Introduction
Breast cancer is one of the most frequently
diagnosed types of cancer in females (1). The risk factors for breast cancer are
age, alcohol consumption, body mass index, hormone replacement
therapy and reproductive factors (2). The majority of females with breast
cancer develop metastasis in common sites, including the bone,
liver and lung (3). Several
reports have revealed that males are also susceptible to breast
cancer in the United States (4).
The progression of breast cancer between an estrogen-dependent,
non-metastatic phenotype and an estrogen-independent, invasive,
metastatic phenotype are accompanied by chemoresistance (5). Treatment for breast cancer includes
several approaches, including surgical and/or pharmacological
approaches by estrogenic signaling in estrogen receptor-positive
breast cancers (6). However, there
is no targeted therapy for estrogen-independent breast cancer
(7). Several studies have
investigated combination therapies to improve the response to
chemotherapy (8,9).
Continuous exposure of cancer cells to drugs leads
to the development of a multidrug resistance (MDR) phenotype. The
resistance of multiple chemotherapeutic drugs has been recognized
as a major contributor to the failure of cancer therapy and
obstacle in the successful treatment of numerous types of
malignancy (10). MDR occurs
through several mechanisms, including increased adenosine
triphosphate (ATP)-dependent efflux (11). The classical cellular mechanism of
MDR involves efflux of the drug by various membrane transport
proteins. ATP-binding cassette (ABC) transporters are a family of
proteins, which mediate MDR via ATP-dependent drug efflux pumps
(12). Several transport proteins
of the ABC superfamily have been characterized and include
P-glycoprotein (P-gp, MDR-1; ABCB-1), multidrug
resistance-associated protein-1 (MRP-1; ABCC-1) and breast cancer
resistance protein (BCRP; ABCG-2) which are overexpressed in
chemoresistant cells (13).
Ionophore is a lipid-soluble molecule, which is
usually produced by a variety of microbes to transport ions across
biological membranes and increase the feeding efficiency of
ruminant animals (14,15). Salinomycin is one of the
monocarboxylic ionophores isolated from Streptomyces albus
(16). Salinomycin has been
demonstrated to cause the death of breast cancer stem cells (CSCs)
more efficiently compared with the anticancer drug, paclitaxel
(17). Several reports have
suggested that salinomycin induces apoptosis via cell cycle arrest
and reactive oxygen species (ROS)-mediated mitochondrial pathways
in a diversity of cancer cells (18,19).
Salinomycin also triggers apoptosis by overcoming ABC
transporter-mediated multidrug and apoptosis resistance in MDR
cancer cells (20). Other reports
have indicated that salinomycin induces apoptosis through the
overexpression of B-cell lymphoma 2 and enhanced proteolytic
activity, independent of the p53 tumor suppressor protein in MDR
cancer cells (18).
Salinomycin-induced activation of autophagy, with concomitant
generation of ROS and activation of endoplasmic reticulum stress
has also been observed in human cancer cells (21,22).
The effect of salinomycin on the death of CSCs and MDR types of
cancer may demonstrate a novel class of anticancer agents. In the
present study, it was hypothesized that salinomycin may act as a
reverser of MDR and benefit patients receiving chemotherapy. The
reversal effect of salinomycin was investigated and the underlying
mechanism of action was evaluated in human breast cancer
doxorubicin-sensitive MCF-7 and doxorubicin-resistant MCF-7/MDR
cells.
Materials and methods
Reagents and antibodies
Salinomycin, doxorubicin and
3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyl-etrazolium bromide (MTT)
were purchased from Sigma-Aldrich (St. Louis, MO, USA). The
salinomycin and doxorubicin were dissolved in methanol (Samchun
Pure Chemical, Pyeongtaek, Korea) and distilled water as 20 mM
stock solutions, respectively. Antibodies for MDR-1, MRP-1 and
β-actin were purchased from Enzo Life Sciences (Farmingdale, NY,
USA) and Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA),
respectively. Gat-anti-mouseimmunoglobulin (Ig)G was purchased from
Enzo Life Sciences. The ECL Western kit was purchased from GE
Healthcare Bio-Sciences (Pittsburgh, PA, USA).
