Introduction
Osteoarthritis (OA) is a common degenerative and
inflammatory joint disease (1). To
date, no drugs are available which are able to structurally modify
OA processes or prevent progression of the disease (2). Mesenchymal stem cells (MSCs), differ
from the single-effect function of drugs and secrete numerous
bioactive agents that inhibit inflammation, suppress immune
recognition and stimulate host progenitors to divide and
differentiate into functional regenerative units (3). MSCs have been widely used in
cartilage regeneration therapies (4), and certain animal studies and initial
clinical studies have demonstrated the potential of MSC
implantation as an alternative treatment for OA (5–10).
Interleukin-1β (IL-1β), one of the most significant
pro-inflammatory cytokines involved in OA, diminishes the
expression of type II collagen (Col2) and aggrecan through the
expression of matrix metalloproteinases (MMPs) and cyclooxygenase-2
(COX-2) (11,12). This effect is mediated by the
mitogen-activated protein kinases (MAPKs) and nuclear factor-κB
(NF-κB) pathways, which have been described as the link between
inflammation and joint cartilage degeneration in OA (13–15).
Despite the implication of MSCs in the regulation of
inflammation, little is known regarding the effects of MSCs on
IL-1β-stimulated rat chondrocytes and intracellular signaling
pathways. The present study therefore aimed to investigate the
effects and mechanism of MSCs on IL-1β-treated rat chondrocytes and
on cartilage in a rat model of OA induced by anterior cruciate
ligament transection and medial meniscectomy. The effects of MSCs
on IL-1β-treated chondrocytes and cartilage in this model were
evaluated by analyzing the expression of Col2, aggrecan, MMP-13 and
COX-2, as well as the activation of extracellular signal-regulated
kinases 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), p38 MAPK and
NF-κB pathways.
Materials and methods
Ethical approval
All experimental procedures involving animals were
conducted in accordance with the National Institutes of Health
Guidelines for the Care and Use of Laboratory Animals (www.nap.edu/openbook.php?record_id=5140) and were
approved by the Committee for the Administration of Experimental
Animals, Nanjing Medical University (Nanjing, China). The
experiments were additionally approved by the Animal Ethical And
Welfare Committee of Nanjing Medical University.
Preparation of MSCs
Sprague-Dawley (SD) rat bone marrow MSCs were
harvested and cultured as described previously (16). Briefly, the femurs and tibias were
dissected away from the attached soft tissue under aseptic
conditions and the epiphyses were removed. The bone marrow was
flushed out with phosphate-buffered saline (PBS) containing heparin
(Gibco Life Technologies, Carlsbad, CA, USA). The mixture was
separated by density centrifugation through lymphocyte separation
solution (1.073 g/ml; Gibco Life Technologies) at 1000 × g for 15
min at 24°C. Subsequently the mononuclear fraction interphase was
collected, resuspended in Dulbecco’s modified Eagle’s medium
(DMEM)/F12 (Gibco Life Technologies) supplemented with 10% fetal
bovine serum (FBS), 100 U/ml penicillin G and 100 µg/ml
streptomycin (all from Gibco Life Technologies) and seeded into
culture flasks (cultivation area, 25-cm2) containing 5
ml culture medium (DMEM/F12). The cells were cultured at 37°C in 5%
CO2 for three days, following which the non-adherent
cell population was removed. The medium was subsequently changed
every 48 h, and cells of passage three were induced to
differentiate into adipogenic [DMEM/F12, 10% FBS, 1 µmol/l
dexamethasone (DEX), 0.5 mmol/l : 3-isobutyl-1-methylxanthine, 5
mg/l insulin, 100 µmol/l indomethacin; (17)], osteogenic [DMEM/F12, 10% FBS,
100nmol/l DEX, 10 mmol/l β-sodium glycerophosphate, 50 µg/ml
vitamin C (Vc); (17)] and
chondrogenic [DMEM/F12, 10% FBS and 1% insulin-transferrin-selenium
all purchased from Sigma-Aldrich (San Francisco, CA, USA), and 10
ng/ml transforming growth factor (TGF)-β1 (Gibco Life
Technologies), 0.1 µmol/l DEX, 50 µg/ml Vc; (18)] lineages.
Chondrocyte culture and treatment
Chondrocytes were harvested from rat articular
cartilage as described previously (19). In brief, under sterile conditions,
cartilage tissues derived from the limb joints of 2-week-old SD
rats were cut into small sections (<1 mm3) and
digested with 0.2% trypsin (Gibco Life Technologies) and 0.2% type
II collagenase (Gibco Life Technologies) for 30 min and 2 h,
respectively. The isolated chondrocytes were resuspended in
DMEM/F12 supplemented with 10% FBS, 100 U/ml penicillin G and 100
µg/ml streptomycin. The culture medium was changed every
other day. Following passage two, the cells reached ~70–80%
confluence and the medium was replaced with DMEM/F12 supplemented
with 0.5% FBS and antibiotics for 12 h. Following synchronization,
10 ng/ml IL-1β (R&D Systems, Minneapolis, MN, USA) was added to
the culture medium and the cells were cultured for a further 24 h.
At the end of this treatment period, the cells were cultured with
normal medium for 3, 6 and 12 days (for analysis of Col2 and
aggrecan) or for 1, 2 and 4 days (for COX-2 and MMP-13 analysis).
Total RNA and protein were collected for reverse
transcription-quantitative polymerase chain reaction and western
blot analysis. Proteins were extracted from cells by washing
chondrocyte monolayers three times with PBS, and extracting whole
cell proteins by incubation with lysis buffer (50 mM Tris/HCl pH
7.2, 150 mM NaCl, l% (v/v) Triton X-100, 1 mM sodium orthovanadate,
50 mM sodium pyrophosphate, 100 mM sodium fluoride, 0.01% (v/v)
aprotinin, 4 µg/ml pepstatin A, 10 µg/ml leupeptin
and 1 mM phenylmethanesulfonyl fluoride, all purchased from
Beyotime Institute of Biotechnology, Jiangsu, China) on ice for 30
min and removal of cell debris by centrifugation. The supernatants
were then stored at −70°C. Proteins were extracted from cartilage
by crushing the tissue, then incubating the tissue with lysis
buffer as described above. RNA was collected according to the
protocol outlined at www.biomart.cn/experiment/430/443/728/65628.htm.
Additionally, following serum starvation, IL-1β (10 ng/ml) was
added to the culture medium for 15, 30 or 60 min to investigate
ERK1/2, JNK and p38 MAPK signaling, as well as IκBα and NF-κB p65.
Controls were cultured in DMEM/F12 with 0.5% FBS without IL-1β.
Protein was collected for western blot analysis.
