Introduction
Acute lung injury (ALI) is a common complication
following sepsis. Despite advances in supportive care and the
pharmacological treatment of the condition, the mortality rates
from ALI remain high (1). Previous
studies have demonstrated that mesenchymal stem cells (MSCs) have
therapeutic effects in the treatment of ALI, by reducing surface
tension, thus preventing the alveoli from collapsing (2). MSCs can be engrafted in injured lung
tissue and induced to differentiate into alveolar epithelial cells
type II (ATII), which are vital in the maintenance of respiratory
function through a reduction in surface tension, preventing
collapse of the alveoli (3). In
addition, it has also been revealed that MSCs may differentiate
into alveolar epithelial cells type I (ATI), which are also
associated with a reduction of pulmonary edema (4). However, the methods and mechanisms of
improvement in the differentiation efficiency of MSCs into ATI
remain to be elucidated.
Danshen [Salvia (S.)
miltiorrhiza], a well-established Chinese Herbal Medicine,
has been widely used in medicinal preparations for the treatment of
ALI. Salvianolic acid B (Sal B) is one of the major constituents of
the water-soluble extracts of S. miltiorrhiza Bunge, which
has been reported to promote the differentiation of stem cells into
cells of other lineages (5–7). It
was thus hypothesized that Sal B possesses a promoting effect on
the differentiation of stem cells into alveolar epithelial
cells.
It has been reported that certain signaling pathways
contribute to the induction of differentiation of MSCs into
alveolar epithelial cells; therefore, it is important to examine
the potential mechanisms in vitro. The Wnt signaling
pathways have various roles in the differentiation of MSCs,
dependent upon the specific ligands and receptors involved, as well
as the target cells and the developmental stages (2–4). A
previous study also demonstrated that the Wnt/β-catenin signaling
pathway is one of the fundamental pathways in cell proliferation,
fate determination and the differentiation of MSCs, including the
differentiation of MSCs into cells of an epithelial lineage
(8). In addition, the
Wnt/β-catenin signaling pathway is a crucial regulator in tissue
remodeling associated with lung diseases, including asthma,
pulmonary fibrosis and chronic obstructive pulmonary disease
(9,10). Thus, it was hypothesized that the
differentiation of stem cells into ATI occurs through the Wnt
signaling pathways.
Considering the prospective effects of Sal B and Wnt
signaling on the differentiation of bone marrow-derived mesenchymal
stem cells (BMSCs), the present study was undertaken to investigate
whether Sal B promoted the differentiation of BMSCs into ATI and
whether this process was mediated by the activation of the Wnt
signaling pathways.
Materials and methods
Ethical statement
All animal experimental procedures were approved by
the Institutional Animal Care Committee of Dalian Medical
University (Dalian, China). The present study was conducted
according to institutional guidelines under an approved protocol. A
total of 18 male Sprague Dawley (SD) rats (80–100 g body weight;
3–5 weeks; Specific Pathogen Free Laboratory Animal Center of
Dalian Medical University, Dalian, China) were used in the present
study.
Isolation and culture of BMSCs
BMSCs were isolated from the femur and tibia of two
male SD rats as previously described (11). Briefly, the ends of the bones were
cut, and the marrow was extruded with medium using a needle and
syringe. The cell suspensions were plated on 25-cm2
plastic flasks and supplemented with Dulbecco’s modified Eagle’s
medium (DMEM) with 10% fetal bovine serum (FBS) and 1% glutamine
(Cyagen Biosciences, Santa Clara, CA, USA). The cells were then
incubated at 37°C with 5% CO2. After incubation for 24
h, the non-adherent cells were removed by replacing the medium. All
experiments described in the present study were performed using
cells from passages 5–8.
Flow cytometric analysis
Flow cytometric analysis of the expression of a
panel of surface markers was performed using a BD Biosciences
fluorescence-activated cell sorting machine (FACSAria2) using
standard techniques (BD Biosciences, Mountain View, CA, USA). The
BMSCs were harvested and washed with phosphate-buffered saline,
then stained using antibodies against CD29, CD34, CD71, CD90 and
CD106 (BD Biosciences).
BMSC differentiation assays
The lung homogenate was attained from the right lung
of four SD rats (80–100 g), and was cultured without serum for 24
h. Subsequently, the medium was concentrated using ultrafiltration
units with a 3 kDa molecular weight cutoff (Amicon, Billerica, MA,
USA). The BMSCs were plated and supplemented with conditioned
medium (CM), which contained a mixture of concentrated medium (1
ml) and DMEM stem cell culture medium with 10% FBS and 1% glutamine
(1 ml) in equal volumes (1:1). The groups were as follows: i) CM
group: BMSCs were supplemented with CM; ii) lithium chloride (LiCl;
Amresco LLC, Solon, OH, USA) group: BMSCs were supplemented with CM
and 5 mM LiCl; iii) Sal B group: BMSCs were supplemented with CM
and 10 mM Sal B (Sigma-Aldrich, St. Louis, MO, USA). All samples
were collected and assessed on days 7 and 14.
