Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common and aggressive malignancy worldwide, accounting for >90%
of all primary liver cancers and representing the third leading
cause of liver cancer mortality (1). Annually, HCC is responsible for ~1
million mortalities worldwide (2).
HCC is also known as adult primary liver cancer and develops from
an abnormal mass of tumor nodules, metastasizing to adjoining parts
of the liver and resulting in malignancy (3). An increasing body of evidence has
suggested that >0.5 million novel cases of HCC are diagnosed
annually (4). Patient prognoses
following surgical resection of HCC remain poor due to the high
rate of recurrence and dearth of effective adjuvant therapies
(5). Tumor recurrence occurs in
>70% of cases within five years (6), and the five-year survival rate is
just 60–70%. The clinical therapies currently available require
extensive immunosuppressive pre-treatments, which frequently result
in undesirable physiological side-effects (7). The identification of patients with a
higher risk of recurrence and the development of more effective and
targeted therapeutic strategies are required in order to improve
patient prognoses. Therefore, the development of novel therapeutic
strategies for the treatment of HCC, with fewer adverse effects, is
urgently required for the improvement of clinical practices and HCC
patient prognosis.
Camellia oleifera (C. oleifera) Abel. is
cultivated in numerous regions throughout China (8). C. oleifera seeds are used in
the production of oil. Sasanquasaponin (SQS) is a biologically
active ingredient, which may be extracted from C. oleifera
Abel. SQS has numerous pharmacological properties, including
anti-inflammatory, anti-hyperlipidemic and anti-effusive effects
(9). Studies have revealed that
SQS is able to induce apoptosis in human leukemia Jurkat cells and
HepG2 hepatoma cells (10,11). The present study therefore aimed to
determine whether SQS was able to induce cell cycle arrest and
apoptosis in the HepG2 HCC cell line, and investigate the potential
mechanisms underlying these effects.
Materials and methods
Materials and reagents
RPMI-1640, fetal bovine serum (FBS),
penicillin-streptomycin, Trypsin-EDTA and TRIzol were obtained from
Invitrogen Life Technologies (Carlsbad, CA, USA). SuperScript II
reverse transcriptase was obtained from Promega Corp. (Madison, WI,
USA). B-cell lymphoma 2 (Bcl-2), Bcl-2-associated x protein Bax and
β-actin primary antibodies, as well as horseradish
peroxidase-conjugated secondary antibodies were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). 5-FU was obtained
from Zhejian Wanma Pharm., Co. (Hangzhou, China). All other
reagents, unless otherwise stated, were obtained from Sigma-Aldrich
(St. Louis, MO, USA).
SQS drug preparation
Enrichment experiments were performed in glass
columns (2.5×60 cm) packed with AB-8 (Shanghai Resin Factory Co.,
Ltd., Shanghai, China). C. olifeira was obtained from
Sanming City, Fujian, China. A total of ~500 g seeds of C.
olifeira were refluxed with 70% ethanol. The extracted solution
was concentrated to 500 ml without ethanol. The ethanolic extract
was adjusted to pH 5.0, and the sample flowed through the glass
column at a rate of 1.0 bed volumes (BV)/h, while 0.1% NaOH
solution was added via the separatory funnel, in order to elute
impurities and pigment. Following clarification, the sample was
sequentially eluted with distilled water to neutral, and then with
90% ethanol (Sinopharm Chemical Reagent Co., Ltd., Beijing, China)
solution (containing 1% NaOH; Sinopharm Chemical Reagent Co., Ltd.)
at a constant flow rate of 2.0 BV/h. Each fraction was collected
and evaporated to remove the ethanol using a rotary evaporator
(RE52AA; Yarong Equipment Co., Shanghai, China) under reduced
pressure at 55°C. Subsequently, 500 ml acetone (Sinopharm Chemical
Reagent Co., Ltd.) was added to the concentrated liquid and left to
stand overnight, followed by centrifugation at 1,006 x g for 30
min. The sediment was dissolved in 70% ethanol, placed in the
fridge at 4°C overnight, filtered and dried at 60°C to a constant
weight (500 g) to produce high-purity SQS crystals (5 g) (12).
Cell culture and treatment
Immortalized HepG2 human hepatocellular carcinoma
cells were obtained from the Live Cancer Institute of Zhongshan
Hospital (Shanghai, China) and maintained in Dulbecco’s modified
Eagle’s medium containing 10% FBS and 1% penicillin/streptomycin
solution at 37°C in 5% CO2. All experiments were
performed while the cells were in an exponential growth phase
(13).
Cytotoxicity assay
HepG2 cells were seeded into standard 96-well
culture plates at a density of 2.0×104 cells/well and
cultured to ~70% confluence. Cells were maintained in medium
containing various concentrations (0, 10, 20 or 30 µg/ml) of
SQS and 30 µg/ml 5-FU for 6, 12 or 24 h prior to the
addition of 20 µl MTT solution (5 mg/ml MTT) to each well
and cultured for a further 4 h. The medium was subsequently removed
and 150 µl/well dimethyl sulfoxide was added and incubated
for 10 min whilst shaking gently to stop the reaction. The optical
density (OD) at 570 nm was read using an ELISA reader (ELX800;
BioTek Instruments, Inc., Winooski, VT, USA). Each independent
experiment was performed in triplicate (14).
