Introduction
Osteoporosis is a disease characterized by loss of
bone mass and degeneration of the bone microstructure (1). It is estimated that it affects one in
two women >50 years of age in the UK (2). Osteoporosis also represents a major
public health concern in Asian countries, particularly China
(3), resulting in a requirement
for investigation into osteoporosis treatment methodology.
Resveratrol (3,5,4-tri-hydroxystilbene; RESV) is
synthesized by various species of plant (4). It is a phytoalexin antimicrobial,
which has broad-spectrum beneficial health effects, including
anti-infective, antioxidant and cardioprotective functions
(5). Furthermore, RESV has
demonstrated promise in delaying the onset of a variety of
age-related diseases (6). RESV has
been revealed to stimulate bone cell proliferation and
differentiation (7). Liu et
al (8) demonstrated that RESV
increases the bone mineral density (BMD) of the epiphyses of
ovariectomized (OVX) female rats. However, further investigation is
required in order to fully elucidate the mechanisms underlying the
effects of RESV in the treatment of osteoporosis. MicroRNAs
(miRNA/miR) are short, single-stranded RNAs consisting of 20–25
nucleotides. They are able to bind complementary sequences in the
3′-untranslated regions (UTR) of target genes to induce mRNA
degradation or suppress translation (9).
The purpose of the present study was to determine
the effects of RESV on BMD and the microarchitecture of the bone in
OVX rats, and examine their effects on the bone by investigating
the expression of bone-specific genes. In addition, the mechanisms
underlying the effects of RESV were determined.
Materials and methods
Animals
The experiments were performed using 200 8-week-old
female Wistar rats (BetterBiotechnology Co., Ltd., Nanjing, China)
weighing between 180 and 200 g. Throughout the experiment, the
animals were housed in groups in a room with controlled temperature
(22°C) and humidity (50%) with a 12 h light-12 h dark cycle.
Standard rat chow (BetterBiotechnology Co., Ltd.) and tap water
were available ad libitum. All procedures involving the
animals were approved by the ethics committee of China Medical
University (Shenyang, China).
OVX and treatment
The OVX was performed under isoflurane (10%, 0.35
ml/100 g) anesthesia. A single dorsal incision was made in the skin
and, through this, two lateral incisions were made in the muscle
layer ~0.5 cm below each kidney. The ovaries were extruded through
the incision, ligated off and removed. At 20 days post-surgery, a
500 mg/kg dose of RESV was prepared by mixing RESV (Sigma-Aldrich,
St. Louis, MO, USA) with deionized water at a ratio of 3:7, and was
administered to the OVX female rats through tail intravenous
injection. The OVX rats were divided into four groups (n=10 per
group) as follows: Untreated rats; OVX rats OVX rats with
phosphate-buffered saline (PBS; Sigma-Aldrich); and OVX rats with
RESV. The subcutaneous fat and the uterus were also harvested,
rinsed with physiological saline, and weighed.
BMD
The BMD was assessed using dual-energy X-ray
absorptiometry with a Hologic QDR-4500A X-ray bone densitometer
(Hologic, Inc., Danbury, CT, USA). The total body BMD was assessed
on the 30th and 60th day following the administration of RESV.
Bone turnover markers in serum and
urine
The concentrations of calcium, phosphorus and
creatinine in the serum and urine were measured using a commercial
kit (Quanti Chrom™Bioassay Systems, Hayward, CA, USA). The levels
of serum alkaline phosphatase (ALP) and osteocalcin were measured
using a commercial ALP kit (Asan Pharm, Seoul, Korea) and Rat-MID™
Osteocalcin EIA kit (Immunodiagnostic systems Inc., Stoughton, MA,
USA), respectively. The level of urinary deoxypyridinoline (DPD)
was measured using a METRA™ DPD EIA kit (Quidel Corporation, San
Diego, CA, USA). All experiments were performed according to the
manufacturer’s instructions and the results were analyzed using a
microplate reader (Bio-Rad Laboratories, Inc., Richmond, CA, USA).
The values for DPD were adjusted for volume by determining the
level of urinary creatinine (Quanti Chrom™ Bioassay Systems), with
concentrations presented as nmol DPD/mmol creatinine.
miRNA expression array
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA), and further
purified using an mirVana miRNA isolation kit (Life Technologies,
Grand Island, NY, USA). Microarray analysis was performed using an
Agilent Whole Rat Genome Array (Agilent, Santa Clara, CA, USA),
according to the manufacturer’s instructions. The integrity of the
total RNA was assessed using an Agilent 2100 Bioanalyzer (Agilent).
cDNA was synthesized from RNA by reverse transcription using the
PrimeScript™ II 1st strand cDNA synthesis kit (cat. no. 6210B;
Takara Bio Inc., Dalian, China). The cDNA and biotinylated cRNA
were synthesized and hybridized to the array. The slides were
scanned using an Agilent Microarray Scanner (cat no. G2565BA;
Agilent) and Feature Extraction software 10.7 (Agilent) with
default settings. The raw data were normalized by Quantile
algorithm, Gene Spring software 11.0 (Agilent).
Western blot analysis
The rats were sacrificed using ketamine (70 mg/kg)
and xylazine (6 mg/kg) purchased from Sigma-Aldrich. The femur
tissues were obtained from the rat models and lysed in 20 mM
Tris-HCl buffer (pH 7.4), containing 150 mM NaCl, 2 mM EDTA, 1%
Nonidet P-40, 50 mM NaF, 1 mM Na3VO4, 1 mM
Na2MoO4, 10 μm leupeptin (Roche,
Basel, Switzerland). The protein concentration was quantified using
a bicinchoninic protein assay kit (CW Biotech, Beijing, China). The
crude lysates were then centrifuged at 14,000 × g for 10 min,
following which the cleared lysates were collected and were
separated by 8% SDS-polyacrylamide gel electrophoresis
(Sigma-Aldrich) and transferred onto nitrocellulose membranes
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membranes were
blocked with 5% milk-Tris-buffered saline with 5% Tween-20
(Sigma-Aldrich) and incubated with primary antibodies at room
temperature overnight. Rabbit polyclonal anti-runt-related
transcription factor (RUNX)2 (1:200; cat. no. sc-10758) and mouse
monoclonal anti-β-actin (1:200; cat. no. sc-47778) antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The secondary antibodies were purchased from Beyotime
Biotechnology (Beijing, China). The secondary antibodies included
anti mouse IgG (cat. no. A0216), anti rabbit IgG (cat. no. A0239)
or anti goat IgG (cat. no. A0181). The membranes were incubated
with the secondary antibodies for 2 h at room temperature. The
membranes were then developed and visualized using a Pierce ECL kit
(Thermo Fisher Scientific Inc., Waltham, MA, USA). Densitometric
quantification for western blotting was performed using Scion 4.03
image software (Scion Corporation, Bethesda, MD, USA).
