Introduction
The corneal endothelium exhibits no regenerative
capacity, therefore decompensation of the corneal endothelium
induced by a decreased cell density will fail to maintain corneal
hydration, thickness and transparency (1–3).
Once irreversible decompensation of the corneal endothelium occurs,
corneal transplantation is required to restore visual function.
Currently, endothelial keratoplasty is the most
frequently used surgical treatment for corneal endothelial diseases
(4,5). However, low endothelial cell count,
possible age-related diseases, cultural, logistical and technical
difficulties, long postmortem time and severe damage occurring
during the handling of fragile donor corneas affect the
availability of donor tissues (6,7).
These challenges contribute to the global shortage of suitable
transplant-grade corneal tissues.
Corneal tissue engineering has recently emerged as a
promising option to overcome these challenges. In addition to
collagen, gelatin membranes and amniotic membrane, Descemet’s
membrane (DM) is one of the most effective choices as a natural
scaffold for tissue-engineered endothelium, although worldwide
demand for human donor corneas far exceeds supply (8). Compared with other matrices, the
porcine cornea appears particularly attractive as a possible
scaffold due to its similar anatomic and biomechanical parameters
to the human cornea (9–11); in addition, it shows great promise
in providing a virtually limitless supply of cells, tissues and
organs for a variety of therapeutic procedures.
There are two main obstacles, however, preventing
pig-to-human xenotransplantation. One is the immunological
hyperacute or delayed rejections induced by xenotransplantation
α-gal epitopes (12–15), which are expressed in the cell
membranes of all mammals except those of humans and Old World
monkeys. The other is the transmission of porcine micro-organisms,
particularly the infection of porcine endogenous retroviruses
(PERVs) to the human xenotransplantation recipient (16,17).
These issues must be resolved before xenotransplantation becomes a
clinical possibility.
At present, there are no studies that describe α-gal
and PERVs in porcine DM for corneal transplantation. The purpose of
this study was to evaluate the safety and feasibility of porcine DM
as a carrier for generating tissue-engineered corneal
endothelium.
Materials and methods
Animals
Fresh porcine corneas were obtained from a local
slaughter house. Human corneas were obtained from the Peking
University Eye Bank, originating from three females and three males
aged 72–83 years old. All experiments adhered to the ARVO Statement
for the Use of Animals in Ophthalmic and Vision Research and the
Declaration of Helsinki. The study was approved by the Ethics
Committee of Peking University Third Hospital, China. All reagents
were obtained from Sigma-Aldrich (St. Louis, MO, USA) unless
otherwise stated.
Separation of different sections of the
porcine cornea
Corneal epithelia were scraped by a microkeratome.