Co-culture
For co-culture without direct cell-cell contact,
passage three MSCs were seeded onto Transwell inserts (six-well
plates; BD Biosciences, Franklin Lakes, NJ, USA) with a
0.4-µm porous membrane (BD Biosciences) and lowered into
wells seeded with passage two chondrocytes. Following IL-1β
stimulation, the chondrocytes were cultured with or without MSCs
for three days (for analysis of Col2 and aggrecan) or for one day
(for COX-2 and MMP-13 analysis). The number of chondrocytes and
MSCs used was 3.0×104 and 1.5×104,
respectively, and the ratio was that previously identified as the
optimal ratio (19). Total RNA and
protein were collected for reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blot analysis. In
addition, subconfluent monolayers of IL-1β-induced chondrocytes
were cultured with or without MSC-conditioned medium (5 ml) for 15
min (for analysis of IκBα and NF-κB p65) or for 30 min (for ERK1/2,
JNK and p38 MAPK signaling analysis). The MSCs and IL-1β-induced
chondrocytes were co-cultured for 24 h and the medium was collected
as MSC-conditioned medium. Protein was collected for western blot
analysis. The incubation times were based on previous analysis of
cultured chondrocytes by our group (20).
Animal study
Thirty-six male SD rats (Central Lab Animal Inc.,
Nanjing Medical University, Nanjing, China), weighing 300–325 g,
were randomly divided into three groups (n=12 per group) as
follows: Group A, the sham operation group; group B, the vehicle
(PBS)-treated group and group C, the MSC-treated group. The rats
were maintained under the following conditions: 24°C with 50%
humidity; 7 am –7 pm light; 40–60 g/day food (18% protein, 4% fat,
5% fiber, 8% ash, 10% water) and 50–70 ml/day water. Surgical
procedures were performed as described previously (21). Briefly, the 24 animals in groups B
and C underwent open surgery under 2% sodium pentobarbital
anesthesia (0.2 ml/100 g) on their right knees, involving anterior
cruciate ligament transection and medial meniscectomy via an
incision on the medial aspect of the joint capsule, anterior to the
medial collateral ligament. The animals in group A underwent a sham
operation on their right knees, in which a similar incision was
made but the anterior cruciate ligament transection and medial
meniscectomy were not performed. Following surgery, all the rats
were intramuscularly administered antibiotics for three days and
were allowed free activity without immobilization. Eight weeks
post-surgery, the animals of group C received an intra-articular
injection of 0.3 ml allogeneic MSCs (~5.0×105 cells)
into the right knee, while 0.3 ml PBS was injected into the right
knees of groups A and B as a control. All rats were sacrificed by
cervical dislocation at 14 weeks post-surgery. Half of the rats in
each group were used for histology and the remainder were used for
western blot analysis.
The femoral condyles were retrieved and fixed in 10%
neutral-buffered formalin (Shenzhen Ketian Plastic Co., Ltd.,
Shenzhen, China) at 4°C for 48 h, then decalcified with EDTA
(Sigma-Aldrich, St. Louis, MO, USA) for three weeks. The
decalcified specimens were subsequently dehydrated in alcohol
(Shenzhen Ketian Plastic Co., Ltd.), embedded in paraffin blocks
(Shenzhen Ketian Plastic Co., Ltd.) and cut into 5-µm
sections. Serial sections, including the severely degenerated area,
were stained with hematoxylin and eosin (HE), Safranin-O and
toluidine blue (TB) (All from Sigma-Aldrich) using standard methods
(http://protocolsonline.com/histology/dyes-and-stains/haematoxylin-eosin-he-staining/;
https://www.medialabinc.net/spg531612/). The degree of
cartilage degradation was scored using the modified Mankin score
system (22). Histological
analysis was performed by two blinded independent researchers.
The femoral condyle articular cartilage was
pulverized into powder in liquid nitrogen (Nuotaishige, Nanjing,
China), then lysis buffer (Sigma-Aldrich) was added and the mixture
was centrifuged at 16,000 × g for 10 min at 4°C. Total protein
extracted from the cartilage of the three groups was assessed by
western blot.
Western blot analysis
The proteins from chondrocytes and cartilage were
isolated using the Total Protein Extraction kit (Beyotime Institute
of Biotechnology) according to the manufacturer’s instructions.
Harvested protein was subjected to SDS-PAGE and transferred onto
polyvinylidene difluoride membranes (Invitrogen Life Technologies,
Carlsbad, CA, USA). Following blocking in Tris-buffered saline
(TBS; pH 7.6) containing 5% non-fat dried milk and 0.1% Tween-20,
the membranes were probed with the following antibodies against:
Col2 (1:100-1:200; ab185430; Abcam, Cambridge, MA, USA), rabbit
polyclonal aggrecan (1:1,000; ab36861; Abcam), rabbit polyclonal
COX-2 (1:300-1:1,000; ab52234; Abcam), rabbit polyclonal MMP-13
(1:3,000-1:6,000; ab39012; Abcam), mouse monoclonal β-actin
(1:5,000-1:16,000; ab6276; Abcam), mouse monoclonal p-ERK1/2
(1:2,000; #9106; Cell Signaling Technology, Inc.), mouse monoclonal
ERK1/2 (1:1,000; #9107; Cell Signaling Technology, Inc., Danvers,
MA, USA), human polyclonal p-JNK (1:1,000; #9251; Cell Signaling
Technology, Inc.), human polyclonal JNK (1:1,000; #9252; Cell
Signaling Technology, Inc.), p-p38 (1:2,000; #9216; Cell Signaling
Technology, Inc.), rabbit monoclonal p38 (1:1,000; #8690; Cell
Signaling Technology, Inc.), mouse monoclonal p-NF-κB p65 (1:1,000;
#13346; Cell Signaling Technology, Inc.), rabbit monoclonal NF-κB
p65 (1:1,000; #8242; Cell Signaling Technology, Inc.) or mouse
monoclonal IκBα (1:1,000; #4814; Cell Signaling Technology, Inc.)
in blocking buffer and incubated overnight at 4°C. The membranes
were subsequently washed three times with TBS and 0.1% Tween-20,
incubated at 25°C for 2 h with goat anti-mouse (#170-6516), goat
anti-rabbit (#170-6515) and goat anti-human (#172-1033) horseradish
peroxidase-conjugated IgG secondary antibodies (1:5,000; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and probed using a Super
Signal West Pico chemiluminescent substrate kit (Pierce
Biotechnology, Rockford, IL, USA). The membranes were scanned using
a GS800 Densitometer Scanner (Bio-Rad Laboratories, Inc.), followed
by data analysis using PDQuest 7.2.0 software (Bio-Rad
Laboratories, Inc.).
RNA extraction and RT-qPCR
Total RNA was extracted from chondrocytes using 1 ml
TRIzol reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions, dissolved in 0.1% diethylpyrocarbonate
water and quantified by spectrophotometry at 260 nm absorbance
using a Nucleic Quantitative Instrument (BioPhotometer plus;
Eppendorf, Hamburg, Germany). A total of 1 µg RNA was used
to synthesize complementary DNA (cDNA) by reverse transcription
using a PrimeScript™ RT reagent kit (Takara Bio, Inc., Otsu, Japan)
according to the manufacturer’s instructions. Subsequently, the
samples were subjected to qPCR using an ABI Prism 7500 detection
system (Applied Biosystems Life Technologies, Foster City, CA,
USA). Reactions in triplicate were conducted in 20 µl
reaction volume containing 1 µl cDNA, 10 µl Power
SYBR Green PCR Master Mix (Applied Biosystems Life Technologies)
and 250 nM of each primer. The primers were designed by Takara
Biotechnology Co., Ltd (Dailan, China). The gene for GAPDH acted as
an endogenous reference for normalization of fluorescence
thresholds (Ct) values of target genes. PCR was performed using
specific primers designed from the published sequence of each cDNA
as follows: GAPDH sense, 5′-GGTGGACCTCATGGCCTACAT-3′ and antisense,
5′-GCCTCTCTCTTGCTCTCAGTATCCT-3′; Col2 sense,
5′-ACGCTCAAGTCGCTGAACAA-3′ and antisense,
5′-TCAATCCAGTAGTCTCCGCTCT-3′; aggrecan sense,
5′-TCCAAACCAACCCGACAAT-3′ and antisense,
5′-TCTCATAGCGATCTTTCTTCTGC-3′; MMP-13, sense,
5′-TGGTCCCTGCCCCTTCCCT-3′ and antisense,
5′-CCGCAAGAGTCACAGGATGGTAGT-3′; COX-2 sense,
5′-CCATCCTCCTTGAACACGG-3′ and antisense, 5′-TGCCACTGCTTGTACAGCG-3′.
