Introduction
Small ubiquitin-like modifiers (SUMOs) are
covalently bonded proteins, and SUMOylation is a post-translational
protein modification, which regulates the activities of a wide
spectrum of substrate proteins (1), which are involved in the regulation
of gene expression, signal transduction, chromosome integrity, DNA
replication and repair, cell division, nuclear trafficking and
mitochondrial function (2–8). Similar to ubiquitination, SUMOylation
is catalyzed by the E1-activating enzyme complex, E2-conjugating
enzyme and E3 ligases, however, the covalent bonding of the SUMO
proteins can be reversed by sentrin/SUMO-specific proteases (SENPs)
(9). SENP-mediated de-SUMOylation
is involved in various cell processes, including hypoxia, cell
cycle regulation and cell division, development and
differentiation, neurodegeneration, rRNA processing and androgen
receptor signaling (10–18). The balance between SUMOylation and
de-SUMOylation has been suggested to be crucial for cellular
health, and disturbaces in homeostasis are considered to facilitate
the development and progression of cancer (19). Oxidative stress is mediated through
the production of reactive oxygen species (ROS), which are induced
by a number of endogenous and exogenous processes, including
temperature and pH changes, osmotic pressure, oxygen tension and
high sugar concentrations (20).
Homeostatic ROS control is one of the key determinants for
maintaining cell growth and proliferation (20), and SENP5, which is a
SUMO2/3-specific protease, has been reported to be important in
cellular adaptive responses to the production of ROS, by regulating
the balance between SUMOylation and de-SUMOylation (21–23).
Oral squamous cell carcinoma (OSCC), is the eighth most prevalent
type of cancer and accounts for 2% of all cancer-associated
mortality worldwide (24). OSCC is
often diagnosed at an advanced stage and the overall 5-year
survival rate is <50% (25,26).
The mechanisms involved in the tumorigenesis of OSCC remain to be
fully elucidated, however, a number of studies have demonstrated
that the development of OSCC is correlated with oxidative stress
(27,28). Although SUMO2/3 conjugation is a
response to oxidative stress, its involvement in OSCC has not been
previously demonstrated. Therefore, the aims of the present study
were to investigate the activities of SENP5 in an OSCC cell line
and to determine its correlation with oxidative stress.
Materials and methods
Cell culture
CAL-27 cells were obtained from the Laboratory of
Oral Oncology, Ninth People’s Hospital, School of Medicine,
Shanghai Jiaotong University (Shanghai, China) and maintained in
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
fetal bovine serum (FBS; Gibco Life Technologies, Carslbad, CA,
USA),100 U/ml penicillin and 100 mg/l streptomycin (Beyotime
Institute of Biotechnology, Nantong, China). The CAL-27 cells were
cultured in a humidified atmosphere of 5% CO2 at 37°C.
To investigate associations with ROS, the cells were treated with
H2O2 and N-acetyl cysteine (NAC; Beyotime
Institute of Biotechnology, Haimen, China).
Tissue samples
Archived paraffin-embedded tissue specimens from 31
previously untreated patients were obtained from the Department of
Pathology, Zhongshan Hospital, Fudan University (Shanghai, China).
Of the 31 patients, 9 were female and 22 were male and the median
age was 61.8 years (range, 45–88 years). A total of 26 tumors
originated from the tongue and 5 tumors were buccal. Written
informed consent was obtained from the patients and the patients’
families.
Immunohistochemistry
For immunohistochemistry, the 5 µm sections
(cut with a microtome and mounted onto poly-lysine coated slides)
were treated with xylene (Beyotime Institute of Biotechnology) for
10 min, alcohol hydration (70%; Changshu Yangyuan Chemical Co.,
Ltd., Changshu, China) for 15 min and methanol (Beyotime Institute
of Biotechnology), containing 3% H2O2, for 10
min. The sections were then washed in phosphate-buffered saline
(PBS) containing 0.1% Triton X-100 (Bio Basic, Inc., Toronto, ON,
Canada) for 5 min, blocked with 5% bovine serum albumin (BSA;
Beyotime Institute of Biotechnology) for 1 h at room temperature
and then incubated with rabbit polyclonal antibody against SENP5
(1:80; AP1237a; Abgent, Inc., San Diego, CA, USA) overnight at 4°C.
The sections were then incubated with biotinylated mouse
anti-rabbit monoclonal immunoglobulin (Ig)G (1:1,000; Cell
Signaling Technology, Inc., Boston, MA, USA) for 1 h at room
temperature and then washed twice with PBS, containing 0.01% Tween
20 (Beyotime Institute of Biotechnology). Following incubation with
horseradish peroxidase (HRP)-conjugated avidin in PBS-0.01% Tween
for 20 min at room temperature, developing solution [DAB Detection
kit (polymer); Genetic Technology Co., Ltd., Shanghai, China] was
added and the sections were counterstained with hematoxylin
(Beyotime Institute of Biotechnology) for 10 min and cover-slips
(Beyotime Institute of Biotechnology) were mounted onto the slides
using 50% glycerin (Beyotime Institute of Biotechnology). Images of
the stained samples were captured using an Olympus Fluoview FV100
(Olympus, Tokyo, Japan).