Target gene prediction
The target gene information of mmu-miR-338-3p was
analyzed using microRNA TargetScan software (http://www.microrna.org/mammalian/index.html).
The minimum free energy predicted for hybridization was determined
by BibiServ analysis (http://bibiserv.techfak.uni-bielefeld.de/genefisher2).
Cell lines
Human osteoblast (HOB) cells were purchased from the
Shanghai Institutes for Biological Sciences, Chinese Academy of
Sciences (Shanghai, China) and maintained in osteoblast basal media
(Lonza, Walkersville, MD, USA) supplemented with fetal bovine serum
(Lonza).
Transfection
The anti-miRNA inhibitor (anti-miR-338-3p) and
negative control miRNA (anti-control) were purchased from Ambion
Life Technologies, Carlsbad, CA, USA). For transfection, the cells
were plated at 70–80% confluency in a 6-well dish. After 24 h at
room temperature, the cells were transfected with 1 μg
chemically-synthesized RNA (GenePharma Co., Ltd., Shanghai, China)
using Lipofectamine 2000 (Invitrogen Life Technologies), according
to the manufacturer’s instructions.
Immunofluorescence
The cells were washed with PBS, fixed with 4%
formaldehyde in PBS for 20 min, and finally permeabilized with 0.2%
Triton X-100 solution (Beyotime Biotechnology) for 5 min at room
temperature. The cells were incubated with anti-RUNX2 antibody for
1 h, washed three times with PBS and then incubated with the
appropriate Alexa Fluor 594-conjugated secondary antibody The cells
were then washed three times in PBS. The slides were mounted with
VECTASHIELD mounting medium (Vector Laboratories, Inc., Burlingame,
CA, USA). For every cover-slip, the cells were visualized and
images were captured in five random fields using an Olympus CX71
fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Cell proliferation
The HOBs were plated at a density of
1×103 cells/well in 96-well plates. The mitogenic
activity following treatment with RESV was assayed using a
colorimetric 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymetho
xyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (Promega,
Madison, WI, USA). Following treatment of the different groups, 20
μl MTS was added to each well for 4 h at room temperature,
and the light absorbance at 490 nm was measured using a
spectrophotometric microplate reader (Model 680; Bio-Rad
Laboratories, Inc.).
In vitro mineralization assay
The cells were seeded in 6-well plates, in
triplicate, at a density of 3×103/cm2.
Alizarin Red S staining, which detects calcium deposition, was used
as an indicator of mineralization. The cells were rinsed in PBS and
fixed in 70% ice-cold ethanol prior to staining with 40 mM Alizarin
Red S (pH 4.2; Sigma-Aldrich) for 10 min at room temperature. The
calcium content was then quantified by measuring the level of
Alizarin Red S staining, which was bound to the mineralizing
nodules.
Dual-luciferase reporter assay
Dual-luciferase reporter assays were performed in
the HOB cells to determine the binding site of miR-338-3p
within the 3′UTR of RUNX2. Briefly, a pMIR-reporter (Signosis,
Inc., Santa Clara, CA, USA), containing either the wild-type or
mutant 3′-UTR was cotransfected with phRL-TK (Signosis, Inc.) into
the HOB cells. At 48 h post-transfection, the cells were harvested
and analyzed using a dual-luciferase reporter assay (Promega),
according to the manufacturer’s instructions. Briefly, the cell
extract was subjected to dual-luciferase assay as measured by the
Xenogen IVIS 200 imaging system (Caliper Life Sciences, Hopkinton,
MA, USA). The data were presented as luciferase activity/RL-TK
activity.
Reverse transcription-quantitative
polymerase chain reaction (qPCR)
qPCR was performed using specific primers. qPCR
analysis was performed on an ABI prism 7500 sequence detection
system (Applied Biosystems, Foster City, CA, USA), using SYBR Green
PCR master mixture (Takara Bio Inc.). The PCR conditions were as
follows: One cycle at 95°C for 10 min followed by 40 cycles at 95°C
for 15 sec and at 60°C for 1 min. The following primer sets were
used: RUNX2, sense 5′-ATGGCCTGGTCCATCTCCAC-3′ and antisense
5′-GGCAGAGGTGAAAAAGTTGC-3′ and GAPDH, sense
5′-CACTGGCGGTGCAACAAGA-3′ and antisense
5′-CATGGGTGGAATCATATTGGAA-3′. The miRNA-338-3p and U6
primers were purchased from Qiagen (Hilden, Germany). Relative
quantification was calculated using the 2−∆∆Ct method,
where ∆∆Ct = Ct(RUNX2) - Ct(GAPDH) (10). The expression level of the control
group was considered “1”.
Multiple sequence alignment and
phylogenetic analysis
A total of 19 nucleotide sequences of
miR-338-3p from a wide range of organisms were obtained from
the National Center for Biotechnology Information database
(https://www.ncbi.nlm.nih.gov/). Multiple
sequence alignments were performed using the ClustalW programme
(11). To determine the
phylogenetic associations between these sequences, maximum
likelihood, neighbor-joining and Bayesian Markov chain Monte Carlo
approaches were used to infer three individual trees.
Statistical analysis
The data are presented as the mean ± standard
deviation Statistical analysis was performed using a two-tailed
t-test using SPSS 17.0 statistical software (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference. All experiments were repeated at least
three times.