Messenger RNA expression was quantified according to the
2−ΔΔCt method (23).
Statistical analysis
Data are presented as the mean ± standard deviation.
One-way analysis of variance was used to assess the significance of
differences observed between groups. P<0.05 was considered to
indicate a statistically significant difference. All data were
analyzed using SPSS 16.0 software (SPSS Inc., Chicago, IL,
USA).
Results
Characterization of bone marrow-derived
MSCs
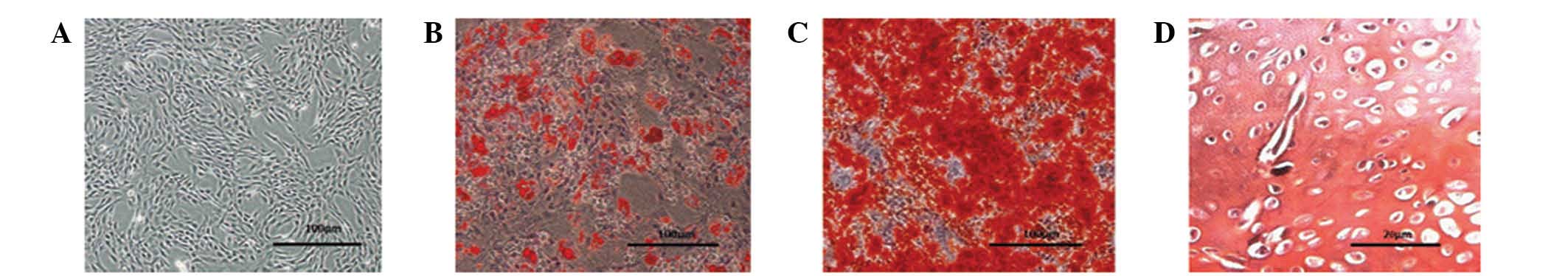
To confirm that the cells isolated from rat bone
marrow (Fig. 1A) were MSCs, their
potential for multilineage differentiation was evaluated (Fig. 1B–D). Following 14 days of culture
in adipogenic induction medium, the cells contained a large number
of neutral lipid vacuoles (lipid droplets) that were positive for
Oil Red O staining. Following three weeks of culture, the cells
grown in osteogenic induction medium were positive for Alizarin red
staining. The cartilaginous phenotype of the induced cells was
confirmed by Safranin O staining following 14 days of culture. The
ability of these cells to successfully differentiate into distinct
cell types verified their status as MSCs.
MSCs alter the gene and protein
expression profiles of rat chondrocytes
Western blot analysis revealed that the levels of
Col2 and aggrecan protein (Fig.
2A) in IL-1β-treated chondrocytes were markedly lower than
those in normal cells at the indicated time-points, while COX-2 and
MMP-13 (Fig. 2A) expression levels
were significantly higher than those in normal chondrocytes. The
gene expression levels of Col2, aggrecan, MMP-13 and COX-2 in
chondrocytes were analogous to those of the protein expression
