Introduction
Corneal endothelial cells (CECs) are crucial for the
maintenance of corneal transparency. Human CECs (hCECs) are a
non-proliferative monolayer of cells. This results in an average
cell loss of 0.3–0.6% per year (1). The decrease may be accelerated as a
result of accidental trauma; certain systemic diseases, such as
diabetes (2); treatment for
glaucoma (3); or endothelial
dystrophies (4). When corneal
endothelial cell density falls below a critical threshold, the
functions of the endothelium are compromised, which leads to the
formation of corneal edema and the loss of visual acuity (5).
In order to promote CEC proliferation, numerous
mechanisms aiming to alter the non-proliferative status of these
cells have been investigated. These include the release of
cell-cell contact (6); growth
factor-induced proliferation (7);
the use of siRNA to reduce cyclin-dependent kinase inhibitors
(8,9), SV40 large-T antigen (10); and induction of cell division by
the human papilloma virus type 16 oncoproteins, E6/E7 (11). However, these results have not yet
been transferred to clinical trials.
The Ras-related small GTPase, Rho, functions as a
molecular switch of various cellular processes by shuttling between
the inactive GDP-bound and active GTP-bound forms (12). Various cellular actions of Rho,
include cell-to-substrate adhesion and motility, G1-S
cell cycle progression and cell transformation, amongst other
roles, by serum response factor (13). In addition, the microinjection of
active RhoA into quiescent Swiss 3T3 fibroblasts (14) was found to induce the
G1-S transition via downstream effectors, such as the
Rho-associated coiled-coil forming protein serine/threonine kinase
(ROCK) family of proteins. In particular, p160ROCK (ROCK-I)
(15) and ROKa/Rho-kinase/ROCK-II
(16–18). These studies verified that active
RhoA induces G1-S transition.
In contrast to the findings from these studies,
Y-27632, a specific inhibitor of the ROCK family of kinases
(19), was used to promote cell
cycle progression (20). However,
these studies did not assess the cell cycle in detail; instead
using changes in positive Ki67 and BrdU expression to measure cell
cycle progression. Therefore, the present study was designed to
further evaluate cell cycle G1-S phase progression and
the effects of Y-27632 on rabbit CECs (rCECs).
Materials and methods
Animals
A total of 15 New Zealand white rabbits (Peking
University Health Science Center, Beijing, China; weight, 1.5–2.0
kg) were used in the present study. The experiments performed were
in accordance with the ARVO Statement for the Use of Animals in
Ophthalmic and Vision Research and the Declaration of Helsinki. All
animal care and experimental protocols complied with the Animal
Management Rules of the Ministry of Health of the People’s Republic
of China and the guide for the Care and Use of the Laboratory
Animals of Peking University. All animal protocols were approved by
the Animal Research Committee of the Peking University Health
Science Center. All efforts were made to minimize animal
suffering.
Primary culture
Descemet membranes were stripped to obtain CECs. The
cells were then incubated in 0.25% trypsin (Sigma-Aldrich, St.
Louis, MO, USA) at 37°C in 5% CO2. After 15 min, the
CECs were resuspended in culture medium and plated in one well of a
12-well plate. All primary cell cultures and passages of CECs were
performed in Dulbecco’s modified Eagle’s medium (DMEM; cat no.
31600-034, Invitrogen Life Technologies, Carlsbad, CA, USA)
supplemented with 15% fetal bovine serum (FBS; cat no. 16000-044,
Gibco-BRL, Carlsbad, CA, USA), 50 U/ml penicillin and 50
µg/ml gentamicin. CECs were maintained in a humidified
atmosphere at 37°C in 5% CO2. Culture medium was changed
every two days. When cells reached confluence, they were
trypsinized and passaged at ratios ranging from 1:2 to 1:4. rCECs
from passage three were used in the present study.
Effect of Y-27632 on cell shape
Passage three rCECs were plated in 24-well plates
(1.1875×104 cells/well). Different concentrations of
Y-27632 (1, 10 or 30 µM) were added simultaneously when
plating or after culture for 72 h. Cells without Y-27632 treatment
were used as the control. After 12, 24 and 72 h, changes in cell
morphology were observed using an inverted microscope (Eclipse Ti;
Nikon Corp., Tokyo, Japan).
Adhesion assay
The adhesion assay was performed as reported
previously (21). Briefly, rCECs
(1.1875×104) with Y-27632 (1, 10 and 30 µM) were
plated in each well of 24-well plates. The cells without Y-27632
were used as controls. There were three wells, each at different
concentrations. Following incubation at 37°C in 5% CO2
for 2 h, the 24-well plate was washed with phosphate-buffered
saline (PBS) and confirmed that there were no residual non-adherent
cells. Subsequently, the non-adherent cells of each well were
removed into centrifuge tubes. The ratios of adherent cells were
calculated using the following formula: adherent ratio =
(1.1875×104 – number of unattached cells) /
1.1875×104
Cell counting kit-8 (CCK-8) assay to
evaluate cell proliferation
Passage three rCECs were seeded in 96-well plates
(2,000 cells/well) and the medium was changed daily. rCECs that
were treated with Y-27632, received this compound either at plating
or after 72 h of cell culture. For rCECs that received Y-27632
during plating, the cells were cultured with Y-27632 (1, 10 or 30
µM) for 12, 24 and 72 h. At these time points, the treatment
medium was then replaced with fresh culture medium that did not
contain Y-27632 and the treatment groups were cultured for a
further 96 h. For rCECs that received Y-27632 following 72 h of
cell growth, 1, 10 and 30 µM concentrations of Y-27632 were
applied for 24 h. In order to assay the number of viable cells
present, complete medium (100 µl) plus CCK-8 (10 µl)
was added to each well. Untreated cells were used as controls and
wells that did not contain cells were used as blank controls. After
2 h at 37°C, culture plates were agitated for 5 min prior to
recording of optical density (OD) values at 450 nm. OD values for
viable cells were calculated using the following formula: Fold
difference in viable cell number =
(ODsample−ODblank)/(ODcontrol−ODblank).