Immunofluorescence staining
The cells were fixed in 4% paraformaldehyde
(Solarbio, Beijing, China) and permeabilized with 0.1% Triton X-100
(Amresco LLC) for 15 min prior to incubation with the primary
antibodies for 2 h at room temperature. The primary antibodies
included rabbit polyclonal aquaporin (AQP)-5 (cat. no. sc-28628;
1:70 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), rabbit polyclonal T1α (cat. no. bs-1048R; 1:70 dilution;
Bioss, Shanghai, China), and fluorescein isothiocyanate-labeled
donkey anti-rabbit secondary antibodies (cat. no. 711-095-152; 1:70
dilution; Jackson Immuno Research Inc., West Grove, PA, USA). The
binding of DAPI (Sigma-Aldrich) to DNA was used to identify the
nucleus. The immune complexes were observed using fluorescence
microscopy (Olympus BX63; Olympus Corporation, Tokyo, Japan).
Western blot analysis
Total proteins were extracted from differentiated
cells. The proteins were loaded onto a linear gradient
polyacrylamide gel, with a polyacrylamide stacking gel, then
electrophoretically transferred onto a polyvinylidene difluoride
membrane following electrophoresis. The membranes were blocked via
incubation with 5% evaporated skimmed milk for blocking, followed
by incubation with the primary antibodies at 4°C overnight. The
primary antibodies, including AQP-5 (1:200 dilution), T1α (1:200
dilution), rabbit polyclonal Wnt-1 (cat. no. bs-1739R; 1:150
dilution; Bioss), rabbit polyclonal Wnt-3a (cat. no. bs-1700R;
1:150 dilution; Bioss) and mouse polyclonal β-actin (cat. no.
sc-47778; 1:400 dilution; Santa Cruz Biotechnology, Inc.) were
used. Following washing, the membranes were incubated with
horseradish peroxidase-linked immunoglobulin G goat anti-rabbit or
goat anti-mouse secondary antibodies (cat. nos. ZB-2305 or ZB-2301;
1:6,000 dilution; ZSGB-BIO, Beijing, China) for 2 h at room
temperature. After standard enhanced chemiluminescence detection
using gel-imaging with ChemiDoc XRS+ (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and Image Lab software, version 4.0 (Bio-Rad
Laboratories, Inc.), the samples were subjected to image analysis
using the ImageJ software, version 2.1 (National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
Values are presented as the mean ± standard error of
the mean and were analyzed using SPSS 19.0 software (IBM, Armonk,
NY, USA). The experimental data were analyzed using a one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of BMSCs in culture
The majority of cells exhibited a spindle-like or
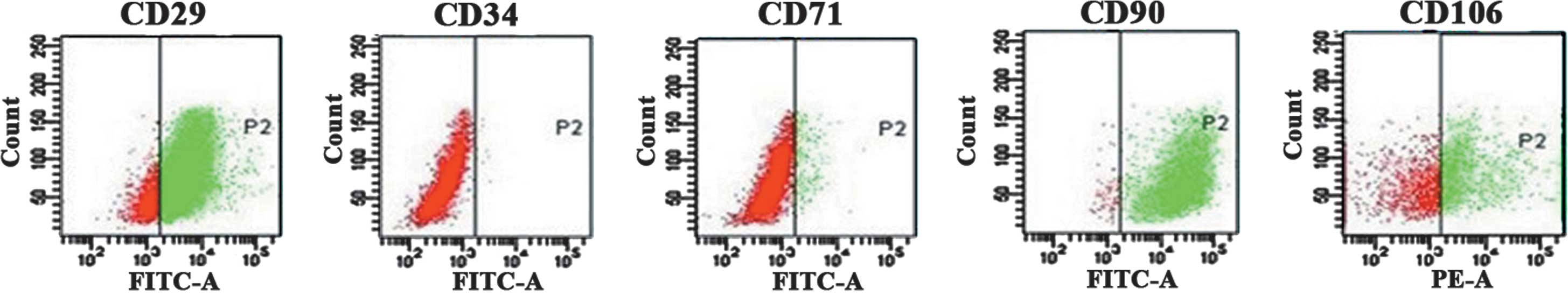
fibroblast-like shape. Flow cytometric analysis demonstrated the
expression of BMSC surface markers. BMSCs were positive for CD29
and CD90, (85.0 and 97.5%, respectively). Expression of CD106 was
present on 47.2% of BMSCs, while CD34 and CD71 expression was rare
(0.18 and 4%, respectively) (Fig.
1).