Cell apoptosis analysis
Annexin V staining (in the presence or absence of
SQS) and cell scanning were conducted using an Annexin
V-fluorescein isothiocyanate (FITC) detection kit (Beyotime
Institute of Biotechnology, Haimen, China). The HepG2 cells were
treated with 0, 10, 20 or 30 µg/ml SQS for 12 h. To detect
apoptosis, 1×105 cells were resuspended in 500 µl
binding buffer in the presence of 5 µl Annexin V-FITC and 5
µl propidium iodide and incubated at room temperature for 15
min in the dark. The fluorescence was measured using a FACScan flow
cytometer (BD Bioscience) and the mean fluorescent intensity of
triplicate samples for each SQS concentration cohort was used to
ascertain the levels of Annexin V. The data were analyzed using
CellQuest software version 1.0 (BD Biosciences, Franklin Lakes, NJ,
USA) (15).
Cell cycle analysis
HepG2 cells were treated with 0, 10, 20 or 30
µg/ml SQS for 12 h. Subsequently, cells were harvested,
washed with solution buffer and fixed in cold 70% ethanol
overnight. A DNA content detection kit (Beyotime Institute of
Biotechnology, Haimen, China) was used for cell cycle analysis. The
cells were collected and suspended in 100 µl solution A and
incubated at room temperature for 10 min. Analogous conditions were
used upon the addition of 100 µl solution B and 150
µl solution C. Cell cycle progression was analyzed using a
FACScan flow cytometer (BD Biosciences). The percentages of cells
in G0/G1, S and G2/M phases were
determined using ModFitLT software version 3.0 (Verity Software
House, Topsham, ME, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR) and gene expression analysis
RT-PCR was performed as described previously
(16). HepG2 cells were seeded
into six-well plates at a density of 1×105 cells/well in
2 ml medium and treated with 0, 10, 20 or 30 µg/ml SQS for
12 h. Total RNA was isolated with TRIzol, and oligo (dT)-primed RNA
(1 µg) was reverse-transcribed using SuperScript II reverse
transcrip-tase (Promega Corp.) according to the manufacturer’s
instructions. The obtained cDNA was used to determine the mRNA
expression levels of Bcl-2, Bax and caspase-3 by PCR analysis.
GAPDH was used as an internal control. The primer sequences used
for the amplification of Bcl-2, Bax, caspase-3 and GAPDH were as
follows: Bcl-2 for wa rd, 5′-TTCTCTCGTCGCTACCGTCGC-3′ and reverse,
5′-CCT CCCCCAGTTCACCCCATC-3′; Bax forward, 5′-CTTTTT
GCTACAGGGTTTCA-3′ and reverse, 5′-CCATGTTGTTGT CCAGTTCAT-3′;
caspase-3 forward, 5′-CAGAAGATACCA GTGGAGGCC-3′ and reverse,
5′-TTCCGGTTAACACGA GTGAGG-3′; GADPH forward, 5′-CGACCACTTTGTCA
AGCTCA-3′ and reverse, 5′-AGGGGTCTACATGGCAA CTG-3′. All primers
were synthesized by Sangon Biotech (Shanghai, China). The reaction
included 1 µl cDNA, 2 µl 10X Taq Buffer, 1.2
µl 25 mM MgCl2, 0.4 µl 10 mM dNTP, 0.8
µl 1U/μL Taq polymerase, 1 µl each primer and DEPC
water upto 20 µl. The PCR conditions were as follows: 95°C
for 3 min and 30 cycles at 95°C for 30 sec, 56°C for 40 sec and
72°C for 40 sec. Samples were analyzed by 1.5% agarose gel
electrophoresis. The DNA bands were evaluated using a Gel
Documentation system (Gel Doc 2000; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Western blot analysis
HepG2 cells were seeded in culture dishes
(2×105/well) and treated with 0, 10, 20 or 30
µg/ml SQS for 12 h. The cells were washed twice with
phosphate-buffered saline (PBS) and lysed with lysis buffer
according to the manufacturer’s instructions. The protein
concentration of the lysate was determined via the bicinchoninic
acid assay (Pierce, Rockford, IL, USA). Samples were analyzed using
10% SDS-PAGE (Dingguo Biotech., Fuzhou, China). Following
electrophoresis, the proteins were transferred onto polyvinylidene
difluoride membranes. The membranes were blocked with 5% skimmed
milk in PBS with Tween 20 (PBST; Xilong Chemical Co., Ltd.,
Shantou, China) for 1.5 h and incubated with rabbit anti-Bcl-2,
anti-Bax and anti-caspase-3 antibodies (1:1,000 dilution) at 4°C
overnight. Following three washes with PBST buffer, the membranes
were incubated with goat anti-rabbit immunoglobulin G conjugated
with horseradish peroxidase for 2 h at room temperature. Following
three washes with PBST, the protein bands were detected using
enhanced chemiluminescence using Clarity Western Substrate
(Bio-Rad, Hercules, CA, USA), according to the manufacturer’s
instructions and images were captured using a ChemiDoc XRS+ system
(Bio-Rad). β-actin was used as the control to confirm equal
loading. Densitometry index analysis of the bands was conducted
using a gel imagery system (17).