Analysis of cell cycle using flow
cytometry
For rCECs treated with Y-27632 at the time of
plating and those treated after 72 h of cell growth, cells were
harvested and resuspended in 2.1 ml absolute ethyl alcohol and 0.9
ml PBS. Cells were then stained for 1 h in darkness with PBS
containing 50 µg/ml propidium iodide (PI) and 100
µg/ml ribonuclease A [included in the Annexin-flurorescein
isothiocyanate (FITC) apoptosis detection kit;cat no. APOAF-50TST;
Sigma-Aldrich]. Cell cycle progression was then evaluated using
flow cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ,
USA). The results shown are representative of three independent
experiments.
Apoptosis analysis
Using an Annexin-FITC apoptosis detection kit, cells
were washed twice with Dulbecco’s PBS (Sigma-Aldrich) and
resuspended in 1X binding buffer at a concentration of
1×106 cells/ml. For each sample, a 500 µl aliquot
was combined with 5 µl Annexin V-FITC and 10 µl PI
solution and incubated at room temperature in darkness for 10 min.
The fluorescence for each sample was then detected and analyzed
using flow cytometry (BD Biosciences).
Statistical analysis
Statistical analyses were performed using SPSS 16.0
statistical software (IBM, Armonk, N Y, USA). A completely
randomized design analysis of variance (ANOVA; one-factor ANOVA)
and independent samples t-test were used to evaluate test data.
Experimental data are expressed as the mean ± standard deviation
(SD). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of Y-27632 on cell shape
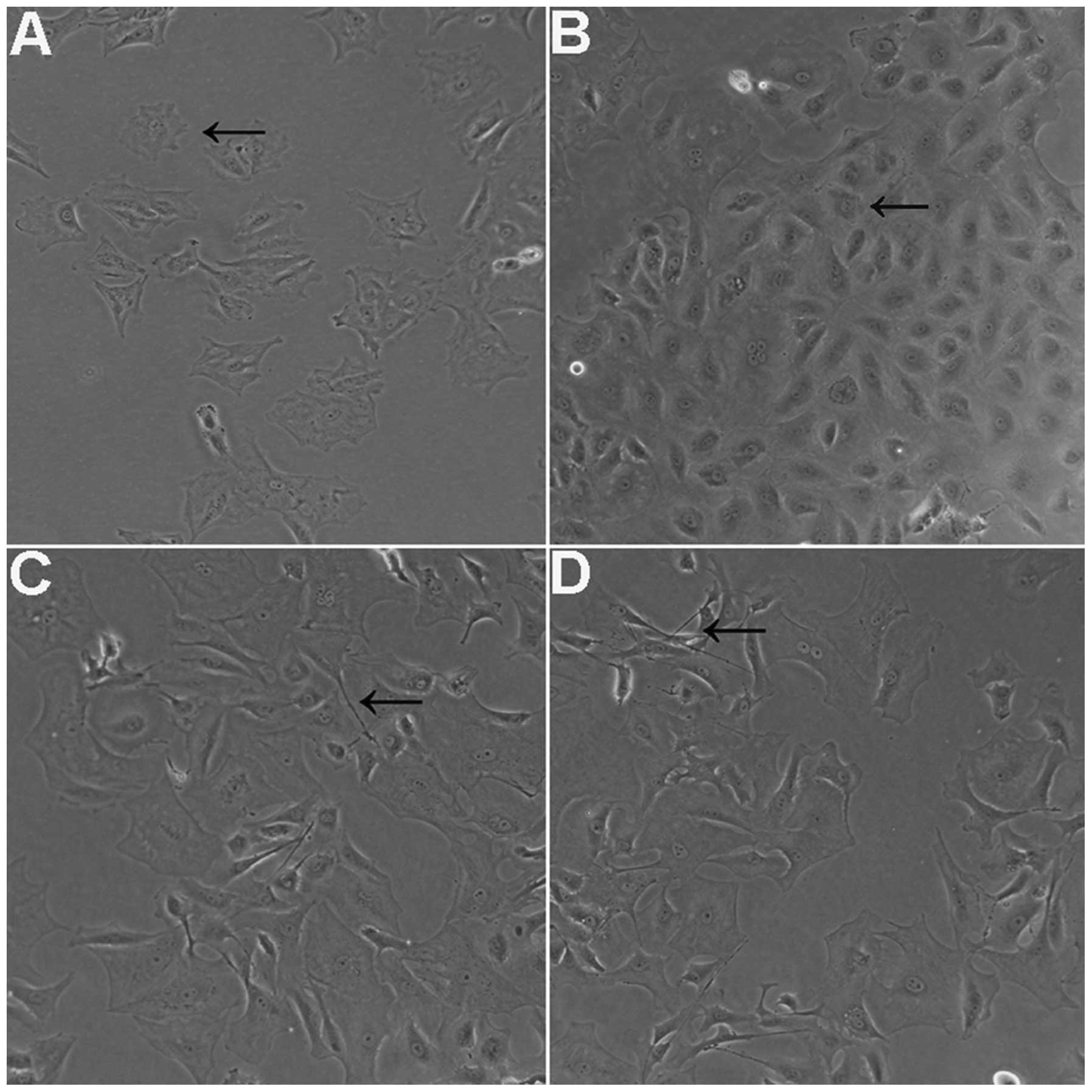
For cells treated with 1 µM (Fig. 1A) and 10 µM (Fig. 1B) Y-27632 at the time of plating,
normal cellular morphology was observed at 24 h. However, for cells
plated with 30 µM Y-27632 for 12 h, thin, long, neurite-like
processes and cell extensions were observed (Fig. 1C). In addition, the cell shapes
observed were not homogenous compared with the control group, or
with cells treated with 1 and 10 µM Y-27632. At 24 h
following removal of Y-27632, cells which had been treated with 30
µM Y-27632 remained irregular in shape, while the cells
treated with 1 and 10 µM Y-27632 retained a generally normal
shape. Furthermore, these changes were found to be concentration-
and time-dependent (Fig. 1D).