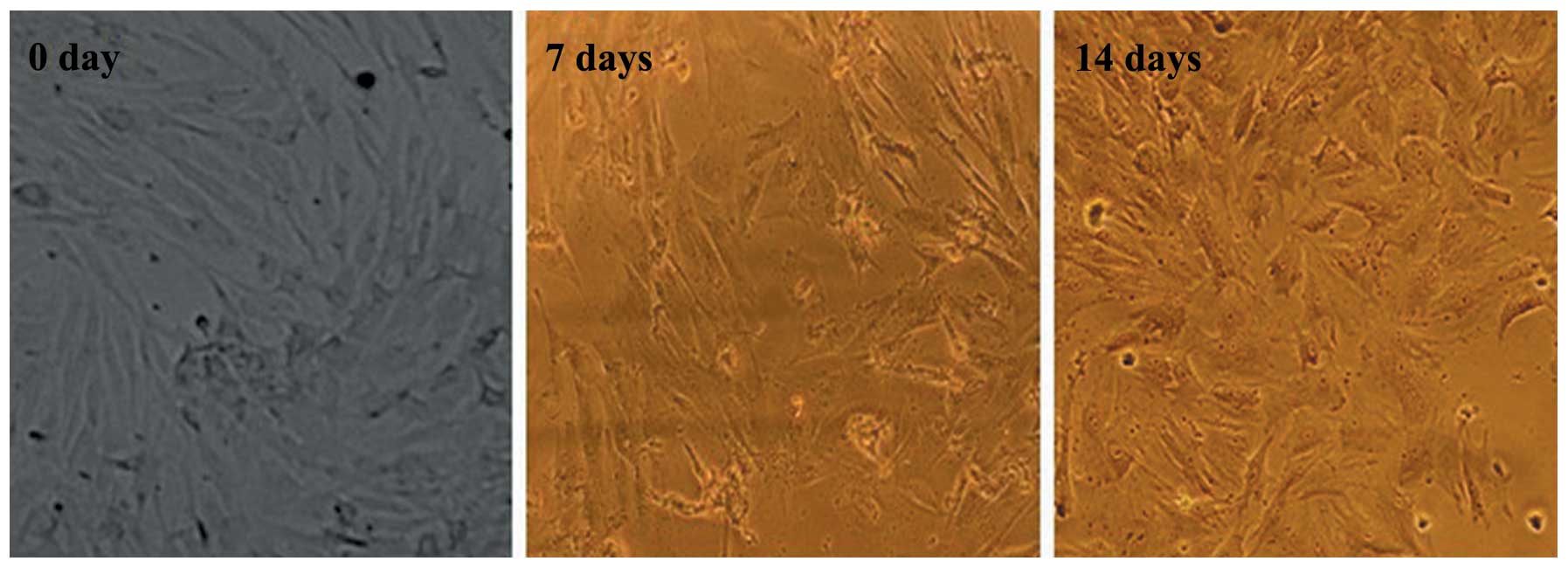
Morphological changes of BMSCs during
differentiation into ATI
Under inductive conditions, the BMSCs lost their
characteristic fibroblast-like shape and gradually began to exhibit
a polygonal morphology, typical of ATI. On day 7, a few BMSCs
exhibited an alveolar epithelial-like shape; however, by day 14,
the majority of BMSCs exhibited the morphology indicative of
differentiation into ATI (Fig.
2).
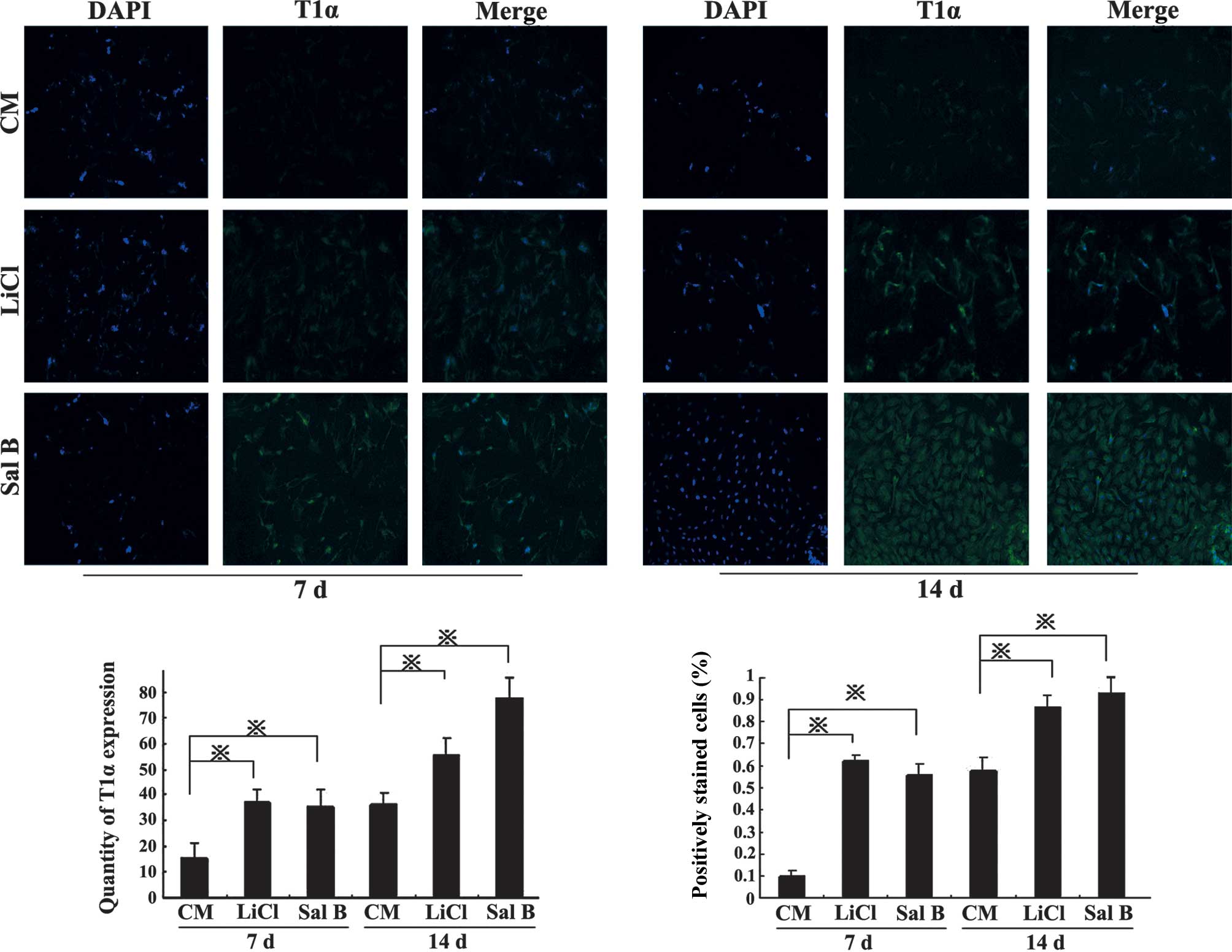
BMSC differentiation and expression of