SENP5 small interfering (si)RNA
construction
siRNA specific for SENP5 and control non-specific
siRNA oligonucleotides were synthesized byGenePharma (Shanghai,
China). The sequences of the siRNA oligonucleotides were as
follows: Short hairpin (sh)RNAI,
5′-TGCTGTTGACAGTGAGCGACCAGTTTACTTGGAATAGACAGTAGTGAAGCCACAGATGTA-3′
and shRNAII,
5′-TGCTGTTGACAGTGAGCGCGCGCAGATGGTTTGTTACTTGAATAGTGAAGCCACAGATGTAT-3′.
The cells were transfected with siRNA oligonucleotides using
Lipofectamine 2000 (Gibco Life Technologies), according to the
manufacturer’s instructions.
Western blotting
The cells (6×105) were lysed in 0.1 ml
sample buffer (0.1% SDS, 1% NP-40, 50 mM HEPES, pH 7.4, 2 mM EDTA,
100 mM NaCl, 5 mM sodium orthovanadate and 1% protease inhibitor
mixture set I; EMD Millipore, San Diego, CA, USA) on ice for 30
min. Following centrifugation at 13,400 × g (Eppendorf Centrifuge
5415 D; Eppendorf AG, Hamburg, Germany) for 15 min, the
supernatants were removed. The proteins were then quantified using
a BCA Protein Assay kit (Pierce Biotechnology, Inc., Rockford, IL,
USA), according to the manufacturer’s instructions. The samples,
adjusted to a total of 50 µg protein, were heated at 100°C
for 5 min in 2X SDS sample buffer, and then separated on 10 or 12%
SDS-PAGE gels (Nanjing KeyGen Biotech. Co., Ltd., Nanjing, China)
and transferred onto polyvinylidene difluoride membranes (Merck
Millipore, Darmstadt, Germany). The membranes were incubated with
blocking buffer (5% BSA), followed by incubation with rabbit
polyclonal antibodies against SENP5 (1:50; AP14400b; Abgent, Inc.)
at 4°C overnight and HRP-conjugated secondary mouse anti-rabbit IgG
(1:1,000; sc-2357; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) for 2 h at room temperature prior to analysis using an
enhanced chemiluminescence system (Beyotime Institute of
Biotechnology).
Immunostaining
The CAL-27 cell monolayers were fixed using 4%
paraformaldehyde (Beyotime Institute of Biotechnology),
permeabilized with 0.2% Triton X-100 and blocked with 5% BSA prior
to incubation with the rabbit polyclonal antibody against SENP5
(1:50; AP1237a; Abgent, Inc.) at 4°C overnight. The cover slides
were then mounted with medium containing
4′,6-diamidino-2-phenylindole (Beyotime Institute of Biotechnology)
for 5 min to visualize the cell nuclei, then subsequently were
incubated with Rhodamin-conjugated goat anti-rabbit antibody
(1:1000; Abcam) for 1.5 h at room temperature prior to washing
twice with PBS. The slides were evaluated using a laser-scanning
confocal microscope (FV100; Olympus). A mitochondria tracker
(GMS10020.1; Shanghai Baoman Biological Technology Co., Ltd.,
Shanghai, China) was used, according to the manufacturer’s
instructions. MitoTracker® stock solution (1 mM) was
diluted to a working concentration of 100 nM in growth medium, then
once the cells reached the desired confluency, the media was
removed from the dish and prewarmed (37°C) staining solution
containing MitoTracker® probe, which was prepared prior to
incubation, was added for 15 min under growth conditions.
Subsequently, fixing, rinsing and permeabilization was
performed.
Flow cytometry
The CAL-27 cells (6×105) were grown in
complete culture medium (DMEM, supplemented with 10% heat
inactivated FBS, 100 IU/ml penicillin and 100 mg/ml streptomycin)
and silenced using SENP5-siRNA, according to the manufacturer’s
instructions. The cells were treated with
H2O2 and SENP5-siRNA, as indicated, and then
fixed using ice-cold ethanol for 20 min, prior to staining with 50
µg/ml propidium iodide (Sigma-Aldrich, St. Louis, MO, USA)
and 100 µg/ml RNase A (Beyotime Institute of Biotechnology)
for 20 min at room temperature. The DNA content of the cells was
then determined by fluorescence-activated cell sorting, using a 488
nm laser (FACSAria; BD Bioscienes, Franklin Lakes, NJ, USA).