Results
Body weight and uterus weight
The baseline body weights of the animal groups were
similar (Table I), however, the
final body weights and subcutaneous fat weights were significantly
higher in the OVX group and the OVX + PBS groups, compared with the
untreated group and the OVX + RESV groups (Tables I and II), while the uterus weight was
significantly lower in the OVX group compared with the OVX + RESV
group (Table III). The body
weights and levels of subcutaneous fat decreased significantly in
the ratstreated with RESV compared with those in the OVX-only group
(Tables I and II).
 | Table IEffects in of OVX and RESV treatment
in rats on body weight (g). |
Table I
Effects in of OVX and RESV treatment
in rats on body weight (g).
| Group | n | Day 0 | Day 30 | Day 60 |
|---|
| Untreated | 10 | 203.26±2.45 | 222.27±2.09 | 238.35±2.72 |
| OVX | 10 | 201.85±1.98 | 252.46±1.93 | 302.24±3.48 |
| OVX + PBS | 10 | 198.42±2.17 | 261.33±2.21 | 292.67±3.22 |
| OVX + RESV | 10 | 202.67±2.24 | 231.92±2.87 | 255.24±1.88 |
 | Table IIEffects in of OVX and RESV treatment
in rats on levels of subcutaneous fat (g). |
Table II
Effects in of OVX and RESV treatment
in rats on levels of subcutaneous fat (g).
| Group | n | Day 0 | Day 30 | Day 60 |
|---|
| Untreated | 10 | 3.75±0.23 | 3.80±0.32 | 4.02±0.31 |
| OVX | 10 | 3.64±0.25 | 4.81±0.43 | 6.92±0.46 |
| OVX + PBS | 10 | 3.63±0.34 | 4.77±0.31 | 6.56±0.28 |
| OVX + RESV | 10 | 3.76±0.29 | 4.09±0.21 | 4.27±0.37 |
 | Table IIIEffects of OVX and RESV treatment on
uterus weight (mg). |
Table III
Effects of OVX and RESV treatment on
uterus weight (mg).
| Group | n | Day 0 | Day 30 | Day 60 |
|---|
| Untreated | 10 | 497.56±25.96 | 500.42±22.58 | 503.57±22.53 |
| OVX | 10 | 498.39±29.03 | 271.85±14.14 | 89.86±4.78 |
| OVX + PBS | 10 | 500.91±27.35 | 288.45±12.35 | 95.67±5.28 |
| OVX + RESV | 10 | 494.75±25.75 | 342.35±13.88 | 309.43±12.94 |
Bone turnover markers
The BMD was significantly lower in the OVX group
compared with the untreated group, and OVX + RESV increased the BMD
(Table IV). When comparing the
OVX group to the untreated group, the calcium/phosphate ratio and
the levels of serum calcium were significantly lower, however, the
levels of serum ALP, urinary calcium excretion and urinary
DPD/creatinine were significantly higher (Tables VTable VITable VIITable VIIITable IX–X). The rats treated with RESV had
significantly increased levels of serum osteocalcin and
calcium/phosphate ratios, however, they exhibited decreased levels
of urinary calcium and DPD excretion compared with the rats in the
OVX group (Tables VTable VITable VIITable VIIITable IX–X). The serum calcium level to increase
and the levels of serum ALP and serum phosphorus tended to decrease
in the rats that treated with RESV (Tables VTable VITable VIITable VIIITable IX–X).
 | Table IVEffects of OVX and RESV treatment on
bone mineral densities of the total body (mg/cm2). |
Table IV
Effects of OVX and RESV treatment on
bone mineral densities of the total body (mg/cm2).
| Group | n | Day 0 | Day 30 | Day 60 |
|---|
| Untreated | 10 | 161.58±11.24 | 178.89±10.79 | 196.49±13.52 |
| OVX | 10 | 159.23±10.25 | 147.42±10.65 | 131.26±11.29 |
| OVX + PBS | 10 | 160.76±13.36 | 145.36±11.44 | 134.95±11.10 |
| OVX + RESV | 10 | 158.64±10.21 | 152.27±10.38 | 148.62±12.93 |
 | Table VEffects of OVX and RESV treatment on
levels of serum calcium (mmol/l). |
Table V
Effects of OVX and RESV treatment on
levels of serum calcium (mmol/l).
| Group | n | Day 0 | Day 30 | Day 60 |
|---|
| Untreated | 10 | 6.24±0.31 | 6.31± 0.32 | 6.32±0.27 |
| OVX | 10 | 6.33±0.32 | 5.12±0.28 | 4.74±0.26 |
| OVX + PBS | 10 | 6.18±0.27 | 5.04±0.25 | 4.66±0.21 |
| OVX + RESV | 10 | 6.27±0.28 | 5.69±0.34 | 5.08±0.35 |
 | Table VIEffects of OVX and RESV treatment on
levels of serum phosphorus (mmol/l). |
Table VI
Effects of OVX and RESV treatment on
levels of serum phosphorus (mmol/l).
| Group | n | 0 day | Day 30 | Day 60 |
|---|
| Untreated | 10 | 4.72±0.57 | 4.84± 0.53 | 4.72±0.44 |
| OVX | 10 | 4.83±0.48 | 5.22±0.41 | 5.71±0.46 |
| OVX + PBS | 10 | 4.68±0.53 | 5.32±0.45 | 5.69±0.47 |
| OVX + RESV | 10 | 4.71±0.47 | 4.97±0.86 | 5.02±0.46 |
 | Table VIIEffects of OVX and RESV treatment on
levels of serum alkaline phosphatase (U/l). |
Table VII
Effects of OVX and RESV treatment on
levels of serum alkaline phosphatase (U/l).
| Group | n | Day 0 | Day 30 | Day 60 |
|---|
| Untreated | 10 | 11.49±1.64 | 11.54±1.79 | 11.52±1.57 |
| OVX | 10 | 11.65±1.57 | 17.53±1.84 | 21.28±1.99 |
| OVX + PBS | 10 | 10.34±1.65 | 18.85±1.41 | 22.05±1.57 |
| OVX + RESV | 10 | 11.75±1.61 | 14.27±1.62 | 16.26±1.93 |
 | Table VIIIEffects of OVX and RESV treatment on
levels of serum osteocalcin (nmol/l). |
Table VIII
Effects of OVX and RESV treatment on
levels of serum osteocalcin (nmol/l).