ATI markers
Under inductive conditions (stimulation with LiCl or
Sal B), the expression of ATI lineage-specific markers, AQP-5 and
T1α, was examined using immunofluorescence. BMSCs under control
conditions expressed these proteins at relatively low levels on day
7; however, when BMSCs differentiated into ATI, AQP-5 and T1α
levels were increased by day 14 and following induction with LiCl
or Sal B compared with the CM group (P<0.05; Figs. 3 and 4).
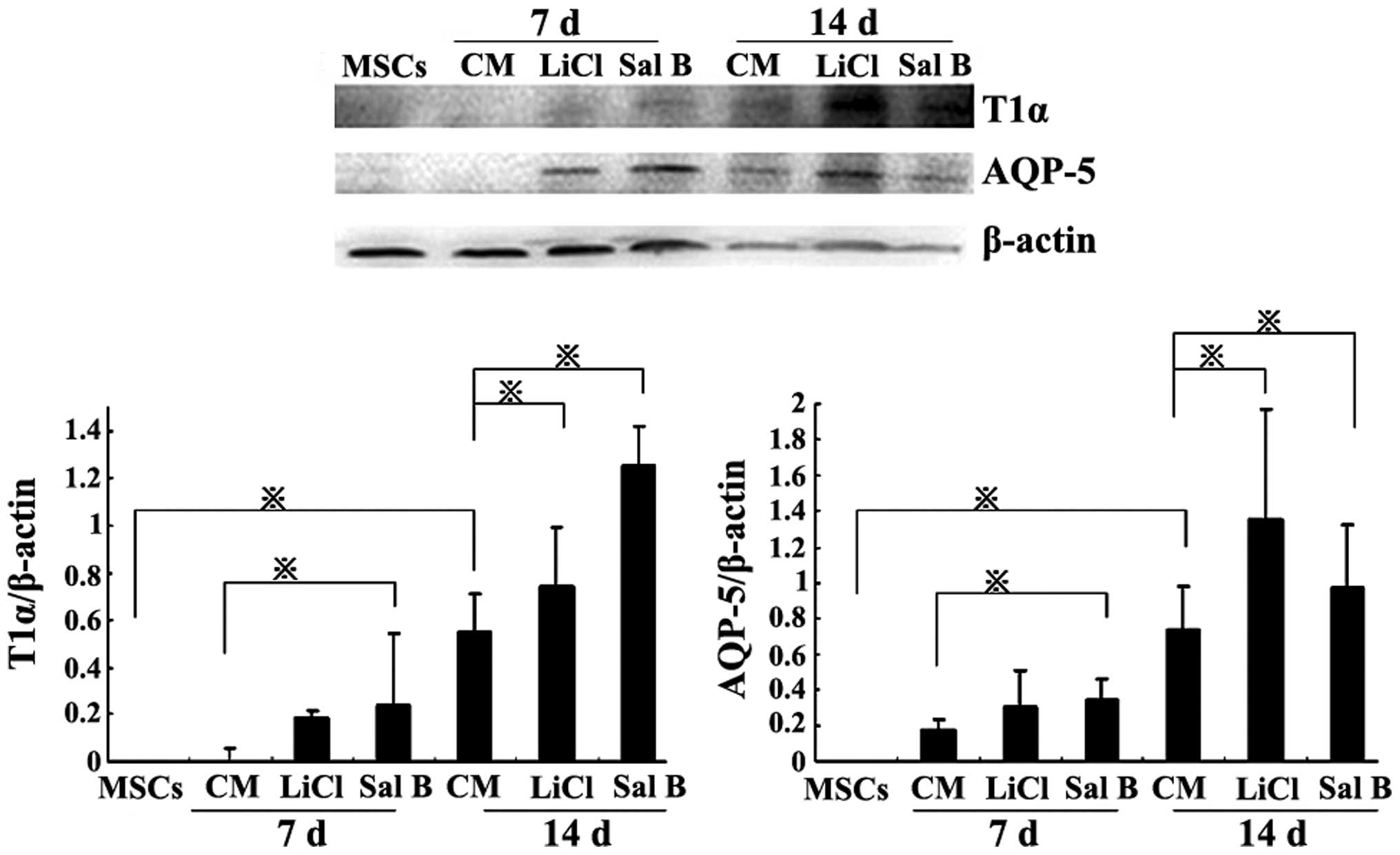