Cell lines and cell culture
The MCF-7 human breast cancer cells lines were
obtained from American Type Culture Collection (Manassas, VA, USA).
The MCF-7/MDR cell lines were generated through sequential exposure
to increasing concentrations of doxorubicin (0.1–1 µM). The
cells were maintained and cultured in Dulbecco’s modified Eagle’s
medium (DMEM; WelGENE Inc., Daegu, Republic of Korea), supplemented
with 10% fetal bovine serum (FBS; WelGENE Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin (WelGENE Inc.) at a
temperature of 37°C in a humidified atmosphere with 5%
CO2. Doxorubicin (1 µM) was added to the culture
medium to maintain the MDR characteristics of the MCF-7/MDR
cells.
Cell viability analysis
The doxorubicin-sensitive MCF-7 and
doxorubicin-resistant MCF-7/MDR cells were seeded at a density of
1×104 cells/well into a 6-well plate. The cells were
treated with doxorubicin (0.1–20 µM) and salinomycin (0.5–20
µM), either alone or in combination for 72 h. Subsequently,
MTT solution (0.5 mg/ml) was added to the culture medium for a
further 4 h incubation in a 37°C and 5% CO2 atmosphere.
The cells were then dissolved in dimethyl sulfoxide (Junsei
Chemical Co., Tokyo, Japan). Colorimetric analysis was performed at
570 nm using an ELISA reader (VERSAmax microplate reader; Molecular
Devices, Toronto, ON, Canada).
Intracellular accumulation of
doxorubicin
Doxorubicin is a well-known P-gp substrate and is
frequently used to treat breast cancer. In the present study, the
MCF-7/MDR cells were seeded at a density of 5×104 cells
in a glass-bottom dish. The cells were treated with 10 µM
doxorubicin, alone or in combination with salinomycin (10–20
µM). Following 3 h incubation at 37°C, the cells were washed
three times with phosphate-buffered saline (PBS). To determine
changes in the efflux of intracellular doxorubicin, a separate set
of samples was incubated for 2 h at 37°C in fresh medium without
doxorubicin or salinomycin, as a control. The cells were visualized
using a laser scanning confocal microscope (Olympus Fluoview
FV1000; Olympus, Tokyo, Japan). To quantify the intracellular
accumulation of doxorubicin, the cells were exposed to doxorubicin,
alone or in combination with salinomycin for 3 h at 37°C. The
control samples were incubated without doxorubicin or salinomycin,
or with salinomycin (10–20 µM) alone for 2 h at 37°C. The
cells were analyzed using flow cytometry (FACScalibur; Becton
Dickinson, Franklin Lakes, NJ, USA) and the accumulation of
doxorubicin was calculated using Cell Quest Pro software on
Mac®OS 9 (Becton Dickinson).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The MCF-7/MDR cells were seeded into 60
cm2 cell culture dishes (1×106 cells).
Following 24 h incubation at 37°C, the cells were treated with
salinomycin (10 or 20 µM) for 3 and 6 h. Subsequently, the
cells were harvested, and total RNA isolation was performed using a
RiboEX_column Total RNA Purification kit (GeneAll, Seoul, Korea).