Statistical analysis
Values are presented as the mean ± standard
deviation. Results were analyzed by one-way analysis of variance.
When significant treatment effects were detected, Tukey-Kramer’s
t-test was performed to make pairwise comparisons between
individual means. Statistical analysis software SPSS 19.0
(International Business Machines, Armonk, NY, USA) was used to
conduct statistical analyses. P<0.05 was considered to indicate
a statistically significant difference.
Results
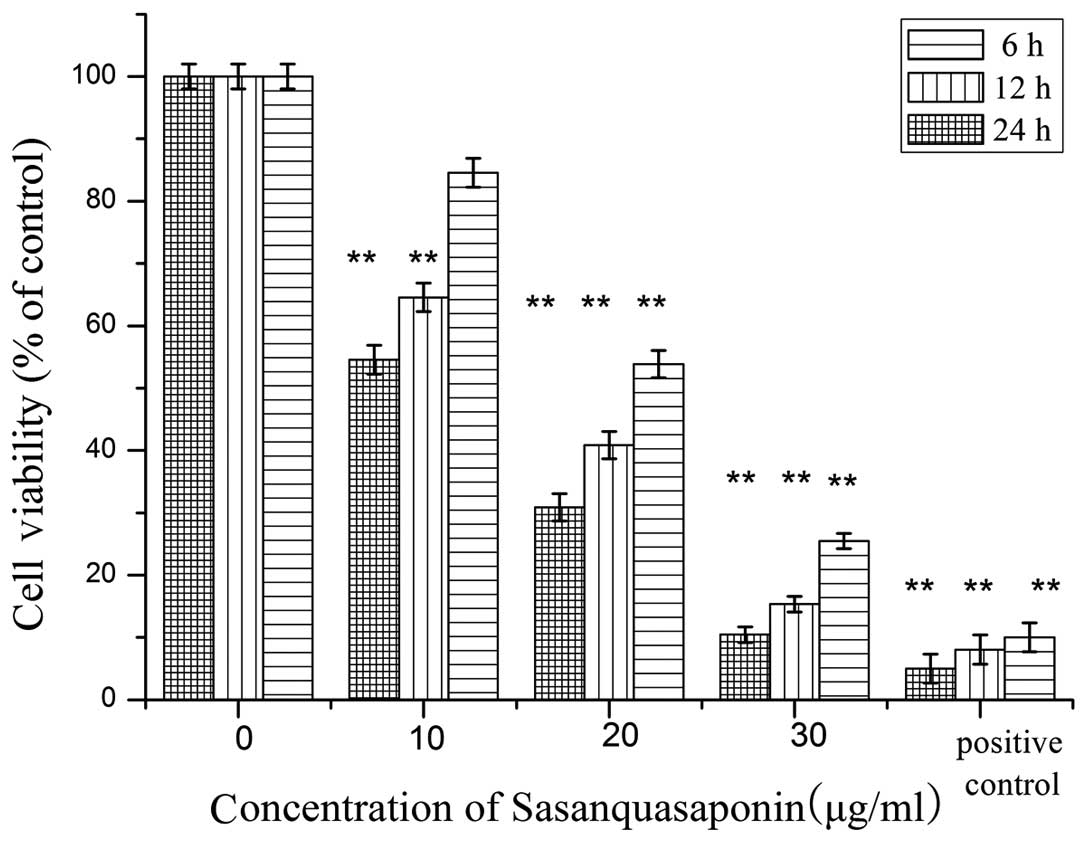
SQS reduces HepG2 cell viability
The MTT assay suggested that cytotoxic effects
occurred 6, 12 and 24 h following incubation with SQS, even at
doses as low as 20 µg/ml SQS. As indicated in Fig. 1, the degree of cytotoxicity was
directly proportional to the concentration of SQS, suggesting a
dose-dependent effect. The results of the MTT test indicated that
following 6 h of incubation with 10, 20 or 30 µg/ml SQS,
cell survival was reduced to 84.56, 53.86 and 25.25%,
respectively.