| Group | n | Day 0 | Day 30 | Day 60 |
|---|
| Untreated | 10 | 2.72±0.37 | 2.86±0.38 | 2.85±0.41 |
| OVX | 10 | 2.82±0.38 | 2.27±0.35 | 1.71±0.42 |
| OVX + PBS | 10 | 2.58±0.41 | 2.32±0.41 | 1.89±0.38 |
| OVX + RESV | 10 | 2.57±0.35 | 2.48±0.48 | 2.52±0.47 |
 | Table IXEffects of OVX and RESV treatment on
levels of urinary calcium (mmol/l). |
Table IX
Effects of OVX and RESV treatment on
levels of urinary calcium (mmol/l).
| Group | n | Day 0 | Day 30 | Day 60 |
|---|
| Untreated | 10 | 1.68±0.29 | 1.76±0.28 | 1.65±0.31 |
| OVX | 10 | 1.72±0.34 | 2.65±0.43 | 2.71±0.54 |
| OVX + PBS | 10 | 1.58±0.22 | 2.68±0.47 | 2.84±0.56 |
| OVX + RESV | 10 | 1.66±0.27 | 2.01±0.48 | 2.32±0.35 |
 | Table XEffects of OVX and RESV treatment on
levels of urinary deoxypyridinoline/creatinine (nmol/mmol). |
Table X
Effects of OVX and RESV treatment on
levels of urinary deoxypyridinoline/creatinine (nmol/mmol).
| Group | n | Day 0 | Day 30 | Day 60 |
|---|
| Untreated | 10 | 147.25±10.09 | 148.75±10.82 | 150.50±11.31 |
| OVX | 10 | 129.75±10.85 | 222.24±14.42 | 271.52±15.88 |
