Introduction
CCAAT/enhancer binding protein α (C/EBPα) is a
uniquely multifunctional transcription factor, which binds with the
promoters of target genes and C/EBP family homodimers or
heterodimers to regulate the transcription of target genes
(1). In addition to its
transcriptional activity, C/EBPα also promotes differentiation and
suppresses proliferation in numerous cell types (2). These antiproliferative and
pro-apoptotic characteristics of C/EBPα provide it with a tumor
suppressive function in multiple tissues and it has been observed
that downregulation of C/EBPα expression or C/EBPα gene mutations
are associated with several human malignancies, including acute
myeloid leukemia (3), lung cancer
(4), breast cancer (5), hepatocellular carcinoma (6), head and neck squamous cell carcinoma
(7) and skin cancer (8). Thus, C/EBPα has been recognized as a
potential tumor suppressor and therapeutic target in human
cancer.
As malignancy develops, the majority of types of
solid tumor are characterized by hypoxic environments, which
accelerate progression and metastasis (9). Bladder carcinoma is one of the common
types of solid tumor associated with a hypoxic environment
(10). The key hypoxic-responsive
regulator of gene expression is hypoxia inducible factor-1 (HIF-1),
which is a heterodimeric protein composed of a constitutively
expressed β subunit and an inducible α subunit (11). Under normoxic conditions, HIF-1α
mRNA is translated, but its protein is rapidly degraded (11). However, under hypoxic conditions,
HIF-1α protein becomes increasingly stabilized and its degradation
is prevented, facilitating the regulation of the expression of a
series of genes, which are involved in cell proliferation,
differentiation, apoptosis, angiogenesis, migration and invasion
(12). The overexpression of
HIF-1α has been identified in patients with bladder carcinoma, and
the expression of HIF-1α is correlated with the progression and
recurrence of bladder carcinoma (13). Consequently, in addition to being a
diagnostic biomarker, HIF-1α may be a novel therapeutic target in
bladder carcinoma. Although the involvement of HIF-1α in the
progression and metastasis of bladder cancer has previously been
reported (14), the potential
regulatory mechanisms of HIF-1α on the proliferation and
differentiation of bladder transitional carcinoma cancer cells has
remained to be elucidated.
Based on the above observations, the present study
hypothesized that HIF-1α regulated the expression of C/EBPα in
hypoxic bladder cancer cells and was involved in the regulation of
the proliferation and differentiation of bladder cancer cells.
Materials and methods
Cell culture
The 5637 and T24 human bladder cancer cell lines
were obtained from the American Type Culture Collection (Manassas,
VA, USA). The cells were grown in RPMI-1640 (Gibco Life
Technologies, Grand Island, NY, USA) with 10% fetal bovine serum.
The cultures were maintained at 37°C under a humidified 5%
CO2 atmosphere.
Hypoxic treatment
Hypoxia was achieved by placing 50% confluent 5637
and T24 cells in an oxygen control incubator (Heal Force, Shanghai,
China), which was flushed with a mixture of 1% O2, 94%
N2 and 5% CO2. The 5637 and T24 cells were
incubated at 37°C for 48 h under the hypoxic conditions (1%
O2, 5% CO2 and 94% N2).
Cell treatment with HIF-1α inhibitor
The HIF-1α inhibitor
3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole (YC-1) was purchased
from Sigma-Aldrich (St. Louis, MO, USA) and resuspended in dimethyl
sulfoxide (DMSO; Sigma-Aldrich). YC-1 was used at a final
concentration of 50 µmol/l. The 5637 and T24 cells were incubated
at 37°C for 12 h under hypoxic conditions (1% O2, 5%
CO2 and 94% N2). YC-1 or DMSO was added to
RPMI-1640 medium prior to incubation for 12 h under hypoxic
conditions. The final volume of YC-1 or DMSO in the medium was
≤0.5% (v/v).