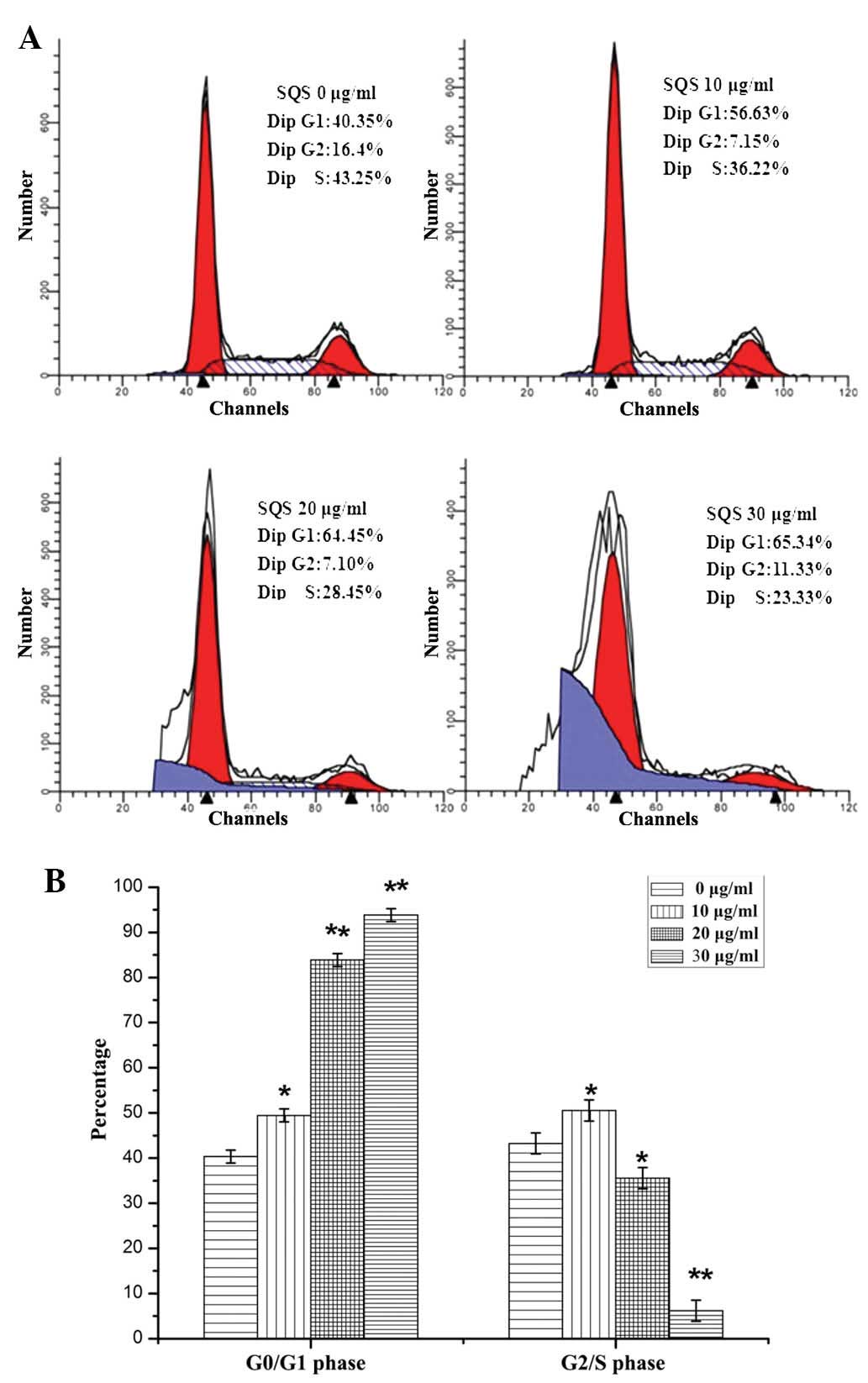
SQS alters HepG2 cell cycle
progression
The cell cycle has a key function in HepG2 cells,
inducing their division and duplication. In addition, the
G1/S-phase transition represents one of the major
checkpoints used in the regulation of cell cycle progression. Once
the cell cycle passes this checkpoint late in G1 phase,
progression through the cell cycle continues with little or no
extracellular stimuli (18). As
indicated in Fig. 2, the
proportion of cells in G0/G1 phase following
treatment with 10, 20 or 30 µg/ml SQS was 56.63±1.76,
64.45±1.47 and 65.34±1.67%, a significantly higher proportion than
that of untreated cells (40.35±1.33%; P<0.05). Concurrently, the