RT-qPCR amplification was performed using primers for MDR1, MRP1
and GAPDH. The PCR primers were designed using the Primer3 programs
(http://frodo.wi.mit.edu) and the sequences were
as follows: MDR1, forward 5′-ATATCAGCAGCCCACATCAT-3′ and reverse
5′-GAAGCACTGGGATGTCCGGT-3′; MRP1, forward
5′-TGTGAGCTGGTCTCTGCCATA-3′ and reverse 5′-CTGGCTCATGCCTGGACTCT-3′
and GAPDH, forward 5′-GCCAAAAGGGTCATCATCTC-3′ and reverse
5′-GTAGAGGCAGGGATGATGTTC-3′. The PCR cycles were as follows: 94°C
for 5 min; 33 cycles at 94°C for 1 min, 58°C for 1 min and 72°C for
1 min; followed by 72°C for 10 min. For The RT-qPCR was performed
using low cycle numbers to avoid saturation, in triplicate
samples.
Western blot analysis
The cell extracts were prepared by incubating the
cells in lysis buffer, containing 150 mM NaCl, 10 mM Tris (pH 7.4),
5 mM EDTA (pH 8.0), 1% Triton X-100, 1 mM PMSF, 20 mg/ml aprotinin,
50 µg/ml leupeptin, 1 mM benzidine and 1 mg/ml pepstatin.
Equal quantities of proteins (40 µg) were
electrophoretically separated using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on an 8% gel,
and were then transferred onto a polyvinylidenefluoride (PVDF)
membrane (GE Healthcare Bio-Sciences). Following blocking with
Tris-buffered saline with Tween 20 (TBS-T) containing 20 mM Tris
(pH 7.4), 150 mM NaCl and 0.1% Tween 20, with5% skim milk, the
membranes were incubated with primary (MDR-1, MRP-1 and β-actin;
1:1,000; 4°C overnight) and secondary (goat-anti-mouse; 1:5,000, 2
h at room temperature) antibodies. The membranes were then washed
with TBS-T buffer and visualized using enhanced chemiluminescence
western blotting detection reagents. The density of each band was
determined using a fluorescence scanner (LAS 3000; FujiFilm, Tokyo,
Japan) and analyzed using Multi Gauge V3.0 software (FujiFilm).
Statistical analysis
The experiments were repeated at least three times
with consistent results. Unless otherwise stated, the data are
expressed as the mean ± standard deviation. Analysis of variance
was used to compare the experimental groups with the control group,
whereas comparisons among multiple groups were performed by Tukey’s
multiple comparison test using Graphpad InStat V3.05. P<0.05 was
considered to indicate a statistically significant difference.
Results
Salinomycin sensitizes MCF-7/MDR cells
against doxorubicin