Parallel experiments with rCECs treated with Y-27632 following 72 h
of cell growth were also performed and similar results were
obtained.
Cell adhesion following Y-27632
treatment
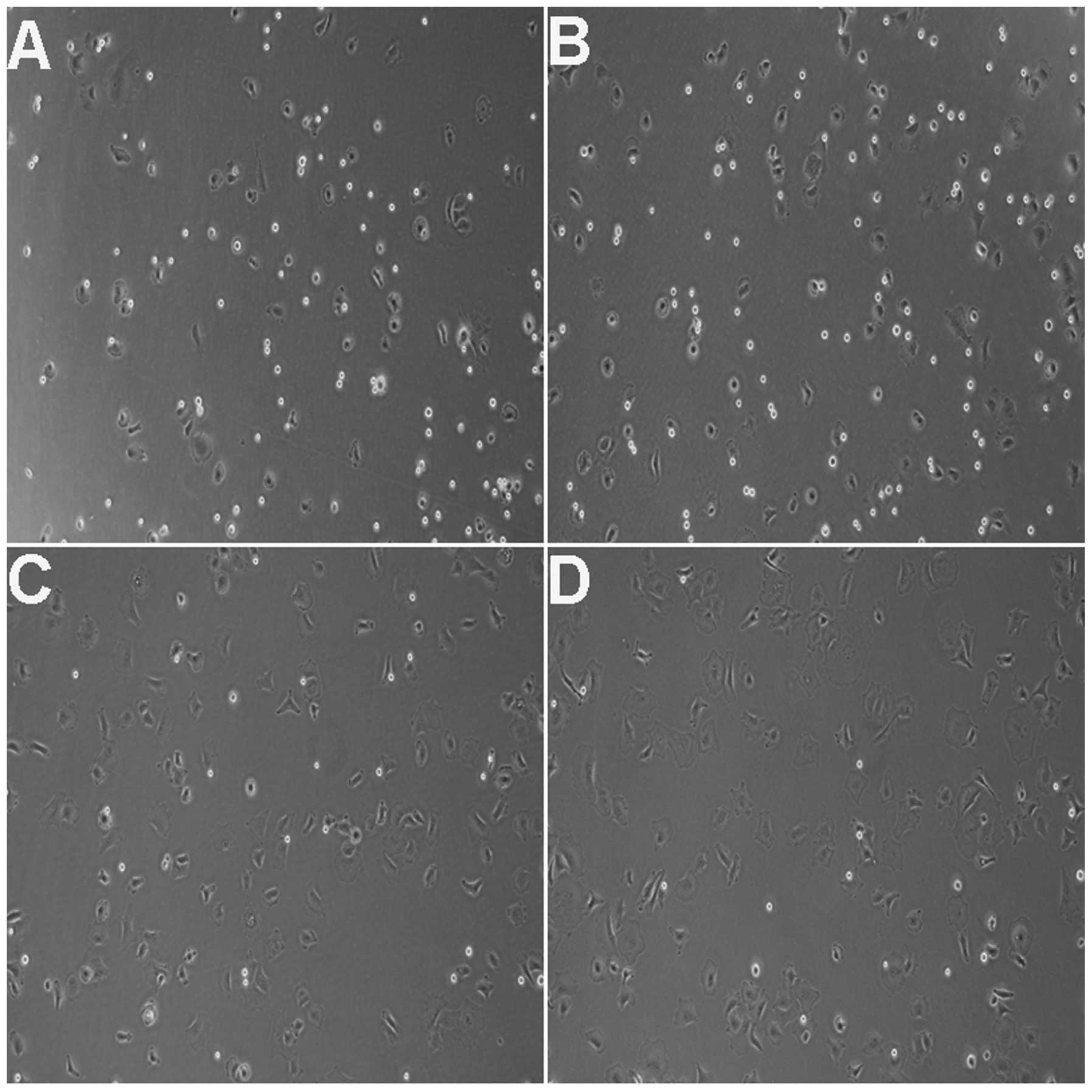
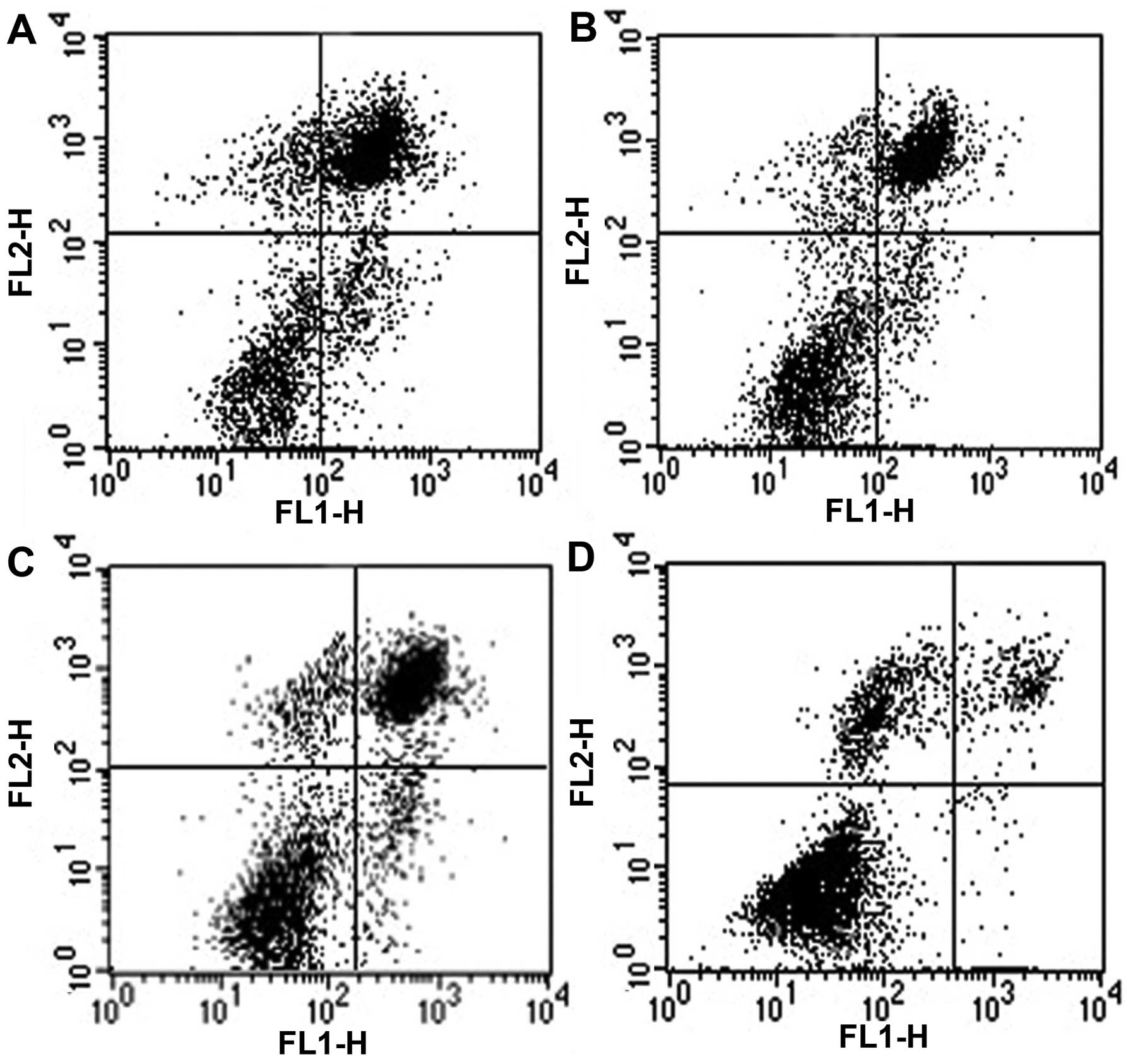
Cell adhesion assays were performed and cell
adhesion ratios were calculated 2 h after the cells were plated. A
ratio of 84.21% (Fig. 2C and D)
was recorded for rCECs treated with 10 and 30 µM Y-27632,
while the ratios for control cells (19.33%; Fig. 2A) and the 1 µM treatment
group (36.84%; Fig. 2B) were
lower. Furthermore, the differences in adhesion ratios between the
treatment groups and the control group, as well as between the 10
and 30 µM treatment groups and the 1 µM group, were
statistically significant (P<0.01 in each case) (Table III).