percentage of cells in S phase following SQS treatment demonstrated
the opposite trend. It was therefore suggested that SQS treatment
may arrest cell cycle progression of HepG2 cells by inhibiting the
G1 to S phase transition.
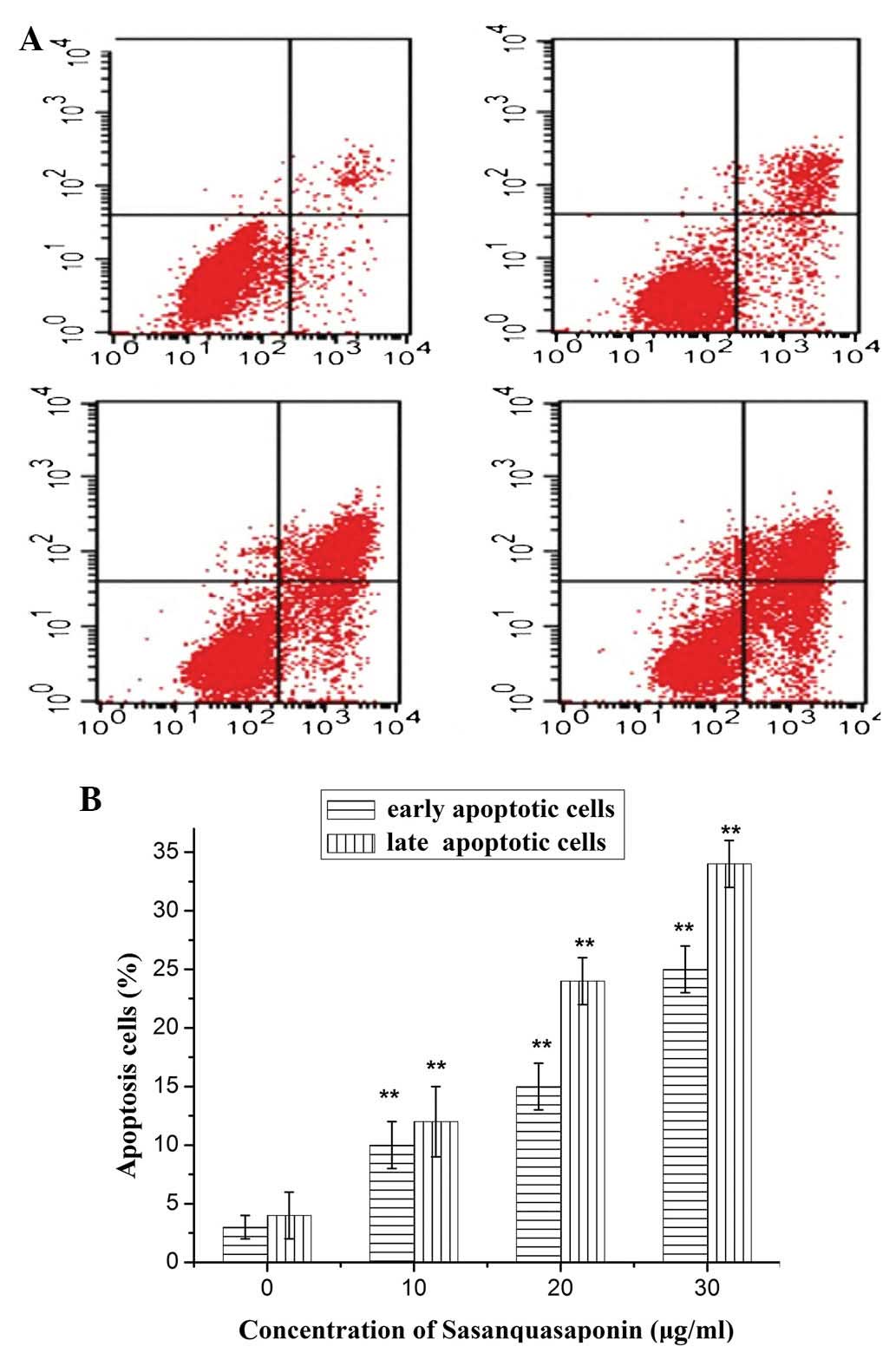
SQS induces apoptosis of HepG2 cells
To determine the mechanisms underlying the effects
of SQS treatment on HepG2 cells, membrane alterations were analyzed
by flow cytometry and double staining with Annexin V-FITC and PI. A
progressive increase in the percentage of apoptotic cells was
observed with increasing SQS concentration, which demonstrated the
dose-dependent cytotoxicty of SQS to HepG2 cells (Fig. 3).
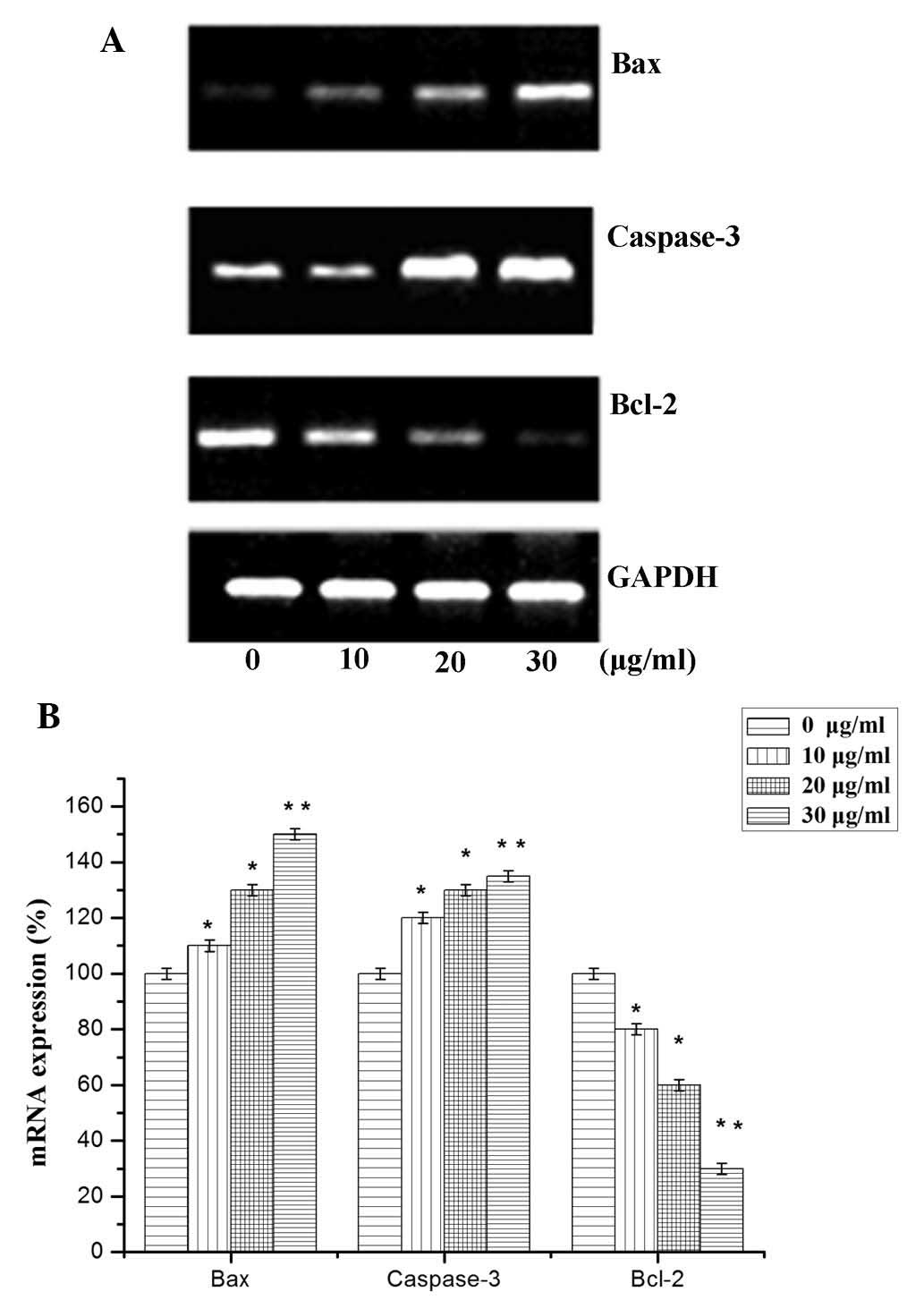
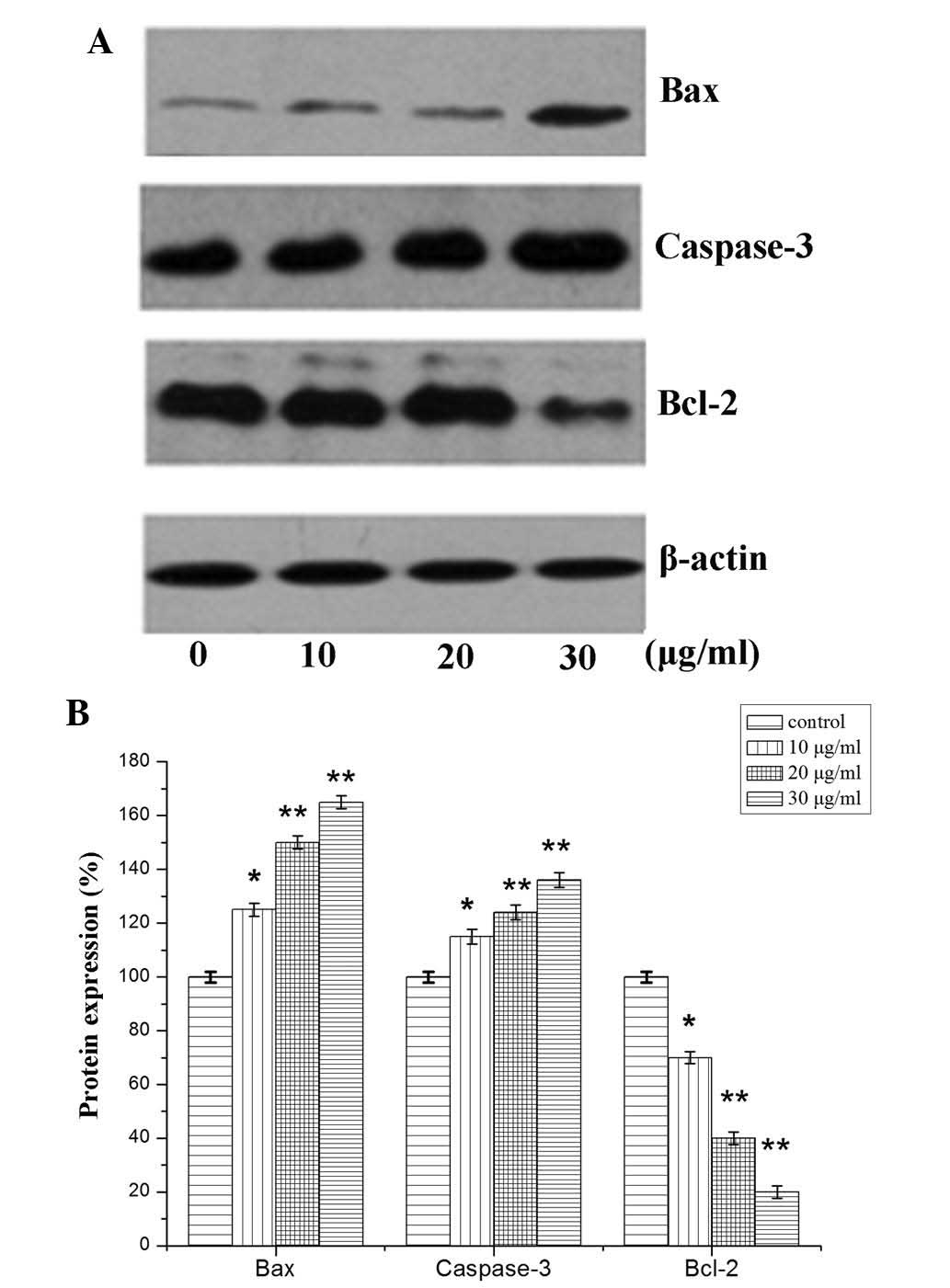
Expression levels of Bcl-2, Bax and
caspase-3
Based on the aforementioned results, the association
between the expression of apoptotic factors and SQS-induced
apoptosis of HepG2 cells was investigated. The mRNA and protein
expression levels of apoptosis-associated factors Bcl-2, Bax and
caspase-3 were therefore evaluated (19). A significant reduction in Bcl-2
mRNA expression levels was observed following treatment with 10, 20
and 30 µg/ml SQS, compared with those in untreated cells
(Fig. 4). Conversely, a
significant increase in caspase 3 and Bax mRNA expression was
observed. An analogous trend was observed in the protein expression
levels of caspase-3, Bcl-2 and Bax following SQS treatment
(Fig. 5).