Small interference RNA (siRNA)
transfection
The HIF-1α siRNA was transiently transfected into
bladder cancer cell lines using X-tremeGENE siRNA transfection
reagent (Roche Diagnostics, Indianapolis, IN, USA). The cells were
trans-fected with HIF-1α siRNA for 12 h, following 24 h hypoxia.
The HIF-1α siRNAs (GenePharma, Shanghai, China) used are listed in
Table I.
 | Table IPrimer and siRNA sequences. |
Table I
Primer and siRNA sequences.
| Gene | Sequence (5′-3′) | Experimental use |
|---|
| C/EBPα |
ATTGGAGCGGTGAGTTTG
TTGGTGCGTCTAAGATGAG | RT-qPCR |
| HIF-1α |
CATCTCCATCTCCTACCCACA
CTTTTCTGCTCTGTTTGGTG | RT-qPCR |
| β-actin |
TCCCTGGAGAAGAGCTACGA
AGCACTGTGTTGGCGTACAG | RT-qPCR |
| siRNA control |
UUCUCCGAACGUGUCACGUTT
ACGUGACACGUUCGGAGAATT | RNAi |
| HIF-1α siRNA |
GCCUCUUUGACAAACUUAATT
UUAAGUUUGUCAAAGAGGCTT | RNAi |
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the bladder cancer cell
lines using TRIzol reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA). First-strand cDNA was then synthesized using a
PrimeScript RT reagent kit (Perfect Real-Time; Takara
Biotechnology, Co., Ltd., Dalian, China). RT-qPCR was performed
using a SYBR Premix Ex Taq™ II (Takara Biotechnology, Co., Ltd.) in
a CFX96 Real-Time PCR Detection system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and the results were normalized against β-actin
as an internal control. The PCR cycling conditions were as follows:
initial denaturation step at 95°C for 30 sec, followed by 40 cycles
at 95°C for 5 sec and 60°C for 30 sec. The PCR primers that were
used are listed in Table I.
Western blotting
The bladder cancer cells were washed with
phosphate-buffered saline (PBS), composed of 140 mM NaCl, 2.7 mM
KCl, 10 mM Na2HPO4, 1.8 mM
KH2PO4 (pH 7.4), and lysed in
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Waltham, MA, USA) containing complete protease inhibitor cocktail
tablets (Roche, one tablet/10 ml aqueous buffer). The protein
concentrations were quantified using a bicinchoninic acid protein
assay kit (Thermo Scientific; Pierce BCA Protein Assay Kit; cat.
no. 23227). All proteins were separated by 10% SDS-PAGE and
transferred onto nitrocellulose membranes (Pall Life Science, Port
Washington, NY, USA). The membranes were incubated overnight at 4°C
with the following primary antibodies: monoclonal rabbit anti-human
C/EBPα antibody (1:500; Abcam; cat. no. ab40761), monoclonal mouse
anti-human HIF-1α antibody (1:500; Abcam; cat. no. ab1) and
monoclonal mouse anti-human β-actin antibody (1:1000; Cell
Signaling Technology, Beverly, MA, USA; cat. no. 3700). Following
washing with TBST, composed of 20 mM Tris, 140 mM NaCl and 0.5%
Tween-20 (pH 7.6), the membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (goat anti- rabbit/mouse
immunoglobulin G; 1:2,000; Cell Signaling Technology; cat. no.
7074/7076) for 1 h at room temperature. Protein expression was
assessed using enhanced chemiluminescent reagents (Pierce
Biotechnology, Inc.).
Immunofluorescence analysis
The 5637 and T24 cells were fixed for 20 min at room
temperature with 4% formaldehyde, permeabilized for 10 min at room
temperature with 0.2% Triton X-100, blocked for 1 h at room
temperature with 5% BSA and incubated overnight at 4°C with
monoclonal rabbit anti-human C/EBPα (Abcam). The cells were
subsequently incubated for 1 h at room temperature with
Cy3-conjugated goat anti-rabbit immunoglobulin G (Invitrogen Life
Technologies). The cellular nuclei were counterstained with
4′,6-diamidino-2-phe-nylindole (Roche Diagnostics) and the cells
were detected using a Nikon Eclipse Ti-S fluorescence microscope
(Nikon Corp., Tokyo, Japan)..