 | Table IIICell adhesion ratios under different
Y-27632 concentrations (1 µm, 10 µm and 30
µm). |
Table III
Cell adhesion ratios under different
Y-27632 concentrations (1 µm, 10 µm and 30
µm).
| Y-27632
concentrations | Cell adhesion
ratios |
|---|
| Control | 19.33% |
| 1 µm | 36.84% |
| 10 µm | 84.21% |
| 30 µm | 84.21% |
Assessment of cell proliferation
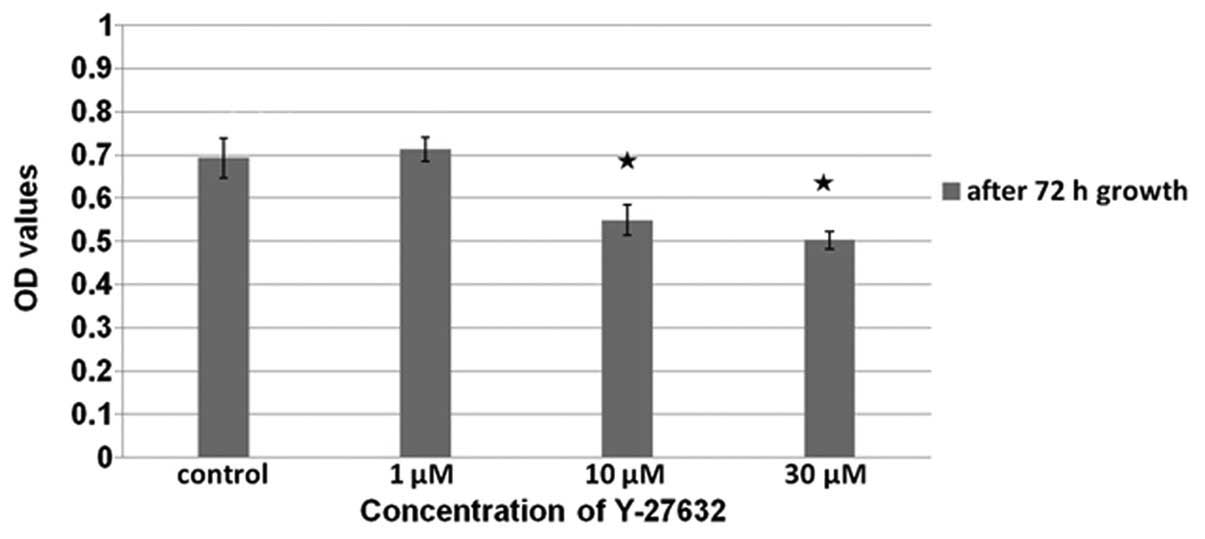
Using a CCK-8 assay, the number of viable cells
present was assayed (Figs. 3 and
4). For rCECs that were treated
with Y-27632 following 72 h of cell growth, the number of viable
cells present was determined at 24 h following treatment with
Y-27632. In these cells, a dose-dependent decrease in viability was
observed. In addition, of the control cells compared with the cells
treated with 1 µM Y-27632 did not differ (P>0.05).