To remove the corneal endothelial cells, each DM was first
incubated with 0.02% ethylenediaminetetraacetic acid (EDTA) at 37°C
for 45 min. A cell scraper was used to remove the corneal
endothelial cells from the DM (the denuded DM). A 30-gauge needle
bent bevel-up was attached to a 1-ml syringe filled with air and
was inserted into the posterior stroma with the entry point located
just outside of the Schwalbe line. The needle was advanced between
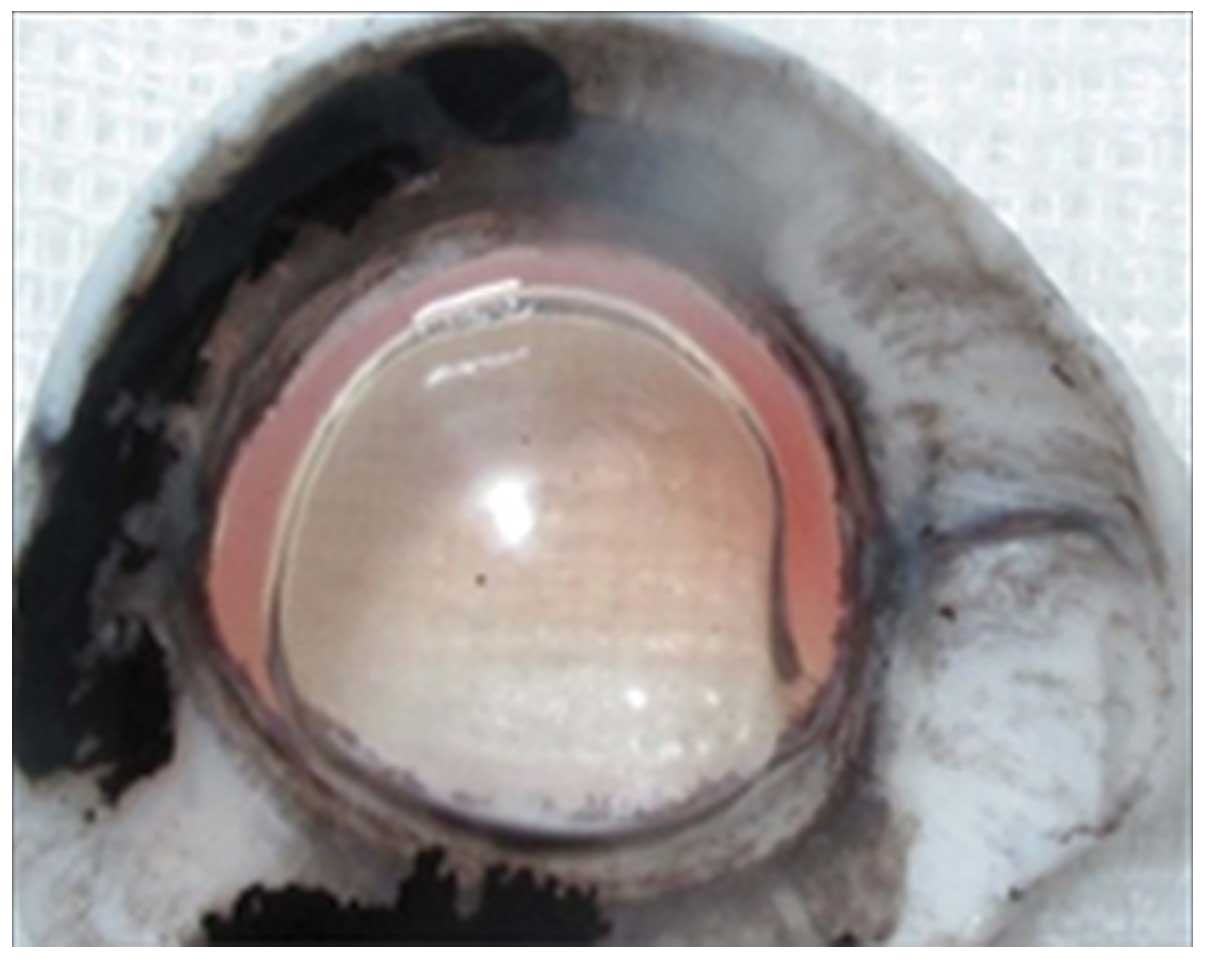
the stroma and the DM. Air was gently injected, causing corneal
emphysema. The rapidly formed air bubble coalesced into a large
bubble (Fig. 1), thus detaching
the DM from the posterior stoma (18).
Histological examination of porcine and
human DMs
For hematoxylin and eosin (H&E) staining, six
human and six porcine corneas were fixed in 4% formaldehyde,
dehydrated in a series of ethanol solutions and embedded in
paraffin. Cross-sections of 4 µm were cut, stained with
H&E and examined under a light microscope (dm4000B; Leica,
Wetzlar, Germany).
Scanning electron microscopy
The denuded porcine DM was fixed in 2.5%
glutaraldehyde in 0.1 M phosphate-buffered saline (PBS), washed
three times for 15 min in PBS, postfixed for 2 h in 2% osmium
tetroxide and washed three more times in PBS. Following dehydration
through a series of graded ethanol solutions (50, 70, 80, 90, 95
and 100%), specimens were transferred to hexamethyldisilazane for
2×10 min and allowed to air dry. When dry, the specimens were
mounted on aluminum stubs and sputter-coated with gold prior to
examination using a scanning electron microscope (JSM-5600; Jeol,
Tokyo, Japan).
Immunohistochemical evaluation of the
α-gal epitope
The location of the α-gal epitope in the DM was
determined by immunohistochemical staining using paraffin-embedded
tissue sections, following the method of Gonzalez-Andrades et
al (19). First, paraffin was
removed from the tissue sections with xylene, then the samples were
rehydrated in water through a graded series of alcohol solutions
(100, 95, 90, 85, 80 and 70%) and washed 3 times with PBS for 5
min. Endogenous peroxidase activity was blocked with 3% hydrogen
peroxide (PV-6002, two-step IHC detection reagent; ZSGB-BIO,
Beijing, China) for 10 min at room temperature, and the tissue
samples were rinsed three times with PBS for 5 min. The tissue
samples were treated with a trypsin solution (0.125%) maintained at
37°C for 45 min for antigen retrieval and then washed three times
for 5 min with PBS. The samples were incubated with monoclonal
mouse antibody (ALX-801-090-1, clone M86; Alexis Biochemicals,
Farmingdale, NY, USA) against the α-gal epitope at a 1:100 dilution
at 4°C overnight. Then, the samples were equilibrated to room
temperature. Incubation with secondary antibodies (pv-9002;
ZSGB-BIO) was carried out for 45 min using anti-mouse secondary
antibodies. After removing the secondary antibody, the slides were
washed three times for 5 min with PBS. Next, the slides were
treated with DAB (ZSGB-BIO) as the chromogenic agent for 3 min.
