Introduction
The hepatitis C virus (HCV) is a major cause of
chronic hepatitis, cirrhosis and hepatocellular carcinoma (1,2). An
estimated 185 million people around the world are infected with
HCV, with three to four million new cases each year (3). However, the pathogenesis of HCV
remains to be fully elucidated. The standard of care (SOC) for HCV
is the combined application of pegylated interferon α and
ribavirin, with a sustained virological response (SVR) rate of up
to 50%; however, the various genetic subtypes of HCV exhibit
differing response rates to treatment (4). In addition, the treatment cost is
high and there is a significant risk of side effects (5,6). The
development of small molecule inhibitors targeting HCV
replication-associated enzymes has recently achieved success in HCV
therapy, with a significant increase in SVR (7–10).
Despite this advance, the issue of resistance remains a problem
(11,12), even when used in combination
therapy with the SOC. Therefore, the development of novel antiviral
strategies is required in order to more effectively treat HCV
infection.
RNA interference (RNAi) is a post-transcriptional
cellular process mediated by short (21–25 nucleotides)
double-stranded RNA molecules (13), which are capable of gene silencing
(14–17). MicroRNAs (miRNAs) are members of
this group of small RNAs. The primary miRNAs are processed by the
Drosha ribozyme and DGCR8 into precursor miRNAs consisting of 60–70
nucleotides (18). These miRNAs
are subsequently exported from the nucleus by Exportin-5 (19), and processed into mature miRNAs by
the ribonuclease-III enzyme Dicer (18). The guiding strand of the mature
miRNA is then loaded into the RNA-induced silencing complex
(20) to form the miRNA-containing
ribonucleoprotein complex (miRNP), which is able to mediate the
cleavage of target mRNA or result in translational repression
(16). Artificial miRNAs (amiRNAs)
comprise a class of artificially synthesized RNAs (21), similar to cellular miRNAs, which
may be harnessed to silence mRNAs encoding pathogenic proteins for
use in therapeutic strategies and functional genomics (22).
The HCV genome is a 9.6 kb positive-stranded RNA
molecule containing a long open reading frame encoding a
polyprotein precursor, which is processed by cellular and viral
proteases into structural (Core, E1 and E2) and nonstructural (p7,
NS2, NS3, NS4A, NS4B, NS5A and NS5B) proteins. The HCV core protein
is a conserved protein, which comprises the viral nucleocapsid and
is able to bind viral RNA. The HCV core protein is also a
multifunctional, influencing gene transcription, lipid metabolism,
apoptosis and a variety of signal transduction pathways in host
cells (23–26). In addition, the core protein has
been implicated in HCV-associated steatosis and carcinogenesis in
transgenic mice (27,28). This protein is hypothesized to
serve a function in the assembly of HCV (29). HCVNS4B is a hydrophobic integral
membrane protein that has a central function in the formation of
the membranous web; a specific membrane alteration, which serves as
a scaffold for the HCV replication complex (30–32).
In addition, NS4B possesses NTPase (33,34)
and RNA-binding activities (35),
as well as contributing to viral assembly and release (36,37).
Therefore, HCV core and NS4B mRNAs may be used as targets for
RNAi.
In the present study, two sets of amiRNA expression
vectors were designed and constructed against the HCV1b genotype
core and NS4B mRNAs, in order to evaluate the interference
efficiency of amiRNAs on HCV core and NS4B gene expression in HepG2
cells. In addition, the present study aimed to screen the optimum
miRNA interference vectors for use in subsequent studies on the
functions and pathogenesis of HCV core and NS4B genes, as well as
the examination of novel classes of inhibitors for HCV
infection.
Materials and methods
Collection of serum samples
A total of 15 patients with HCV1b were enrolled in
the present study from the First Affiliated Hospital of The
University of South China (Hengyang, China). The patients included
7 males and 8 females, and were aged between 27 and 66 years old.
The venous blood samples of the patients were collected by a
trained nurse at the Research Center of Liver Diseases (First
Affiliated Hospital of the University of South China). After
centrifugation at 241 × g for 10 min at room temperature, the serum
was obtained and preserved at −80°C until further use. The
quantification and genotyping of HCV RNA were performed by the
Research Center of Liver Diseases. The present study was conducted
in accordance with the Declaration of Helsinki, and with approval
from the Ethics Committee of Central South University (Changsha,
China). Written informed consent was obtained from all
participants.
Construction of plasmids
TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) was used to extract HCV RNA from the serum of
HCV1b patients. The serum was then reverse transcribed into cDNA
using the SuperScript III First-strand synthesis system (Invitrogen
Life Technologies). Subsequently, polymerase chain reaction (PCR)
amplification was performed with gene-specific primers (Shanghai
Sangon Biological Technology and Services Co., Ltd., Shanghai,
China). The primer sequences of the core gene amplification in the
first round were as follows: HCV1b sense, 5′-GAGTAGYGT
TGGGTCGCGAAAGG-3′ and antisense, 5′-TTGAGTTGGAGCAGTCGTTCGTGA-3′. In
the second round, the PCR primer sequences were as follows: HCV1b
sense, 5′-GCCACGAAG CTTGCATGAGCACRAATCCWAAAC-3′ (AAGCTT is the
enzyme restriction site of HindIII) and antisense,
5′-TAGATTGGATCCAARGCGGAAGCTGGGRTGGTC-3′ (GGATCC is the enzyme
restriction site of BamHI). The primer sequences of NS4B
gene amplification in the first round were as follows: NS4B sense,
5-′GAGTGCGCYTCRCACCTCCCT TA-3′ and antisense,
5′-CGGAGCAYGGYGTGGAGCAG-3′. In the second round, the PCR sense
primer sequences were as follows: NS4B sense,
5′-GTCCAGAAGCTTGCATGGCYTCRCACCTCCCTTA-3′ (AAGCTT is the enzyme
restriction site of HindIII) and antisense,
5′-TAGATTGGATCCAAGCAYGGYGTGGAGCAGTCCT-3′ (GGATCC is the enzyme
restriction site of BamHI). The PCR conditions consisted of
pre-denaturation, 94°C for 3 min; denaturation, 94°C for 30 sec;
annealing, 61°C for 30 sec and extension, 72°C for 1 min. This
cycle was repeated 35 times with a final extension at 72°C for 10
min. The amplified HCV1bCore and HCV1bNS4B genes contained the
double-enzyme restriction sites of HindIII and BamHI
(Fermentas International Inc., Burlington, ON, Canada), which were
then used to digest the two aforementioned target fragments and
vector pDsRed-monomer-N1 (Clontech, Mountain View, CA, USA).
Following purification, the two digested fragments were connected
with the vectors using T4 DNA ligase (Fermentas International
Inc.), and were then transformed into DH5α competent cells
(preserved in the Laboratory of Infectious Diseases, Xiangya
Hospital, Changsha, China) to construct the plasmids of
pDsRed-monomer-Core and pDsRed-monomer-NS4B. The constructed
plasmids were then subjected to double digestion and sequencing
(Invitrogen Life Technologies, Shanghai, China).