Statistical analysis
All experiments were performed at least three times.
The data are expressed as the mean ± standard error of the mean and
were analyzed using SPSS 19.0 (IBM SPSS, Armonk, NY, USA) and
GraphPad Prism 5 software (GraphPad Software, Inc., San Diego, CA,
USA). Statistical evaluations were determined using Student’s
two-tailed unpaired t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Hypoxia induces changes in the gene
expression of HIF-1α and C/EBPα in bladder cancer cells
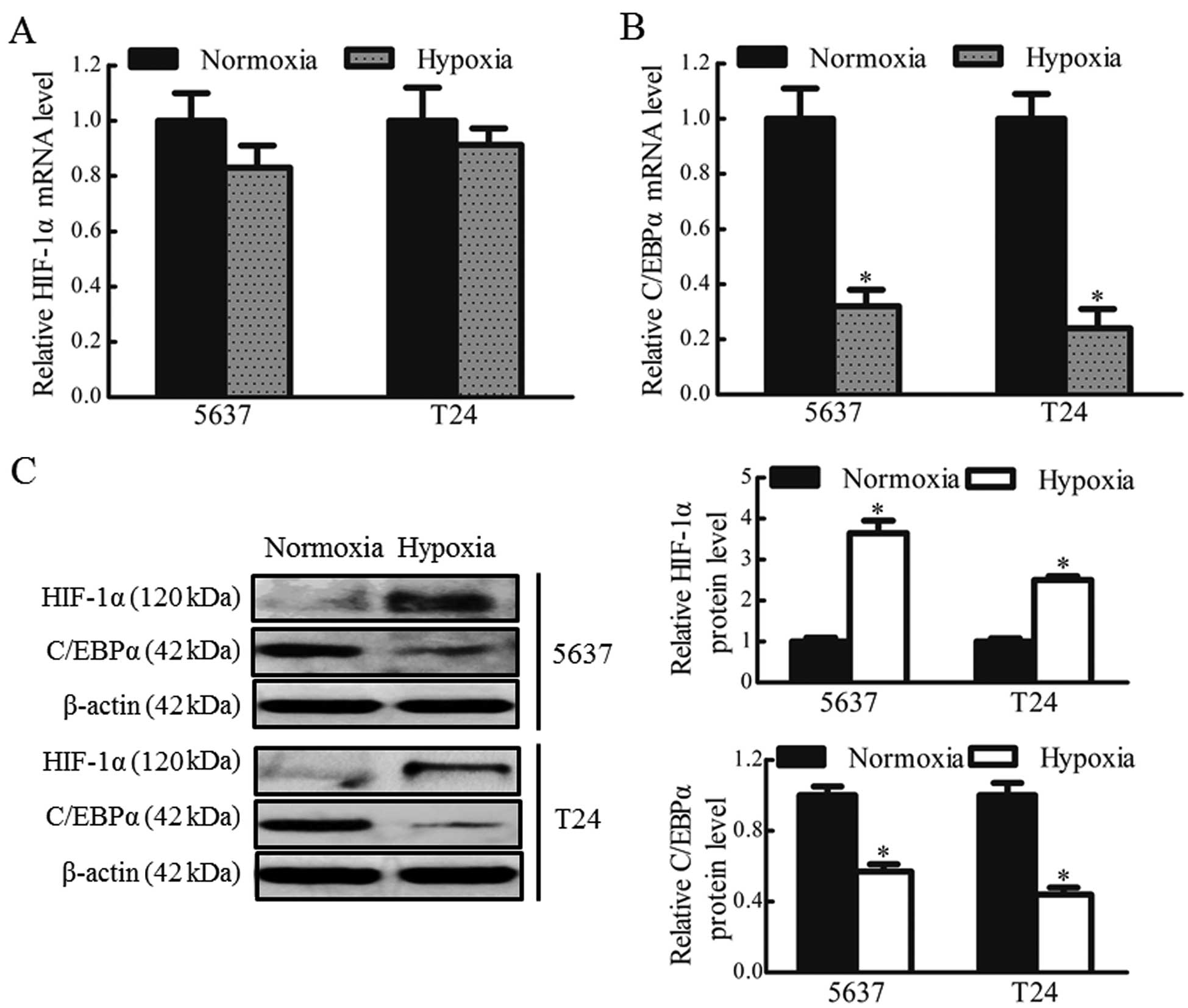
To confirm whether hypoxia regulated the expression
of C/EBPα in bladder cancer cells, the mRNA and protein expression
levels of HIF-1α and C/EBPα in the 5637 and T24 cells under hypoxic
and normoxic conditions were determined. No significant differences
were observed in the expression levels of HIF-1α mRNA between the
hypoxic and normoxic groups (Fig.