Results
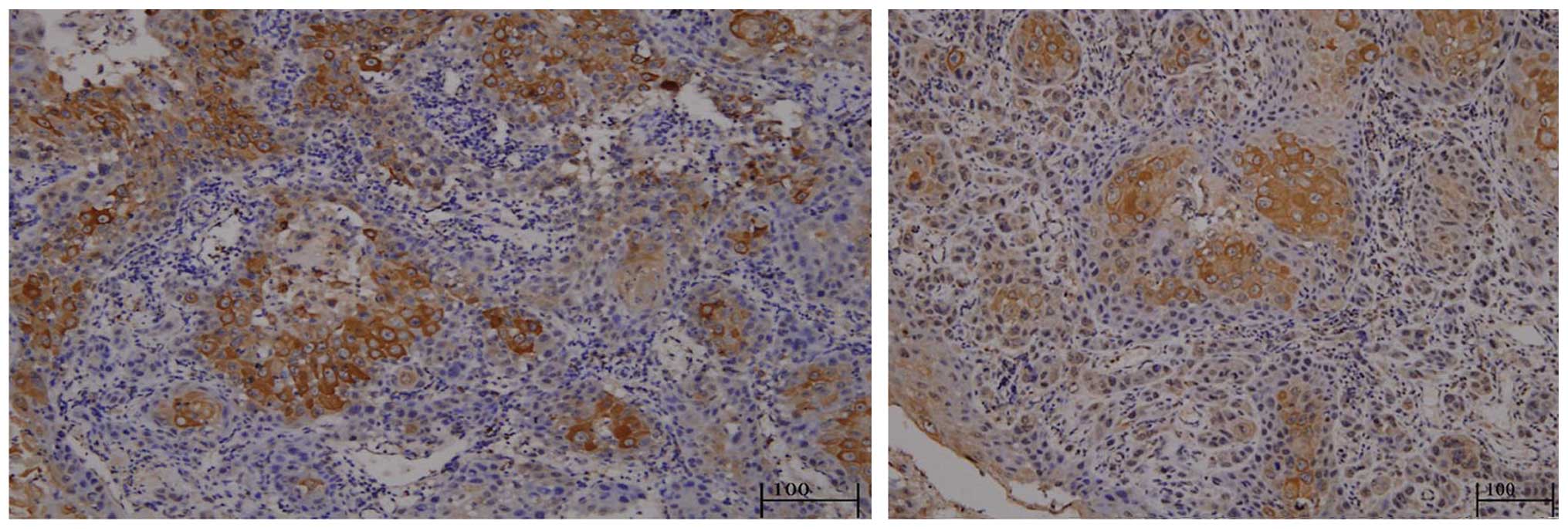
The results of the SENP5 staining in the 31 tissue
specimens revealed that SENP5 was predominantly expressed in the
cytosols of the inner layer tumor cells (Fig. 1).
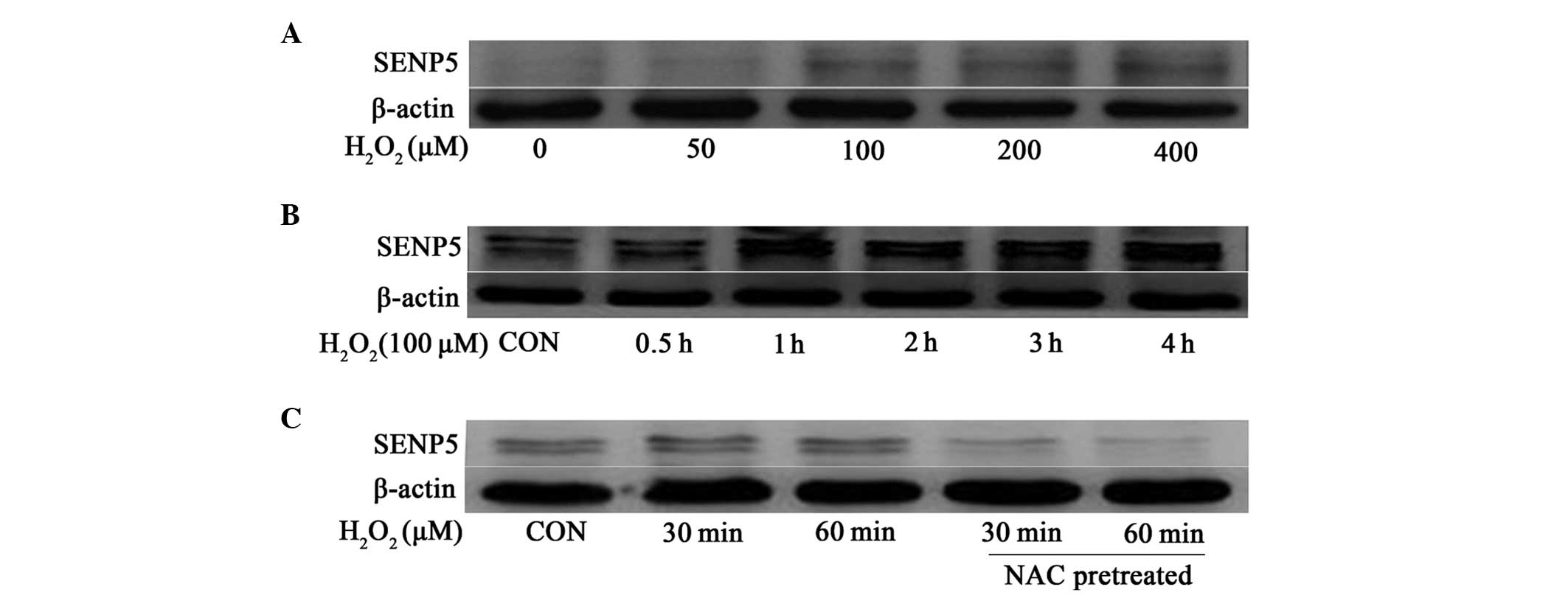
Mild oxidative stress induces rapid
protein stabilization of SENP5
ROS generation is common to various stress inducers,
including hypoxia, low pH and ultraviolet radiation (29). In order to examine the association
between SENP5 and ROS in the present study, CAL-27 OSCC cells were
exposed to varying concentrations of H2O2,
and the expression of SENP5 was evaluated. Initially, the cells
were incubated with increasing concentrations of
H2O2 for 1 h and SENP5 protein accumulated in
the cells in a dose dependent manner, beginning at 100 µM
H2O2 (Fig.