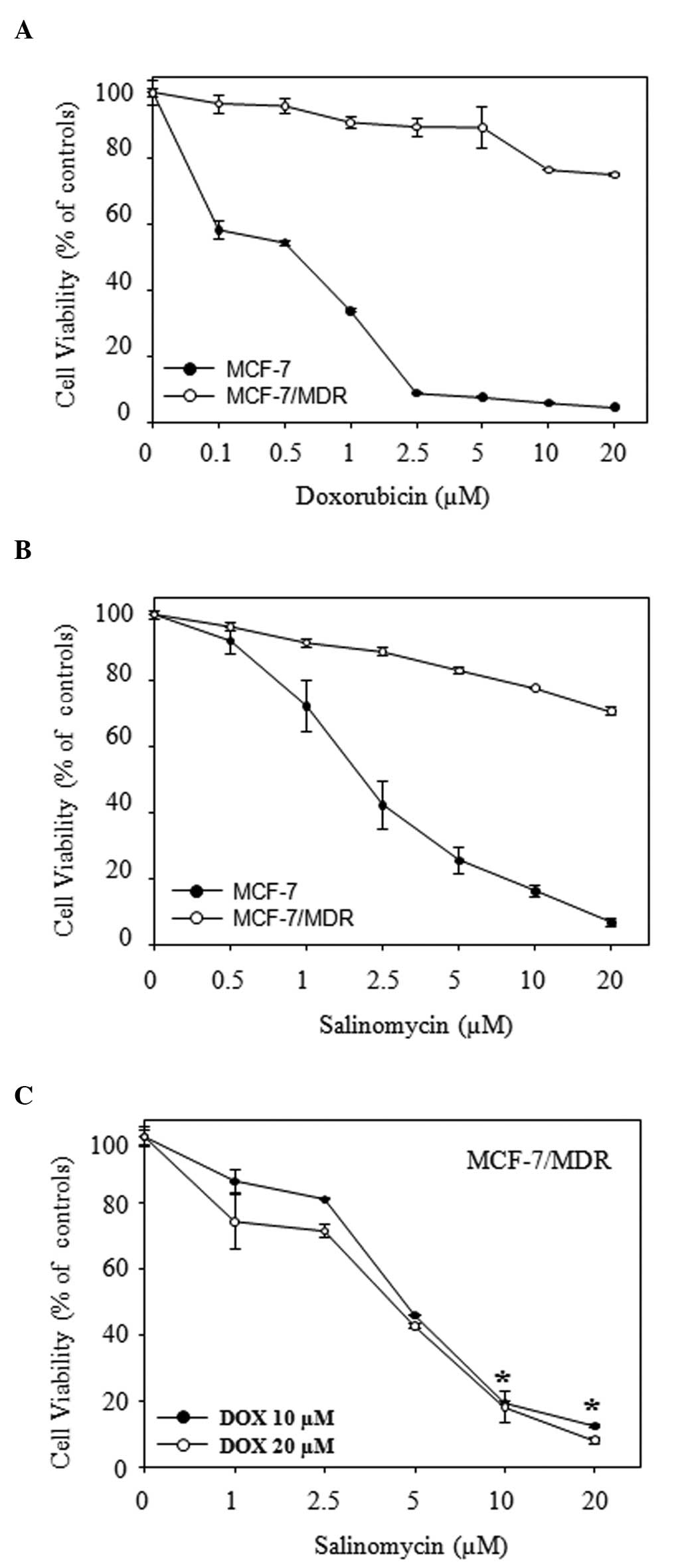
The effect of salinomycin on cytotoxicity was
investigated using an MTT assay on the doxorubicin-sensitivite
MCF-7 or doxorubicin-resistant MCF-7/MDR cells. Following treatment
with various concentrations of doxorubicin for 72 h, the viability
of cells against MCF-7 was reduced in a dose-dependent manner. The
half maximal inhibitory concentration of doxorubicin was <1
µM. By contrast, the MCF-7/MDR cells were highly resistant
to doxorubicin (Fig. 1A). In
addition, salinomycin significantly inhibited cell viability in the
MCF-7 cells, however MCF-7/MDR cells exhibited only mild
cytotoxicity compared with the MCF-7 cells when the cells were
exposed to salinomycin alone, at the levels up to 20 µM
(Fig. 1B). Salinomycin
significantly enhanced the cytotoxicity of doxorubicin on the
MCF-7/MDR cells in the dose-dependent manner (Fig. 1C). In the presence of 10 µM
salinomycin, the viabilities of these cells following 72 h
treatment with 10 or 20 µM doxorubicin were 19.4 and 18.2%,
respectively, However, in the presence of 20 µM salinomycin,
the viabilities at the same concentrations of doxorubicin were 12.4
and 8.1%, respectively. No significant effects of salinomycin on
doxorubicin cytotoxicity were observed on the MCF-7 cells (data not
shown).
Salinomycin increases doxorubicin
accumulation and decreases efflux in MCF-7/MDR cells
The intracellular localization and accumulation of
doxorubicin in the MCF-7/MDR cells were observed using laser
scanning confocal microscopy. Red fluorescence was observed in the
cells (Fig. 2A) following
treatment with 10 µM doxorubicin alone for 3 h. The
fluorescence observed following treatment with the combination of
either 10 or 20 µM doxorubicin with salinomycin led to a
marginal increase in the accumulation of doxorubicin, and was
dependent on the salinomycin concentration (Fig. 2B and C). At 2 h post-removal of
doxorubicin and salinomycin, the fluorescence of the cytoplasmic
signal in the control cells treated with doxorubicin alone was
almost absent following its removal (Fig. 2D), however, the fluorescence
remained evident in the cells pretreated with salinomycin (Fig. 2E and F). These results were
consistent with the cell viability data, indicating that
salinomycin induced the efflux of doxorubicin from the cells.