Construction of miRNA expression
vectors
The HCV core and NS4B genetic information was
submitted through the BLOCK-iT™ RNAi Express database (http://rnaidesigner.invitrogen.com/rnaiexpress/rnaiExpress.jsp)
to select the interference sites. The gene specificity of all
interference sites was also assessed using the Basic Local
Alignment Search Tool (BLAST; http://blast.ncbi.nlm.nih.gov/Blast.cgi). A total of
two sets of miRNA interference sequences were designed and the
single-stranded DNA oligonucleotides (Invitrogen Life Technologies)
of eight pairs of miRNAs and those of a negative control miRNA,
were synthesized. The sequences are shown in Table I. The eight pairs of
single-stranded DNA oligonucleotides were then annealed and the
double strands were formed. The vector construction kit BLOCK-iT™
Pol II miR RNAi expression vector kit with Emerald Green
Fluorescent Protein (EmGFP) (Invitrogen Life Technologies,
Carlsbad, CA, USA) was then used to recombine the clones. A total
of eight pairs of double-stranded oligonucleotides were inserted
into the miRNA expression vector pcDNA™ 6.2-GW/EmGFP-miR to
construct eight miRNA expression plasmids for core silencing
(pmiRE-C-mi1 to -mi4) and NS4B silencing (pmiRE-NS4B-mi1 to -mi4).
The above plasmids were transformed into DH5α competent cells.
Following agitation extraction using TIANprep Mini Plasmid kit
(Tiangen Biotech (Beijing) Co., Ltd., Beijing, China) in a SHZ-88
Thermostatic Oscillator (Jintan Medical Instrument Factory,
Changzhou, China), the extracted plasmids were sequenced, using the
reverse sequencing primer 5′-CTCTAGATCAACCACTTTGT-3′, to verify
whether the fragment sequence inserted into the recombinant clones
was consistent with the designed single-stranded DNA
oligonucleotide sequence.
 | Table ISequences of oligonucleotides for
microRNAs directed against hepatitis C virus 1b core and
non-structural protein 4B genes. |
Table I
Sequences of oligonucleotides for
microRNAs directed against hepatitis C virus 1b core and
non-structural protein 4B genes.
| Oligo name | Oligo
single-stranded DNA sequence 5′ to 3′ |
|---|
| C-mi1F |
TGCTGATAGGTTGTCGCCTTCCACGAGTTTTGGCCACTGACTGACTCGTGGAACGACAACCTAT |
| C-mi1R |
CCTGATAGGTTGTCGTTCCACGAGTCAGTCAGTGGCCAAAACTCGTGGAAGGCGACAACCTATC |
| C-mi2F |
TGCTGTATCGATGACCTTACCCAAATGTTTTGGCCACTGACTGACATTTGGGTGGTCATCGATA |
| C-mi2R |
CCTGTATCGATGACCACCCAAATGTCAGTCAGTGGCCAAAACATTTGGGTAAGGTCATCGATAC |
| C-mi3F |
TGCTGATTCCCTGTTGCGTAGTTCACGTTTTGGCCACTGACTGACGTGAACTACAACAGGGAAT |
| C-mi3R |
CCTGATTCCCTGTTGTAGTTCACGTCAGTCAGTGGCCAAAACGTGAACTACGCAACAGGGAATC |
| C-mi4F |
TGCTGCAAACAGGACAGCAGAGCCAAGTTTTGGCCACTGACTGACTTGGCTCTTGTCCTGTTTG |
| C-mi4R |
CCTGCAAACAGGACAAGAGCCAAGTCAGTCAGTGGCCAAAACTTGGCTCTGCTGTCCTGTTTGC |
| NS4B-mi1F |
TGCTGAGTGCCTTCTGCTTGAATTGTGTTTTGGCCACTGACTGACACAATTCACAGAAGGCACT |
| NS4B-mi1R |
CCTGAGTGCCTTCTGTGAATTGTGTCAGTCAGTGGCCAAAACACAATTCAAGCAGAAGGCACTC |
| NS4B-mi2F |
TGCTGTAAGCCTGCTAAGTACTGTATGTTTTGGCCACTGACTGACATACAGTATAGCAGGCTTA |
| NS4B-mi2R |
CCTGTAAGCCTGCTATACTGTATGTCAGTCAGTGGCCAAAACATACAGTACTTAGCAGGCTTAC |
| NS4B-mi3F |
TGCTGTTAAACAGGAGGGTGTGTTGGGTTTTGGCCACTGACTGACCCAACACACTCCTGTTTAA |
| NS4B-mi3R |
CCTGTTAAACAGGAGTGTGTTGGGTCAGTCAGTGGCCAAAACCCAACACACCCTCCTGTTTAAC |
| NS4B-mi4F |
TGCTGTTCAGTAACTGAGTGATGGTAGTTTTGGCCACTGACTGACTACCATCACAGTTACTGAA |
| NS4B-mi4R |
CCTGTTCAGTAACTGTGATGGTAGTCAGTCAGTGGCCAAAACTACCATCACTCAGTTACTGAAC |
| Negative-F |
TGCTGAAATGTACTGCGCGTGGAGACGTTTTGGCCACTGACTGACGTCTCCACGCAGTACATTT |
| Negative-R |
CCTGAAATGTACTGCGTGGAGACGTCAGTCAGTGGCCAAAACGTCTCCACGCGCAGTACATTTC |
Cell culture and transfection
HepG2 cells (preserved in the Laboratory of
Infectious Diseases, Xiangya Hospital) were cultivated in
Dulbecco’s modified Eagle’s medium supplemented with 10% fetal
bovine serum (FBS; Invitrogen Life Technologies) in a humidified 5%
CO2 atmosphere at 37°C. HepG2 cells at the logarithmic
growth phase were seeded into six-well plates at a density of
~1.0×106 cells/well. When the cell density reached ~80%,
1 µg target gene plasmid and 3 µg interference
plasmid or negative control plasmid were mixed with
Lipofectamine® 2000 (Invitrogen Life Technologies). The
transfection was performed according to the Lipofectamine 2000
manufacturer’s instructions. Following 48 h, the
transfected-cellular fluorescent protein expression was observed
under a fluorescence microscope (Olympus IX71; Olympus Corp.,
Tokyo, Japan).
Reverse transcription quantitative PCR
(RT-qPCR)
At 48 h post-transfection, the total RNA of each
group was extracted using TRIzol reagent (Invitrogen Life
Technologies). Subsequently, 5 µg of RNA was reverse
transcribed into cDNA with SuperScript III reverse transcriptase
(Invitrogen Life Technologies). The obtained cDNA was then used as
the template for the PCR reaction. The primer sequences were as
follows: HCV core sense, 5′-TACGGCAACGAGGGCTTAG-3′ and antisense,
5′-ATGGTCAAACAGGACAGCAGAG-3′; NS4B sense, 5′-CTCGCCGAACAATTCAAGC-3′
and antisense, 5′-GTTCCCAGGCAGAGTGGATAA-3′; and GAPDH sense,
5′-GAAGGTCGGAGTCAACGGATT-3′ and antisense,
5′-CGCTCCTGGAAGATGGTGAT-3′ (Shanghai Sangon Biological Technology
and Services Co., Ltd.). The PCR reaction mixture consisted of 1.2
µl cDNA, 2.5 µl 10X PCR buffer, 0.2 µl dNTPs
(25 mM), 0.5 µl each of upstream and downstream primers (10
µM), 0.5 µl SYBR (50X), 0.3 µl of Taq enzyme
(5u/µl), 2 µl of Mg2+ (25 mM) and 17.3
µl of ultrapure water (Shanghai Sangon Biological Technology
and Services Co., Ltd.). The total volume of the reaction mixture
was 25 µl. The PCR reaction conditions were as follows: 95°C
for 2 min; 95°C for 10 sec, 60°C for 30 sec and 70°C for 45 sec for
40 cycles. All RT-qPCR experiments were performed in triplicate on
a 7500 Fast real-time PCR system (Applied Biosystems, Foster City,
CA, USA). Based on the RT-qPCR reaction curve, the Ct values of the
target gene and reference gene of each sample were obtained using
the 2−ΔΔCt method for the relative quantification. The
interference efficiency of the target gene was
1–2−ΔΔCt.