2A). The following time-course experiments revealed that the
protein levels of SENP5 started to increase following 1 h exposure
to 100 µM H2O2 and remained stable.
Notably, hypoxia had a similar effect on the protein levels of
SENP5 (data not shown). Subsequently, the present study examined
whether the increased protein levels of SENP5 were inhibited by
antioxidants, including NAC. As shown in Fig. 2C, the addition of NAC to the medium
reversed the H2O2-induced accumulation of
SENP5 in the CAL-27 cells, suggesting that the protein levels of
SENP5 were regulated by changes in redox states.
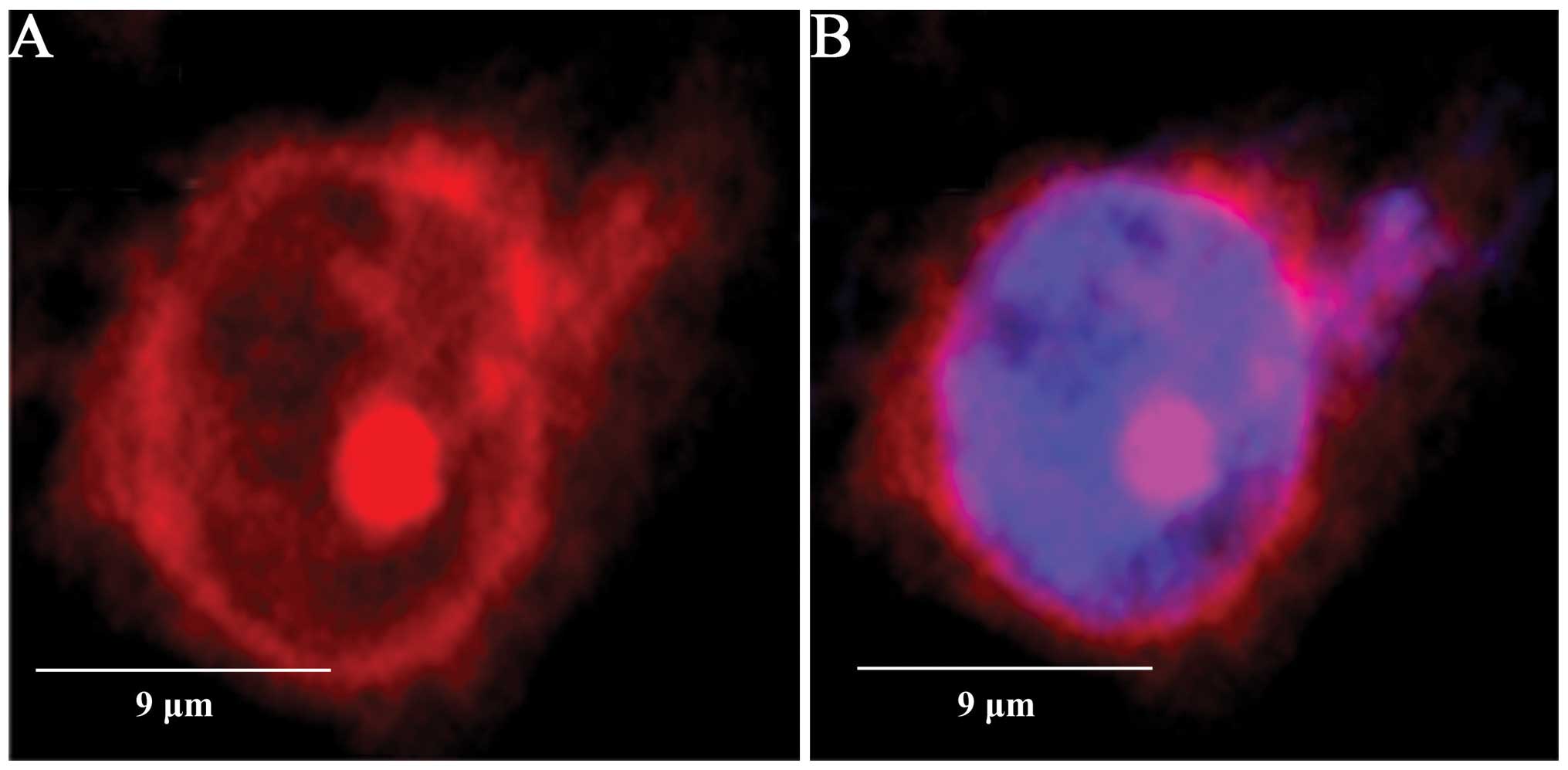
SENP5 is predominantly localized in the
cytosol of CAL-27 cells
The de-SUMOylation activity of SENPs is directed by
their subcellular distribution (30), and SENP5 has been reported to be
preferentially expressed in the nucleoli (31). In the present study, however, SENP5
was also found to be localized in the cytoplasm in 84.2±2.6% of the
CAL-27 cells (Fig. 3), which was
not affected by H2O2 application (data not
shown).