Finally, the sections were counterstained with hematoxylin and
photographed under a light microscope.
DNA extraction
Genomic DNA was extracted from the samples by
following the procedure listed in the genomic DNA extraction kit
instructions (catalog no. 51304; Qiagen, Hamburg, Germany). The
tissues were weighed three times to verify that they were ≤25 mg,
as per the kit instructions. Details are shown in Table I. The DNA extractions were repeated
three times. Extracted genomic DNA concentrations were determined
by a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific,
Waltham, MA, USA).
 | Table IWeights of various porcine
tissues. |
Table I
Weights of various porcine
tissues.
| Weight
(g)/tissue | Sample 1 | Sample 2 | Sample 3 | Mean ± SD |
|---|
| Full-thickness
cornea | 0.0227 | 0.0215 | 0.0195 |
0.0212±0.0016a |
| Epithelium | 0.0229 | 0.0214 | 0.0191 |
0.0211±0.0019a |
| Stroma | 0.0229 | 0.0219 | 0.0195 |
0.0214±0.0017a |
| Descemet’s
membrane | 0.0227 | 0.0218 | 0.0193 |
0.0213±0.0018a |
| Descemet’s membrane
and epithelium | 0.0227 | 0.0218 | 0.0194 |
0.0213±0.0017a |
| Iris | 0.0224 | 0.0219 | 0.0195 |
0.0213±0.0016a |
| Aqueous humor | 0.0226 | 0.0211 | 0.0197 |
0.0211±0.0215a |
Polymerase chain reaction (PCR)
In this process, gag, pol and envelope
genes env-A, env-B and env-C were selected as
the PERV-specific genes. The previously described primer sequences
were as follows (20):
gag-F (5′-CCCGATCAGGAGCCCTATATCCTTACGTG-3′) and gag-R
(5′-CGCAGCGGTAATATCGCGATCTCGT-3′) (GenBank: AF038599.1);
pol-F (5′-AGCTCCGGGAGGCCTACTC-3′) and pol-R
(5′-ACAGCCGTTGGTGTGGTCA-3′) (GenBank: Y17013.1) (21); env-A-F
(5′-GAGATGGAAAGATTGGCAACAGCG-3′) and env-A-R
(5′-AGTGATGTTAGGCTCAGTGGGGAC-3′) (GenBank: HQ688785.1);
env-B-F (5′-AATTCTCCTTTGTCAATTCCGGCCC-3′) and env-B-R
(5′-CCAGTACTTTATCGGGTCCCACTG-3′) (GenBank: AY056035.1); and
env-C-F (5′-CTGACCTGGATTAGAACTGGAAGC-3′) and env-C-R
(5′-GTTATGTTAGAGGATGGTCCTGGTC-3′) (GenBank: AY534304.1). The
housekeeping gene GAPDH was selected as an internal
reference gene using the following primers: GAPDH-F
(5′-ACATGGCCTCCAAGGAGTAAGA-3′) and GAPDH-R
(5′-GATCGAGTTGGGGCTGTGACT-3′) (AF_017079.1). A PCR kit was used for
this procedure (6210A; Takara, Dalian, China). The PCR mixture
included 0.4 µl Takara Taq (5 U/µl; Takara), 5
µl 10X PCR buffer, 2.5 mM dNTP mixture, 10 ng DNA sample, 1
µl PCR forward primer (20 µM) and 1 µl PCR
reverse primer (20 µM); finally dH2O was added
for a total volume of 50 µl. PCR assays were performed with
a PCR system (T-Gradient Thermoblock, serial no. 2009179, Biometra,
Göttingen, Germany). DNA templates were amplified with 30 cycles at
94°C for 30 sec, at 59°C (GAPDH, pol) or 64°C
(gag, env-A, env-B and env-C) for 30
sec and at 72°C for 1 min. PCR products were separated on a 3%
agarose gel in Tris-acetate-EDTA buffer.