levels observed with western blot analysis (Fig. 2B–E). In addition, IκBα protein
expression levels were markedly lower than those in normal
chondrocytes, while, phosphorylated ERK1/2 (p-ERK1/2), p-JNK, p-p38
and p-p65 protein expression levels were significantly higher than
those of normal chondrocytes (Fig.
3).
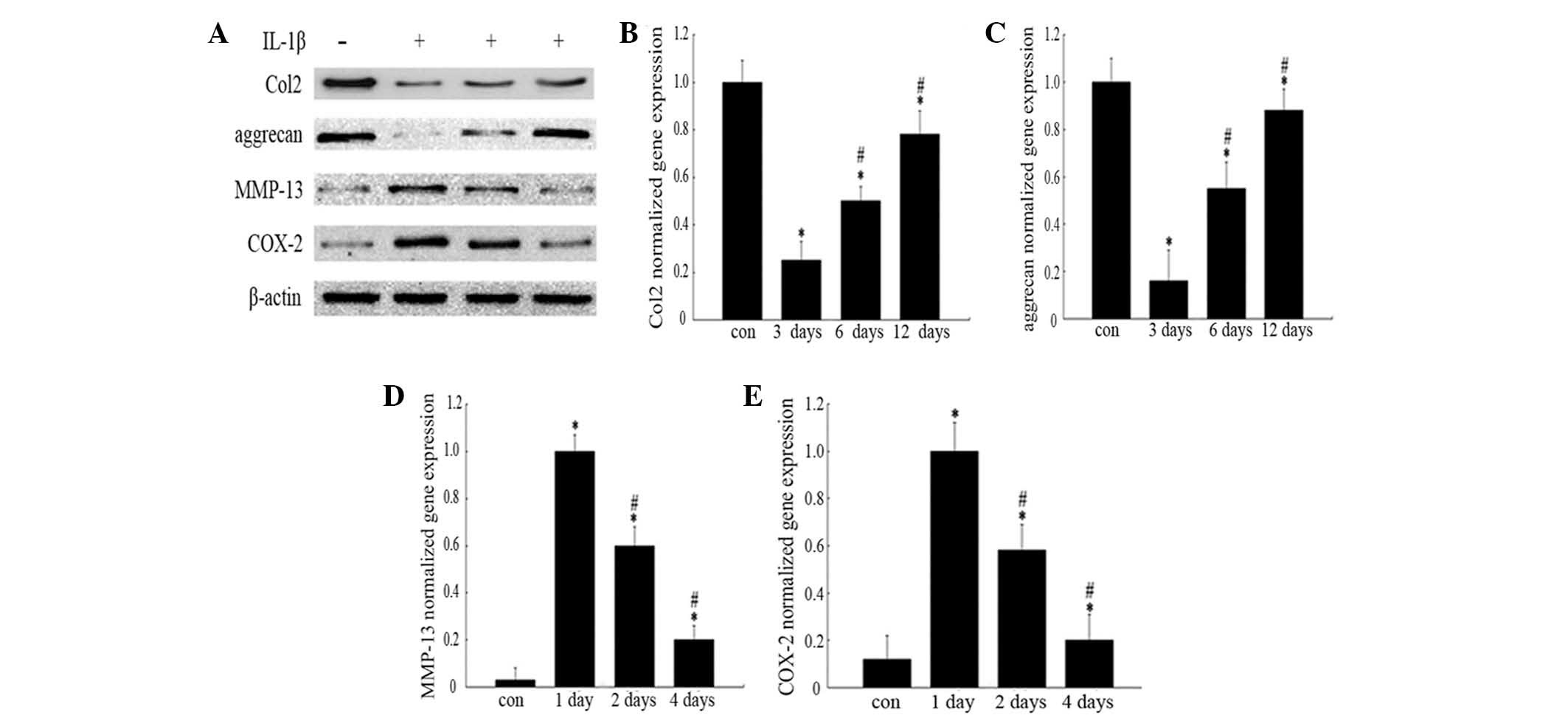 | Figure 2Effects of IL-1β on protein and gene
expression levels. The groups represent chondrocytes without
treatment (con) and chondrocytes cultured with normal medium for
either 3, 6 or 12 days (for analysis of Col2 and aggrecan) or for
1, 2 or 4 days (for COX-2 and MMP-13 analysis) following incubation
with 10 ng/ml IL-1β for 24 h, respectively. (A) Protein expression
was analyzed by western blotting and the blot shown is
representative of typical results (three repeats completed). Gene
expression levels of (B) Col2, (C) aggrecan, (D) COX-2 and (E)
MMP-13 were analyzed by RT-qPCR. Histograms represent the mean of
values relative to the maximum value. RT-qPCR analyses were run in
triplicate and the results are presented as the mean ± standard
deviation. *P<0.05 vs. control group;
#P<0.05 vs. 3/1 day group. IL-1β, interleukin-1β;
Col2, type II collagen; COX-2, cyclooxygenase-2; MMP-13, matrix
metalloproteinase-13; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction. |
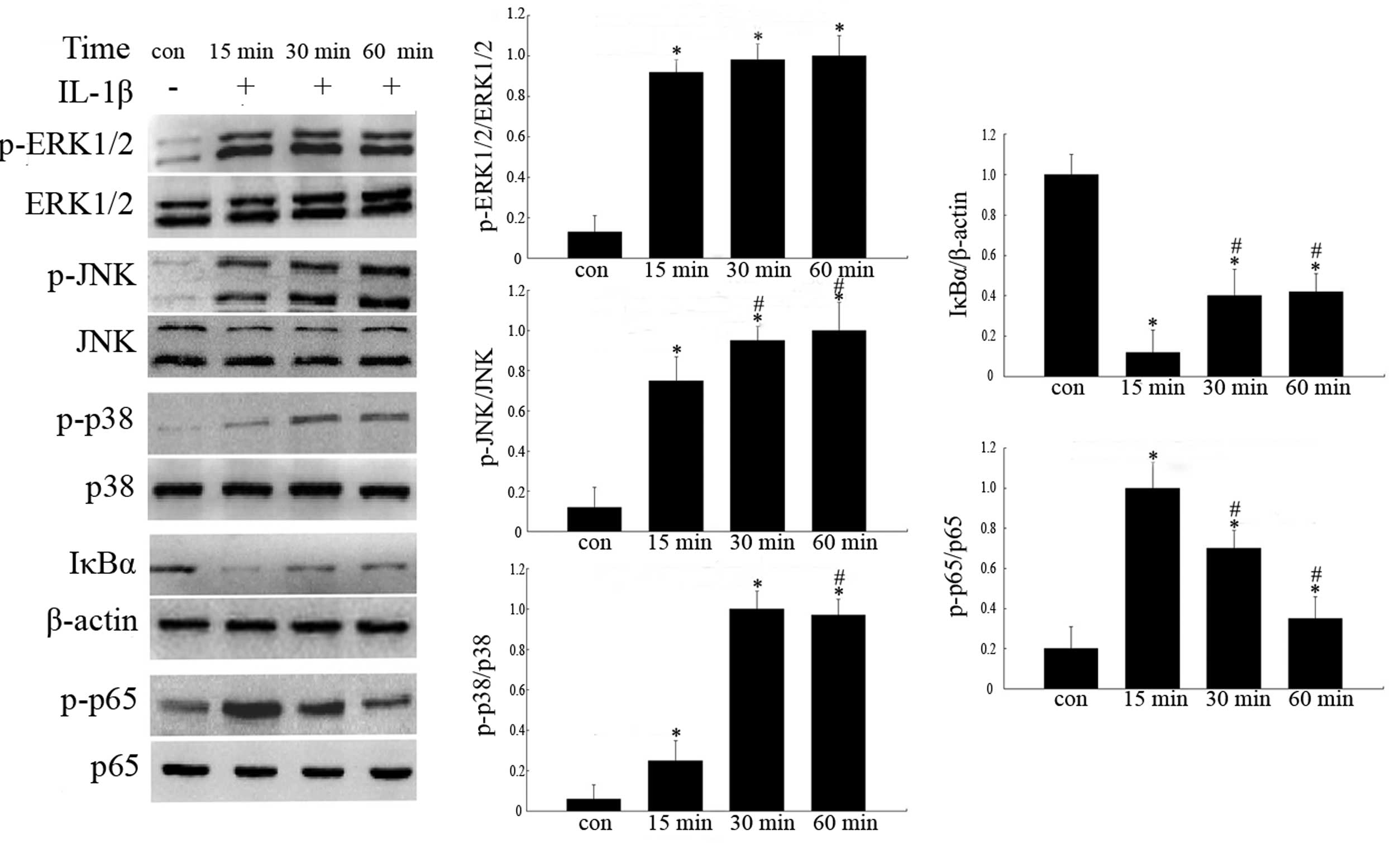 | Figure 3Analysis of expression levels of
IL-1β-induced MAPK and NF-κB pathway-associated proteins. The four
groups represent chondrocytes without treatment (con) and
chondrocytes cultured with 10 ng/ml IL-1β for 15, 30 and 60 min,
respectively. The proteins were analyzed by western blotting and
quantified by densitometry. Histograms represent the mean values