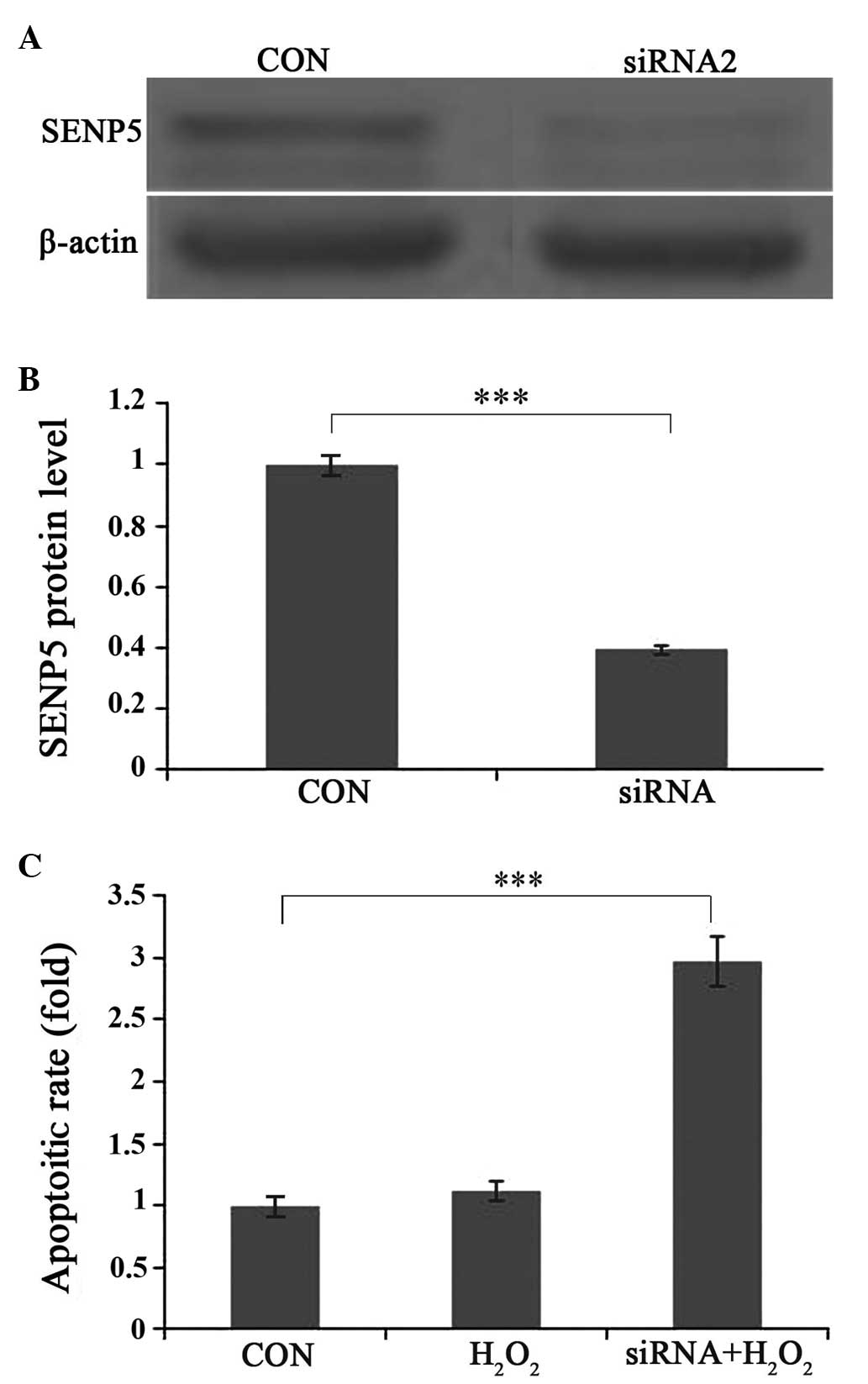
Moderate oxidative stress induces
apoptosis in SENP5-silenced CAL-27 cells
To examine the importance of SENP5 on cell survival,
shRNAs were designed to silence the expression of SENP5. Western
blot analyzes reavealed a 65% reduction in protein expression
(Fig. 4A and B). To assess whether
knockdown of SENP5 had an effect on apoptosis, the cells were
divided into control; 1 h 100 µm H2O2
exposure; and 1 h 100 µm H2O2 +
siRNA-SENP5 groups. As shown in Fig.