| OVX + PBS | 10 | 130.46±11.14 | 225.36±14.47 | 274.98±14.92 |
| OVX + RESV | 10 | 148.89±11.33 | 191.67±14.24 | 207.68±14.45 |
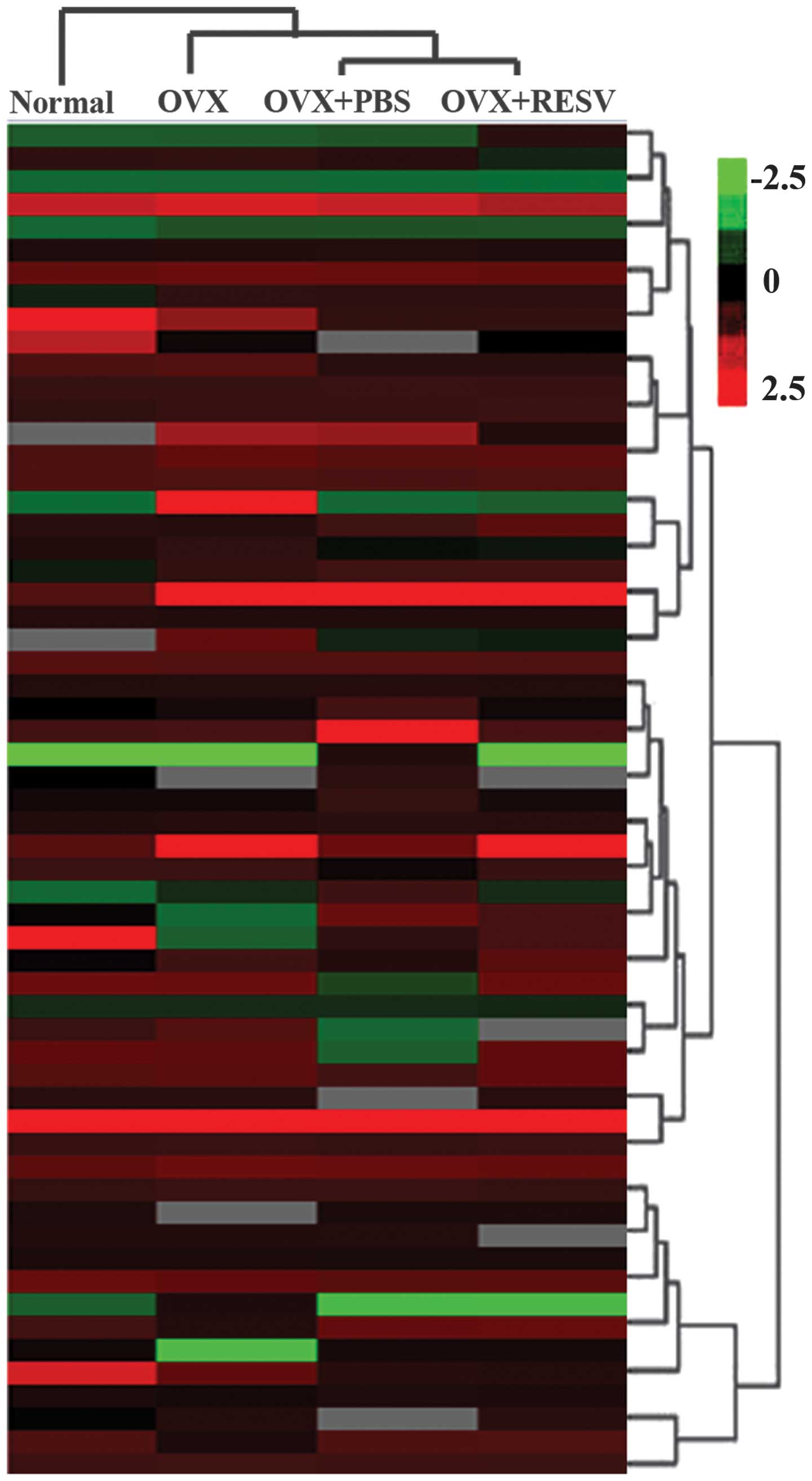
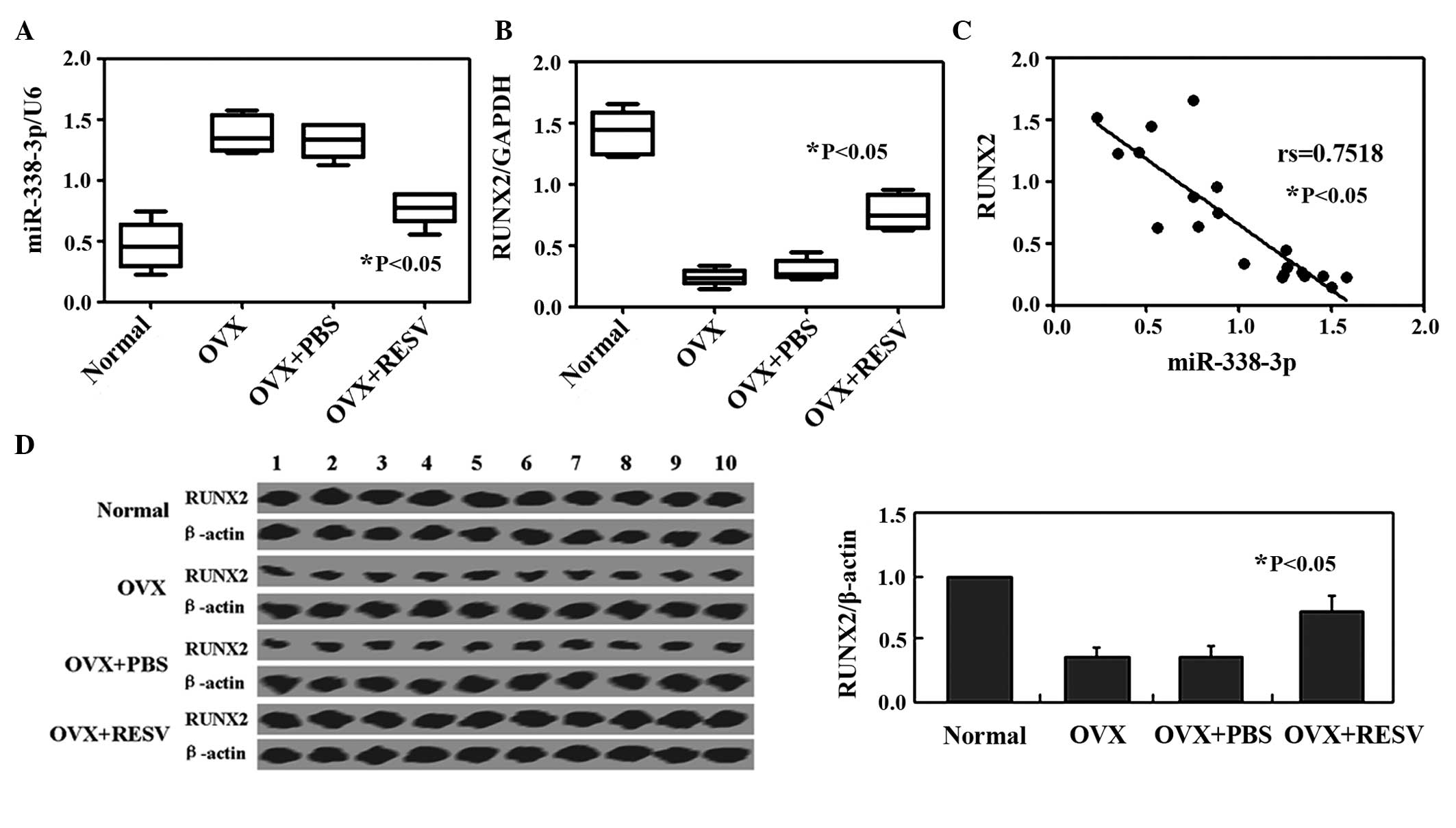
RESV downregulates the expression of
miR-338-3p in OVX rats
To identfiy miRNAs, which may have been involved in
RESV-induced differentiation in the OVX group, array analysis was
used to screen for miRNAs, which were differentially expressed
between the OVX group and the OVX + RESV group (Fig. 1). Changes of several miRNAs were
identified, of which miR-338-3p was examined in the present study
as it exhibited the most marked change in expression. qPCR analysis
confirmed that miR-338-3p was significantly decreased in the OVX +
RESV treatment compared with the OVX group (Fig. 2A). Furthermore, qPCR and western
blot analysis were performed in order to determine the mRNA and
protein levels of RUNX2 in each group, respectively (Fig. 2B and C). The mRNA expression of
RUNX2 was negatively correlated with that of miR-338-3p
(rs=−0.7518; P<0.05; Fig.
2D).
