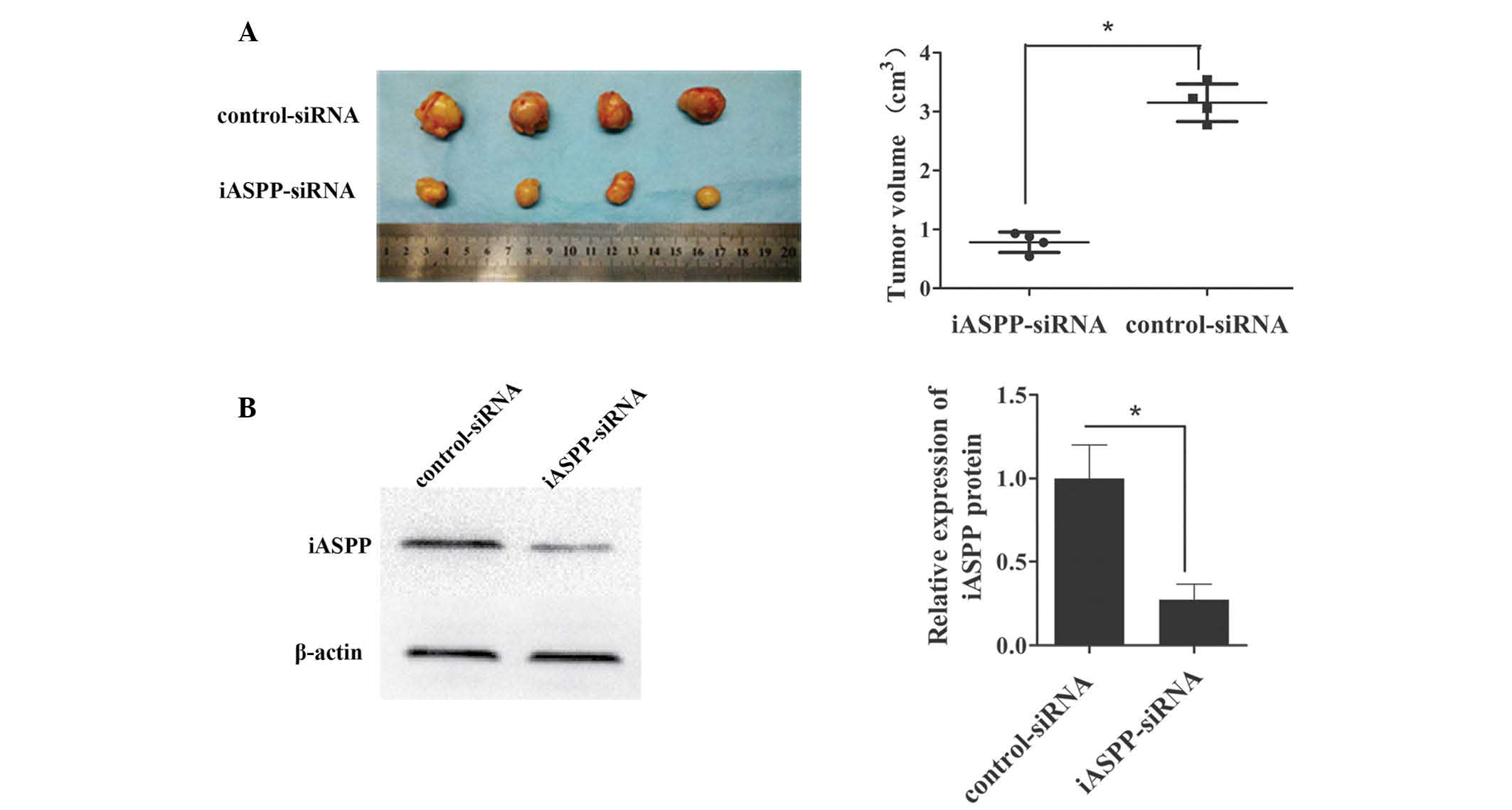
Inhibition of iASPP decreases tumor
growth in vivo
To investigate the role of iASPP in tumor growth
in vivo, nude mice were subcutaneously injected with an
equal quantity of MKN-45 cells, which were transfected with
iASPP-siRNA lentivirus or control-siRNA lentivirus (106
cells/mouse). Tumors appeared in all mice. As shown in Fig. 4, forced downregulation of iASPP
significantly inhibited tumor growth in vivo. iASPP
expression in the xenograft tumors was measured using western
blotting and it was identified that iASPP expression was
significantly decreased in the tumor cells transfected with the
iASPP-siRNA lentivirus as compared that in the control tumors.