Flow cytometric analysis
The two RT-qPCR-screened miRNA vectors with the
greatest interfering effects against the HCV core and NS4B genes,
as well as the negative control miRNA vector, were co-transfected
with the cells containing the target gene for 48 h. The cells were
then digested with 0.25% trypsin (Fuzhou Maixin Biotechnology
Development Co., Ltd., Fuzhou, China), washed twice with
phosphate-buffered saline (PBS; Fuzhou Maixin Biotechnology
Development Co., Ltd.) and resuspended in PBS. Samples were
subsequently assessed using a FACS Calibur flow cytometer (Becton
Dickinson, Franklin Lakes, NJ, USA) to calculate the percentage of
fluorescent cells and the mean fluorescence intensity.
Western blot analysis
The two screened miRNA vectors with the optimum
interfering effects, as well as the negative control miRNA vector,
were co-transfected into the cells with the target gene for 48 h.
The cells were then lysed with radioimmunoprecipitation assay
buffer (Beyotime Institute of Biotechnology, Jiangsu, China).
Following centrifugation at 1,048 × g for 5 min at 4°C, the
supernatant was collected for protein concentration detection.
Subsequently, 60 µg of protein was separated using 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred onto a nitrocellulose membrane (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) under semi-dry conditions. Blots were
blocked with 5% non-fat milk (Beyotime Institute of Biotechnology)
in Tris-buffered saline with 0.1% Tween 20 (Sigma-Aldrich, St.
Louis, MO, USA) at room temperature for 2 h. The membrane was
incubated overnight at 4°C with the following primary antibodies:
Mouse anti-HCV core antigen antibody (cat. no. ab2740; 1:1,000
dilution; Abcam, Cambridge, UK), mouse anti-HCV NS4B monoclonal
antibody (cat. no. ab24283; 1:1,000 dilution; Abcam) and β-actin