1A). Low protein expression levels of HIF-1α were observed in
the 5637 and T24 cells under normoxic culture conditions. However,
the protein levels of HIF-1α were markedly upregulated (~3-fold
increase) in the 5637 and T24 cells under hypoxic conditions, while
the mRNA and protein levels of C/EBPα were significantly reduced,
by 70 and 50% in the 5637 and T24 cells, respectively, under
hypoxic conditions (P<0.05; Fig. 1B
and C). These data indicated that the downregulation of C/EBPα
may be associated with the stable expression of HIF-1α in hypoxic
bladder cancer cells.
Hypoxia reduces the expression of C/EBPα
in bladder cancer cells
To further evaluate the differences in the
expression of C/EBPα in hypoxia, the present study also analyzed
the subcellular localization and protein levels of C/EBPα in the
5637 and T24 cells under hypoxic and normoxic culture conditions.
The protein expression and subcellular localization of C/EBPα was
detected using an immunofluorescence assay. Under normoxic culture
conditions, the protein expression of C/EBPα was marked in the
nucleus and cytoplasm of the bladder cancer cells (Fig. 2). However, under hypoxic
conditions, the protein expression of C/EBPα was markedly decreased
in the cytoplasm and, in particular, the nuclei of the 5637 and T24
bladder cancer cells. These data suggested that hypoxia reduced the
nuclear localization of C/EBPα protein in the bladder cancer
cells.
siRNA-mediated silencing of HIF-1α
enhances the expression of C/EBPα in hypoxic bladder cancer
cells
In order to further confirm that HIF-1α regulated
the expression of C/EBPα in hypoxic bladder cancer cells, the 5637
and T24 cells were transfected with HIF-1α siRNA or scrambled siRNA
(siRNA control) prior to culture under hypoxic conditions.
Transfection with the HIF-1α siRNA led to a significant reduction
in the mRNA and protein expression levels of HIF-1α, by ~80 and
~50%, respectively (Fig. 3A and
B). Silencing of HIF-1α may, therefore, prevent the
downregulation of C/EBPα in hypoxic bladder cancer cells. Following
treatment with HIF-1α siRNA, the mRNA and protein levels of C/EBPα
were rescued in the 5637 and T24 cells, exhibiting a 1.4 and
1.5-fold increase, respectively (Fig.
3B and C). These results suggested that HIF-1α was involved in
the downregulation of C/EBPα in bladder cancer cells under hypoxic
culture conditions.