However, the proliferation of the control cells was higher than
that of the cells treated with 10 and 30 µM Y-27632
(P<0.01 in each case). Fig. 4
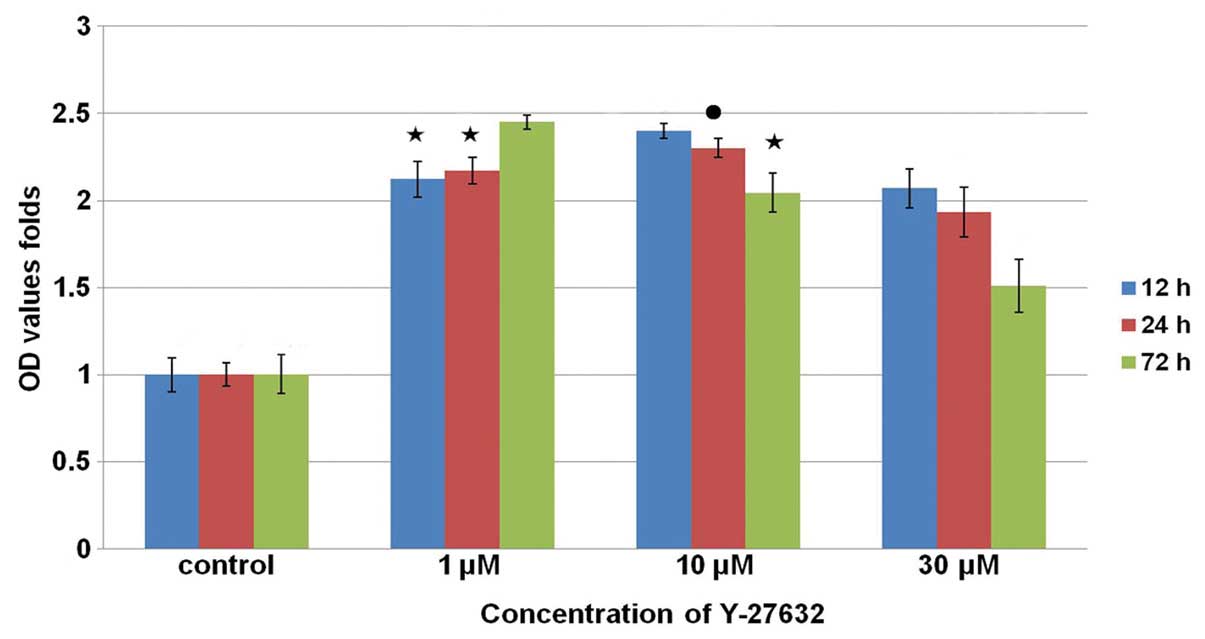
shows the results of the cell counting assays performed in
parallel, with rCECs treated with Y-27632 at the time of plating.
The number of viable cells was subsequently assayed at 12 and 24 h
time points. The highest and lowest levels of proliferation were
observed in the group that received treatment with 10 µM
Y-27632 (P<0.01) and in the control cells (P<0.01),
respectively. However, at the 72 h time point, the highest
proliferation was observed for the rCECs treated with 1 µM
Y-27632 (P<0.01). In addition, at each of the time points
assayed, the proliferation levels of the cells treated with 30
µM Y-27632 were lower than those for cells that received 1
or 10 µM Y-27632. Proliferation was highest at the 72 h time
point following treatment of rCECs with 1 μM Y-27632
(P<0.01). A difference in cell proliferation was also observed
at the 12 and 24 h time points, although the difference was not
significant (P>0.05). Furthermore, for cells receiving 10 or 30
µM Y-27632, a time-dependent decrease in proliferation was
observed (P<0.01).
Apoptotic analysis
Levels of apoptosis were assayed following the
treatment of rCECs with 10 µM Y-27632 (Table I and Fig. 5). Using Annexin V/PI staining, the
percentage of cells undergoing apoptosis following treatment with
Y-27632 during plating was 47.91±0.37% after 12 h. For rCECs
treated with Y-27632 following 72 h of cell growth, the percentage
of cells undergoing apoptosis at 24 h following the administration
of Y-27632 was 8.48±0.98%. Cell apoptosis ratios were also lower
for these two treatment groups compared with control cells, at
61.8±0.92 and 46.41±0.98%, respectively (P<0.01).
 | Table IApoptosis assay of rCECs treated with
Y-27632. |
Table I
Apoptosis assay of rCECs treated with
Y-27632.