antibody (cat. no. sc-47778; 1:1,000 dilution; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Following washing the
membrane, the blots were incubated with horseradish
peroxidase-conjugated goat anti-mouse immunoglobulin G (cat. no.
A0216; 1:5,000 dilution; Beyotime Institute of Biotechnology) at
room temperature for 2 h. The signals were revealed with the
BeyoECL Plus enhanced chemiluminescent kit (Beyotime Institute of
Biotechnology).
Statistical analysis
Statistical analyses were carried out using SPSS
version 16.0 software (SPSS Inc., Chicago, IL, USA). Data are
expressed as the mean ± standard deviation. Significance between
groups was determined by one-way analysis of variance followed by a
Student-Newman-Keuls post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Construction of plasmids
The target gene-expressing plasmids,
pDsRed-monomer-Core and pDsRed-monomer-NS4B, were subjected to PCR
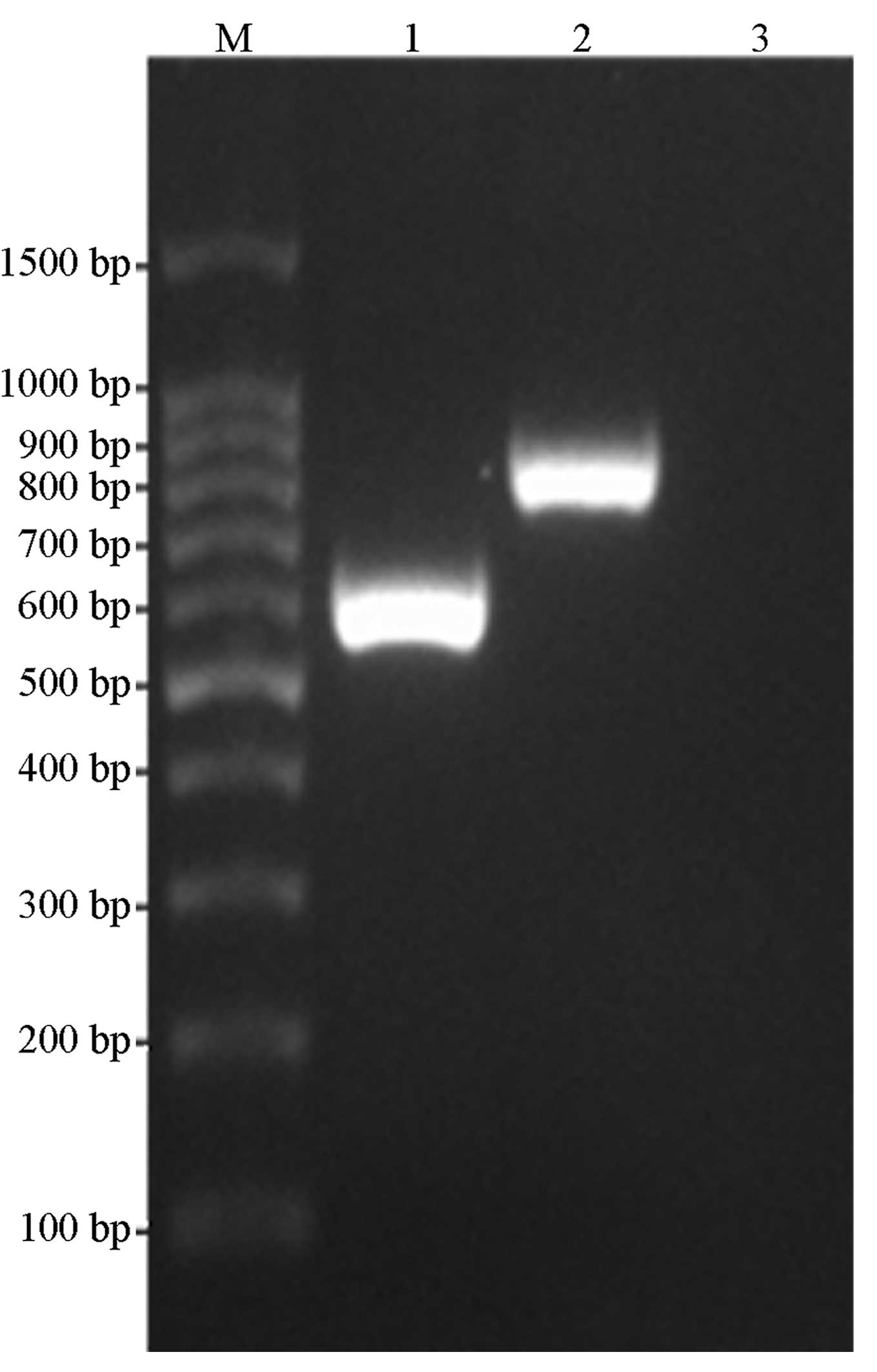
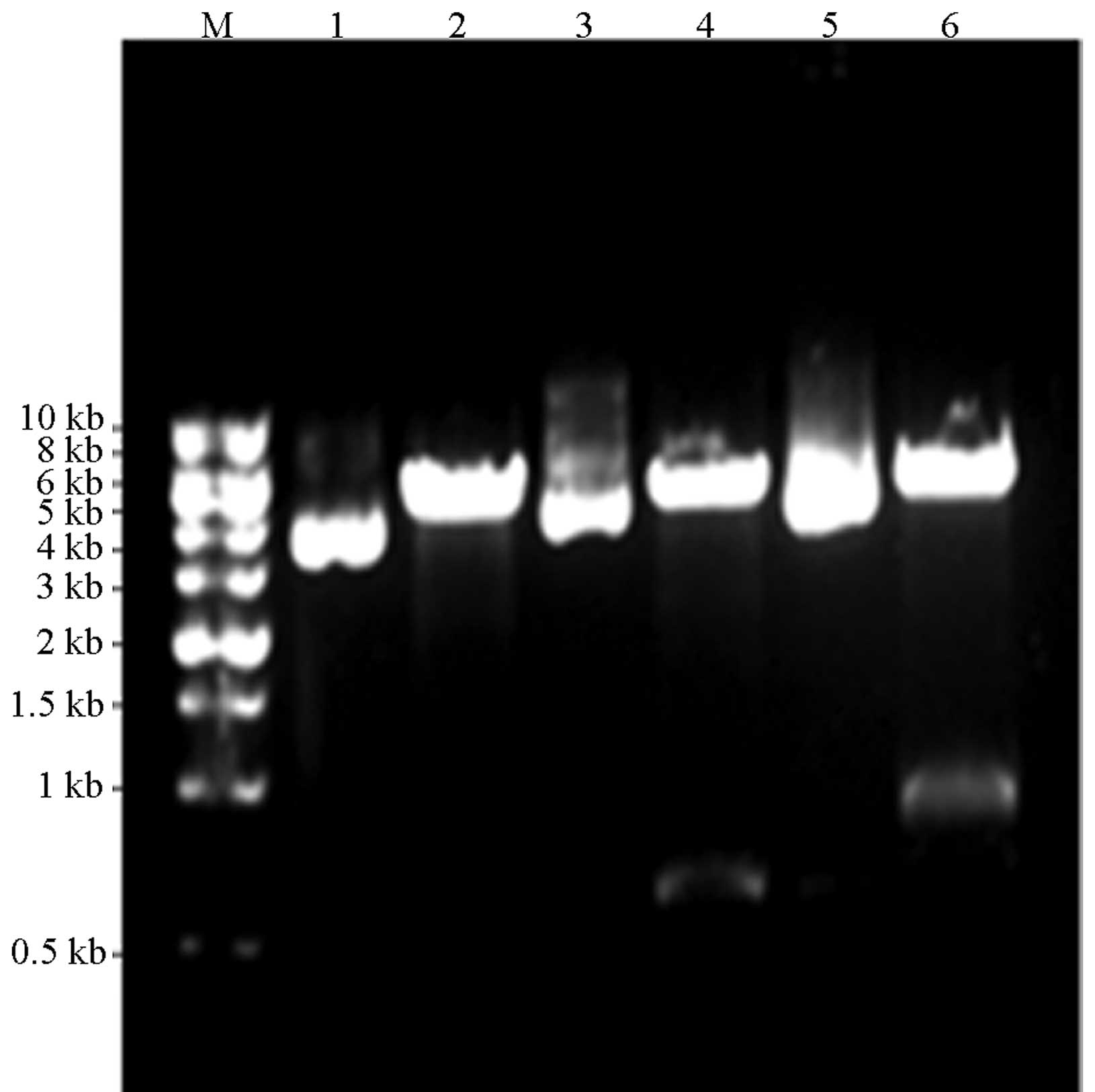
amplification, double-enzyme digestion and sequencing. The results
were consistent with the expected values, whereby the PCR gene
products of the HCV core and NS4B proteins were ~580 and 790 bp,
respectively and the digested fragment of pDsRed-monomer-N1 was
~4.7kb, as shown in Figs. 1 and
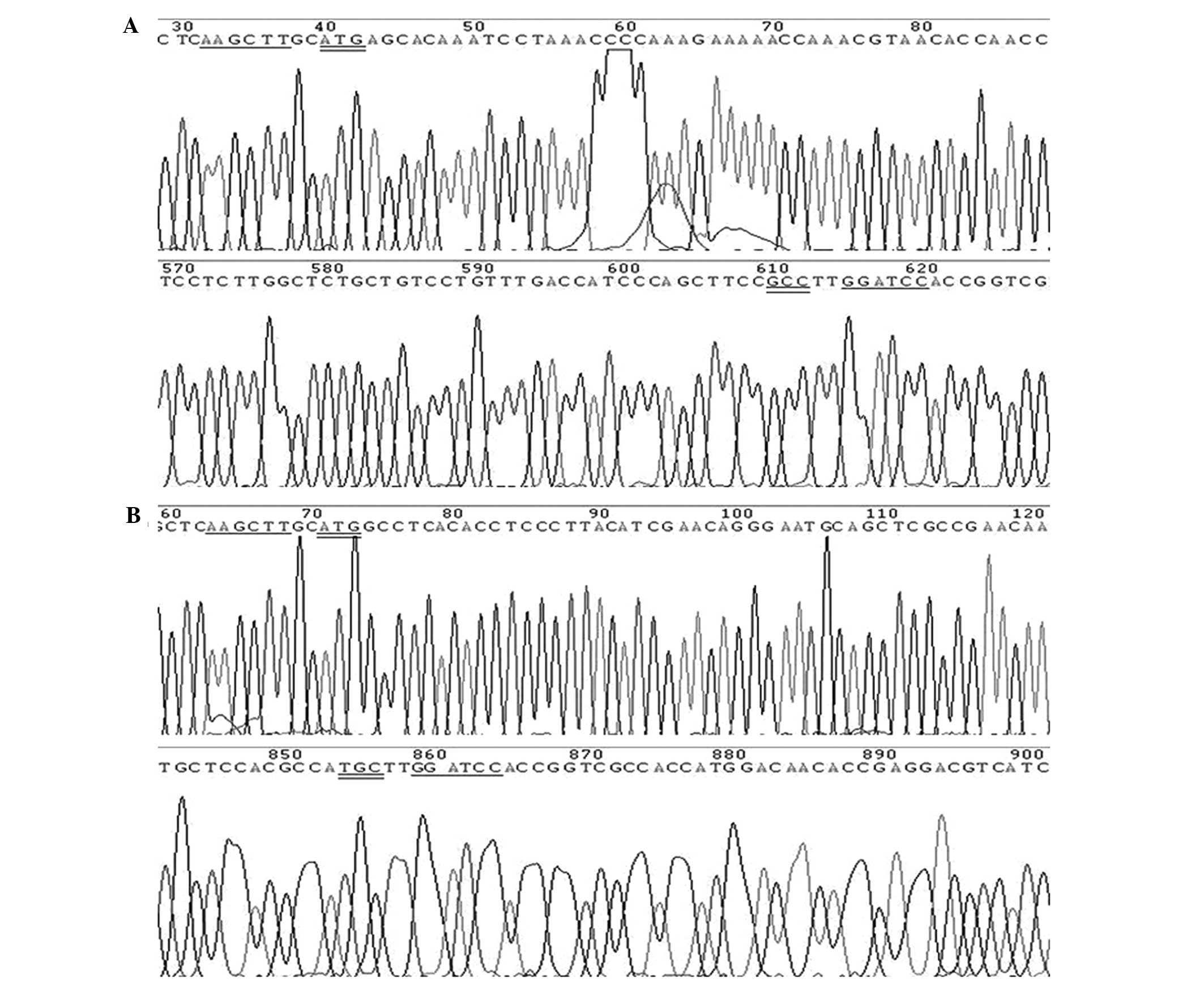
2. The DNA sequencing results
confirmed that two recombinant plasmids were efficiently
constructed. Partial sequencing peak patterns of the recombinant
pDsRed-monomer-Core and pDsRed-monomer-NS4B plasmids are shown in
Fig. 3.
Construction of miRNA expression
vectors
A total of eight miRNA expression plasmids,
pmiRE-C-mi1 to -mi4 and pmiRE-NS4B-mi1 to -mi4, were verified to
contain identical designed single-stranded DNA oligonucleotide
sequences using the sequencing test, relative to the inserted
fragment sequences (data not shown).