relative to the maximum value. Experiments were performed in
triplicate and the bands shown represent typical results. Data are
presented as the mean ± standard deviation. *P<0.05
vs. control group; #P<0.05 vs. 15 min group. IL-1β,
interleukin-1β; MAPK, mitogen-activated protein kinase; NF-κB,
nuclear factor κB, p-, phosphorylated; ERK1/2, extracellular
signal-regulated kinases 1/2; JNK, c-Jun N-terminal kinase; IκBα,
inhibitory-κ-B-α. |
In order to evaluate whether factors secreted by
MSCs effected inflammatory and catabolic processes in rat
chondrocytes, chondrocytes were co-cultured with MSCs in a
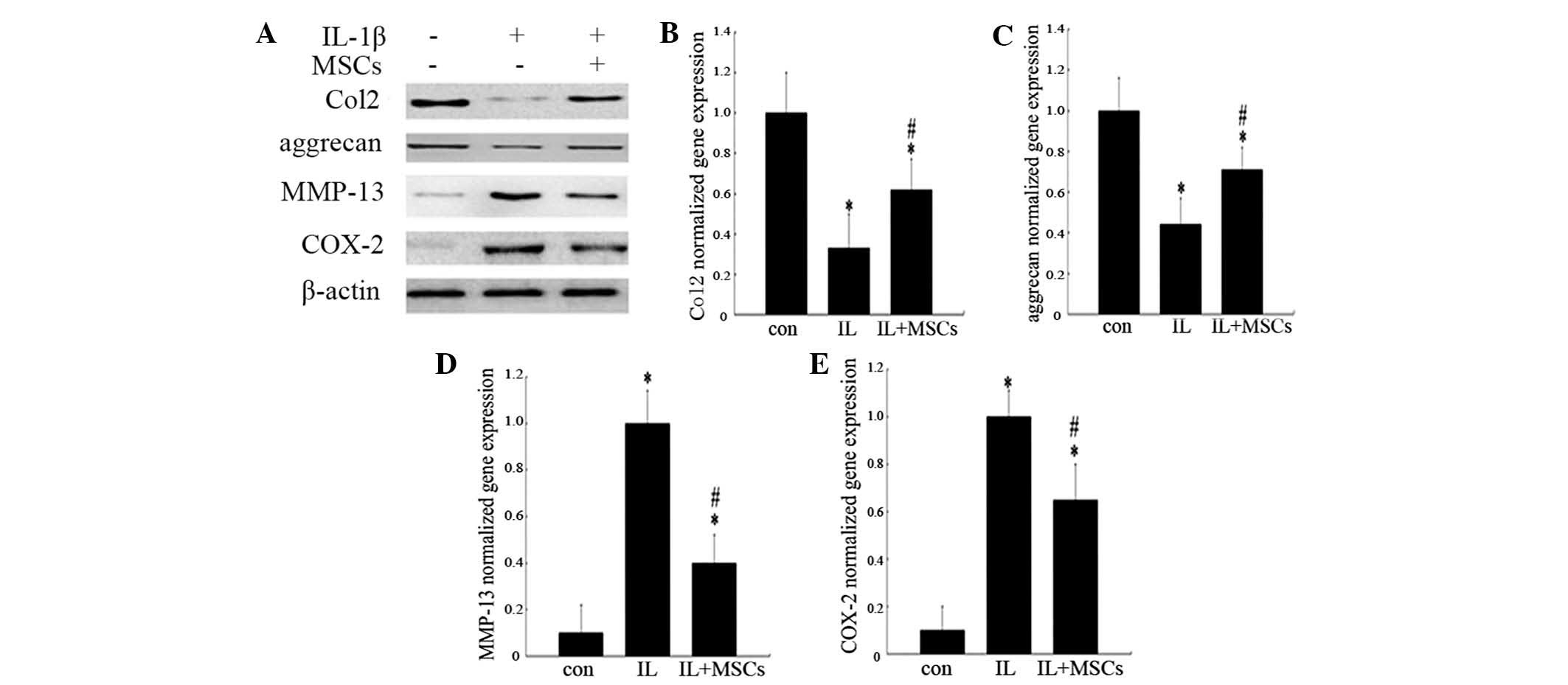
Transwell membrane system. As shown in Fig. 4A, co-culture of IL-1β-treated
chondrocytes with MSCs resulted in a reduction in MMP-13 and COX-2
expression, compared with that of chondrocytes stimulated with
IL-1β alone. By contrast, the protein expression levels of Col2 and
aggrecan in chondrocytes co-cultured with MSCs were higher than
those in chondrocytes treated with IL-1β alone. Changes to the gene
expression levels of Col2, aggrecan, MMP-13 and COX-2 in
chondrocytes were analogous to the results observed in the western
blot analysis (Fig. 4B–E).
In order to evaluate potential signaling pathways
influenced by MSCs, the expression of p-ERK1/2, p-JNK, p-p38, p-p65
and IκBα proteins were analyzed by western blotting.
MSC-conditioned medium did not influence the phosphorylation of
ERK1/2, JNK or p38 MAPK at the indicated time-points (Fig. 5); however, treatment with
MSC-conditioned medium reduced the expression of p-p65 and
increased the levels of IκBα (Fig.
5).
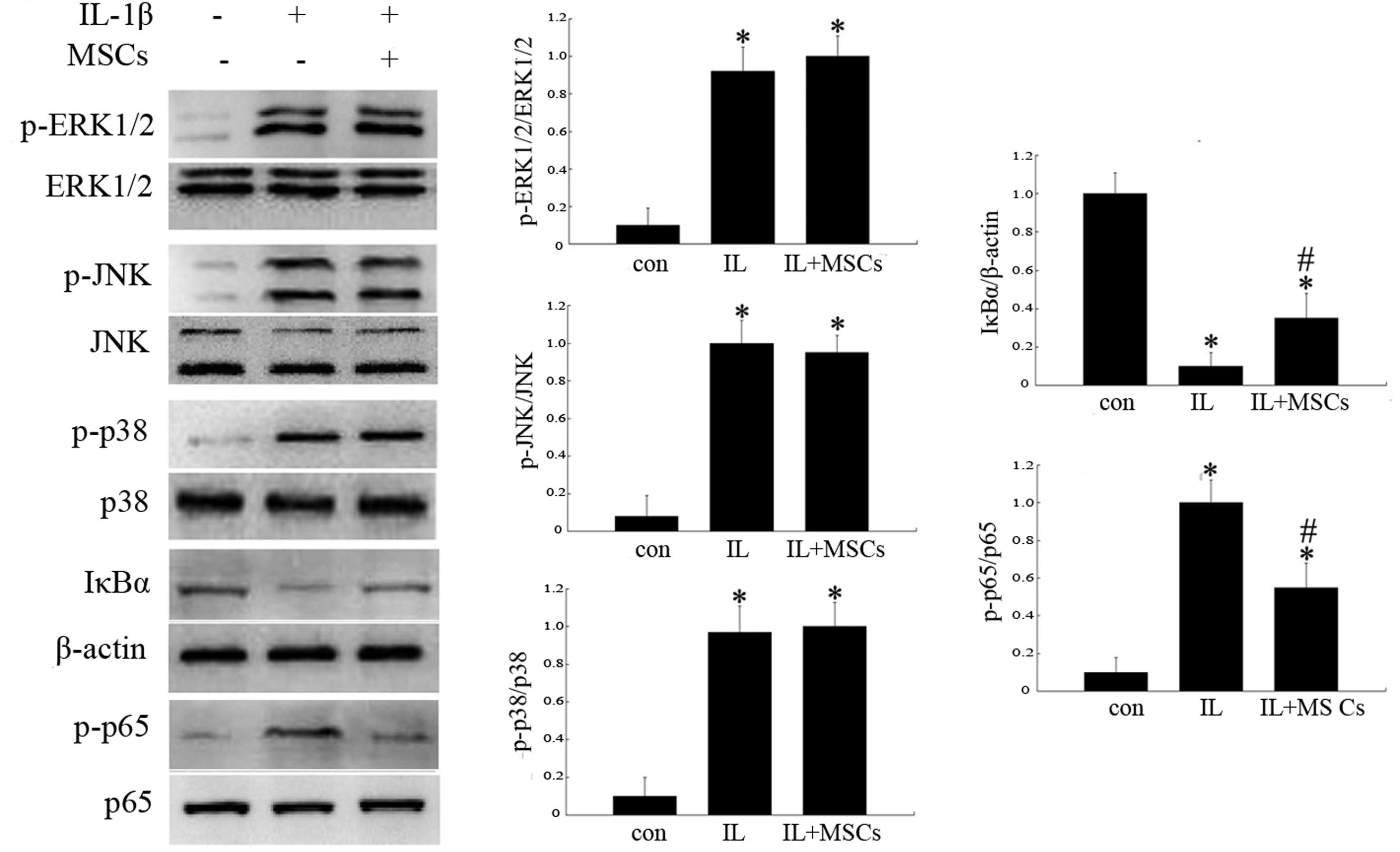 | Figure 5Effects of MSCs on the expression of
MAPK and NF-κB pathway-associated proteins in rat chondrocytes