Salinomycin regulates the cellular uptake
and efflux of doxorubicin in MCF-7/MDR cells
To determine whether salinomycin mediated the net
uptake and efflux of doxorubicin in the MCF-7/MDR cells, the flow
cytometric intensities were analyzed. These results demonstrated
significant increases, similar to those observed using confocal
microscopy, in the net uptake of doxorubicin, and decreased efflux
of doxorubicin by salinomycin (Fig. 3A
and B). In the case of net uptake, at 3 h post-treatment of the
MCF-7/MDR cells with 10 µM doxorubicin with either 10 or 20
µM salinomycin, the fluorescence signal intensities were
1.6- or 3.8-fold higher, respectively, compared with treatment with
10 µM doxorubicin alone. Following treatment with 20
µM doxorubicin with either 10 or 20 µM salinomycin,
the intensities were 1.7- and 4.9-fold higher, respectively,
compared with treatment with 20 µM doxorubicin alone.
 | Figure 3Salinomycin regulates the uptake and
efflux of doxorubicin in MCF-7/MDR cells. (A and B) Effects of
salinomycin on the accumulation of doxorubicin. The MCF-7/MDR cells
were treated with doxorubicin, alone or in combination with
salinomycin (10 or 20 µM) for 3 h. (C and D) Effects of
salinomycin on the efflux of doxorubicin. The cells were removed
from exposure to doxorubicin, but not salinomycin, for 2 h. (A and
C) Flow cytometric analysis. 1, untreated; 2, doxorubicin (10
µM); 3, doxorubicin (10 µM) + salinomycin (10
µM); 4, doxorubicin (10 µM) + salinomycin (20
µM); 5, doxorubicin (20 µM); 6, doxorubicin (20
µM) + salinomycin (10 µM); 7, doxorubicin (20
µM) + salinomycin (20 µM). All data are
representative of at least three times independent experiment(B)
Fold change in the intracellular fluorescent intensity. (D)
Percentage loss of intracellular fluorescent intensity.. Data in B
and D are expressed as the mean ± standard deviation
(#P<0.05). MDR, multidrug resistance; Sal,
salinomycin. |
In terms of the efflux of intracellular doxorubicin
following removal of the drug from the culture media, the
intracellular signal loss was significantly lower in the cells
treated with a combination of doxorubicin and salinomycin, compared
with that of the cells treated with doxorubicin alone (Fig. 3C and D). Compared with the signal
prior to drug removal, the losses in intracellular doxorubicin 2 h
post-treatment were 75.1 and 69.3% following 3 h treatment with 10
or 20 µM doxorubicin, respectively. The losses in
intracellular doxorubicin were only 63.0 and 46.1% following
treatment with 10 or 20 µM salinomycin, compared with those
observed following combined treatment with salinomycin and 10
µM doxorubicin, respectively. In the cells treated with a
combination of salinomycin and 20 µM doxorubicin, the 63.3%
loss of intracellular doxorubicin was reduced to 37.5%. These
results demonstrated that salinomycin enhanced doxorubicin-induced
cytotoxicity by increasing the influx of doxorubicin and decreasing
the efflux of doxorubicin in the MCF-7/MDR cells.