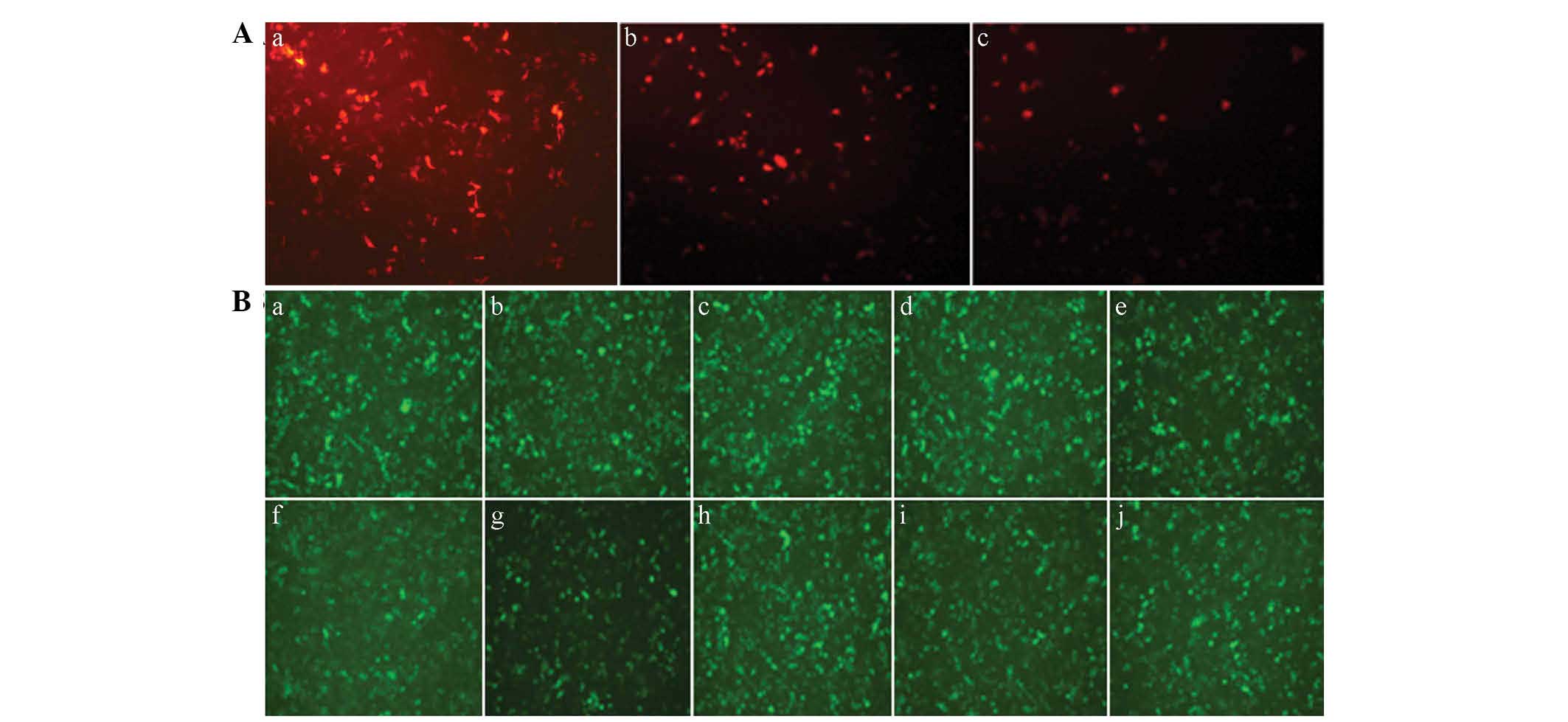
Expression of fluorescent proteins
A fluorescence microscope was used to observe the
fluorescent protein expression 48 h after the cells were
transfected (Fig. 4). The
fluorescence of the pDesRed-monomer-N1 (53.80±3.56%) was more
evident, as compared with that of the pDsRed-monomer-Core
(32.17±3.82%) and the pDsRed-monomer-NS4B (32.73±3.45%),
respectively (P<0.01). At 48 h post-transfection of the eight
pairs of miRNA expression plasmids, the cells of each group
exhibited green fluorescent protein expression indicating
successful transfection.
Expression of HCV core and NS4B
mRNAs
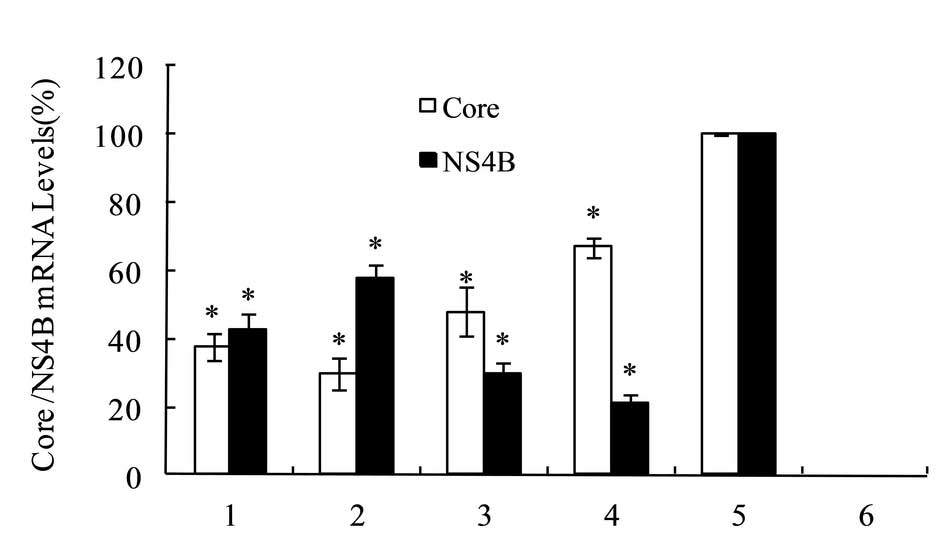
RT-qPCR was performed to assess the mRNA expression
levels of HCV core or NS4B in the HepG2 cells. The expression
levels of core or NS4B gene in HepG2 cells, which had been
co-transfected by the negative control miRNA and
pDsRed-monomer-Core or pDsRed-monomer-NS4B were set as 100%.
pmiRE-C-mi1 to -mi4 was co-transfected into the HepG2 cells with
pDsRed-monomer-Core. The relative expression levels of core mRNA
were 38.0±4.0, 30.0±4.6, 48.0±7.2 and 67.0±3.0%, respectively. The
pmiRE-NS4B-mi1 to -mi4 was co-transfected into the HepG2 cells with
pDsRed-monomer-NS4B, and the relative expression levels of NS4B
mRNA were 43.0±4.6, 58.0±4.0, 30.0±3.0 and 21.0±3.0%, respectively.
The results are shown in Fig. 5.
The results indicated that among the HCV core interference vectors,
the optimum gene silencing effect of was a reduction of 70%,
exerted by pmiRE-C-mi2; whereas, among the HCVNS4B interference
vectors, the optimum gene silencing effect was a decrease of 79%,
induced by pmiRE-NS4B-mi4.