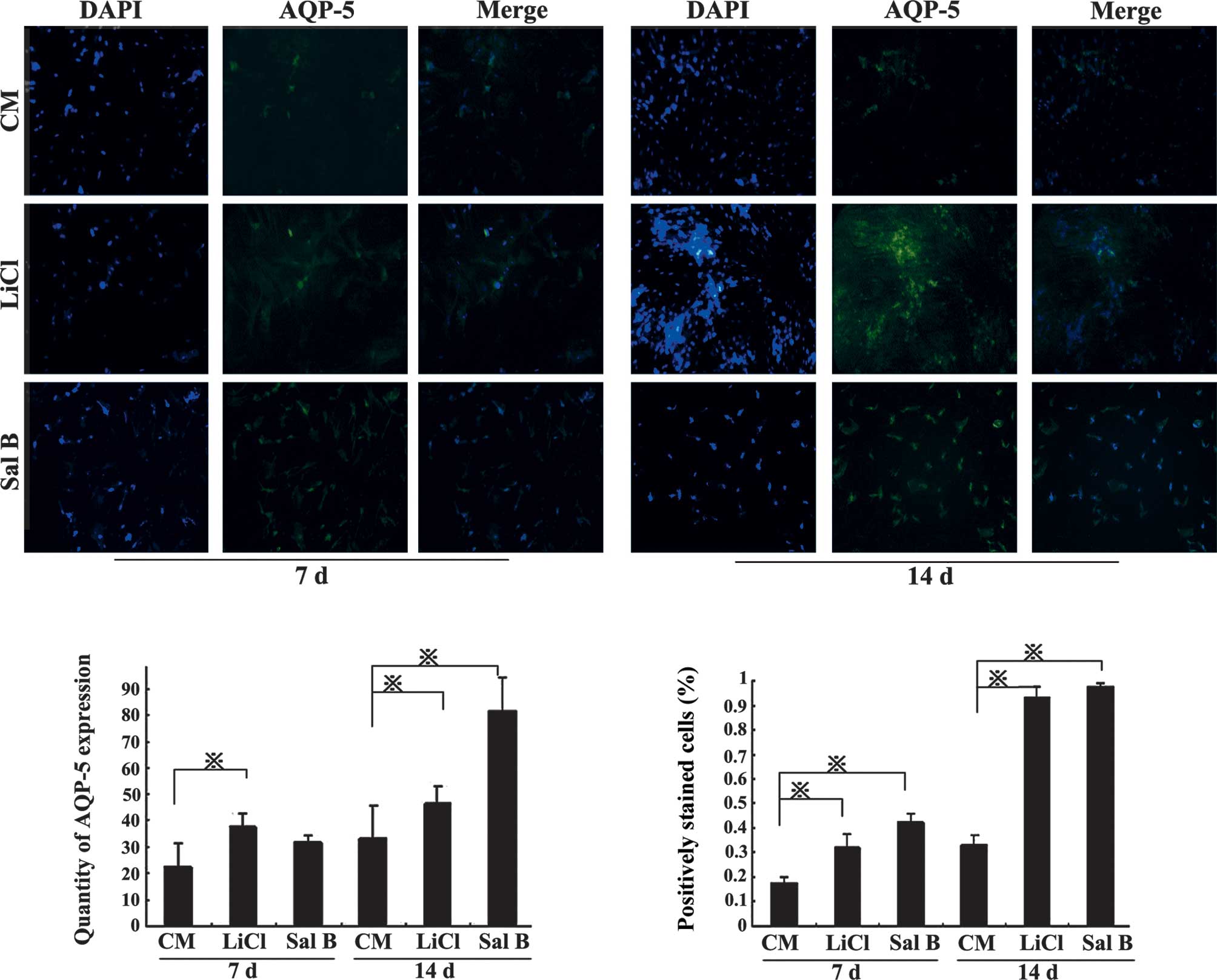 | Figure 4Bone marrow-derived mesenchymal stem
cell differentiation increases expression of AQP-5. Under inductive
conditions, the markers of the ATI lineage-specific marker, AQP-5,
were examined. Induction with LiCl and Sal B increased AQP-5
expression. In the LiCl group, the AQP-5 expression was
significantly higher than that in the CM group at 7 and 14 days. In
the Sal B group, the AQP-5 expression was significantly higher than
that in the CM group on day 14. Magnification, ×40.