Salinomycin regulates efflux and influx
independently of the gene and protein expression levels of MDR1 and
MRP1 in MCF-7/MDR cells
To determine the mechanism underlying the reversal
effect of salinomycin on the resistance of cancer cells, the
present study examined the mRNA and protein expression levels of
MDR1 and MRP1 in the MCF-7 and MCF-7/MDR cells. The mRNA and
protein levels were estimated using RT-qPCR and western blot
analysis. Following treatment with salimycin (10 or 20 µM)
for 3 and 6 h, no significant changes were detected in either the
mRNA or protein expression levels (Fig. 4), compared with the cells not
exposed to salinomycin. However, the MDR1 and MRP1 proteins were
not detected in the MCF-7 cells. These results support the
suggested that salinomycin did not affect the mRNA and protein
expression levels of MDR1 and MRP1 mRNA and protein.
Discussion
MDR in cancer cells is a significant cause of
failure in the chemotherapeutic treatment of several types of
cancer (23). MDR involves
cross-resistance to unassociated compounds by exposure to an
anticancer agent (24). MDR cancer
cells often exhibit elevated abilities of increased efflux
(ATP-dependent efflux pumps) or decreased influx. The development
of MDR is mediated by different mechanisms, and conventional
resistance to anticancer drugs has been linked predominantly to the
overexpression of ABC transporters, including P-gp, multi-drug
resistance-associated protein-1 and the breast cancer resistance
protein (12).
Doxorubicin is an anthracycline antibiotic and is
commonly used as an effective agent inthe treatment of several
types of cancer, including bladder, breast and stomach cancer and
multiple myeloma (25). Notably,
the resistance of drugs, including doxorubicin, cisplatin and
vinblastine, represents a major obstacle in the successful
treatment of MDR cancer (26).
There have been several reports on the use of doxorubicin in
combination with certain other drugs, including fluorouracil and
cyclophosphamide (27). The
identification of novel combination drugs, which correlate with
treatment response, has the potential to improve the success of MDR
cancer therapy.
Salinomycin is an ionophore, which is used as a
therapeutic antibacterial and coccidiostat (16). A previous study reported that
salinomycin causes breast cancer stem cell death, by screening
16,000 different chemical compounds targeting cancer stem cell
metastasis and relapse (17). In
our previous study, salinomycin was observed to induce apoptosis
via the ROS-mediated mitochondrial pathway (19). Other studies have supported these
results in variety of cancer cells (23). It has also been demonstrated that
salinomycin, as P-gp inhibitors, exhibit potent antiproliferative
activity against MDR cancer cells (20).
The present study hypothesized that salinomycin may
effectively inhibit binding with P-gp and thus decrease the efflux
of anticancer agents from the MDR cancer cells. To investigate
this, doxorubicin-resistant MCF-7/MDR cells were treated with
doxorubicin, alone or in combination with salinomycin, to assess
cell viability. The results revealed the enhancement of
salinomycin-mediated cytotoxicity in the MCF-7/MDR cells. This
finding was consistent with those of the confocal microscope
analysis, in which the doxorubicin fluorescent signals of the
salinomycin-treated cells were higher compared with those in the
cells treated with doxorubicin alone. In several previous studies,
salinomycin has been revealed as a substrate with increased
P-gp-dependent transport in MDR cancer cells, as evidenced through
drug efflux assays in MDR cancer cell lines (28). Use of a conformational P-gp assay
in a previous study provided evidence that the inhibitory effect of
salinomycin on P-gp function may be mediated by the induction of
ATP transporter conformational change (28). Using flow cytometric analysis, the
present study confirmed that salinomycin significantly increased
the net cellular uptake and decreased the cellular efflux of
doxorubicin. Even 2 h following the removal of doxorubicin and
salinomycin, the efflux rate of intracellular doxorubicin remained.
Another important feature of salinomycin is that it facilitates
bidirectional ion flux through the lipid barrier of membranes,
acting as channel blockers to inhibit cell proliferation (29). A similar competitive mechanism has
been observed in the reversal of MDR by P-gp inhibitors, including
verapamil and cyclosporine A (30,31).