Expression of HCV core and NS4B
proteins
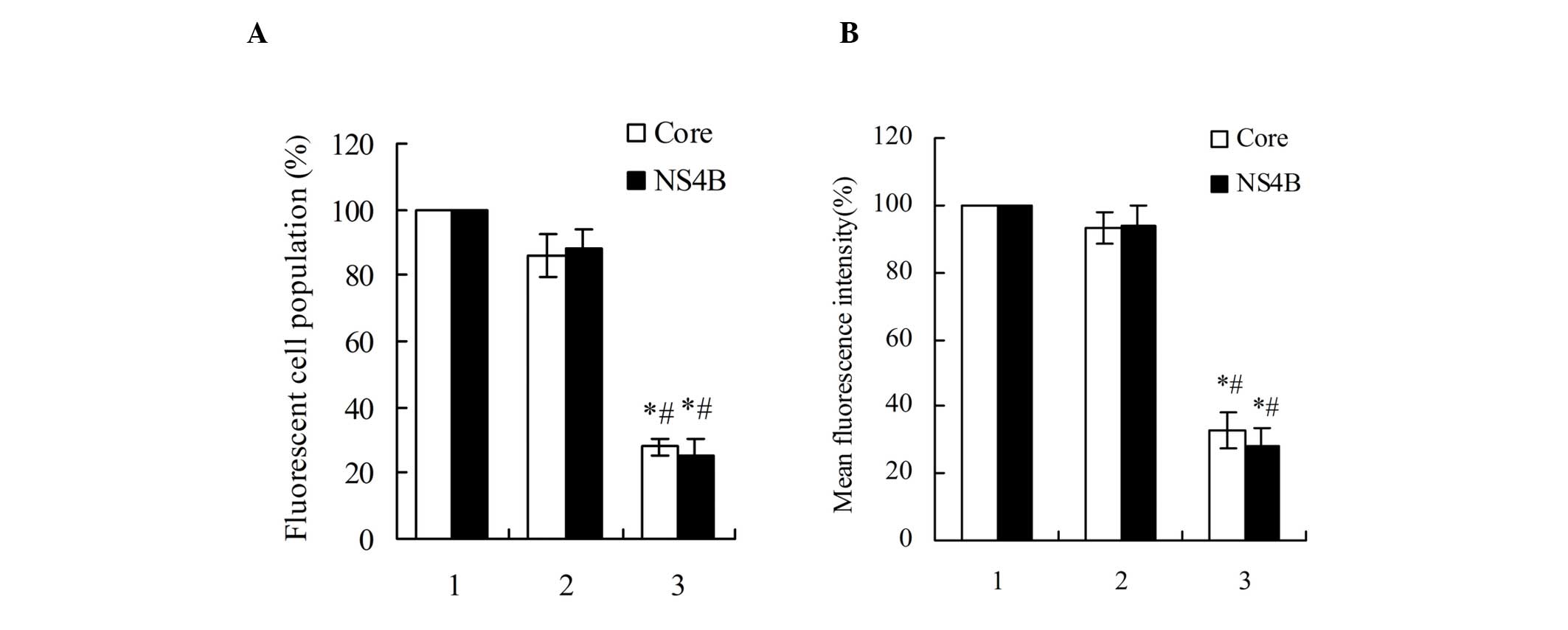
Flow cytometric analysis revealed that the single
transfection of pDsRed-monomer-Core or pDsRed-monomer-NS4B into the
HepG2 cells, when compared with the HepG2 cells that were
co-transfected by the negative control miRNA and
pDsRed-monomer-Core or pDsRed-monomer-NS4B, exhibited no
significant differences in terms of red fluorescent protein
percentage and average fluorescence intensity. In addition, the
pDsRed-monomer-Core and pmiRE-C-mi2 co-transfected HepG2 cells
exhibited a reduced percentage of red fluorescent protein
expression and average fluorescence intensity by 2.5 and 2.0-fold,
respectively. The red fluorescent protein percentage and average
fluorescence intensity in the pDsRed-monomer-NS4B and
pmiRE-NS4B-mi4 co-transfected HepG2 cells were also decreased by
3.0 and 2.6-fold, respectively, as shown in Fig. 6.
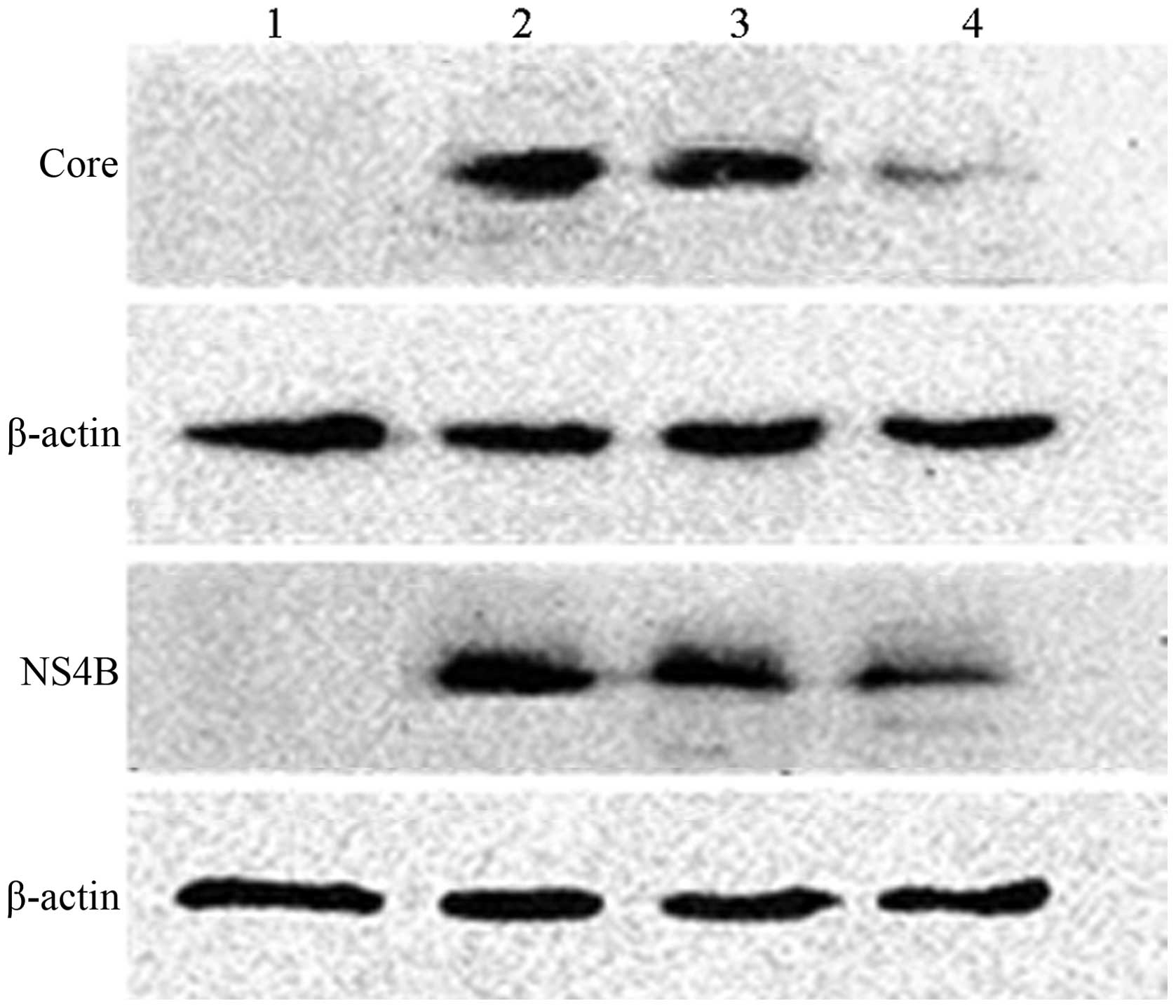
Western blot analysis revealed that, compared with
the HepG2 cells that were transfected by the pDsRed-monomer-Core
only, the pDsRed-monomer-Core and pmiRE-C-mi2 co-transfected HepG2
cells exhibited reduced expression of the HCV core protein; whereas
expression levels in the pDsRed-monomer-Core and negative control
miRNA co-transfected HepG2 cells were not decreased. Similar
results were also observed in the expression of NS4B protein, as
shown in Fig. 7.