incubated with IL-1β. The groups represent chondrocytes without
treatment (con) and chondrocytes cultured with (IL+MSCs) or without
(IL) MSC-conditioned medium for 15 min (for analysis of IκBα and
NF-κB p65) or 30 min (for ERK1/2, JNK and p38 analysis) following
incubation with 10 ng/ml IL-1β for 24 h, respectively. Protein
expression was analyzed by western blotting and quantified by
densitometry. Histograms represent the mean of values relative to
the maximum value. Experiments were performed in triplicate and the
bands shown represent typical results. Data are presented as the
mean ± standard deviation. *P<0.05 vs. control group;
#P<0.05 vs. IL group. IL-1β, interleukin-1β; MAPK,
mitogen-activated protein kinase; NF-κB, nuclear factor κB, p-,
phosphorylated; ERK1/2, extracellular signal-regulated kinases 1/2;
JNK, c-Jun N-terminal kinase; IκBα, inhibitory-κ-B-α. |
MSC treatment alters the morphology and
protein expression of cartilage
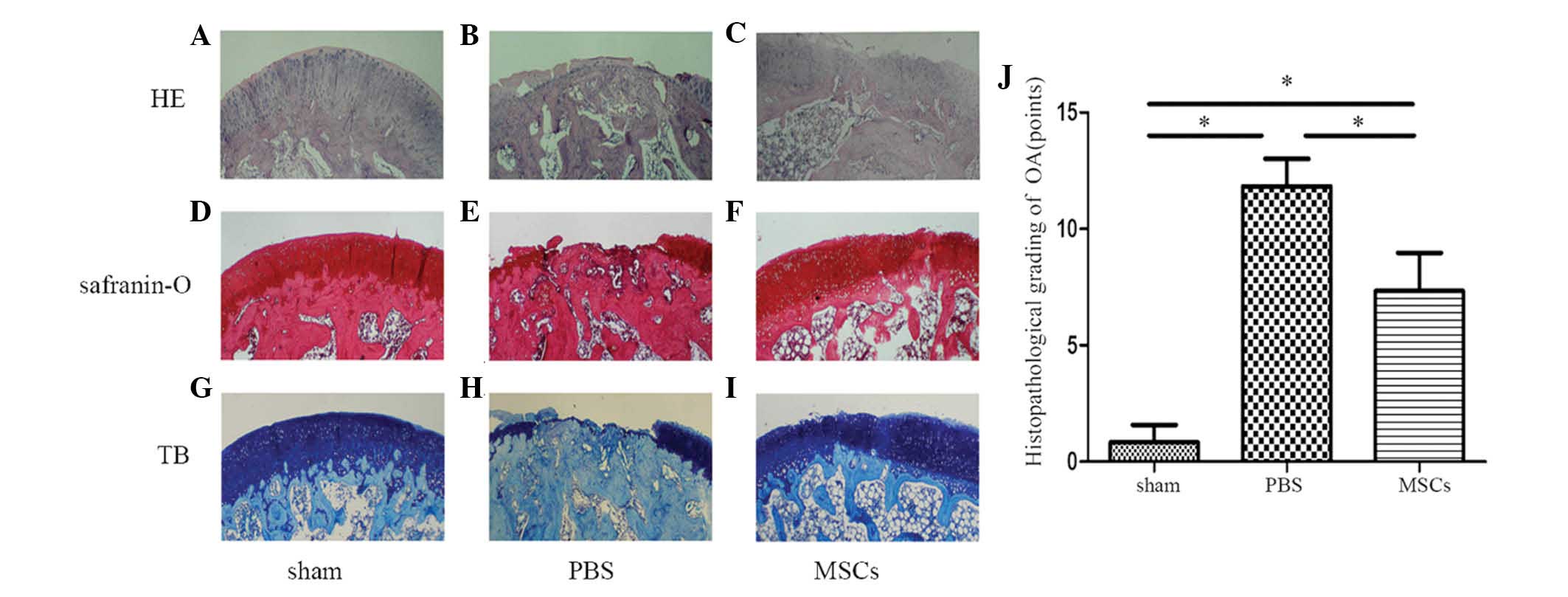
Six weeks following the injection of MSCs, femoral
condylar cartilage samples from the three groups were stained with
HE, Safranin-O and TB and analyzed by western blotting for Col2,
aggrecan, MMP-13, COX-2, IκBα and p-p65 (Fig. 6A–I). In the sham operation group,
the articular cartilage was smooth, with intact superficial, mid
and deep zones. The matrix surrounding the chondrocytes was
arranged in columns which were smooth and evenly stained (Fig. 6A, D and G). The joints that were
injected with PBS exhibited cartilage loss, almost exposing the
bony layer. There was almost complete denudation of the articular
cartilage with loss of the extracellular matrix, and no evidence of
regeneration (Fig. 6B, E and H).