4C, H2O2 incubation alone enhanced
apoptosis in the CAL-27 cells, whereas combined
H2O2 incubation and SENP5 silencing increased
the apoptotic rate significantly (P<0.001).
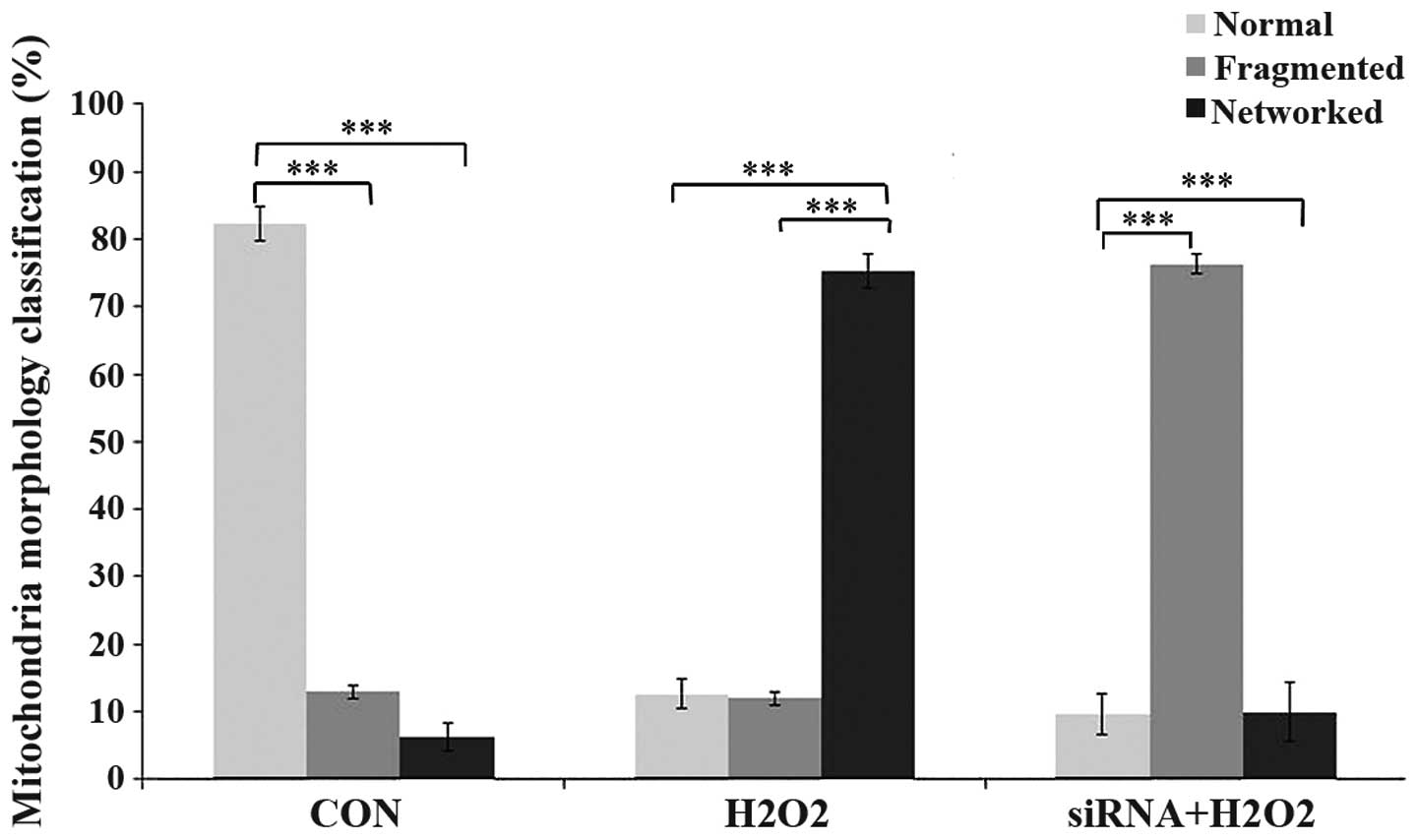
SENP5 is associated with mitochondria
stabilization of H2O2-exposed CAL-27
cells
In order to further analyze the function of SENP5 in
CAL-27 cell mitochondria, the present study determined the
mitochondrial structures following either control siRNA
application, the addition of 100 µm
H2O2 for 1 h, or the addition of 100
µm H2O2 for the final 1 h of a 72 h
period with SENP5 silencing using specific siRNA. Increased fused
mitochondria were observed in the
H2O2-treated group, compared with the control
group, which suggested that ROS are associated with mitochondrial
morphology. When SENP5 was silenced, exposure to
H2O2 led to fragmentation of the mitochondria
(Fig. 5).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis was performed using SPSS software, version
11.0 (SPSS, Inc., Chicago, IL, USA). The difference between two
groups was analyzed using Student’s t-test or analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Discussion
In our previous study, SENP5 was observed to be
predominantly expressed in well-differentiated cells, located at
the inner layer of carcinoma nests, and was associated with the
pathological degree of OSCC (32).