Real-time PCR assay for pol gene
sequences
The expression levels of pol (pol-F
5′-AGCTCCGGGAGGCCTACTC-3′, pol-R 5′-ACAGCCGTTGGTGTGGTCA-3′)
(GenBank: Y17013.1) (22) were
detected by real-time PCR assays, and a full-thickness cornea was
used as a control. The housekeeping gene porcine transferrin
receptor (tfrc) (tfrc-F 5′-GAGACAGAAACTTTCGAAGC-3′,
tfrc-R 5′-GAAGTCTGTGGTATCCAATCC-3′) (NM_214001.1) (22) was selected as an internal reference
gene. This reaction system in a total volume of 20 µl using
the SYBR Premix Ex Taq™ kit (DRR420A, Takara) included 10 µl
SYBR Premix Ex Taq (2X), 0.4 µl PCR forward primer, 0.4
µl PCR reverse primer, 0.4 µl ROX reference dye II
(50X; Takara), 2 µl DNA sample and 6.8 µl
dH2O. The PCR conditions involved initial denaturation
at 95°C for 30 sec followed by 95°C for 5 sec and 59°C for 34 sec.
This process was performed with a 7500 Real-Time PCR system (ABI
7500, Applied Biosystems, Grand Island, NY, USA). The assay was
performed in triplicate and repeated three times. The results were
analyzed using the 2−∆∆Ct method of Livak and Schmittgen
(23).
Chemical treatment of porcine DM
The porcine DM was chemically stabilized with 5%
ethylene glycol diglycidyl ether (EDGE; CAS: 2224-15-9; Tokyo
Chemical Industry Co., Ltd., Tokyo, Japan) solution in phosphate
buffer (pH 7.46). Twenty samples were incubated at 25°C for 2
weeks. The EDGE solution was changed after 1 and 6 days of
fixation. Specimens for molecular analysis were collected after 14
days. All samples were rinsed with sterile physiological saline
solution for 15 min to remove residual substances, then they were
stored frozen at −80°C for 24 h until DNA extraction (24).
Statistical analysis
Statistical analyses were performed using SPSS 16.0
statistical software (SPSS Inc., Chicago, IL, USA). A completely
randomized design analysis of variance (ANOVA, one-factor ANOVA)
was used to evaluate the test data by the Student-Newman-Keuls and
least significant difference tests. Experimental data are expressed
as the mean ± standard deviation. P<0.05 was considered to
indicate a statistically significant difference.
Results
Histological examination
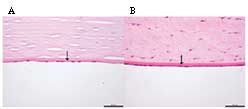
As shown in Fig. 2,
the histological examination by H&E staining confirmed that
porcine DM was a basal lamina, which was similar to the structure
of human DM. The scanning electron microscope examination further
revealed that the porcine DM was a tight membrane through the
longitudinal section (Fig. 3A),
while no residual corneal endothelial cells were observed on
porcine DM following EDTA treatment (Fig. 3B).
Immunohistochemical localization of α-gal
epitope in porcine cornea
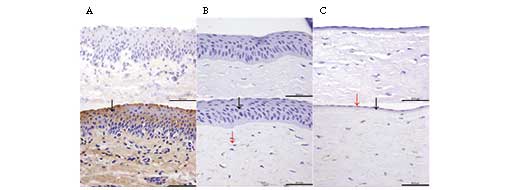
The localization of the α-gal epitope in the porcine
cornea was detected using immunohistochemical staining. The
staining of the α-gal epitope in the conjunctiva was used as a
positive control, and samples with no primary antibody were used as
negative controls. As shown in Fig.
4, positive immunohistochemical localization of the α-gal
epitope in porcine tissues was identified in conjunctiva and stroma
cells, but no positive staining was observed in the porcine DM.