*P<0.05. Results are presented as the mean ± standard
deviation. CM, conditioned medium; ATI, alveolar epithelial cells
type I; Sal B, salvianolic acid B; AQP, aquaporin. |
The western blot analysis revealed similar results
to those of the immunofluorescence staining. In the Sal B group,
the T1α and AQP-5 protein levels were significantly increased
compared with the CM group on days 7 and 14 (P<0.05). In the
LiCl group, the T1α and AQP-5 protein levels were significantly
increased compared with those in the CM group on day 14 (P<0.05;
Fig. 5).
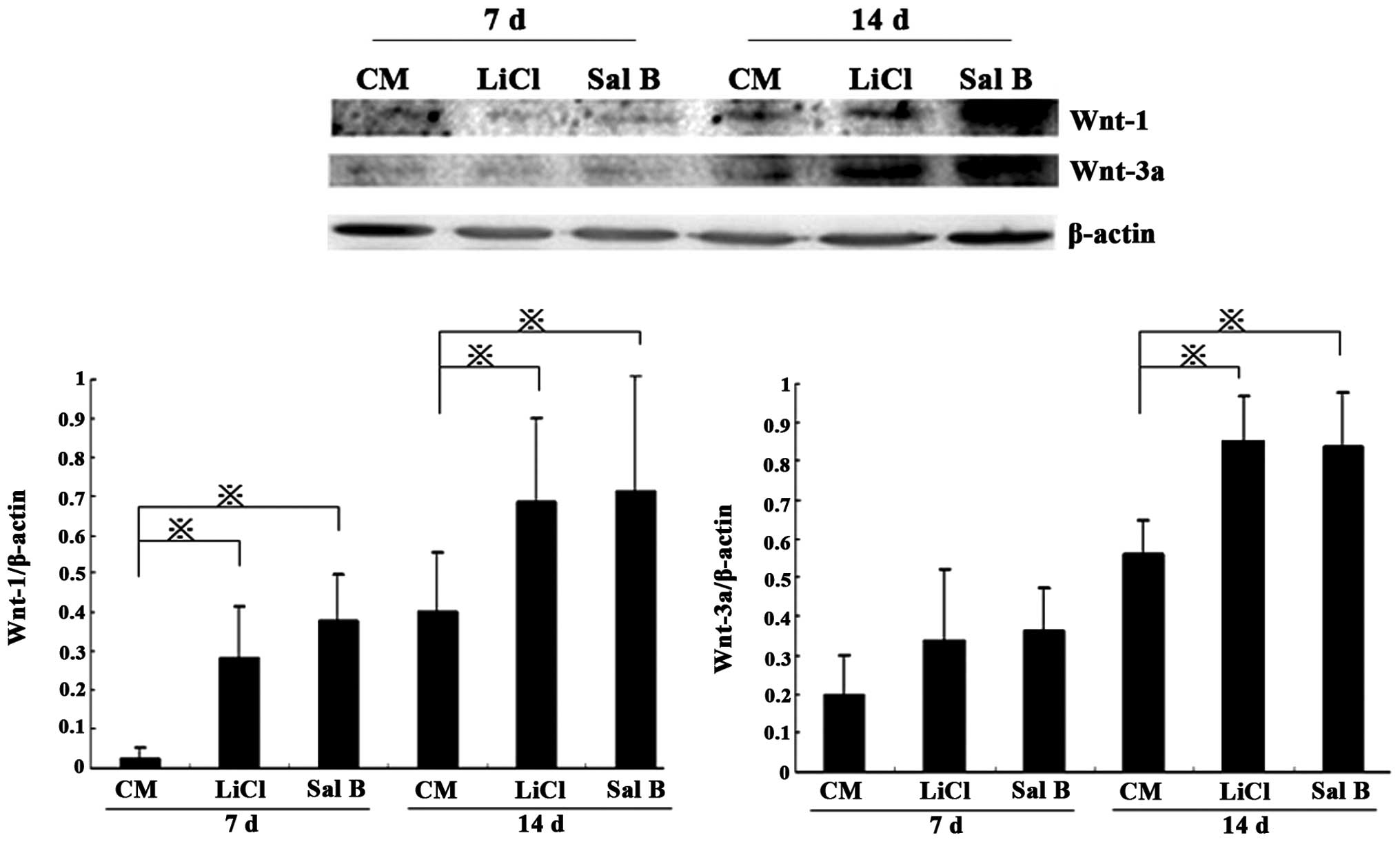
Wnt pathway activation during the
differentiation of BMSCs into ATI
Differentiation of BMSCs into ATI activated Wnt
signaling, as indicated by western blot analysis of Wnt-1 and -3a.