Discussion
RNAi has become a powerful tool, with the potential
to knock down the expression of disease-associated genes or inhibit
viral gene expression (38). HCV
is comprised of a positive-stranded RNA virus, containing a
single-stranded RNA genome, which functions as mRNA for the
translation of viral proteins and the template for RNA replication
(39). As replication occurs in
the cytoplasm of liver cells without integration into the host
genome DNA, HCV has emerged as a target of RNAi (40). Certain in vivo and in
vitro experiments have demonstrated that exogenously
administered short-interfering RNAs are able to exert replication
inhibition against a variety of viruses (41–44).
In the present study, two sets of amiRNA expression vectors were
designed and constructed against HCV1b genotype core and NS4B
mRNAs, and it was investigated whether these amiRNAs were capable
of efficiently inhibiting the expression of the HCV core and NS4B
genes.
miRNAs are small non-coding RNA molecules,
consisting of ~22 nucleotides, which may be incorporated into miRNP
complexes and are able to induce mRNA degradation or translational
repression by binding to the 3′-untranslated regions of their
target mRNAs (45–48). The HCV core consists of three
distinct domains: An N-terminal hydrophilic domain of ~120 amino
acids, termed domain D1; a C-terminal hydrophobic domain of ~50
amino acids, termed domain D2; and the last 20 amino acids are
designated as the signal peptide of the downstream protein E1
(49). The domain D1 is mainly
involved in RNA binding and nucleocapsid formation. The domain D2
is responsible for core association with lipid droplets in
mammalian cells and with the membrane of the endoplasmic reticulum
(50). Previous studies have
revealed that expression levels of the HCV core protein are
correlated with the occurrence of hepatic steatosis, oxidative
stress and hepatocellular carcinoma (27,28,51).
The HCV core protein may also have a function in HCV assembly
(29). NS4B is an integral
membrane protein, which performs an essential function in HCV
replication (52–54). The NS4B protein may induce the
formation of the membranous web structure, which was hypothesized
to represent the platform upon which HCV replication is performed
(30–32). The NS4B protein also interacts with
NS4A, and indirectly interacts with NS3 and NS5A (55). In addition, NS4B has been found to
exhibit NTPase (33,34) and RNA binding (35) activities. Recently, NS4B has been
reported to regulate HCV genome encapsidation (36). In the present study, the silencing
effects of amiRNAs on the expression of HCV core and NS4B genes
were investigated. The RT-qPCR method was used to detect the mRNA
levels of HCV core and NS4B genes and the results demonstrated that
in the miRNA-transfected HepG2 cells, the mRNA levels of HCV core
and NS4B genes were effectively reduced. C-mi2 was identified for
its efficient silencing effect toward the core gene C-terminal
domain (amino acids 117–124), which is required for the folding and
stability of the core protein (49), reducing the expression of the core
gene to 70% at the transcriptional level. NS4B-mi4 was also
identified for its effective silencing effect toward the NS4B gene
C-terminal domain (amino acids 240–247), which can mediate membrane
association (56) and reduce the
expression of the NS4B gene to 79% at the transcriptional
level.
To clarify whether amiRNAs were able to effectively
inhibit the expression of target gene proteins in HepG2 cells, flow
cytometric analysis was applied. It was revealed that the red
fluorescent protein percentage and average fluorescence intensity
in the miRNA-transfected HepG2 cells were significantly reduced,
compared with those of the negative control miRNA-transfected HepG2
cells. Western blot analysis revealed that the core and NS4B
protein levels in the miRNA-transfected cells were also
significantly reduced compared with the negative control
miRNA-transfected cells, further confirming that the HCV core and
NS4B-targeting miRNAs were able to effectively inhibit the
expression of target genes. The results of the present study are
supported by multiple studies, demonstrating that amiRNAs are able
to effectively inhibit the replication of the rabies virus
(57), adenoviruses (58), the human immunodeficiency virus
(59) and the HCV (60).
The results of the present study also demonstrated
that the amiRNAs designed against various domains of the HCV core
and NS4B genes exhibited differing silencing effects against the
target genes in the HepG2 cells, which may be attributed to the
specific conformations between the miRNP and target mRNAs.
Therefore, the biological activities varied and influenced the
interference efficiency. Specific amiRNAs exhibit differing
silencing effects, such that the expressed miRNAs cannot completely
inhibit the expression of target genes. For this reason, targeting
multiple domains of the HCV genome associated with HCV replication
may be an effective method for the inhibition of viral gene
expression and prevention of the generation of viral
resistance.
In conclusion, the present data indicated that the
expression of amiRNAs may effectively and specifically inhibit the
expression of target HCV core and NS4B genes in HepG2 cells in
vitro, providing a powerful tool for follow-up studies
regarding HCV core and NS4B gene functions and pathogenesis. This
approach is expected to become a novel therapeutic strategy for
anti-HCV treatment.
References
|
1
|
Alter MJ: Epidemiology of hepatitis C
virus infection. World J Gastroenterol. 13:2436–2441. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lavanchy D: The global burden of hepatitis
C. Liver Int. 29:74–81. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mohd Hanafiah K, Groeger J, Flaxman AD and
Wiersma ST: Global epidemiology of hepatitis C virus infection: New
estimates of age-specific antibody to HCV seroprevalence.
Hepatology. 57:1333–1342. 2013. View Article : Google Scholar
|
|
4
|
Zhu Y and Chen S: Antiviral treatment of
hepatitis C virus infection and factors affecting efficacy. World J
Gastroenterol. 19:8963–8973. 2013. View Article : Google Scholar :
|
|
5
|
Sarasin-Filipowicz M: Interferon therapy
of hepatitis C: molecular insights into success and failure. Swiss
Med Wkly. 140:3–11. 2010.
|
|
6
|
Feld JJ: The beginning of the end: what is
the future of interferon therapy for chronic hepatitis C? Antiviral
Res. 105:32–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Poordad F, McCone J Jr, Bacon BR, et al:
Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J
Med. 364:1195–1206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jacobson IM, McHutchison JG, Dusheiko G,
et al: Telaprevir for previously untreated chronic hepatitis C
virus infection. N Engl J Med. 364:2405–2416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gane EJ, Stedman CA, Hyland RH, et al:
Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for
hepatitis C. N Engl J Med. 368:34–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lawitz E, Mangia A, Wyles D, et al:
Sofosbuvir for previously untreated chronic hepatitis C infection.
N Engl J Med. 368:1878–1887. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pawlotsky JM: Treatment failure and
resistance with direct-acting antiviral drugs against hepatitis C
virus. Hepatology. 53:1742–1751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wyles DL: Beyond telaprevir and
boceprevir: resistance and new agents for hepatitis C virus
infection. Top Antivir Med. 20:139–145. 2012.PubMed/NCBI
|
|
13
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fire A: RNA-triggered gene silencing.
Trends Genet. 15:358–363. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huntzinger E and Izaurralde E: Gene
silencing by microRNAs: contributions of translational repression
and mRNA decay. Nat Rev Genet. 12:99–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawamata T and Tomari Y: Making RISC.