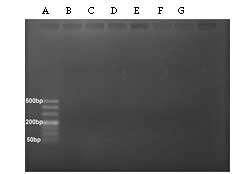
PERV expression in porcine DM
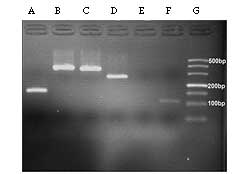
The PCR results revealed that PERV sequences of
pol, gag, env-A and env-B were
expressed in normal porcine DM, but env-C was not detected
(Fig. 5). In addition, the
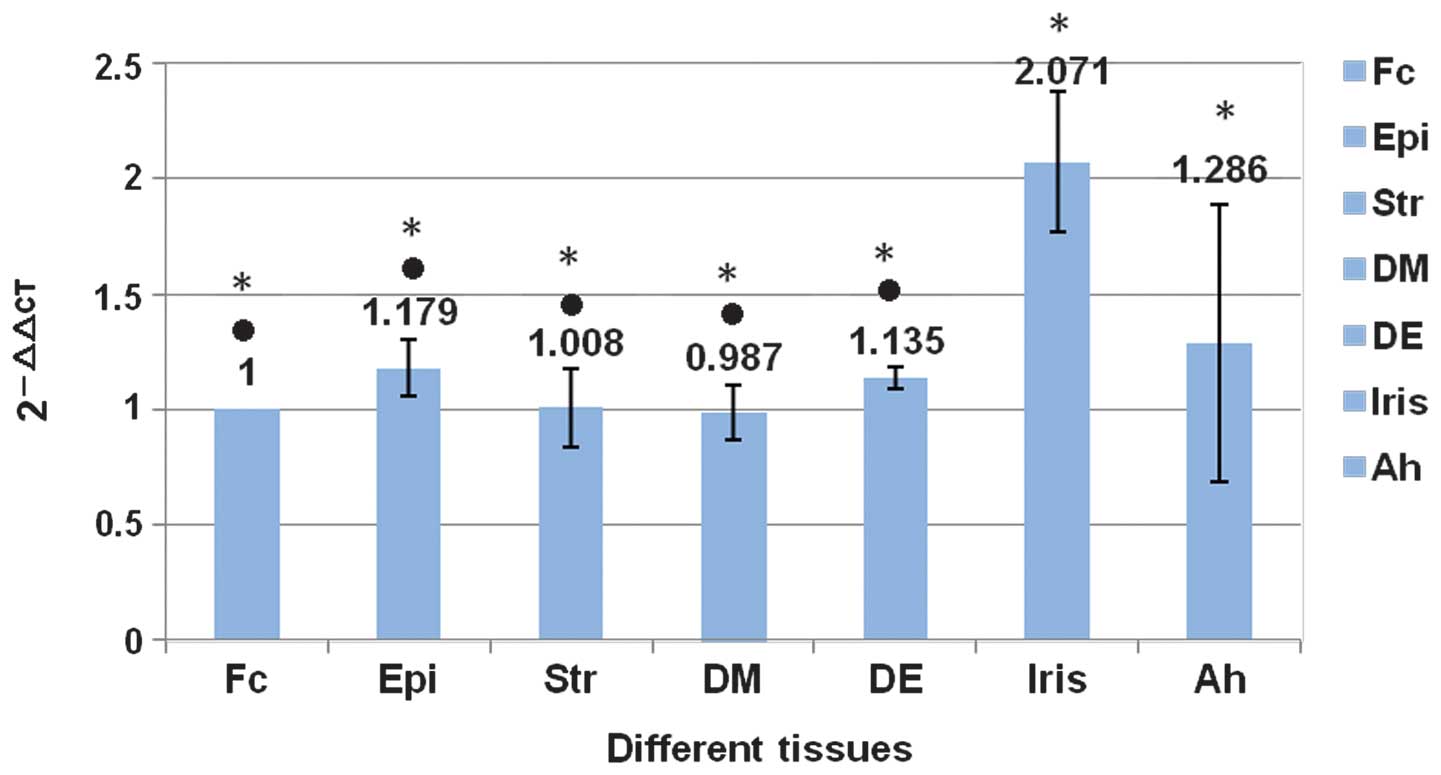
expression of PERVs was negative in porcine DM-EDGE (Fig. 6). The expression of pol in
porcine DM was compared with other corneal tissues using real-time
PCR, and no statistical difference was noted (P>0.05). However,
comparing the iris and the aqueous humor, the expression of
pol was greater in the iris (P<0.01). There were no
statistically significant differences among the various parts of
the porcine cornea (P>0.05; Fig.
7).
Discussion
Endothelial keratoplasty has been increasingly
performed in recent years in patients exhibiting endothelial
dysfunctions from surgical trauma (5), corneal endothelial diseases (4,25) or
age-related pathologies. Therefore, good-quality corneal
endothelial donors are urgently required. The concept of a
tissue-engineered endothelium provides new hope for overcoming
these challenges. Seed cells, scaffolds and functional evaluation
following transplantation are the main obstacles preventing the use
of a tissue-engineered endothelium in human patients. This study
was conducted to evaluate the feasibility and safety of porcine DM
as a tissue-engineered endothelial scaffold by analyzing PERVs and
the α-gal epitope. Our results revealed that porcine DM was
appropriate as a tissue-engineered endothelial scaffold in terms of
its anatomical, immunological and etiological characteristics.