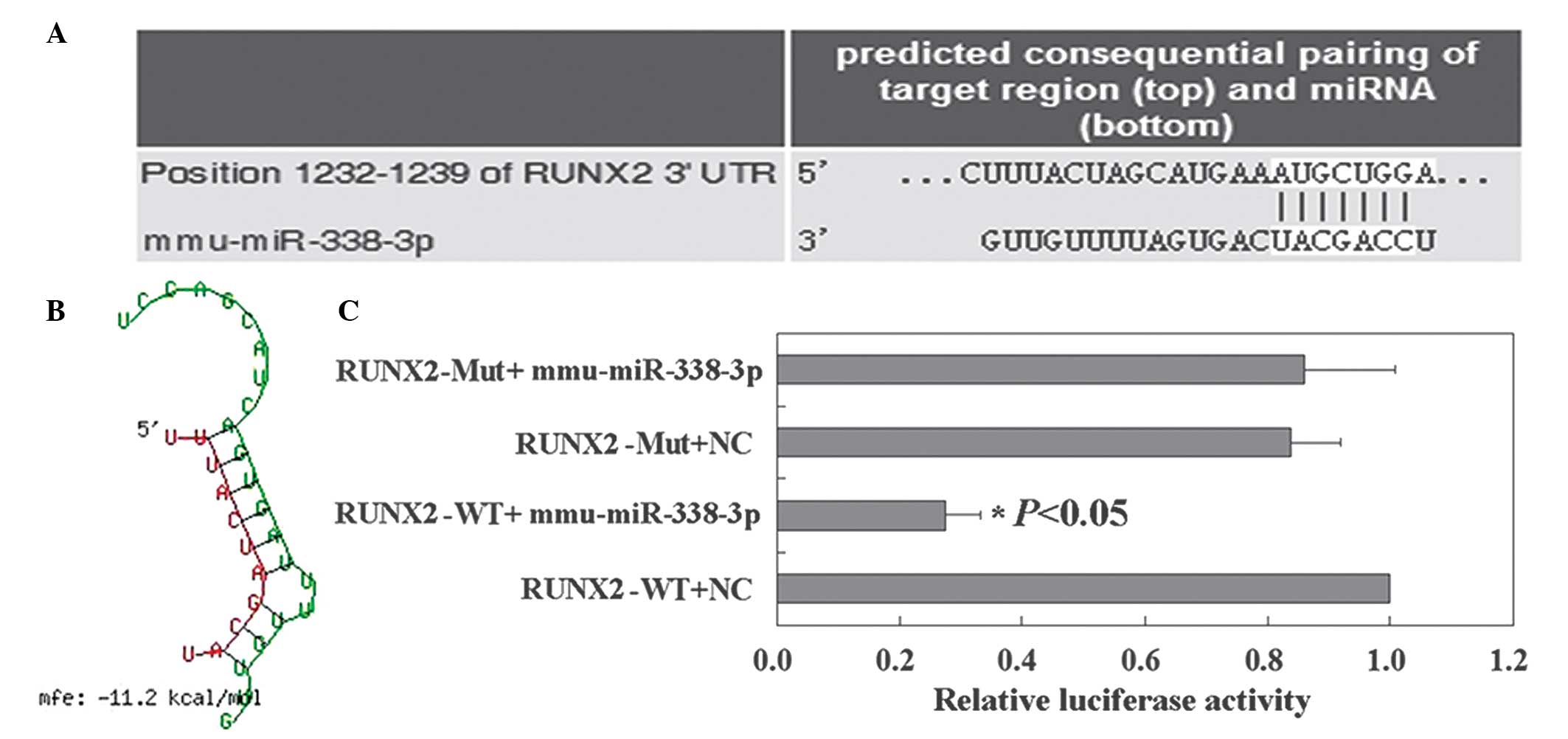
RUNX2 is a direct target of
miR-338-3p
TargetScan was used to identify the potential
targets of miR-338-3p. Runx2 was identified as a target of
miR-338-3p (Fig. 3A). BibiServ
analysis determined the free energy for the RUNX2 3′UTR region and
miR-338-3p hybrid was ~−11.2 kcal/mol (Fig. 3B). The relative luciferase activity
results revealed that the RUNX2 reporter was suppressed by
miR-338-3p (Fig. 3C). These
results demonstrated that miR-338-3p specifically targeted the
3′UTR region of RUNX2.
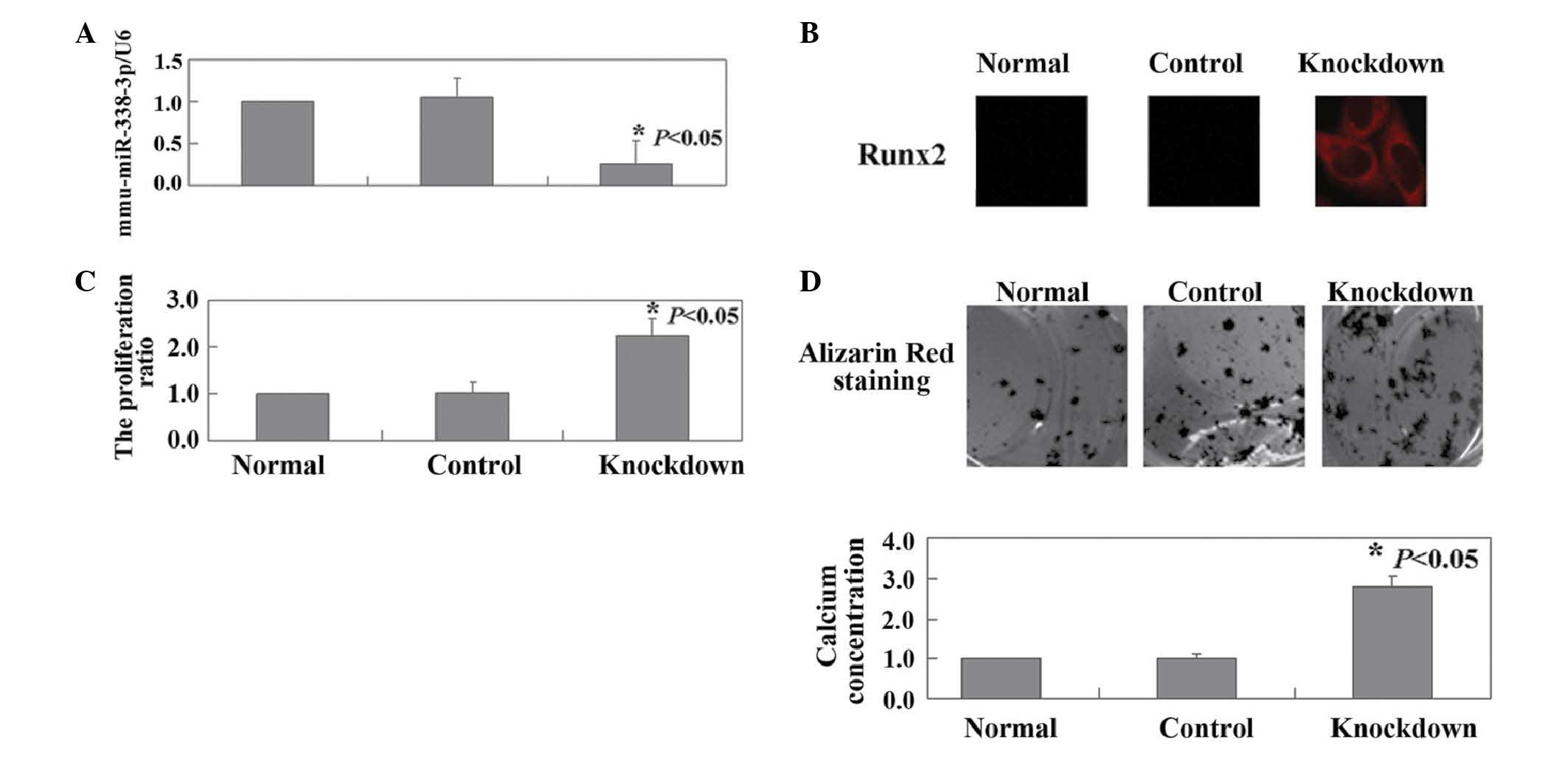
Inhibition of endogenous miR-338-3p
induces the proliferation and differentiation of HOB cells
The present study also investigated the effects of
miR-338-3p knockdown in HOB cells, by transfection of the HOB cells
with miR-338-3p inhibitor. As shown in Fig. 4A, the results of qPCR confirmed the