| Treatment
group | UR+LR (%) |
|---|
| A-12 h-con | 61.8±0.92 |
| A-12 h | 47.91±0.37a |
| B-24 h-con | 46.41±0.98 |
| B-24 h | 8.48±0.98a |
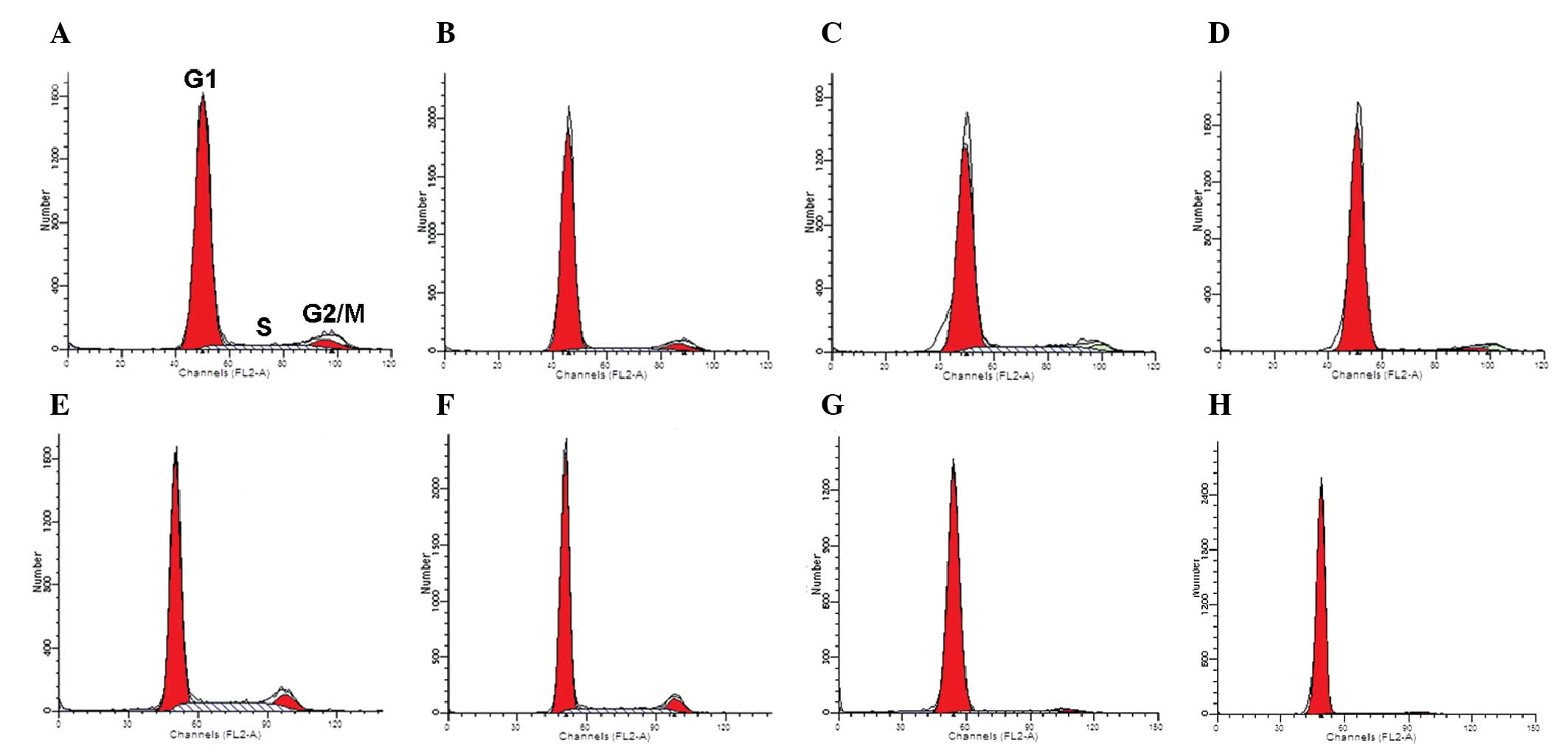
Cell cycle progression
The distribution of cells between various phases of
the cell cycle was assayed using flow cytometry (Table II and Fig. 6). For rCECs treated with 10
µM Y-27632 during plating, a reduced number of cells
transitioning from G1 phase to S/G2 phase was
observed at 12 h and 72 h, compared with control cells examined at
the same time points. In addition, the differences at the two time
points were significant, and the percentage of cells in the
G1 phase at 72 h was greater than the number of cells in
the G1 phase at 12 h (P<0.01). For cells that were
treated with Y-27632 following 72 h of cell growth, 10 µM
Y-27632 induced the transition from S phase to G1 phase
after 72 h (P<0.01).
 | Table IICell cycle profiles for rCECs treated
with Y-27632. |
Table II
Cell cycle profiles for rCECs treated
with Y-27632.
| Treatment
group | G1
(%) | S/G2
(%) |
|---|
| A-12 h-con | 83.91±0.71 | 16.09±0.71 |
| A-12 h | 86.77±0.89a | 13.23±0.89a |
| A-72 h-con | 83.59±1.9 | 16.41±1.9 |
| A-72 h | 91.98±1.2b | 8.020±1.2b |
| B-12 h-con | 72.24±0.53 | 27.75±0.54 |
| B-12 h | 76.34±0.61b | 23.66±0.61b |
| B-72 h-con | 93.06±0.76 | 6.930±0.75 |
| B-72 h | 95.70±0.33b | 4.300±0.33b |
Discussion
In the present study, treatment of rCECs with 10
µM Y-27632 maintained an improved cell shape and cell
adhesion compared with treatment with 30 and 1 µM Y-27632.
Previously, it has been demonstrated that CECs, in particular
hCECs, require the promotion of their attachment for cell growth
(22). For example, extracellular
matrices (ECM) plated on coated culture surfaces, including
collagen (23), ECM derived from
bovine CECs (24), a mixture of
laminin and chondroitin sulfate (25), and a commercially available FNC
coating mix containing fibronectin, collagen and albumin (26), have been reported to promote cell
adhesion. Although the underlying mechanisms responsible for
enhanced cell adhesion have not been well-characterized, it has
been hypothesized that the actin cytoskeleton has a critical role
in combination with integrins (27–29).
Vinculin, which was involved in the linkage of the integrin
adhesion complex to the actin cytoskeleton, was upregulated in ROCK
inhibitor-treated CECs. However, additional studies are required to
further elucidate the mechanisms that mediate enhanced adhesion
induced by ROCK inhibitors.