Discussion
The results of the present study revealed that SQS
inhibited human HepG2 hepatocellular carcinoma cell growth,
indicated by a decrease in cell density following SQS treatment.
Furthermore, the mRNA and protein expression levels of key
apoptotic regulators Bax, Bcl-2 and caspase-3 were found to be
altered following SQS treatment (20).
It has previously been demonstrated that the
intracellular signaling pathways influenced by SQS and its
metabolites are involved in the regulation of numerous cell
signaling pathways, which contribute to the activation or
inhibition of various cellular processes (21). SQS influences not only the
transduction of signals from the extracellular space, but also
functions as a mediator and modulator of intercellular and
intracellular signaling networks (22–24).
The results of the present study indicated that
pre-treatment of HepG2 cells with SQS resulted in Annexin
V-positive staining. These results suggested that SQS may block the
signal transduction pathway required for cell survival and induce
HepG2 cell apoptosis. The mRNA and protein expression levels of
Bcl-2, caspase-3 and Bax were therefore examined. Bax is a
pro-apoptotic Bcl-2 protein, which contains BH1, BH2 and BH3
domains and initiates apoptotic signaling. The expression of Bax is
upregulated by the tumor suppressor protein p53, and Bax has been
shown to be involved in p53-mediated apoptosis. The results of the
present study demonstrated that SQS treatment increased Bax
expression in HepG2 cells. Caspase-3 is encoded by the CASP3
gene and interacts with caspase-8 and -9. In apoptotic cells,
caspase-3 activation may occur via extrinsic (death ligand) or
intrinsic (mitochondrial) pathways. Of note, Bax is involved in the
intrinsic pathway. Activated caspase-3 induces caspase-8 activation
and subsequently activates Bax, which stimulates cytochrome
C and induces apoptosis (25). An identical signaling pathway was
identified in the HepG2 cells evaluated in the present study.
Caspase-3 activation was previously demonstrated to be increased
following treatment of cells with SQS (26,27).
SQS interacts with mitochondrial membranes and
alters their permeability by opening transition pores and
decreasing the mitochondrial membrane potential (28). SQS therefore induces structural
changes and increases mitochondrial membrane unsaturation.
These mitochondrial alteration may influence the
activity of pro- and anti-apoptotic proteins of the Bcl-2 family.
It was previously demonstrated that SQS contributes to the
down-regulation of Bcl-2 expression, a well-known anti-apoptotic
molecule, and blocks lipid peroxidation; therefore, inhibiting the
induction of apoptosis (29). The
results of the present study indicated that SQS suppressed Bcl-2
expression.
Based on previously published studies and the
results of the present study, it was hypothesized that SQS induces
human hepatocellular carcinoma cell apoptosis. The signal
transduction occurs via the caspase-3 signaling pathway. Further
study on this signaling pathway is required to elucidate the
function of SQS in tumor invasion and survival.
Acknowledgments
The present study was supported by the Key Projects
Science Foundation of Fujian province (grant no. 2011J05073) and
the Natural Science Foundation of Fujian Province (grant no.
2011J05073).
References
|
1
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Y, Liu A, Xu Z, et al: XZH-5 inhibits
STAT3 phosphorylation and causes apoptosis in human hepatocellular
carcinoma cells. Apoptosis. 3:502–510. 2011. View Article : Google Scholar
|
|
4
|
Ercolani G, Grazi GL, Ravaioli M, et al:
Liver resection for hepatocellular carcinoma on cirrhosis:
Univariate and multivariate analysis of risk factors for
intrahepatic recurrence. Ann Surg. 237:536–543. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhong W, Peng J, He H, et al: Ki-67 and
PCNA expression in prostate cancer and benign prostatic
hyperplasia. Clin Invest Med. 31:E8–E15. 2008.PubMed/NCBI
|
|
6
|
Longley DB, Allen WL and Johnston PG: Drug
resistance, predictive markers and pharmacogenomics in colorectal
cancer. Biochim Biophys Acta. 1766:184–196. 2006.PubMed/NCBI
|
|
7
|
Pizova K, Tomankova K, Daskova A, et al:
Photodynamic therapy for enhancing antitumour immunity. Biomed Pap
Med Fac Univ Palacky Olomouc Czech Repub. 156:93–102. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang XF, Han YY, Bao GH, et al: A new
saponin from tea seed pomace (Camellia oleifera Abel.) and its
protective effect on PC12 cells. Molecules. 17:11721–11728. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sagesaka YM, Uemura T, Suzuki Y, et al:
Antimicrobial and anti-inflammatory actions of tea-leaf saponin.