Trends Biochem Sci. 35:368–376. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cullen BR: Transcription and processing of
human microRNA precursors. Mol Cell. 16:861–865. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yi R, Qin Y, Macara IG and Cullen BR:
Exportin-5 mediates the nuclear export of pre-microRNAs and short
hairpin RNAs. Genes Dev. 17:3011–3016. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sontheimer EJ: Assembly and function of
RNA silencing complexes. Nat Rev Mol Cell Biol. 6:127–138. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng Y, Wagner EJ and Cullen BR: Both
natural and designed microRNAs can inhibit the expression of
cognate mRNAs when expressed in human cells. Mol Cell. 9:1327–1333.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baumann V and Winkler J: miRNA-based
therapies: Strategies and delivery platforms for oligonucleotide
and non-oligonucleotide agents. Future Med Chem. 6:1967–1984. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fukutomi T, Zhou Y, Kawai S, Eguchi H,
Wands JR and Li J: Hepatitis C virus core protein stimulates
hepatocyte growth: correlation with upregulation of wnt-1
expression. Hepatology. 41:1096–1105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boulant S, Douglas MW, Moody L, Budkowska
A, Targett-Adams P and McLauchlan J: Hepatitis C virus core protein
induces lipid droplet redistribution in a microtubule- and
dynein-dependent manner. Traffic. 9:1268–1282. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sato Y, Kato J, Takimoto R, et al:
Hepatitis C virus core protein promotes proliferation of human
hepatoma cells through enhancement of transforming growth factor α
expression via activation of nuclear factor-κB. Gut. 55:1801–1808.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park J, Kang W, Ryu SW, et al: Hepatitis C
virus infection enhances TNF-α-induced cell death via suppression
of nuclear factor-κB. Hepatology. 56:831–840. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lerat H, Honda M, Beard MR, et al:
Steatosis and liver cancer in transgenic mice expressing the
structural and nonstructural proteins of hepatitis C virus.
Gastroenterology. 122:352–365. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moriya K, Fujie H, Shintani Y, et al: The
core protein of hepatitis C virus induce hepatocellular carcinoma
in transgenic mice. Nat Med. 4:1065–1067. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Neveu G, Barouch-Bentov R, Ziv-Av A,
Gerber D, Jacob Y and Einav S: Identification and targeting of an
interaction between a tyrosine motif within hepatitis C virus core
protein and AP2M1 essential for viral assembly. PLoS Pathog.
8:e10028452012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Egger D, Wölk B, Gosert R, Bianchi L, Blum
HE, Moradpour D and Bienz K: Expression of hepatitis C virus
proteins induces distinct membrane alterations including a
candidate viral replication complex. J Virol. 76:5974–5984. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hügle T, Fehrmann F, Bieck E, et al: The
hepatitis C virus nonstructural protein 4B is an integral
endoplasmic reticulum membrane protein. Virology. 284:70–81. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gosert R, Egger D, Lohmann V,
Bartenschlager R, Blum HE, Bien K and Moradpour D: Identification
of the hepatitis C virus RNA replication complex in Huh7 cells
harboring subgenomic replicons. J Virol. 77:5487–5492. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Einav S, Elazar M, Danieli T and Glenn JS:
A nucleotide binding motif in hepatitis C virus (HCV) NS4B mediates
HCV RNA replication. J Virol. 78:11288–11295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thompson AA, Zou A, Yan J, et al:
Biochemical characterization of recombinant hepatitis C virus
nonstructural protein 4B: evidence for ATP/GTP hydrolysis and
adenylate kinase activity. Biochemistry. 48:906–916. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Einav S, Gerber D, Bryson PD, et al:
Discovery of a hepatitis C target and its pharmacological
inhibitors by microfluidic affinity analysis. Nat Biotechnol.
26:1019–1027. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Han Q, Manna D, Belton K, Cole R and Konan
KV: Modulation of hepatitis C virus genome encapsidation by
nonstructural protein 4B. J Virol. 87:7409–7422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jones DM, Patel AH, Targett-Adams P and
McLauchlan J: The hepatitis C virus NS4B protein can
trans-complement viral RNA replication and modulates production of
infectious virus. J Virol. 83:2163–2177. 2009. View Article : Google Scholar :
|
|
38
|
Davidson BL and McCray PB Jr: Current
prospects for RNA interference-based therapies. Nat Rev Genet.
12:329–340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang H and Grisé H: Cellular and molecular
biology of HCV infection and hepatitis. Clin Sci (Lond). 117:49–65.
2009. View Article : Google Scholar
|
|
40
|
Arbuthnot P: Harnessing RNA interference
for the treatment of viral infections. Drug News Perspect.
23:341–350. 2010.PubMed/NCBI
|
|
41
|
Zhou J and Rossi JJ: Progress in
RNAi-based antiviral therapeutics. Methods Mol Biol. 721:67–75.
2011.PubMed/NCBI
|
|
42
|
Khaliq S, Jahan S, Ijaz B, et al:
Inhibition of core gene of HCV 3a genotype using synthetic and
vector derived siRNAs. Virol J. 7:3182010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ali Ashfaq U, Ansar M, Sarwar MT, Javed T,
Rehman S and Riazuddin S: Post-transcriptional inhibition of
hepatitis C virus replication through small interference RNA. Virol
J. 8:1122011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
DeVincenzo JP: The promise, pitfalls and
progress of RNA-interference-based antiviral therapy for
respiratory viruses. Antivir Ther. 17(1 Pt B): 213–225. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang R and Su B: Small but influential:
the role of microRNAs on gene regulatory network and 3′UTR
evolution. J Genet Genomics. 36:1–6. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pillai RS, Bhattacharyya SN, Artus CG, et
al: Inhibition of translational initiation by Let-7 MicroRNA in
human cells. Science. 309:1573–1576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shukla GC, Singh J and Barik S: MicroRNAs:
processing, maturation, target recognition and regulatory
functions. Mol Cell Pharmacol. 3:83–92. 2011.PubMed/NCBI
|
|
49
|
Boulant S, Vanbelle C, Ebel C, Penin F and
Lavergne JP: Hepatitis C virus core protein is a dimeric
alpha-helical protein exhibiting membrane protein features. J
Virol. 79:11353–11365. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Boulant S, Montserret R, Hope RG, et al:
Structural determinants that target the hepatitis C virus core
protein to lipid droplets. J Biol Chem. 281:22236–22247. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Moriya K, Nakagawa K, Santa T, et al:
Oxidative stress in the absence of inflammation in a mouse model
for hepatitis C virus-associated hepatocarcinogenesis. Cancer Res.
61:4365–4370. 2001.PubMed/NCBI
|
|
52
|
Dvory-Sobol H, Pang PS and Glenn JS: The
future of HCV therapy: NS4B as an antiviral target. Viruses.
2:2481–2492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gouttenoire J, Penin F and Moradpour D:
Hepatitis C virus nonstructural protein 4B: a journey into
unexplored territory. Rev Med Virol. 20:117–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rai R and Deval J: New opportunities in
anti-hepatitis C virus drug discovery: targeting NS4B. Antiviral
Res. 90:93–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lin C, Wu JW, Hsiao K and Su MS: The
hepatitis C virus NS4A protein: interactions with the NS4B and NS5A
proteins. J Virol. 71:6465–6471. 1997.PubMed/NCBI
|
|
56
|
Gouttenoire J, Montserret R, Kennel A,
Penin F and Moradpour D: An amphipathic α-helix at the C terminus
of hepatitis C virus nonstructural protein 4B mediates membrane
association. J Virol. 83:11378–11384. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Israsena N, Supavonwong P, Ratanasetyuth
N, Khawplod P and Hemachudha T: Inhibition of rabies virus
replication by multiple artificial microRNAs. Antiviral Res.
84:76–83. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ibrišimović M, Kneidinger D, Lion T and
Klein R: An adenoviral vector-based expression and delivery system
for the inhibition of wild-type adenovirus replication by
artificial microRNAs. Antiviral Res. 97:10–23. 2013. View Article : Google Scholar
|
|
59
|
Kato K, Senoki T and Takaku H: Inhibition
of HIV-1 replication by RNA with a microRNA-like function. Int J
Mol Med. 31:252–258. 2013.
|
|
60
|
Yang X, Haurigot V, Zhou S, Luo G and
Couto LB: Inhibition of hepatitis C virus replication using
adeno-associated virus vector delivery of an exogenous
anti-hepatitis C virus microRNA cluster. Hepatology. 52:1877–1887.
2010. View Article : Google Scholar : PubMed/NCBI
|