In the CCK-8 assays performed 24 h after the
addition of Y-27632, it was initially observed that rCECs treated
with 10 µM Y-27632 following 72 h of cell growth exhibited
lower OD values than control cells. These results suggested that
treatment with 10 µM Y-27632 did not promote rCEC
proliferation; and instead either induced cell apoptosis or delayed
cell cycle progression. This did not coincide with the results of a
previous study (30), which
reported that rCECs and monkey CECs treated with Y-27632 exhibited
an increased number of proliferating cells. It was inferred that
treatment different time points may produced different results.
Therefore, the effects of Y-27632 added at the time of plating were
then evaluated. These results demonstrated that OD value folds of
Y-27632 (1, 10 and 30 µM) were markedly higher than that of
the control groups. It appeared that Y-27632, when added at the
same time as plating increased cell proliferation. However, due to
the concomitant significant increase in cell adhesion, it was
hypothesized that the increase in OD values was a result of the
increased number of adherent cells.
In order to test this hypothesis, the effects of
Y-27632 on cell cycle and apoptosis in rCECs were assessed using
flow cytometry. As shown in the present study, with regard to cell
shape, cell adhesion and the results of the CCK-8 assay, as well as
the results from other studies (5,30–32),
10 µM Y-27632 was the optimal concentration for treatment of
rCECs. Therefore, in the following study, 10 µM Y-27632 was
used to investigate its effects on the cell cycle and apoptosis. In
cells treated at the time of plating, at 12 and 72 h a delay in
G1-S cell cycle progression was observed, and the
percentage of G1-S transition at 72 h was lower than it
was at 12 h. The effects of Y-27632 on the cell cycle appeared to
occur in a time-dependent manner.
In cells treated following 72 h of cell growth, at
12 and 24 h, reduced G1-S phase cell cycle progression
was also observed. This indicated that 10 µM Y-27632 did not
promote G1-S cell cycle progression. Furthermore, the
apoptosis assay revealed that 10 µM Y-27632 inhibited cell
apoptosis. These results suggested that Y-27632 significantly
promoted cell adhesion and inhibited cell apoptosis, and did not
induce proliferation of rCECs. It was inferred that in previous
studies, the apparent increase in rCECs proliferation may have been
due to an increase of the numbers of attached cells, leading to an
increase in the numbers of Ki67- and Brdu-positive cells. The
results of the present study, corresponded with the results of
previous studies, which demonstrated the effects of Y-27632 on
hCECs (5), vascular smooth muscle
cell (33), Swiss 3T3 cells
(12) and myofibroblasts (34). A previous study reported that
Y-27632 significantly inhibited thrombin-induced DNA synthesis in
cultured aortic smooth muscle cells at 10 mM (33). This may explain why Y-27632 delayed
cell cycle progression.
Notably, in the CCK-8 assay, it was shown that the
OD value folds of cells treated with 1 µM Y-27632 increased
with increasing time from the time of plating. However, in cells
treated with 10 and 30 µM, the OD value folds decreased with
increasing time from the time of plating. Furthermore, the OD value
folds in cells treated with 1 µM at 72 h were higher than
those in cells treated with 10 µM at 12 h (P<0.05) and 24
h (P<0.01). It was also observed that the OD values of cells
treated with 1 µM Y-27632 following 72 h cell proliferation
was increased in comparison with control. Therefore, 1 µM
Y-27632 may be the optimal dose.
In 2008, Yin and Yu (35) reported that Y-27632 induced a
reduction of CEC proliferation. The beneficial effects of Y-27632
on the eye remained to be elucidated. Therefore, the present
results suggest that the use of Y-27632, either as a cell culture
additive or in the form of eye drops, should be thoroughly
investigated.
In conclusion, in the present study, Y-27632
significantly enhanced the adhesion of rCECs, inhibited cell
apoptosis and delayed cell cycle progression. The mechanisms
underlying the effect of Y-27632 on CECs and the other tissues of
the eye remain to be elucidated and warrant further investigation
prior to progression to clinical trials.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 31271045).
References
|
1
|
Murphy C, Alvarado J, Juster R and Maglio
M: Prenatal and postnatal cellularity of the human corneal
endothelium. A quantitative histologic study. Invest Ophthalmol Vis
Sci. 25:312–322. 1984.PubMed/NCBI
|
|
2
|
Schultz RO, Matsuda M, Yee RW, et al:
Corneal endothelial changes in type I and type II diabetes
mellitus. Am J Ophthalmol. 98:401–410. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bigar F and Witmer R: Corneal endothelial
changes in primary acute angle-closure glaucoma. Ophthalmol.
89:596–599. 1982. View Article : Google Scholar
|
|
4
|
Stainer GA, Akers PH, Binder PS and Zavala
EY: Correlative microscopy and tissue culture of congenital
hereditary endothelial dystrophy. Am J Ophthalmol. 93:456–465.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pipparelli A, Arsenijevic Y, Thuret G,
Gain P, Nicolas M and Majo F: ROCK inhibitor enhances adhesion and
wound healing of human corneal endothelial cells. PloS One.
8:e620952013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gerdes J, Schwab U, Lemke H and Stein H:
Production of a mouse monoclonal antibody reactive with a human
nuclear antigen associated with cell proliferation. Int J Cancer.
31:13–20. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haj FG, Markova B, Klaman LD, Bohmer FD
and Neel BG: Regulation of receptor tyrosine kinase signaling by
protein tyrosine phosphatase-1B. J Biol Chem. 278:739–744. 2003.
View Article : Google Scholar
|
|
8
|
Kikuchi M, Zhu C, Senoo T, Obara Y and
Joyce NC: p27kip1 siRNA induces proliferation in corneal
endothelial cells from young but not older donors. Invest
Ophthalmol Vis Sci. 47:4803–4809. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Joyce NC and Harris DL: Decreasing
expression of the G1-phase inhibitors, p21Cip1 and p16INK4a,
promotes division of corneal endothelial cells from older donors.