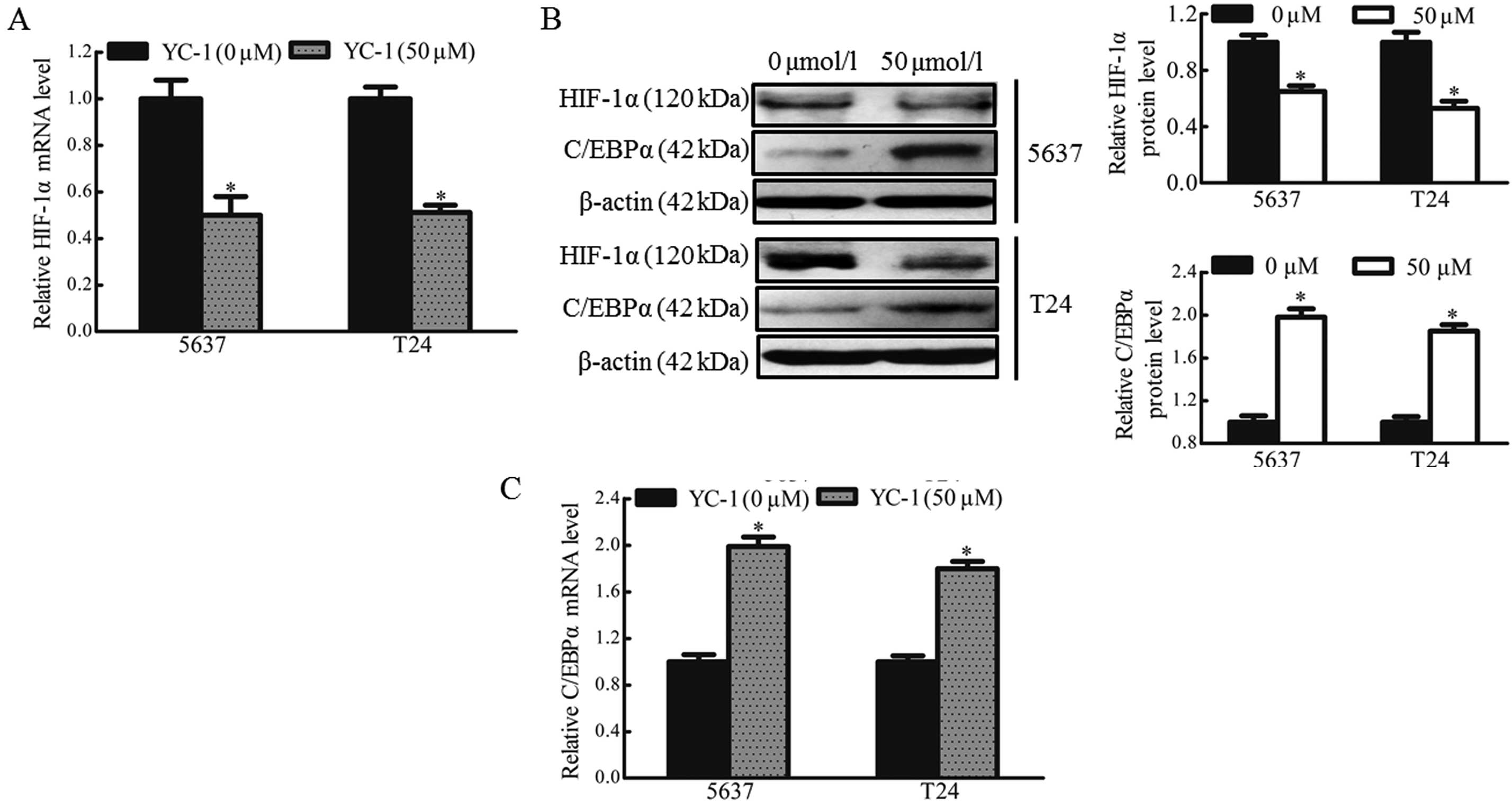
HIF-1α inhibitor YC-1 enhances the
expression of C/EBPα in hypoxic bladder cancer cells
YC-1 has been reported to be a novel anticancer drug
targeting HIF-1α, which may also inhibit the expression of HIF-1α
(15,16). Therefore, the present study
investigated whether bladder cancer cells treated with YC-1
exhibited alterations in the expression of HIF-1α and C/EBPα under
hypoxic conditions. The 5637 and T24 cells were treated with YC-1
(50 µmol/l) for 12 h under hypoxic conditions. The results revealed
that YC-1 reduced the mRNA (~50%) and protein (~50%) expression of
HIF-1α under hypoxic conditions (Fig.