The MSC-treated group exhibited reasonable cartilage regeneration
compared with that of the PBS-treated group, but still exhibited
surface discontinuity, including shallow vertical fissures through
the cartilage superficial zone at numerous points across the
surface and delamination of the superficial zone (Fig. 6C, F and I). At six weeks
post-transplantation, significant differences were identified
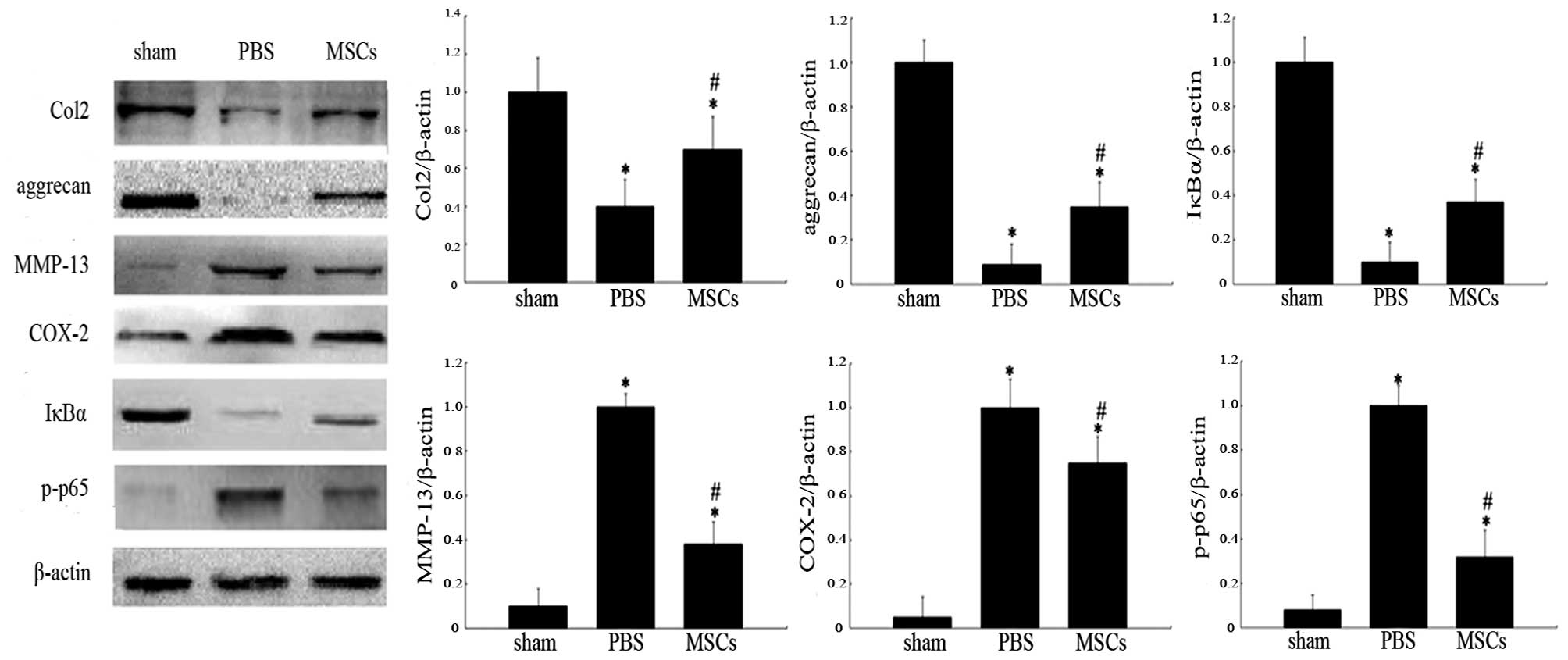
between the Mankin scores of the three groups (Fig. 6J). Western blot analysis revealed
higher levels of Col2, aggrecan and IκBα expression in the
MSC-treated group compared with that of PBS-treated group (Fig. 7). Furthermore, the bands of MMP-13,
COX-2 and p-p65 were weaker in the MSC-treated group than those of
the PBS-treated group. The results demonstrated that injection of
MSCs downregulated MMP-13, COX-2 and p-p65 protein expression in
osteoarthritic cartilage relative to the PBS group (Fig. 7).
Discussion
Since Murphy et al (5) revealed that adult MSCs retard
progressive cartilage destruction in a sheep OA model, MSC therapy
has exhibited extensive potential for the treatment of OA (6–9). In
the present study, it was demonstrated that MSCs promoted Col2 and
aggrecan synthesis and reduced the inflammatory response in
IL-1β-treated chondrocytes and a rat OA model. Previously, studies
have focused on investigating the promotion of tissue repair by
factors synthesized and secreted by MSCs (22,24–27).
These trophic effects are distinct from the direct differentiation
of MSCs into repair tissue, and have numerous advantages in
regenerative medicine, including reducing the time and cost of cell
amplification in vitro (28). Zuo et al (28) reported that the protein expression
levels of Col2 and aggrecan were significantly upregulated in MSCs
and chondrocytes co-cultured with or without direct cell-cell
contact, compared with those of chondrocytes or MSCs cultured
alone. The results of the present study confirmed that IL-1β
increased COX-2 and MMP-13 expression and reduced Col2 and aggrecan
expression. In addition, the present study aimed to investigate
whether MSCs exerted chondroprotective effects via inhibition of
COX-2 and MMP-13 in IL-1β-induced rat chondrocytes. As expected,
the chondrocytes co-cultured indirectly with MSCs exhibited reduced
expression of COX-2 and MMP-13 and upregulated expression of Col2
and aggrecan.
IL-1β is able to activate runt-related transcription
factor 2, activator protein 1 and c-Maf, factors which
significantly promote MMP-13 and COX-2 transcription, via the MAPK
and NF-κB signaling pathways (13–15).
The MAPK signaling pathways transduces numerous external signals,
leading to a variety of cellular responses, including growth,
differentiation, inflammation and apoptosis (29). The three subgroups of the MAPK
family, the ERKs, JNKs and p38-MAPKs are structurally similar and
have key roles in transmitting signals from the cell surface to the
nucleus. NF-κB is retained in the cytoplasm during IκBα inactivity,
while IL-1β activates NF-κB by triggering IκBα degradation. NF-κB
activation results in the upregulation of a group of responsive
genes that contribute to inflammation (30). Consequently, the present study
aimed to investigate whether the MAPK or NF-κB pathways were
involved in the expression of COX-2 and MMP-13 in IL-1β-treated
chondrocytes cultured with MSC-conditioned medium. The results
indicated that IL-1β upregulated the phosphorylation of ERK1/2,
JNK, p38 and NF-κB p65 and downregulated the expression of IκBα.
MSC-conditioned medium did not influence ERK1/2, JNK and p38 MAPK
phosphorylation, but increased the levels of IκBα and reduced
p-p65. Taken together, these results indicated that MSCs inhibit
NF-κB activation in IL-1β-induced chondrocytes. It was therefore
hypothesized that the inhibitory effect of MSCs on COX-2 and MMP-13
expression was, to some extent, attributable to their inhibition of
the NF-κB pathway. Owing to the limited incubation times
investigated in the present study, the possibility that the
inhibitory effect of MSCs may occur via the MAPK pathway was not
excluded entirely.
The identification of which specific factors
secreted by MSCs contribute to the anti-inflammatory effect
observed remains to be elucidated. Proteins secreted by MSCs of
mouse and human origin have been analyzed by a variety of methods,
and found to include chemokines, cytokines, growth factors and
protease inhibitors (24). Many of
these, including IL-4, -10 and -13, transforming growth factor-β
(TGF-β), macrophage migration inhibitory factor, leukocyte
migration inhibitory factor and metalloproteinase inhibitors,
possess the ability to inhibit the release of inflammatory
molecules (31). The
pro-inflammatory cytokines and other signals expressed by injured
cells induce MSCs to secrete anti-inflammatory factors, including
tumor necrosis factor-α stimulated gene/protein 6, prostaglandin E2
and IL-1 receptor antagonist, that mediate the activation of
resident macrophages or decrease the downstream effects of
pro-inflammatory cytokines. The net effect is to decrease the
activation of NF-κB in resident macrophages by parenchymal cells,
via the secretion of IL-6, chemokine (C-X-C motif) ligand 1 and
associated factors, and to decrease the recruitment of neutrophils
(32). In a previous study by our
group (unpublished), the expression of TGF-β by MSCs was blocked by
transfection with slow virus and the results revealed that the
expression of Col2 and aggrecan in co-cultured chondrocytes was
decreased compared with those co-cultured with normal MSCs, which
suggested a significant association between TGF-β and the
regeneration of injured cartilage. There are numerous bioactive
factors, but the identification of which of these are involved in
inhibiting the degeneration of chondrocytes remains to be
elucidated. Further studies are required to clarify the specific
mechanism underlying the modulation of MMP-13 and COX-2 by
MSCs.