One of the barriers of corneal endothelial
xenotransplantation from pigs to humans has been immunological
rejections induced by anti-α-gal antibodies in humans and α-gal
epitopes in pigs (26,27). α-gal epitopes and their precursors
have been identified in all pigs (28). In our study, immunohistochemical
analysis revealed that α-gal epitopes were not expressed in the
porcine DM, which is consistent with several previously published
studies (29–32). Hence, porcine DM may have the
potential to act as a carrier of tissue-engineered corneal
endothelial cells, avoiding the immunological rejections induced by
xenotransplantation α-gal epitopes, and be immunologically accepted
by a human host once implanted in vivo. These results
suggest that porcine DM may be as safe as xenotrans-plantation in
terms of immunological response. However, the corneal stroma
exhibited greater expression of α-gal epitopes in the
immunohistochemical study, so the effects of the α-gal epitopes on
porcine corneal stroma as a tissue-engineered carrier substitute
must be considered.
Aside from the risks associated with graft
rejections, cross-species transmission of porcine pathogens,
particularly PERVs, is also a concern. In light of their
differences in the construction of the env genes and their
ability to penetrate cells of various organisms, three subtypes
were identified: env-A, env-B and env-C. The
sequences of the polymerase genes pol and gag are
conserved in env-A, env-B and env-C; thus,
they represent the expression of all types of PERVs. It is known
that up to 100 integrated proviral copies of PERVs are noted in the
pig genome (16,17,33).
This number may vary among pig breeds and also within pigs of the
same breed (34–38).
In studies of other porcine tissues (e.g., liver,
heart, kidney and nerve cells) xenotransplanted to humans, no
evidence of PERV expression has been detected; in addition, human
serum is reported to have a role of inactivation on PERVs (39–42).
These studies suggest the possibility of porcine DM to act as a
carrier of tissue-engineered corneal endothelium.
To reduce the risk of infection with PERVs, certain
strategies, including antiretroviral therapy (43–45)
and the use of RNA interference mechanisms (46,47)
have been considered. Moza et al (48) reported in 2001 that complete
degradation of PERV DNA was observed following glutaraldehyde (GA)
fixation of porcine heart valves, but there were also disadvantages
that could induce calcification, inflammation and cytotoxicity.
Biological materials treated with epoxy compounds have greater
resistance to enzymatic degradation, have reduced cytotoxicity, and
are less prone to calcification when compared with GA-fixed heart
valves. EDGE is a biofunctional cross-linker with an epoxide
structure. Cyganek-Niemiec et al (24) demonstrated that EDGE fixation
induces complete degradation of PERV genetic material in porcine
aortic heart valves. This study suggests that epoxy compounds may
be used in the preparation of bioprosthetic heart valves.
Our PCR results indicated that porcine DM-EDGE could
avoid cross-species transmission of PERVs. Thus, it may be possible
to increase implantation safety using tissues obtained from pigs.
As a result, porcine DM could become an effective
xenotransplantation carrier of tissue-engineered endothelium.
Previously, it has been reported that anterior chamber-associated
immune deviation (ACAID) plays a pivotal role in avoiding herpes
simplex virus-1 corneal endotheliitis (49). We considered whether ACAID might
play the same role in preventing the potential risk of PERVs in
endothelial keratoplasty. It may be possible to use direct porcine
endothelial grafts (including porcine DM and porcine endothelium)
as a donor endothelium, thus solving the global endothelial donor
shortage. Our next study will test the biological compatibility of
porcine DM treated with EDGE and evaluate the risk of PERV
transmission in vivo by endothelial keratoplasty in animal
models.
In conclusion, our results demonstrate that porcine
DM may be a viable carrier of tissue-engineered corneal endothelium
in terms of its structural, immunological and etiological
characteristics. Porcine DM could solve the shortage of
tissue-engineered corneal endothelium and could also be used as a
carrier of tissue-engineered materials for other purposes.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (no. 31140025 and
31271045).
References
|
1
|
Waring GO III, Bourne WM, Edelhauser HF
and Kenyon KR: The corneal endothelium. Normal and pathologic
structure and function. Ophthalmology. 89:531–590. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaufman HE, Capella JA and Robbins JE: The
human corneal endothelium. Am J Ophthalmol. 61:835–841. 1966.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Svedbergh B and Bill A: Scanning electron
microscopic studies of the corneal endothelium in man and monkeys.
Acta Ophthalmol (Copenh). 50:321–336. 1972. View Article : Google Scholar
|
|
4
|
Gagnon MM, Boisjoly HM, Brunette I,
Charest M and Amyot M: Corneal endothelial cell density in
glaucoma. Cornea. 16:314–318. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rao GN, Shaw EL, Arthur E and Aquavella
JV: Morphological appearance of the healing corneal endothelium.