4A and B). YC-1 also prevented the downregulation of C/EBPα in
the hypoxic bladder cancer cells. The mRNA and protein levels of
C/EBPα were enhanced ~1.8 and 2-fold, respectively, following
treatment with YC-1 in the 5637 and T24 cells (Fig. 4B and C). These data confirmed that
YC-1 inhibited the expression of HIF-1α and enhanced the expression
of C/EBPα in hypoxic bladder cancer cells.
Discussion
Hypoxia is one of the most fundamental and common
biological phenomena found in various types of solid tumor,
including bladder cancer (17). As
a response to the hypoxic environment, HIF-1α is activated in
cancer cells, and contributes to cellular adaptation and survival
(12). A number of genes, which
have been implicated in angiogenesis, invasion, metastasis, cell
proliferation, differentiation and apoptosis, are regulated by
HIF-1α (18). The roles of HIF-1α
in tumor angiogenesis, invasion and metastasis have previously been
investigated, whereas the molecular regulatory mechanisms
underlying the involvement of HIF-1α in tumor cell proliferation
and differentiation have remained to be fully elucidated. Previous
studies have demonstrated that, in breast cancer cells, hypoxia
induces downregulation of the expression of C/EBPα (19,20),
which indicated that hypoxia modulates breast cancer cell
proliferation and differentiation, potentially through
downregulation of C/EBPα. However, to the best of our knowledge,
there is no current evidence indicating that hypoxia regulates the
expression and subcellular localization of C/EBPα in bladder
carcinoma. In the present study, hypoxia was found to down-regulate
the expression and nuclear localization of the tumor suppressor
C/EBPα through an HIF-1α-dependent mechanism in bladder
transitional carcinoma cells. Therefore, HIF-1α may be involved in
regulating the differentiation and proliferation of bladder cancer
cells by controlling the expression of C/EBPα.
C/EBPα inhibits proliferation and induces
differentiation in several cell types through protein-protein
interactions (21-25). Several mechanisms for these effects
have been proposed, including stabilization of p21 (21,22),
repression of E2F (23), direct
inhibition or degradation of Cdk2 and Cdk4 (24) and interaction with the SWI/SNF
chromatin remodeling complex (25). Therefore, C/EBPα, through multiple
mechanisms, functions as a tumor suppressor in tumorigenesis,
however, further investigation is required to determine the exact
function and mechanism of C/EBPα in bladder cancer cell
differentiation and proliferation.
The functional activity of C/EBPα is regulated by
modulation of its intracellular localization (26). C/EBPα requires localization in the
nuclear region to exert its capacity for transcriptional activation
and its biological function (19).
Previous studies have demonstrated that C/EBPα is local-ized in the
nucleus in normal epithelial cells; however, in cancer cells,
C/EBPα is localized in the nucleus and cytoplasm (27). The present study also found that
C/EBPα exhibited mixed nuclear and cytoplasmic localization in the
bladder cancer cells. However, hypoxia markedly decreased the
expression of C/EBPα in the nuclear region of the 5637 and T24
cells (Fig. 2B). The reduction of
C/EBPα within the bladder cancer cell nuclei under hypoxia may
contribute to a decrease in the biological activity of this
protein. Thus, hypoxia regulated the biological effects of C/EBPα
by modulating the nuclear localization of C/EBPα.
YC-1 is reported to be a novel antitumor agent,
which decreases the expression of HIF-1α, inhibiting cell
proliferation and inducing apoptosis in hypoxic bladder cancer
cells (15,16). However, the antitumor mechanism of
YC-1 in bladder cancer remains to be fully elucidated. The present
study observed that YC-1 inhibited the expression of HIF-1α and
prevented the downregulation of C/EBPα in hypoxic bladder cancer
cells. These findings indicated that YC-1 may exert antitumor
effects through the HIF-1α and C/EBPα pathway in hypoxic bladder
cancer cells.