SENP5 regulates the formation of SUMO-2 or SUMO-3 conjugates and,
to a less extent, SUMO-1 modifications (31). Dynamin-1-like protein (Drp1) has
been identified as a cytosolic substrate of SENP5 (33) and SUMO1-conjugated Drp1 is
stabilized and involved in mitochondrial fragmentation,
particularly during mitosis (34).
However, SENP5 deSUMOylation leads to Drp1 inactivation due to
transformation into its instable form. In COS-7 cells, Drp1 was
stably mono-SUMOylated, however, a reduction of SENP5 resulted in
increased free radical production, which was reversed by silencing
Drp1 (33). It has been
demonstrated that Drp1 protein is also essential for apoptotic
mitochondrial fission (35,36).
In the present study SENP5 was observed to rescue CAL-27 cells from
ROS-induced apoptosis (Fig. 4C),
which can be explained by its destabilization of Drp1 and is in
agreement with a previous report regarding resistance to
H2O2-induced cell death in a cell line
containing an activity mutation of Drp1 (37). There have been few reports
regarding SENP5 overexpression in OSCC cells (38), however, Katayama et al
reported the overexpression of SUMO1 in OSCC cells (38). Since overexpression of SUMO1 leads
to excessive Drp1 SUMOylation and results in mitochondrial
fragmentation (39), and as SENP5
has been noted to localize predominantly in the nucleolus (15,40),
its cytosolic accumulation in OSCC cells may represent a
counter-reaction of the cells against enhanced susceptibility to
ROS-induced apoptosis. Due to the rapid growth of tumor cells, the
oxygen supply is inadequate in the center of cancer nests, and
increased ROS development during hypoxia is common (41–43).
A similar mechanism has been suggested for the overexpression of
SENP1 in prostate cancer, and is suggested to be important for the
protein stabilization of hypoxia-induced hypoxia-inducible factor
1α (44).
In conclusion, the present study demonstrated that
SENP5 was overexpressed and accumulated in the cytosols of OSCC
cells. Mild oxidative stress stabilized SENP5 in the CAL-27 cells,
but did not enhance their apoptotic rates, whereas combined SENP5
silencing and mild oxidative stress led to mitochondria
fragmentation and significantly increased cell apoptosis.
The findings of the current study demonstrate that
SENP5 protected OSCC cells from oxidative stress-induced apoptosis,
which may be of clinical importance for further treatment
strategies for OSCC.
Acknowledgments
The current study was supported by the National
Natural Science Foundation of China (no. 81001202).
References
|
1
|
Hay RT: SUMO: a history of modification.
Mol Cell. 18:1–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andreou AM and Tavernarakis N: SUMOylation
and cell signalling. Biotechnol J. 4:1740–1752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dou H, Huang C, Van Nguyen T, Lu LS and
Yeh ET: SUMOylation and de-SUMOylation in response to DNA damage.
FEBS Lett. 585:2891–2896. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Finkbeiner E, Haindl M, Raman N and Muller
S: SUMO routes ribosome maturation. Nucleus. 2:527–532. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lomelí H and Vázquez M: Emerging roles of
the SUMO pathway in development. Cell Mol Life Sci. 68:4045–4064.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scorrano L and Liu D: The SUMO arena goes
mitochondrial with MAPL. EMBO Rep. 10:694–696. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ulrich HD: Ubiquitin and SUMO in DNA
repair at a glance. J Cell Sci. 125:249–254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wan J, Subramonian D and Zhang XD:
SUMOylation in control of accurate chromosome segregation during
mitosis. Curr Protein Pept Sci. 13:467–481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yeh ET, Gong L and Kamitani T:
Ubiquitin-like proteins: new wines in new bottles. Gene. 248:1–14.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng J, Kang X, Zhang S and Yeh ET:
SUMO-specific protease 1 is essential for stabilization of
HIF1alpha during hypoxia. Cell. 131:584–595. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng J, Perkins ND and Yeh ET:
Differential regulation of c-Jun-dependent transcription by
SUMO-specific proteases. J Biol Chem. 280:14492–14498. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng J, Wang D, Wang Z and Yeh ET: SENP1
enhances androgen receptor-dependent transcription through
desumoylation of histone deacetylase 1. Mol Cell Biol.
24:6021–6028. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Degerny C, Monte D, Beaudoin C, et al:
SUMO modification of the Ets-related transcription factor ERM
inhibits its transcriptional activity. J Biol Chem.
280:24330–24338. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deyrieux AF, Rosas-Acosta G, Ozbun MA and
Wilson VG: Sumoylation dynamics during keratinocyte
differentiation. J Cell Sci. 120:125–136. 2007. View Article : Google Scholar
|
|
15
|
Di Bacco A, Ouyang J, Lee HY, et al: The
SUMO-specific protease SENP5 is required for cell division. Mol
Cell Biol. 26:4489–4498. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dorval V and Fraser PE: SUMO on the road
to neurodegeneration. Biochim Biophys Acta. 1773:694–706. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haindl M, Harasim T, Eick D and Muller S:
The nucleolar SUMO-specific protease SENP3 reverses SUMO
modification of nucleophosmin and is required for rRNA processing.