Discussion
iASPP is an evolutionarily conserved inhibitor of
p53, and overexpression of iASPP has been observed in several types
of human cancer (8–12). The present study examined GC cell
lines and tumor samples to demonstrate that the expression levels
of iASPP were higher in GC tissues and GC cell lines compared with
those in their adjacent normal tissues and normal gastric mucosal
cells. The present study suggested that abnormal expression of
iASPP may be an important step in the development of GC and it may
therefore be a useful molecular marker for the diagnosis of GC.
Li et al (16) observed that downregulation of iASPP
is able to inhibit proliferation of the p53-mutant glioblastoma
cell line U251. Zhang et al (17) demonstrated that a reduction of
iASPP inhibited cell growth and induced apoptosis in p53-defective
prostate cancer cells. Lin et al (18) reported that small hairpin
RNA-mediated downregulation of iASPP repressed hepatocellular
carcinoma cell proliferation and colony formation in vitro
and inhibited the growth of tumors in vivo. Inhibition of
iASPP also induced apoptosis in breast cancer cells (19). To the best of our knowledge, no
studies have previously investigated the potential role of iASPP in
the proliferation and apoptosis of GC cell lines. Therefore, in the
present study, the expression of iASPP was inhibited via
transfection with an iASPP-siRNA lentivirus. Following the
transfection, the proliferation, colony formation and apoptotic
rate were assessed. The results revealed that following the
downregulation of iASPP expression using iASPP-siRNA, the two cell
lines exhibited a reduction in the proliferation and colony forming
ability. This indicated that knockdown of iASPP is able to
significantly inhibit the growth of GC cells and may therefore be a
useful approach for anti-tumor therapy. In addition, knockdown of
iASPP expression induced apoptosis in the two GC cell lines, which
indicates an oncogenic function of iASPP, as an imbalance between
proliferation and apoptosis contributes to the formation and
development of human tumors (20).
Additionally, an in vivo xenograft experiment identified
that tumor growth was significantly inhibited by knockdown of
iASPP. Thus, it was concluded that iASPP may act as a potential
oncogene in GC and that iASPP may be an effective target in the
treatment of GC.
p53 is critical in apoptosis, having a high
frequency of mutations in various types of human cancer (21). However, mutations in the p53 gene
do not appear to be a necessary event in human carcinomas.
Wild-type p53 is retained in ~50% of human tumors (22); however, its tumor suppressive
function appears to be inhibited in tumor cells. The identification
of the ASPP family provided novel insight into the mechanism
underlying the suppression of p53 activity in cancer cells. In the
present study, overexpression of iASPP in the p53 wild-type MKN-45
cell line inhibited the apoptotic function of p53, promoting the
progression of GC. The present study revealed that expression of
iASPP in p53 mutant SGC-7901 cells was also upregulated. However,
iASPP is unable to interact with the mutated form of p53 (8). This finding raises questions
regarding the mechanism of action of the iASPP gene. iASPP has been
observed to bind to the NF-κB subunit RELA/p65 and inhibit its
transcriptional activity, which has important roles in the control
of cell proliferation and apoptosis (6,7).
Furthermore, iASPP may also interact with p63 and p73 and affect
their functions (4). Dissimilar to
p53, p63 and p73 are not commonly mutated in human tumors (23,24).
In conclusion, the present study suggested that
iASPP may have an oncogenic function in GC. The results also
indicated that inhibition of iASPP is important in the
downregulation of cell proliferation and the activation of
apoptosis. These findings indicated that iASPP may be a potential
target for GC therapy. However, the specific mechanism whereby
iASPP affects the biological behavior of tumor cells remains to be
fully elucidated and its upstream and downstream factors remain to
be identified. Further genetic studies are required to examine the
signals of iASPP that are able to regulate the biological behavior
of cancer cells.
Acknowledgments
The authors would like to thank the Second
Affiliated Hospitals of Chongqing Medical University (Chongqing,
China) for providing gastric tissue specimens. In addition, the
authors would like to thank their mentor, Dr Xiao-Qiu Xiao
(Institute for Biological Sciences, Chongqing Medical University),
for his support, incisive comments and useful suggestions. The
present study was supported by the Research Projects of the
Chongqing Municipal Health Bureau (grant no. 2013-1-022).
References
|
1
|
Takahashi T, Saikawa Y and Kitagawa Y:
Gastric cancer: current status of diagnosis and treatment. Cancers
(Basel). 5:48–63. 2013. View Article : Google Scholar
|
|
2
|
Samuels-Lev Y, O’Connor DJ, Bergamaschi D,
et al: ASPP proteins specifically stimulate the apoptotic function
of p53. Mol Cell. 8:781–794. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laska MJ, Vogel UB, Jensen UB and Nexø BA:
p53 and PPP1R13L (alias iASPP or RAI) form a feedback loop to
regulate genotoxic stress responses. Biochim Biophys Acta.