In conclusion, the results of the present study
demonstrated that MSCs suppress the IL-1β-induced Col2 and aggrecan
degeneration, as well as the expression of MMP-13 and COX-2 in rat
chondrocytes and in the cartilage of a rat osteoarthritic model, in
part via the NF-κB signaling pathway.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81272033 and
81271986).
References
|
1
|
Feldmann M: Pathogenesis of arthritis:
recent research progress. Nat Immunol. 2:771–773. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harvey WF and Hunter DJ: The role of
analgesics and intra-articular injections in disease management.
Med Clin North Am. 93:201–211. 2009. View Article : Google Scholar
|
|
3
|
Caplan AI and Dennis JE: Mesenchymal stem
cells as trophic mediators. J Cell Biochem. 98:1076–1084. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coleman CM, Curtin C, Barry FP,
O’Flatharta C and Murphy JM: Mesenchymal stem cells and
osteoarthritis: remedy or accomplice? Hum Gene Ther. 21:1239–1250.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murphy JM, Fink DJ, Hunziker EB and Barry
FP: Stem cell therapy in a caprine model of osteoarthritis.
Arthritis Rheum. 48:3464–3474. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsumoto T, Cooper GM, Gharaibeh B, et
al: Cartilage repair in a rat model of osteoarthritis through
intraarticular transplantation of muscle-derived stem cells
expressing bone morphogenetic protein 4 and soluble Flt-1.
Arthritis Rheum. 60:1390–1405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horie M, Choi H, Lee RH, et al:
Intra-articular injection of human mesenchymal stem cells (MSCs)
promote rat meniscal regeneration by being activated to express
Indian hedgehog that enhances expression of type II collagen.
Osteoarthritis Cartilage. 20:1197–1207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Centeno CJ, Busse D, Kisiday J, Keohan C,
Freeman M and Karli D: Increased knee cartilage volume in
degenerative joint disease using percutaneously implanted,
autologous mesenchymal stem cells. Pain Physician. 11:343–353.
2008.PubMed/NCBI
|
|
9
|
Wakitani S, Imoto K, Yamamoto T, Saito M,
Murata N and Yoneda M: Human autologous culture expanded bone
marrow mesenchymal cell transplantation for repair of cartilage
defects in osteoarthritic knees. Osteoarthritis Cartilage.
10:199–206. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Agung M, Ochi M, Yanada S, et al:
Mobilization of bone marrow-derived mesenchymal stem cells into the
injured tissues after intraarticular injection and their
contribution to tissue regeneration. Knee Surg Sports Traumatol
Arthrosc. 14:1307–1314. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karan A, Karan MA, Vural P, et al:
Synovial fluid nitric oxide levels in patients with knee
osteoarthritis. Clin Rheumatol. 22:397–399. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kobayashi M, Squires GR, Mousa A, et al:
Role of interleukin-1 and tumor necrosis factor alpha in matrix
degradation of human osteoarthritic cartilage. Arthritis Rheum.
52:128–135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan Z, Söder S, Oehler S, Fundel K and
Aigner T: Activation of interleukin-1 signaling cascades in normal
and osteoarthritic articular cartilage. Am J Pathol. 171:938–946.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldring MB, Otero M, Plumb DA, et al:
Roles of inflammatory and anabolic cytokines in cartilage
metabolism: signals and multiple effectors converge upon MMP-13
regulation in osteoarthritis. Eur Cell Mater. 21:202–220.
2011.PubMed/NCBI
|
|
15
|
Raveenthiran SP and Chowdhury TT: Dynamic
compression inhibits fibronectin fragment induced iNOS and COX-2
expression in chondrocyte/agarose constructs. Biomech Model
Mechanobiol. 8:273–283. 2009. View Article : Google Scholar
|
|
16
|
Wei A, Chung SA, Tao H, et al:
Differentiation of rodent bone marrow mesenchymal stem cells into
intervertebral disc-like cells following coculture with rat disc
tissue. Tissue Eng Part A. 15:2581–2595. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schilling T, Nöth U, Klein-Hitpass L,
Jakob F and Schutze N: Plasticity in adipogenesis and osteogenesis
of human mesenchymal stem cells. Mol Cell Endocrinol. 271:1–17.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnstone B, Hering TM, Caplan AI,
Goldberg VM and Yoo JU: In vitro chondrogenesis of bone
marrow-derived mesenchymal progenitor cells. Exp Cell Res.
238:265–272. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qing C, Wei-ding C and Wei-min F:
Co-culture of chondrocytes and bone marrow mesenchymal stem cells
in vitro enhances the expression of cartilaginous extracellular
matrix components. Braz J Med Biol Res. 44:303–310. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Appleton CT, McErlain DD, Pitelka V, et
al: Forced mobilization accelerates pathogenesis: characterization
of a preclinical surgical model of osteoarthritis. Arthritis Res
Ther. 9:R132007. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Armstrong S, Read R and Ghosh P: The
effects of intraarticular hyaluronan on cartilage and subchondral
bone changes in an ovine model of early osteoarthritis. J
Rheumatol. 21:680–688. 1994.PubMed/NCBI
|
|
22
|
Prockop DJ, Kota DJ, Bazhanov N and Reger
RL: Evolving paradigms for repair of tissues by adult
stem/progenitor cells (MSCs). J Cell Mol Med. 14:2190–2199. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmittgen TD and Zakrajsek BA: Effect of
experimental treatment on housekeeping gene expression: Validation
by real-time, quantitative RT-PCR. J Biochem Biophys Methods.
46:69–81. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sze SK, de Kleijn DP, Lai RC, et al:
Elucidating the secretion proteome of human embryonic stem
cell-derived mesenchymal stem cells. Mol Cell Proteomics.
6:1680–1689. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
So A, De Smedt T, Revaz S and Tschopp J: A
pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis
Res Ther. 9:R282007. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gabay C, Lamacchia C and Palmer G: IL-1
pathways in inflammation and human diseases. Nat Rev Rheumatol.
6:232–241. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi H, Lee RH, Bazhanov N, Oh JY and
Prockop DJ: Anti-inflammatory protein TSG-6 secreted by activated
MSCs attenuates zymosan-induced mouse peritonitis by decreasing
TLR2/NF-κB signaling in resident macrophages. Blood. 118:330–338.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zuo Q, Cui W, Liu F, Wang Q, Chen Z and
Fan W: Co-cultivated mesenchymal stem cells support chondrocytic
differentiation of articular chondrocytes. Int Orthop. 37:747–752.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roman-Blas JA and Jimenez SA: NF-kappaB as
a potential therapeutic target in osteoarthritis and rheumatoid
arthritis. Osteoarthritis Cartilage. 14:839–848. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Skalnikova H, Motlik J, Gadher SJ and
Kovarova H: Mapping of the secretome of primary isolates of
mammalian cells, stem cells and derived cell lines. Proteomics.
11:691–708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Prockop DJ and Oh JY: Mesenchymal
stem/stromal cells (MSCs): role as guardians of inflammation. Mol
Ther. 20:14–20. 2012. View Article : Google Scholar :
|