EMBO Rep. 9:273–279. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Halliwell B: Oxidative stress and cancer:
have we moved forward? Biochem J. 401:1–11. 2007. View Article : Google Scholar
|
|
19
|
Bawa-Khalfe T and Yeh ET: SUMO losing
balance: SUMO proteases disrupt SUMO homeostasis to facilitate
cancer development and progression. Genes Cancer. 1:748–752. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Halliwell B: Oxidative stress in cell
culture: an under-appreciated problem? FEBS Lett. 540:3–6. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo C, Hildick KL, Luo J, et al:
SENP3-mediated deSUMOylation of dynamin-related protein 1 promotes
cell death following ischaemia. EMBO J. 32:1514–1528. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han Y, Huang C, Sun X, et al:
SENP3-mediated de-conjugation of SUMO2/3 from promyelocytic
leukemia is correlated with accelerated cell proliferation under
mild oxidative stress. J Biol Chem. 285:12906–12915. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Yang J, Yang K, et al: The
biphasic redox sensing of SENP3 accounts for the HIF-1
transcriptional activity shift by oxidative stress. Acta Pharmacol
Sin. 33:953–963. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kademani D: Oral cancer. Mayo Clin Proc.
82:878–887. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Petersen PE: The World Oral Health Report
2003: continuous improvement of oral health in the 21st century-the
approach of the WHO Global Oral Health Programme. Community Dent
Oral Epidemiol. 31(Suppl 1): 3–23. 2003. View Article : Google Scholar
|
|
27
|
Agha-Hosseini F, Mirzaii-Dizgah I,
Farmanbar N and Abdollahi M: Oxidative stress status and DNA damage
in saliva of human subjects with oral lichen planus and oral
squamous cell carcinoma. J Oral Pathol Med. 41:736–740. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hassona Y, Cirillo N, Lim KP, et al:
Progression of genotype-specific oral cancer leads to senescence of
cancer-associated fibroblasts and is mediated by oxidative stress
and TGF-β. Carcinogenesis. 34:1286–1295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bell EL, Klimova TA, Eisenbart J, et al:
The Qo site of the mitochondrial complex III is required for the
transduction of hypoxic signaling via reactive oxygen species
production. J Cell Biol. 177:1029–1036. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim JH and Baek SH: Emerging roles of
desumoylating enzymes. Biochim Biophys Acta. 1792:155–162. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gong L and Yeh ET: Characterization of a
family of nucleolar SUMO-specific proteases with preference for
SUMO-2 or SUMO-3. J Biol Chem. 281:15869–15877. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ding X, Sun J, Wang L, et al:
Overexpression of SENP5 in oral squamous cell carcinoma and its
association with differentiation. Oncol Rep. 20:1041–1045.
2008.PubMed/NCBI
|
|
33
|
Zunino R, Schauss A, Rippstein P,
Andrade-Navarro M and McBride HM: The SUMO protease SENP5 is
required to maintain mitochondrial morphology and function. J Cell
Sci. 120:1178–1188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zunino R, Braschi E, Xu L and McBride HM:
Translocation of SenP5 from the nucleoli to the mitochondria
modulates DRP1-dependent fission during mitosis. J Biol Chem.
284:17783–17795. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Frank S, Gaume B, Bergmann-Leitner ES, et
al: The role of dynamin-related protein 1, a mediator of
mitochondrial fission, in apoptosis. Dev Cell. 1:515–525. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Barsoum MJ, Yuan H, Gerencser AA, et al:
Nitric oxide-induced mitochondrial fission is regulated by
dynamin-related GTPases in neurons. EMBO J. 25:3900–3911. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tanaka A, Kobayashi S and Fujiki Y:
Peroxisome division is impaired in a CHO cell mutant with an
inactivating point-mutation in dynamin-like protein 1 gene. Exp
Cell Res. 312:1671–1684. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Katayama A, Ogino T, Bandoh N, et al:
Overexpression of small ubiquitin-related modifier-1 and sumoylated
Mdm2 in oral squamous cell carcinoma: possible involvement in tumor
proliferation and prognosis. Int J Oncol. 31:517–524.
2007.PubMed/NCBI
|
|
39
|
Harder Z, Zunino R and McBride H: Sumo1
conjugates mitochondrial substrates and participates in
mitochondrial fission. Curr Biol. 14:340–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hay RT: SUMO-specific proteases: a twist
in the tail. Trends Cell Biol. 17:370–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chandel NS, Maltepe E, Goldwasser E, et
al: Mitochondrial reactive oxygen species trigger hypoxia-induced
transcription. Proc Natl Acad Sci USA. 95:11715–11720. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Galanis A, Pappa A, Giannakakis A, et al:
Reactive oxygen species and HIF-1 signalling in cancer. Cancer
Lett. 266:12–20. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guzy RD and Schumacker PT: Oxygen sensing
by mitochondria at complex III: the paradox of increased reactive
oxygen species during hypoxia. Exp Physiol. 91:807–819. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zuo Y and Cheng JK: Small ubiquitin-like
modifier protein-specific protease 1 and prostate cancer. Asian J
Androl. 11:36–38. 2009. View Article : Google Scholar
|