In the LiCl and Sal B groups, Wnt-1 levels were significantly
higher that those in the CM group on day 7 (P<0.05), and Wnt-1
and -3a levels were further increased by day 14 (P<0.05;
Fig. 6).
Discussion
Damage to the alveolar epithelium has been
documented in ~90% of patients with lung injury, according to data
collected from lung biopsies (12). Lung tissue may be recovered with
adequate repair and remodeling of the alveolar epithelia, but not
in the case of severe injury and without the rapid activation of
repair mechanisms. As stem cells are considered to differentiate
into ATI as a replacement of injured alveolar epithelial cells, it
was important to elucidate whether the BMSCs may be a suitable
source of MSCs (3). BMSCs as a
source of multipotent cells can be easily identified by detection
of cell surface antigens; they are positive for CD29 and CD90, have
low expression of CD106, and are negative for CD34 and CD71. The
results of the present study confirmed findings from a previous
study (11).
The supernatant of the lung tissue homogenate was
used in the present study to investigate the mechanism of
increasing the differentiation efficiency of stem cells into ATI by
induction with Sal B. The results revealed that the cultured BMSCs
had a polygonal morphology and expressed T1α and AQP-5, which are
specific markers for ATI. It was suggested that the differentiated
cells serve as a precursor to the population of T1α-positive
cultured cells in vivo (4).
AQP-5 is an important mediator of the movement of water across the
cell membranes of vascular endothelial cells and ATI via a
transcellular pathway. In addition, in the case of pulmonary edema
following injury, AQP-5 is expressed decreasingly in lung tissue
(13). Therefore, it is expected
that, by expressing AQP-5 and exhibiting the specific features of
ATI, the stem cells (BMSCs) may differentiate into ATI and possibly
be involved in the movement of water across the cell membranes to
reduce alveolar edema in response to ALI.
In the present study, the effect of Sal B on the
differentiation of BMSCs into ATI was examined. The natural product
Sal B is an active component of S. miltiorrhiza, an herb
widely used in Traditional Chinese Medicine. Accumulating evidence
has suggested that Sal B has protective effects under various
medical conditions. It has been reported that Sal B is able to
significantly attenuate the decrease in viability of BMSCs
subjected to damage through administration of potentially lethal
H2O2, exhibiting time-dependent protective
effects (14). The marked
oxidative stress status in vivo also prevented the implanted
stem cells from migrating and differentiating, eventually inducing
apoptosis, resulting in an impaired effect of this promising
therapy in the treatment of disease. The major pharmacological
action of Sal B is to protect the pulmonary microcirculation from
disturbance, inflammation and apoptosis (15,16).
In addition, Sal B increases the expression of AQP-5 in lung tissue
in response to ALI (17). Sal B
has been revealed to improve the differentiation capacity of stem
cells into cells of other lineages, including neurons and
cardiomyocytes (6,7). In the present study, it was
demonstrated that Sal B improves the differentiation efficiency of
BMSCs into ATI.
It has been well-established that the Wnt signaling
pathways are fundamental in cell proliferation, cell polarity and
determination during embryonic development, and tissue homeostasis
(18). This pathway may be
activated when Wnt ligands, including Wnt-1, -2, or -3a, are bound
to the co-receptors of Frizzled and low-density lipoprotein
receptor-related protein 5 or 6. Furthermore, these binding events
are able to lead to the inhibition of the Axin complex and glycogen
synthase kinase (GSK)3β, resulting in the dephosphorylation and
stabilization of β-catenin in the cytoplasm, which subsequently
translocates into the nucleus to regulate target gene expression
(19). It is well-established that
the accumulation of β-catenin is critical in inducing the Wnt
signaling cascades (20). It was
observed in the present study that Wnt-1 and -3a may be detected
during the differentiation of BMSCs into ATI between 7 and 14 days.