Mol Vis. 16:897–906. 2010.PubMed/NCBI
|
|
10
|
Wilson SE, Lloyd SA, He YG and McCash CS:
Extended life of human corneal endothelial cells transfected with
the SV40 large T antigen. Invest Ophthalmol Vis Sci. 34:2112–2123.
1993.PubMed/NCBI
|
|
11
|
Wilson SE, Weng J, Blair S, He YG and
Lloyd S: Expression of E6/E7 or SV40 large T antigen-coding
oncogenes in human corneal endothelial cells indicates regulated
high-proliferative capacity. Invest Ophthalmol Vis Sci. 36:32–40.
1995.PubMed/NCBI
|
|
12
|
Ishizaki T, Uehata M, Tamechika I, et al:
Pharmacological properties of Y-27632, a specific inhibitor of
Rho-associated kinases. Mol Pharmacol. 57:976–983. 2000.PubMed/NCBI
|
|
13
|
Narumiya S: The small GTPase Rho: cellular
functions and signal transduction. J Biochem. 120:215–228. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Olson MF, Ashworth A and Hall A: An
essential role for Rho, Rac and Cdc42 GTPases in cell cycle
progression through G1. Science. 269:1270–1272. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ishizaki T, Maekawa M, Fujisawa K, et al:
The small GTP-binding protein Rho binds to and activates a 160 kDa
Ser/Thr protein kinase homologous to myotonic dystrophy kinase.
EMBO J. 15:1885–1893. 1996.PubMed/NCBI
|
|
16
|
Leung T, Manser E, Tan L and Lim L: A
novel serine/threonine kinase binding the Ras-related RhoA GTPase
which translocates the kinase to peripheral membranes. J Biol Chem.
270:29051–29054. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsui T, Amano M, Yamamoto T, et al:
Rho-associated kinase, a novel serine/threonine kinase, as a
putative target for small GTP binding protein Rho. EMBO J.
15:2208–2216. 1996.PubMed/NCBI
|
|
18
|
Nakagawa O, Fujisawa K, Ishizaki T, Saito
Y, Nakao K and Narumiya S: ROCK-I and ROCK-II, two isoforms of
Rho-associated coiled-coil forming protein serine/threonine kinase
in mice. FEBS Lett. 392:189–193. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uehata M, Ishizaki T, Satoh H, et al:
Calcium sensitization of smooth muscle mediated by a Rho-associated
protein kinase in hypertension. Nature. 389:990–994. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okumura N, Koizumi N, Ueno M, et al: The
new therapeutic concept of using a rho kinase inhibitor for the
treatment of corneal endothelial dysfunction. Cornea. 30(Suppl 1):
54–59. 2011. View Article : Google Scholar
|
|
21
|
Bi YL, Zhou Q, Du F, Wu MF, Xu GT and Sui
GQ: Regulation of functional corneal endothelial cells isolated
from sphere colonies by Rho-associated protein kinase inhibitor.
Exp Ther Med. 5:433–437. 2013.PubMed/NCBI
|
|
22
|
Peh GS, Beuerman RW, Colman A, Tan DT and
Mehta JS: Human corneal endothelial cell expansion for corneal
endothelium transplantation: an overview. Transplantation.
91:811–819. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li W, Sabater AL, Chen YT, et al: A novel
method of isolation, preservation and expansion of human corneal
endothelial cells. Invest Ophthalmol Vis Sci. 48:614–620. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Blake DA, Yu H, Young DL and Caldwell DR:
Matrix stimulates the proliferation of human corneal endothelial
cells in culture. Invest Ophthalmol Vis Sci. 38:1119–1129.
1997.PubMed/NCBI
|
|
25
|
Engelmann K, Bohnke M and Friedl P:
Isolation and long-term cultivation of human corneal endothelial
cells. Invest Ophthalmol Vis Sci. 29:1656–1662. 1988.PubMed/NCBI
|
|
26
|
Zhu C and Joyce NC: Proliferative response
of corneal endothelial cells from young and older donors. Invest
Ophthalmol Vis Sci. 45:1743–1751. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sastry SK and Burridge K: Focal adhesions:
a nexus for intracellular signaling and cytoskeletal dynamics. Exp
Cell Res. 261:25–36. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Worthylake RA, Lemoine S, Watson JM and
Burridge K: RhoA is required for monocyte tail retraction during
transendothelial migration. J Cell Biol. 154:147–160. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Worthylake RA and Burridge K: RhoA and
ROCK promote migration by limiting membrane protrusions. J Biol
Chem. 278:13578–13584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okumura N, Ueno M, Koizumi N, et al:
Enhancement on primate corneal endothelial cell survival in vitro
by a ROCK inhibitor. Invest Ophthalmol Vis Sci. 50:3680–3687. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okumura N, Koizumi N, Ueno M, et al: ROCK
Inhibitor converts corneal endothelial cells into a phenotype
capable of regenerating in vivo endothelial tissue. Am J Pathol.
181:268–277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu X, Ory V, Chapman S, et al: ROCK
inhibitor and feeder cells induce the conditional reprogramming of
epithelial cells. Am J Pathol. 180:599–607. 2012. View Article : Google Scholar :
|
|
33
|
Seasholtz TM, Majumdar M, Kaplan DD and
Brown JH: Rho and Rho kinase mediate thrombin-stimulated vascular
smooth muscle cell DNA synthesis and migration. Circ Res.
84:1186–1193. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Porter KE, Turner NA, O’Regan DJ,
Balmforth AJ and Ball SG: Simvastatin reduces human atrial
myofibroblast proliferation independently of cholesterol lowering
via inhibition of RhoA. Cardiovasc Res. 61:745–755. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yin J and Yu FS: Rho kinases regulate
corneal epithelial wound healing. Am J Physiol Cell Physiol.
295:C378–C387. 2008. View Article : Google Scholar : PubMed/NCBI
|