Yakugaku Zasshi. 116:238–243. 1996.In Japanese. PubMed/NCBI
|
|
10
|
Ma LY, Li L, Jiang Y, et al: Study on
Youchasaponin-induced apoptosis of HepG2 cells through the
endoplasmic reticulum stress. Chin Pharmacol Bull. 27:1523–1527.
2011.
|
|
11
|
Ma LY, Li L, Jiang Y, et al: Youchasaponin
induces apoptosis of human leukemia Jurkat cells in vitro and its
possible mechanism. Tumor. 31:1072–1076. 2011.
|
|
12
|
Yang JJ, Hsu HY, Ho YH and Lin CC:
Comparative study on the immunocompetent activity of three
different kinds of Peh- Hue-Juwa-Chi-Cao, Hedyotis diffusa, H.
corymbosa and Mollugo pentaphylla after sublethal whole body
X-irradiation. Phytother Res. 11:428–432. 1997. View Article : Google Scholar
|
|
13
|
Lin JM, Wei LH, Xu W, et al: Effect of
Hedyotis diffusa Willd extract on tumor angiogenesis. Mol Med Rep.
4:1283–1288. 2011.PubMed/NCBI
|
|
14
|
Boos G and Stopper H: Genotoxicity of
several clinically used topoisomerase II inhibitors. Toxicol Lett.
116:7–16. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kelloff GJ: Perspectives on cancer
chemoprevention research and drug development. Adv Cancer Res.
78:199–334. 2000.
|
|
16
|
Singh S, Johnson J and Chellappan S: Small
molecule regulators of Rb-E2F pathway as modulators of
transcription. Biochim Biophys Acta. 1799:788–794. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shortkroff S and Yates KE: Alteration of
matrix glycosami-noglycans diminishes articular chondrocytes’
response to a canonical Wnt signal. Osteoarthritis Cartilage.
15:147–154. 2007. View Article : Google Scholar
|
|
18
|
Chen Y, Robles AI, Martinez LA, et al:
Expression of G1 cyclins, cyclin-dependent kinases, and
cyclin-dependent kinase inhibitors in androgen-induced prostate
proliferation in castrated rats. Cell Growth Differ. 7:1571–1578.
1996.PubMed/NCBI
|
|
19
|
Graña X and Reddy EP: Cell cycle control
in mammalian cells: role of cyclins, cyclin dependent kinases
(CDKs), growth suppressor genes and cyclin-dependent kinase
inhibitors (CKIs). Oncogene. 11:211–219. 1995.PubMed/NCBI
|
|
20
|
Chen HP, He M, Mei ZJ, et al:
Sasanquasaponin up-regulates anion exchanger 3 expression and
elicits cardioprotection via NO/RAS/ERK1/2 pathway. Can J Physiol
Pharmacol. 90:873–880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aszodi A, Hunziker EB, Brakebusch C and
Fässler R: Beta1 integrins regulate chondrocyte rotation, G1
progression, and cytokinesis. Genes Dev. 17:2465–2479. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li R, Zhao HR and Lin YM: Anti-tumor
effect and protective effect on chemotherapeutic damage of water
soluble extracts from Hedyotis diffusa. J Chin Pharm Sci. 11:54–58.
2002.
|
|
23
|
Lin J, Chen Y, Wei L, et al: Hedyotis
diffusa Willd extract induces apoptosis via activation of the
mitochondrion-dependent pathway in human colon carcinoma cells. Int
J Oncol. 37:1331–1338. 2010.PubMed/NCBI
|
|
24
|
Handayani T, Sakinah S, Nallappan M, et
al: Regulation of p53-, Bcl-2 and caspase-dependent signaling
pathway in xanthor-rhizol-induced apoptosis of HepG2 hepatoma
cells. Anticancer Res. 27:965–971. 2007.PubMed/NCBI
|
|
25
|
Lee HP, Chen YL, Shen HC, Lo WH and Hu YC:
Baculovirus transduction of rat articular chondrocytes: roles of
cell cycle. J Gene Med. 9:33–43. 2007. View
Article : Google Scholar
|
|
26
|
Löwenheim H, Reichl J, Winter H, et al: In
vitro expansion of human nasoseptal chondrocytes reveals distinct
expression profiles of G1 cell cycle inhibitors for replicative,
quiescent, and senescent culture stages. Tissue Eng. 11:64–75.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen L, Chen J and Xu H: Sasanquasaponin
from Camellia oleifera Abel. induces cell cycle arrest and
apoptosis in human breast cancer MCF-7 cells. Fitoterapia.
84:123–129. 2013. View Article : Google Scholar
|
|
28
|
Harper JW, Elledge SJ, Keyomarsi K, et al:
Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell.
6:387–400. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hwang SG, Song SM, Kim JR, et al:
Regulation of type II collagen expression by cyclin-dependent
kinase 6, cyclin D1, and p21 in articular chondrocytes. IUBMB Life.
59:90–98. 2007. View Article : Google Scholar : PubMed/NCBI
|