LiCl activates Wnt signaling by inhibiting GSK3β and consequently
stabilizing free cytosolic β-catenin. Therefore, LiCl was used to
investigate the regulation of the Wnt/β-catenin signaling pathway
in the differentiation of BMSCs into ATI. It was demonstrated that
LiCl improved the differentiation efficiency of BMSCs into ATI,
regulated by the Wnt signaling pathway. In addition, Sal B also
markedly increased the differentiation efficiency of BMSCs through
the activation of Wnt signaling pathways.
In the present study, it was suggested that Sal B
possesses promotory effects on the differentiation of BMSCs into
ATI, which is likely to be mediated by the activation of Wnt
signaling.
Acknowledgments
The present study was supported by the National
Nature Science Fund of China (grant no. 81273923).
References
|
1
|
Rubenfeld GD, Caldwell E, Peabody E, et
al: Incidence and outcomes of acute lung injury. N Engl J Med.
353:1685–1693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ortiz LA, Gambelli F, McBride C, et al:
Mesenchymal stem cell engraftment in lung is enhanced in response
to bleomycin exposure and ameliorates its fibrotic effects. Proc
Natl Acad Sci USA. 100:8407–8411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rojas M, Xu J, Woods CR, et al: Bone
marrow derived mesenchymal stem cells in repair of the injured
lung. Am J Respir Cell Mol Biol. 33:145–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kotton DN, Ma BY, Cardoso WV, Sanderson
EA, Summer RS, et al: Bone marrow-derived cells as progenitors of
lung alveolar epithelium. Development. 128:5181–5188.
2001.PubMed/NCBI
|
|
5
|
Zhuang P, Zhang Y, Cui G, Bian Y, Zhang M,
Zhang J, Liu Y, Yang X, Isaiah AO, Lin Y, et al: Direct stimulation
of adult neural stem/progenitor cells in vitro and neurogenesis in
vivo by salvianolic acid B. PLoS One. 7:e356362012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo G, Li B, Wang Y, Shan A, Shen W, Yuan
L and Zhong S: Effects of salvianolic acid B on proliferation,
neurite outgrowth and differentiation of neural stem cells derived
from the cerebral cortex of embryonic mice. Sci China Life Sci.
53:653–662. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan SS, Chen JH, Hwang SM, Wang IJ, Li
HJ, Lee RT and Hsieh PC: Salvianolic acid B-vitamin C synergy in
cardiac differentiation from embryonic stem cells. Biochem Biophys
Res Commun. 387:723–728. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Sun Z, Qiu X, Li Y, Qin J and Han
X: Roles of Wnt/beta-catenin signaling in epithelial
differentiation of mesenchymal stem cells. Biochem Biophys Res
Commun. 390:1309–1314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee H, Bae S, Choi BW and Yoon Y:
WNT/β-catenin pathway is modulated in asthma patients and
LPS-stimulated RAW264.7 macrophage cell line. Immunopharmacol
Immunotoxicol. 34:56–65. 2012. View Article : Google Scholar
|
|
10
|
Baarsma HA, Spanjer AI, Haitsma G, et al:
Activation of WNT/β-catenin signaling in pulmonary fibroblasts by
TGF-β1 is increased in chronic obstructive pulmonary
disease. PLoS One. 6:e254502011. View Article : Google Scholar
|
|
11
|
Ionescu L, Byrne RN, van Haaften T, et al:
Stem cell conditioned medium improves acute lung injury in mice: in
vivo evidence for stem cell paracrine action. Am J Physiol Lung
Cell Mol Physiol. 303:L967–L977. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Parambil JG, Myers JL, Aubry MC and Ryu
JH: Causes and prognosis of diffuse alveolar damage diagnosed on
surgical lung biopsy. Chest. 132:50–57. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao C, Li R, Huan J, et al: Caveolin-1
siRNA increases the pulmonary microvascular and alveolar epithelial
permeability in rats. J Trauma. 70:210–219. 2011. View Article : Google Scholar
|
|
14
|
Lu B, Ye Z, Deng Y, Wu H and Feng J:
MEK/ERK pathway mediates cytoprotection of salvianolic acid B
against oxidative stress-induced apoptosis in rat bone marrow stem
cells. Cell Biol Int. 34:1063–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lay IS, Hsieh CC, Chiu JH, Shiao MS, Lui
WY and Wu CW: Salvianolic acid B enhances in vitro angiogenesis and
improves skin flap survival in Sprague-Dawley rats. J Surg Res.
115:279–285. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen YL, Hu CS, Lin FY, et al: Salvianolic
acid B attenuates cyclooxygenase-2 expression in vitro in
LPS-treated human aortic smooth muscle cells and in vivo in the
apolipoprotein-E-deficient mouse aorta. J Cell Biochem. 98:618–631.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin F, Liu YY, Xu B, et al: Salvianolic
acid B protects from pulmonary microcirculation disturbance induced
by lipopolysaccharide in rat. Shock. 39:317–325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gordon MD and Nusse R: Wnt signaling:
Multiple pathways, multiple receptors, and multiple transcription
factors. J Biol Chem. 281:22429–22433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|