In the present study, an efflux was examined using RT-qPCR and
western blot analysis, to determine whether this accumulation
effect was directly associated with the gene and protein expression
of MDR1 and MRP. The expression levels of MDR-1 and MRP-1 were not
altered at either the mRNA or protein levels in the cells treated
with salinomycin. These results indicated that salinomycin was
mediated by its ability to promote the increased uptake and
decreased efflux of doxorubicin in the MCF-7/MDR cells.
In analyzing the resistance of MCF-7/MDR cells
treated with doxorubicin, the present study demonstrated that
salinomycin was a significant inhibitor, with a reversal effect on
the resistance of cancer cells. When used in combination with
chemotherapeutic agents in MDR cancer, salinomycin may have
beneficial therapeutic effects.
Acknowledgments
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF), funded by the Ministry of Science, ICT and Future Planning
(grant. no. NRF-2012R1A1A2022587) and an NRF grant funded by the
Korea Government (grant. no. 2012R1A1 A4A01019016).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singletary SE: Rating the risk factors for
breast cancer. Ann Surg. 237:474–482. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berman AT, Thukral AD, Hwang WT, Solin LJ
and Vapiwala N: Incidence and patterns of distant metastases for
patients with early-stage breast cancer after breast conservation
treatment. Clin Breast Cancer. 13:88–94. 2013. View Article : Google Scholar
|
|
4
|
Giordano SH, Cohen DS, Buzdar AU, Perkins
G and Hortobagyi GN: Breast carcinoma in men: a population-based
study. Cancer. 101:51–57. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prat A and Perou CM: Deconstructing the
molecular portraits of breast cancer. Mol Oncol. 5:5–23. 2011.
View Article : Google Scholar
|
|
6
|
Nilsson S, Koehler KF and Gustafsson JA:
Development of subtype-selective oestrogen receptor-based
therapeutics. Nat Rev Drug Discov. 10:778–792. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Higgins MJ and Baselga J: Targeted
therapies for breast cancer. J Clin Invest. 121:3797–3803. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim TH, Shin YJ, Won AJ, Lee BM, Choi WS,
Jung JH, Chung HY and Kim HS: Resveratrol enhances chemosensitivity
of doxorubicin in multidrug-resistant human breast cancer cells via
increased cellular influx of doxorubicin. Biochim Biophys Acta.
1840:615–625. 2014. View Article : Google Scholar
|
|
10
|
Ullah MF: Cancer multidrug resistance
(MDR): a major impediment to effective chemotherapy. Asian Pac J
Cancer Prev. 9:1–6. 2008.PubMed/NCBI
|
|
11
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sharom FJ: ABC multidrug transporters:
structure, function and role in chemoresistance. Pharmacogenomics.
9:105–127. 2008. View Article : Google Scholar
|
|
13
|
Choudhuri S and Klaassen CD: Structure,
function, expression, genomic organization, and single nucleotide
polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP)
efflux transporters. Int J Toxicol. 25:231–259. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pressman BC: Antibiotic models for
carrier-mediated transport through membranes. Antimicrob. Agents
Chemother (Bethesda). 9:28–34. 1969.
|
|
15
|
Rutkowski J and Brzezinski B: Structures
and properties of naturally occurring polyether antibiotics. Biomed
Res Int. 2013:1–31. 2013. View Article : Google Scholar
|
|
16
|
Naujokat C, Fuchs D and Opelz G:
Salinomycin in cancer: A new mission for an old agent. Mol Med Rep.
3:555–559. 2010. View Article : Google Scholar
|
|
17
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Al Dhaheri Y, Attoub S, Arafat K, Abuqamar
S, Eid A, Al Faresi N and Iratni R: Salinomycin induces apoptosis
and senescence in breast cancer: upregulation of p21,
downregulation of survivin and histone H3 and H4 hyperacetylation.