downregulation of endogenous miR-338-3p in the HOB cells following
transfection (P<0.05). Immunofluorescence analysis revealed an
increase in the expression of RUNX2 in the HOB cells following
transfection (Fig. 4B). Compared
with the untransfected cells, a significant increase was observed
in the proliferation rate of the HOB cells (P<0.05; Fig. 4C). miR-338-3p knockdown also
promoted in vitro calcium deposition in the HOB cells
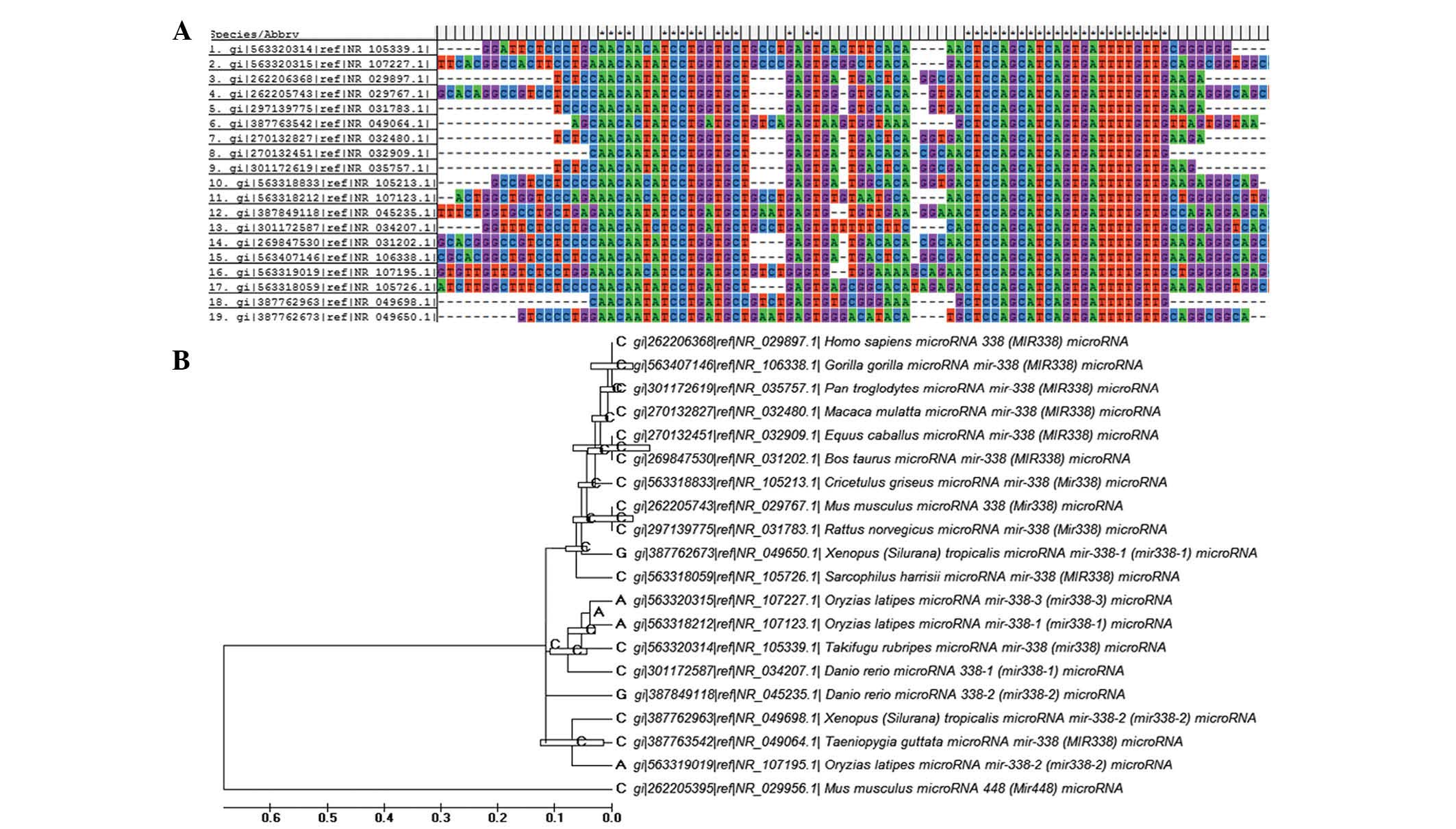
(Fig. 4D). Finally, multiple
sequence alignment and phylogenetic analysis were performed, the
results of which confirmed miR-338-3p as is a highly conserved
microRNA (Fig. 5A and B).
Discussion
RUNX2 is an essential transcription factor, which
controls bone and tooth development by regulating osteoblast and
odontoblast differentiation (12–14).