Arch Ophthalmol. 96:2027–2030. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Engelmann K, Bednarz J and Valtink M:
Prospects for endothelial transplantation. Exp Eye Res. 78:573–578.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ruberti JW and Zieske JD: Prelude to
corneal tissue engineering – gaining control of collagen
organization. Prog Retin Eye Res. 27:549–577. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen J, Chen R and Gao S: Morphological
characteristics and proliferation of keratocytes cultured under
simulated microgravity. Artif Organs. 31:722–731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hara H and Cooper DK: The immunology of
corneal xenotrans-plantation: a review of the literature.
Xenotransplantation. 17:338–349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kampmeier J, Radt B, Birngruber R and
Brinkmann R: Thermal and biomechanical parameters of porcine
cornea. Cornea. 19:355–363. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Danielsen CC: Tensile mechanical and creep
properties of Descemet’s membrane and lens capsule. Exp Eye Res.
79:343–350. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanemura M, Ogawa H, Yin DP, Chen ZC,
DiSesa VJ and Galili U: Elimination of anti-Gal B cells by
alpha-Gal ricin1. Transplantation. 73:1859–1868. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tanemura M, Yin D, Chong AS and Galili U:
Differential immune responses to α-gal epitopes on xenografts and
allografts: implications for accommodation in xenotransplantation.
J Clin Invest. 105:301–310. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Badylak SF: Xenogeneic extracellular
matrix as a scaffold for tissue reconstruction. Transpl Immunol.
12:367–377. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Galili U: The α-gal epitope and the
anti-gal antibody in xenotransplantation and in cancer
immunotherapy. Immunol Cell Biol. 83:674–686. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee D, Lee J, Park N, Oh YK, Kwon M and
Kim YB: Analysis of natural recombination in porcine endogenous
retrovirus envelope genes. J Microbiol Biotechnol. 18:585–590.
2008.PubMed/NCBI
|
|
17
|
Chapman LE, Folks TM, Salomon DR,
Patterson AP, Eggerman TE and Noguchi PD: Xenotransplantation and
xeno-geneic infections. N Engl J Med. 333:1498–1501. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zarei-Ghanavati S, Khakshoor H and
Zarei-Ghanavati M: Reverse big bubble: a new technique for
preparing donor tissue of descemet membrane endothelial
keratoplasty. Br J Ophthalmol. 94:1110–1111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gonzalez-Andrades M, de la Cruz Cardona J,
Ionescu AM, Campos A, del Mar Perez M and Alaminos M: Generation of
bioengineered corneas with decellularized xenografts and human
keratocytes. Invest Ophthalmol Vis Sci. 52:215–222. 2011.
View Article : Google Scholar
|
|
20
|
Bösch S, Arnauld C and Jestin A: Study of
full-length porcine endogenous retrovirus genomes with envelope
gene polymorphism in a specific-pathogen-free large white swine
herd. J Virol. 74:8575–8581. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Le Tissier P, Stoye JP, Takeuchi Y,
Patience C and Weiss RA: Two sets of human-tropic pig retrovirus.
Nature. 389:681–682. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Ren J, Lan L, et al:
Characterization of polymorphisms of transferrin receptor and their
association with susceptibility to ETEC F4ab/ac in pigs. J Anim
Breed Genet. 124:225–229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Cyganek-Niemiec A, Strzalka-Mrozik B,
Pawlus-Lachecka L, et al: Degradation effect of diepoxide fixation
on porcine endogenous retrovirus DNA in heart valves: molecular
aspects. Int J Artif Organs. 35:25–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schultz RO, Matsuda M, Yec RW, et al:
Corneal endothelial changes in type I and type II diabetes
mellitus. Am J Ophthalmol. 98:401–410. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grönlund H, Adédoyin J, Commins SP,
Platts-Mills TA and van Hage M: The carbohydrate galactose-alpha-1,
3-galactose is a major IgE-binding epitope on cat IgA. J Allergy
Clin Immunol. 123:1189–1191. 2009. View Article : Google Scholar
|
|
27
|
Galili U: Evolution and pathophysiology of
the human natural anti-α-galactosyl IgG (anti-gal) antibody.
Springer Semin Immunopathol. 15:155–171. 1993. View Article : Google Scholar
|
|
28
|
Oriol R, Barthod F, Bergemer AM, Ye Y,
Koren E and Cooper DK: Monomorphic and polymorphic carbohydrate
antigens on pig tissues: implications for organ xenotransplantation
in the pig-to-human model. Transpl Int. 7:405–413. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Amano S: Transplantation of cultured human
corneal endothelial cells. Cornea. 22(Suppl 7): S66–S74. 2003.