Biochim Biophys Acta. 1830:3121–3135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim KY, Yu SN, Lee SY, Chun SS, Choi YL,
Park YM, Song CS, Chatterjee B and Ahn SC: Salinomycin-induced
apoptosis of human prostate cancer cells due to accumulated
reactive oxygen species and mitochondrial membrane depolarization.
Biochem Biophys Res Commun. 413:80–86. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fuchs D, Daniel V, Sadeghi M, Opelz G and
Naujokat C: Salinomycin overcomes ABC transporter-mediated
multidrug and apoptosis resistance in human leukemia stem cell-like
KG-1a cells. Biochem Biophys Res Commun. 394:1098–1104. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Verdoodt B, Vogt M, Schmitz I, Liffers ST,
Tannapfel A and Mirmohammadsadegh A: Salinomycin induces autophagy
in colon and breast cancer cells with concomitant generation of
reactive oxygen species. PLoS One. 7:e441322012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoon MJ, Kang YJ, Kim IY, Kim EH, Lee JA,
Lim JH, Kwon TK and Choi KS: Monensin, a polyether ionophore
antibiotic, overcomes TRAIL resistance in glioma cells via
endoplasmic reticulum stress, DR5 upregulation and c-FLIP
downregulation. Carcinogenesis. 34:1918–1928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu CP, Calcagno AM and Ambudkar SV:
Reversal of ABC drug transporter-mediated multidrug resistance in
cancer cells: evaluation of current strategies. Curr Mol Pharmacol.
1:93–105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thomas H and Coley HM: Overcoming
multidrug resistance in cancer: an update on the clinical strategy
of inhibiting p-glycoprotein. Cancer Control. 10:159–165.
2003.PubMed/NCBI
|
|
25
|
Rahman AM, Yusuf SW and Ewer MS:
Anthracycline-induced cardiotoxicity and the cardiac-sparing effect
of liposomal formulation. Int J Nanomedicine. 2:567–583. 2007.
|
|
26
|
Liu Y, Liu H, Han B and Zhang JT:
Identification of 14-3-3sigma as a contributor to drug resistance
in human breast cancer cells using functional proteomic analysis.
Cancer Res. 66:3248–3255. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vulsteke C, Lambrechts D, Dieudonné A, et
al: Genetic variability in the multidrug resistance associated
protein-1 (ABCC1/MRP1) predicts hematological toxicity in breast
cancer patients receiving (neo-) adjuvant chemotherapy with
5-fluorouracil, epirubicin and cyclophosphamide (FEC). Ann Oncol.
24:1513–1525. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Riccioni R, Dupuis ML, Bernabei M,
Petrucci E, Pasquini L, Mariani G, Cianfriglia M and Testa U: The
cancer stem cell selective inhibitor salinomycin is a
p-glycoprotein inhibitor. Blood Cells Mol Dis. 45:86–92. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Le Guennec JY, Ouadid-Ahidouch H, Soriani
O, Besson P, Ahidouch A and Vandier C: Voltage-gated ion channels,
new targets in anti-cancer research. Recent Pat Anticancer Drug
Discov. 2:189–202. 2007. View Article : Google Scholar
|
|
30
|
Tsubaki M, Komai M, Itoh T, Imano M,
Sakamoto K, Shimaoka H, Takeda T, Ogawa N, Mashimo K, Fujiwara D,
Mukai J, Sakaguchi K, Satou T and Nishida S: By inhibiting Src,
verapamil and dasatinib overcome multidrug resistance via increased
expression of Bim and decreased expressions of MDR1 and survivin in
human multidrug-resistant myeloma cells. Leuk Res. 38:121–130.
2014. View Article : Google Scholar
|
|
31
|
De Souza PS, da Cunha Vasconcelos F, Silva
LF and Maia RC: Cyclosporine A enables vincristine-induced
apoptosis during reversal of multidrug resistance phenotype in
chronic myeloid leukemia cells. Tumour Biol. 33:943–956. 2012.
View Article : Google Scholar : PubMed/NCBI
|