1800:1231–1240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murray-Zmijewski F, Lane DP and Bourdon
JC: p53/p63/p73 isoforms: an orchestra of isoforms to harmonise
cell differentiation and response to stress. Cell Death Differ.
13:962–972. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cai Y, Qiu S, Gao X, Gu SZ and Liu ZJ:
iASPP inhibits p53-independent apoptosis by inhibiting
transcriptional activity of p63/p73 on promoters of proapoptotic
genes. Apoptosis. 17:777–783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang JP, Hori M, Sanda T and Okamoto T:
Identification of a novel inhibitor of nuclear factor-kappaB,
RelA-associated inhibitor. J Biol Chem. 274:15662–15670. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Notari M, Hu Y, Koch S, et al: Inhibitor
of apoptosis-stimulating protein of p53 (iASPP) prevents senescence
and is required for epithelial stratification. Proc Natl Acad Sci
USA. 108:16645–16650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bergamaschi D, Samuels Y, O’Neil NJ, et
al: iASPP oncoprotein is a key inhibitor of p53 conserved from worm
to human. Nat Genet. 33:162–167. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang C, Gao CF, Chen Y, Yin J, Wang P and
Lv X: Expression pattern of the apoptosis-stimulating protein of
p53 family in p53+ human breast cancer cell lines.
Cancer Cell Int. 13:1162013. View Article : Google Scholar
|
|
10
|
Liu ZJ, Zhang Y, Zhang XB and Yang X:
Abnormal mRNA expression of ASPP members in leukemia cell lines.
Leukemia. 18:8802004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen J, Xie F, Zhang L and Jiang WG: iASPP
is over-expressed in human non-small cell lung cancer and regulates
the proliferation of lung cancer cells through a p53 associated
pathway. BMC Cancer. 10:6942010. View Article : Google Scholar
|
|
12
|
Lu B, Guo H, Zhao J, et al: Increased
expression of iASPP, regulated by hepatitis B virus X
protein-mediated NF-κB activation, in hepatocellular carcinoma.
Gastroenterology. 139:2183–2194. 2010. View Article : Google Scholar
|
|
13
|
Meng WD, Chu RX, Wang BZ, et al:
Helicobacter pylori infection and expressions of apoptosis-related
proteins p53, ASPP2 and iASPP in gastric cancer and precancerous
lesions. Pathol Biol (Paris). 61:199–202. 2013. View Article : Google Scholar
|
|
14
|
Yokozaki H: Molecular characteristics of
eight gastric cancer cell lines established in Japan. Pathol Int.
50:767–777. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xue Z, Yan H, Li J, et al: Identification
of cancer stem cells in vincristine preconditioned SGC7901 gastric
cancer cell line. J Cell Biochem. 113:302–312. 2012. View Article : Google Scholar
|
|
16
|
Li GL, Wang RZ, Gao J, et al: RNA
interference-mediated silencing of iASPP induces cell proliferation
inhibition and G0/G1 cell cycle arrest in U251 human glioblastoma
cells. Mol Cell Biochem. 350:193–200. 2011. View Article : Google Scholar
|
|
17
|
Zhang B, Xiao HJ, Chen J, Tao X and Cai
LH: Inhibitory member of the apoptosis-stimulating protein of p53
(ASPP) family promotes growth and tumorigenesis in human
p53-deficient prostate cancer cells. Prostate Cancer Prostatic Dis.
14:219–224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin BL, Xie DY, Xie SB, Xie JQ, Zhang XH,
Zhang YF and Gao ZL: Down-regulation of iASPP in human
hepatocellular carcinoma cells inhibits cell proliferation and
tumor growth. Neoplasma. 58:205–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu ZJ, Cai Y, Hou L, et al: Effect of RNA
interference of iASPP on the apoptosis in MCF-7 breast cancer
cells. Cancer Invest. 26:878–882. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu X: p53: a heavily dictated dictator of
life and death. Curr Opin Genet Dev. 15:27–33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki K and Matsubara H: Recent advances
in p53 research and cancer treatment. J Biomed Biotechnol.
2011:9783122011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Patel S, George R, Autore F, Fraternali F,
Ladbury JE and Nikolova PV: Molecular interactions of ASPP1 and
ASPP2 with the p53 protein family and the apoptotic promoters PUMA
and Bax. Nucleic Acids Res. 36:5139–5151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bergamaschi D, Samuels Y, Jin B,
Duraisingham S, Crook T and Lu X: ASPP1 and ASPP2: common
activators of p53 family members. Mol Cell Biol. 24:1341–1350.
2004. View Article : Google Scholar : PubMed/NCBI
|