RUNX2 is important in mediating the bone morphogenetic protein and
transforming growth factor β pathways, which are important for
osteoblast development and growth (15). In the present study, RUNX2 was
found to increase the proliferation and induce the differentiation
of HOB cells.
miR-338-3p, an intronic miRNA, is located within the
eighth intron (intron-8) of the apoptosis-associated tyrosine
kinase gene (16). Liu et
al (17) observed that
miR-338-3p serves as a negative regulator of osteogenic
differentiation in bone marrow stromal cells. Consistent with the
previous study, the present study also confirmed that the
expression of miR-338-3p was decreased during osteoblast
differentiation. Furthermore, the present study found that
miR-338-3p knockdown upregulated RUNX2 at the mRNA and protein
levels. Bioinformatics analysis indicated that RUNX2 may be a
putative target gene of miR-338-3p. Liu et al (17) and Sun et al (18) previously confirmed RUNX2 as a
direct target of miR-338-3p.
Following OVX, the rate of bone turnover is known to
increase (19). The results of the
present study consistently demonstrated that OVX rats exhibited a
decrease in serum levels of ALP and OC. Mizutani et al
(20) found that RESV attenuates
ovariectomy-induced hypertension and bone loss in OVX rats. Casarin
et al (21) also found that
RESV improves bone repair by modulating levels of the bone
morphogenetic protein and osteopontin gene expression in rats.
The present study revealed an intact mechanism by
which RESV acts in OVX rats. The results suggested that RESV
suppressed miR-338-3p, followed by an increase in the expression of
RUNX2 in HOB cells.
References
|
1
|
Guo JD, Li L, Shi YM, Wang HD and Hou SX:
Hydrogen water consumption prevents osteopenia in ovariectomized
rats. Br J Pharmacol. 168:1412–1420. 2013. View Article : Google Scholar :
|
|
2
|
Das S and Crockett JC: Osteoporosis - a
current view of pharmacological prevention and treatment. Drug Des
Devel Ther. 7:435–448. 2013.PubMed/NCBI
|
|
3
|
Yang Y, Wang B, Fei Q, et al: Validation
of an osteoporosis self-assessment tool to identify primary
osteoporosis and new osteoporotic vertebral fractures in
postmenopausal Chinese women in Beijing. BMC Musculoskelet Disord.
14:2712013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pervaiz S: Resveratrol: from grapevines to
mammalian biology. FASEB J. 17:1975–1985. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: the in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karuppagounder SS, Pinto JT, Xu H, Chen
HL, Beal MF and Gibson GE: Dietary supplementation with resveratrol
reduces plaque pathology in a transgenic model of Alzheimer’s
disease. Neurochem Int. 54:111–118. 2009. View Article : Google Scholar
|
|
7
|
Mizutani K, Ikeda K, Kawai Y and Yamori Y:
Resveratrol stimulates the proliferation and differentiation of
osteoblastic MC3T3-E1 cells. Biochem Biophys Res Commun.
253:859–863. 1998. View Article : Google Scholar
|
|
8
|
Liu ZP, Li WX, Yu B, et al: Effects of
trans-resveratrol from Polygonum cuspidatum on bone loss using the
ovariectomized rat model. J Med Food. 8:14–19. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calin GA and Croce CM: MicroRNAs and
chromosomal abnormalities in cancer cells. Oncogene. 25:6202–6210.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kiesslich T, Alinger B, Wolkersdörfer GW,
Ocker M, Neureiter D and Berr F: Active Wnt signalling is
associated with low differentiation and high proliferation in human
biliary tract cancer in vitro and in vivo and is sensitive to
pharmacological inhibition. Int J Oncol. 36:49–58. 2010.
|
|
11
|
Thompson JD, Higgins DG and Gibson TJ:
CLUSTAL W: improving the sensitivity of progressive multiple
sequence alignment through sequence weighting, position-specific
gap penalties and weight matrix choice. Nucleic Acids Res.
22:4673–4680. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
D’Souza RN, Aberg T, Gaikwad J, et al:
Cbfa1 is required for epithelial-mesenchymal interactions
regulating tooth development in mice. Development. 126:2911–2920.
1999.
|
|
13
|
Miyazaki T, Kanatani N, Rokutanda S, et
al: Inhibition of the terminal differentiation of odontoblasts and
their transdifferentiation into osteoblasts in Runx2 transgenic
mice. Arch Histol Cytol. 71:131–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li S, Kong H, Yao N, et al: The role of
runt-related transcription factor 2 (Runx2) in the late stage of
odontoblast differentiation and dentin formation. Biochem Biophys
Res Commun. 410:698–704. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee KS, Kim HJ, Li QL, et al: Runx2 is a
common target of transforming growth factor beta1 and bone
morphogenetic protein 2 and cooperation between Runx2 and Smad5
induces osteoblast-specific gene expression in the pluripotent
mesenchymal precursor cell line C2C12. Mol Cell Biol. 20:8783–8792.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rodriguez A, Griffiths-Jones S, Ashurst JL
and Bradley A: Identification of mammalian microRNA host genes and
transcription units. Genome Res. 14:1902–1910. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu H, Sun Q, Wan C, Li L, Zhang L and
Chen Z: MicroRNA-338-3p regulates osteogenic differentiation of
mouse bone marrow stromal stem cells by targeting Runx2 and Fgfr2.
J Cell Physiol. 229:1494–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun Q, Liu H, Lin H, Yuan G, Zhang L and
Chen Z: MicroRNA-338-3p promotes differentiation of mDPC6T into
odontoblast-like cells by targeting Runx2. Mol Cell Biochem.
377:143–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Govoni KE, Wergedal JE, Chadwick RB,
Srivastava AK and Mohan S: Prepubertal OVX increases IGF-1
expression and bone accretion in C57BL/6 mice. Am J Physiol
Endocrinol Metab. 295:E1172–E1180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mizutani K, Ikeda K, Kawai Y and Yamori Y:
Resveratrol attenuates ovariectomy-induced hypertension and bone
loss in stroke-prone spontaneously hypertensive rats. J Nutr Sci
Vitaminol (Tokyo). 46:78–83. 2000. View Article : Google Scholar
|
|
21
|
Casarin RC, Casati MZ, Pimentel SP, et al:
Resveratrol improves bone repair by modulation of bone
morphogenetic proteins and osteopontin gene expression in rats. Int
J Oral Maxillofac Surg. 43:900–906. 2014. View Article : Google Scholar : PubMed/NCBI
|