In conclusion, the present study demonstrated that,
in bladder cancer cells, hypoxia downregulated the expression and
nuclear localization of C/EBPα, a process which was mediated by
HIF-1α. Furthermore, the HIF-1α specific inhibitor, YC-1, rescued
the downregulation of C/EBPα under hypoxic conditions. Future
investigations using bladder cancer tissue specimens to analyze the
association between the expression of HIF-1α and C/EBPα and
clinical and pathological parameters and patient survival rates are
required to evaluate C/EBPα as a potential therapeutic target in
bladder cancer.
Acknowledgments
This study was supported by grants (nos. 81171970,
81072104 and 30370660) from the National Natural Science Foundation
of China.
References
|
1
|
Schuster MB and Porse BT: C/EBPalpha: a
tumour suppressor in multiple tissues? Biochim Biophys Acta.
1766:88–103. 2006.PubMed/NCBI
|
|
2
|
Lopez RG, Garcia-Silva S, Moore SJ,
Bereshchenko O, Martinez-Cruz AB, Ermakova O, Kurz E, Paramio JM
and Nerlov C: C/EBPalpha and beta couple interfollicular
keratinocyte proliferation arrest to commitment and terminal
differentiation. Nat Cell Biol. 11:1181–1190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pabst T, Mueller BU, Zhang P, Radomska HS,
Narravula S, Schnittger S, Behre G, Hiddemann W and Tenen DG:
Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer
binding protein-alpha (C/EBPalpha), in acute myeloid leukemia. Nat
Genet. 27:263–270. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sato A, Yamada N, Ogawa Y and I kegami M:
CCAAT/enhancer-binding protein-α suppresses lung tumor development
in mice through the p38α MAP kinase pathway. PLoS One.
8:e570132013. View Article : Google Scholar
|
|
5
|
Gery S, Tanosaki S, Bose S, Bose N,
Vadgama J and Koeffler HP: Down-regulation and growth inhibitory
role of C/EBPalpha in breast cancer. Clin Cancer Res. 11:3184–3190.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tseng HH, Hwang YH, Yeh KT, Chang JG, Chen
YL and Yu HS: Reduced expression of C/EBP alpha protein in
hepatocellular carcinoma is associated with advanced tumor stage
and shortened patient survival. J Cancer Res Clin Oncol.
135:241–247. 2009. View Article : Google Scholar
|
|
7
|
Bennett KL, Hackanson B, Smith LT,
Morrison CD, Lang JC, Schuller DE, Weber F, Eng C and Plass C:
Tumor suppressor activity of CCAAT/enhancer binding protein alpha
is epige-netically down-regulated in head and neck squamous cell
carcinoma. Cancer Res. 67:4657–4664. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thompson EA, Zhu S, Hall JR, House JS,
Ranjan R, Burr JA, He YY, Owens DM and Smart RC: C/EBPα expression
is down-regulated in human nonmelanoma skin cancers and
inactivation of C/EBPα confers susceptibility to UVB-induced skin
squamous cell carcinomas. J Invest Dermatol. 131:1339–1346. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Keith B and Simon MC: Hypoxia-inducible
factors, stem cells, and cancer. Cell. 129:465–472. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tickoo SK, Milowsky MI, Dhar N, Dudas ME,
Gallagher DJ, Al-Ahmadie H, Gopalan A, Fine SW, Ishill N, Bajorin
DF and Reuter VE: Hypoxia-inducible factor and mammalian target of
rapamycin pathway markers in urothelial carcinoma of the bladder:
possible therapeutic implications. Bju Int. 107:844–849. 2011.
View Article : Google Scholar
|
|
11
|
Majmundar AJ, Wong WJ and Simon MC:
Hypoxia-inducible factors and the response to hypoxic stress. Mol
Cell. 40:294–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chai CY, Chen WT, Hung WC, Kang WY, Huang
YC, Su YC and Yang CH: Hypoxia-inducible factor-1alpha expression
correlates with focal macrophage infiltration, angiogenesis and
unfavourable prognosis in urothelial carcinoma. J Clin Pathol.