View Article : Google Scholar
|
|
30
|
Goldstein IJ and Winter HG: The Griffonia
simplicifolia I-B4 isolectin. A probe for alpha-D-galactosyl end
groups. Subcell Biochem. 32:127–141. 1999.PubMed/NCBI
|
|
31
|
Wood C, Kabat EA, Murphy LA and Goldstein
IJ: Immunochemical studies of the combining sites of the two
isolectins, A4 and B4, isolated from Bandeiraea simplicifolia. Arch
Biochem Biophys. 198:1–11. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tempel W, Tschampel S and Woods RJ: The
xenograft antigen bound to griffonia simplicifolia lectin 1-B4.
X-ray crystal structure of the complex and molecular dynamics
characterization of the binding site. J Biol Chem. 277:6615–6621.
2002. View Article : Google Scholar
|
|
33
|
Amano S, Shimomura N, Kaji Y, Ishii K,
Yamagami S and Araie M: Antigenicity of porcine cornea as
xenograft. Curr Eye Res. 26:313–318. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma Y, Yang Y, Lv M, et al: Real-time
quantitative polymerase chain reaction with SYBR green i detection
for estimating copy numbers of porcine endogenous retrovirus from
Chinese miniature pigs. Transplant Proc. 42:1949–1952. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garkavenko O, Wynyard S, Nathu D, et al:
Porcine endogenous retrovirus (PERV) and its transmission
characteristics: a study of the New Zealand designated
pathogen-free herd. Cell Transplant. 17:1381–1388. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mang R, Maas J, Chen X, Goudsmit J and van
Der Kuyl AC: Identification of a novel type C porcine endogenous
retrovirus: evidence that copy number of endogenous retroviruses
increases during host inbreeding. J Gen Virol. 82:1829–1834.
2001.PubMed/NCBI
|
|
37
|
Akiyoshi DE, Denaro M, Zhu H, Greenstein
JL, Banerjee P and Fishman JA: Identification of a full-length cDNA
for an endogenous retrovirus of miniature swine. J Virol.
72:4503–4507. 1998.PubMed/NCBI
|
|
38
|
Garkavenko O, Wynyard S, Nathu D, et al:
Porcine endogenous retrovirus transmission characteristics from a
designated pathogen-free herd. Transplant Proc. 40:590–593. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pitkin Z and Mullon C: Evidence of absence
of porcine endogenous retrovirus (PERV) infection in patients
treated with a bioartificial liver support system. Artif Organs.
23:829–833. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Martin U, Steinhoff G, Kiessig V, et al:
Porcine endogenous retrovirus (PERV) was not transmitted from
transplanted porcine endothelial cells to baboons in vivo. Transpl
Int. 11:247–251. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Heneine W, Tibell A, Switzer WM, et al: No
evidence of infection with porcine endogenous retrovirus in
recipients of porcine islet-cell xenografts. Lancet. 352:695–698.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Paradis K, Langford G, Long Z, et al:
Search for cross-species transmission of porcine endogenous
retrovirus in patients treated with living pig tissue The XEN 111
Study Group. Science. 285:1236–1241. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kaulitz D, Fiebig U, Eschricht M,
Wurzbacher C, Kurth R and Denner J: Generation of neutralising
antibodies against porcine endogenous retroviruses (PERVs).
Virology. 411:78–86. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fiebig U, Stephan O, Kurth R and Denner J:
Neutralizing antibodies against conserved domains of p15E of
porcine endogenous retroviruses: basis for a vaccine for
xenotransplantation? Virology. 307:406–413. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qari SH, Magre S, García-Lerma JG, et al:
Susceptibility of the porcine endogenous retrovirus to reverse
transcriptase and protease inhibitors. J Virol. 75:1048–1053. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ramsoondar J, Vaught T, Ball S, et al:
Production of transgenic pigs that express porcine endogenous
retrovirus small interfering RNAs. Xenotransplantation. 16:164–180.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dieckhoff B, Petersen B, Kues WA, Kurth R,
Niemann H and Denner J: Knockdown of porcine endogenous retrovirus
(PERV) expression by PERV-specific shRNA in transgenic pigs.
Xenotransplantation. 15:36–45. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Moza AK, Mertsching H, Herden T, Bader A
and Haverich A: Heart valves from pigs and the porcine endogenous
retrovirus: experimental and clinical data to assess the
probability of porcine endogenous retrovirus infection in human
subjects. J Thorac Cardiovasc Surg. 121:697–701. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zheng X, Yamaguchi M, Goto T, Okamoto S
and Ohashi Y: Experimental corneal endotheliitis in rabbit. Invest
Ophthalmol Vis Sci. 41:377–385. 2000.PubMed/NCBI
|