61:658–664. 2008. View Article : Google Scholar
|
|
14
|
Zhang T, Fan J, Wu K, Zeng J, Sun K, Guan
Z, Wang X, Hiesh JT and He D: Roles of HIF-1α in a novel optical
orthotopic spontaneous metastatic bladder cancer animal model. Urol
Oncol. 30:928–935. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Zhao X, Tang H, Zhong Z, Zhang L, Xu
R, Li S and Wang Y: Effects of YC-1 on hypoxia-inducible factor 1
alpha in hypoxic human bladder transitional carcinoma cell line T24
cells. Urol Int. 88:95–101. 2012. View Article : Google Scholar
|
|
16
|
Sun HL, Liu YN, Huang YT, Pan SL, Huang
DY, Guh JH, Lee FY, Kuo SC and Teng CM: YC-1 inhibits HIF-1
expression in prostate cancer cells: contribution of Akt/NF-kappaB
signaling to HIF-1alpha accumulation during hypoxia. Oncogene.
26:3941–3951. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gospodarowicz M: Combination therapy:
hypoxia modification with radiotherapy for bladder cancer. Nat Rev
Clin Oncol. 8:129–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi SB, Park JB, Song TJ and Choi SY:
Molecular mechanism of HIF-1-independent VEGF expression in a
hepatocellular carcinoma cell line. Int J Mol Med. 28:449–454.
2011.PubMed/NCBI
|
|
19
|
Seifeddine R, Dreiem A, Blanc E,
Fulchignoni-Lataud MC, Le Frère Belda MA, Lecuru F, Mayi TH, Mazure
N, Favaudon V, Massaad C, et al: Hypoxia down-regulates
CCAAT/enhancer binding protein-alpha expression in breast cancer
cells. Cancer Res. 68:2158–2165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seifeddine R, Fulchignoni-Lataud MC and
Massaad-Massade L: Down-regulation of C/EBPα in breast cancer cells
by hypoxia-estrogen combination is mainly due to hypoxia.
Anticancer Res. 29:1227–1231. 2009.PubMed/NCBI
|
|
21
|
Timchenko NA, Harris TE, Wilde M, Bilyeu
TA, Burgess-Beusse BL, Finegold MJ and Darlington GJ:
CCAAT/enhancer binding protein alpha regulates p21 protein and
hepatocyte proliferation in newborn mice. Mol Cell Biol.
17:7353–7361. 1997.PubMed/NCBI
|
|
22
|
Timchenko NA, Wilde M, Nakanishi M, Smith
JR and Darlington GJ: CCAAT/enhancer-binding protein alpha (C/EBP
alpha) inhibits cell proliferation through the p21
(WAF-1/CIP-1/SDI-1) protein. Genes Dev. 10:804–815. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zaragoza K, Bégay V, Schuetz A, Heinemann
U and Leutz A: Repression of transcriptional activity of C/EBPalpha
by E2F-dimerization partner complexes. Mol Cell Biol. 30:2293–2304.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yin H, Lowery M and Glass J: In prostate
cancer C/EBPalpha promotes cell growth by the loss of interactions
with CDK2, CDK4, and E2F and by activation of AKT. Prostate.
69:1001–1016. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Muller C, Calkhoven CF, Sha X and Leutz A:
The CCAAT enhancer-binding protein alpha (C/EBPalpha) requires a
SWI/SNF complex for proliferation arrest. J Biol Chem.
279:7353–7358. 2004. View Article : Google Scholar
|
|
26
|
Zhang J, Wilkinson JE, Gonit M, Keck R,
Selman S and Ratnam M: Expression and sub-cellular localization of
the CCAAT/enhancer binding protein α in relation to postnatal
development and malignancy of the prostate. Prostate. 68:1206–1214.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumagai T, Akagi T, Desmond JC, Kawamata
N, Gery S, Imai Y, Song JH, Gui D, Said J and Koeffler HP:
Epigenetic regulation and molecular characterization of C/EBPalpha
in pancreatic cancer cells. Int J Cancer. 124:827–833. 2009.
View Article : Google Scholar
|