Introduction
Embryonic stem cell (ESC) lines are able to renew
without differentiation and exhibit pluripotency in vitro
under optimal conditions over a long period of time (1). Since ESC lines were first established
from the mouse inner cell mass (ICM) in 1981 (2), attempts to isolate human ESCs have
been successfully performed (1).
In addition, numerous studies have attempted to establish ESC lines
from other species, including sheep (3), hamster (4), mink (5), rabbit (6), pig (7), cattle (8), rat (9) and horse (10). However, to date, authentic ESCs
have only been established from the rat (11,12).
Compared to other animals, pigs are a favored animal
model for translational research regarding humans due to their
similarities in size and physiology (13). There is also great potential for
using genetically modified pigs as organ donors for
xenotransplantation and as a model for human diseases (14). Putative porcine ESC (pESC) lines
were first established in 1990 using porcine blastocysts expanded
in vivo for 7–9 days (15).
Although embryos produced in vivo are of high quality
compared to embryos produced in vitro, this approach is more
expensive and laborious. Therefore, it would be economically
desirable to use blastocysts created in vitro. However, to
date, only few putative ESC lines have been established in farm
animals from material produced in vitro (16).
The production of porcine blastocysts in
vitro was first attempted in 2000 (17). However, it has remained challenging
to establish pESCs from porcine blastocysts produced in
vitro, as there is often no obvious ICM, or only a few cells
are present in porcine embryos (16). One of the reasons for this
difficulty is an incomplete in vitro production system for
porcine embryos compared to the in vivo environment.
Therefore, numerous attempts have been made to improve the
conditions of in vitro systems to the level of what occurs
in vivo. Traditionally, M199 has been used as a common
medium for in vitro maturation (IVM) in most laboratories
(18). Funahashi et al
(19,20) found that the cytoplasmic maturation
of porcine oocytes is significantly affected by the maturation
medium. Supplementation of the maturation medium with cystein
enhances the amount of glutathione in porcine oocytes and improves
the formation of the maternal pronucleus (MPN) after sperm
penetration (21). For in
vitro culture (IVC), porcine zygote medium-5 (PZM5) had been
developed by Yoshioka et al (22) and has been used in numerous studies
(23) as a replacement for porcine
zygote medium-3 (PZM3), the traditional medium for IVC (24). However, the developmental
competence of in vitro culture systems was still below that
observed in vivo.
The aim of the present study was to examine the
effects of resveratrol (RES), granulocyte-macrophage colony
stimulating factor (GM-CSF) and β-mercaptoethanol (β-ME) on the
quality of blastocysts and the efficiency of colony derivation in
the establishment of ESCs. To obtain porcine blastocysts derived
in vitro, an in vitro fertilization (IVF) and
parthenogenetic activation (PA) were performed. The control system
used M199 media for IVM and PZM3 for IVC. By contrast, the novel
system used M199 media with 2 µM RES for IVM and PZM5 with 2
µM RES, 10 ng/ml of porcine GM-CSF (pGM-CSF) and 10
µM β-ME for IVC, while the control system had no additives.
To characterize putative pESCs from PA embryos derived by the novel
and control systems, various parameters were compared, including
typical morphology, alkaline phosphatase (AP) activity analysis,
embryoid body (EB) formation and gene expression analysis (OCT4,
SOX2, Nanog, NESTIN, BLBP, C.actin, SOX17, GATA6 and PECAM).
Materials and methods
Chemicals
All chemicals were purchased from Sigma-Aldrich
Chemical Company (St. Louis, MO, USA) unless stated otherwise.
In vitro maturation (IVM) of porcine
oocytes
Ovaries of gilts were collected from a commercial
abattoir. Porcine cumulus-oocyte complexes (COCs) with follicular
fluid were aspirated from 3- to 6-mm antral follicles using a 10-ml
syringe and an 18-gauge needle. COCs were recovered under a
stereoscopic microscope (SZ51; Olympus, Tokyo, Japan) and washed in
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid-buffered
Tyrode’s medium containing 0.05% (w/v) polyvinyl alcohol (TLH-PVA).
Subsequently, ~60–70 COCs from the control group were cultured in a
four-well dish (Nunc, Roskilde, Denmark) containing 500 µl
tissue culture medium 199 (Invitrogen Life Technologies, Carlsbad,
CA, USA) supplemented with 26 mM sodium bicarbonate, 0.91 mM sodium
pyruvate, 0.57 mM cysteine, 10 ng/ml epidermal growth factor, 0.5
IU/ml porcine luteinizing hormone, 0.5 IU/ml porcine
follicle-stimulating hormone, 10% (v/v) pFF, 75 µg/ml
penicillin-G and 50 µg/ml streptomycin at 39°C under 5%
CO2 in air. After 22 h, COCs were replaced in the same
medium without hormone supplements and then cultured for 20 h under
the same conditions. COCs from the treatment group were cultured in
the same medium with 2 µM RES. After 42 h of IVM, matured
oocytes were selected and used for production of IVF and PA
embryos. In the control system, M199 media was used for IVM, while
the novel system used M199 media with 2 µM RES for IVM.
In vitro fertilization (IVF) of porcine
oocytes
For IVF, liquid semen supplied weekly from the
Veterinary Service Laboratory (Department of Livestock Research,
Yong-in, Korea) and was maintained at 17°C for 5 days prior to use.
After IVM, a denuded oocyte was co-incubated with sperm for 20 min
at 39°C in a humidified atmosphere of 5% CO2 and 95%
air. After co-incubation, attached sperm was removed from the zona
pellucida (ZP) by pipetting. Oocytes were then washed three times
and incubated in modified Tris-buffered medium (mTBM) without sperm
for 5–6 h at 39°C in a humidified atmosphere of 5% CO2
and 95% air. Thereafter, zygotes were washed three times with
embryo culture medium and cultured in PZM3 or modified PZM5. The
control system used PZM3 for IVC, while the novel system used PZM5
with 2 µM RES, 10 ng/ml pGM-CSF and 10 µM β-ME for
IVC. The embryos with culture medium were covered with pre-warmed
mineral oil and incubated at 39°C for seven days under a humidified
atmosphere of 5% O2, 5% CO2 and 90%
N2.
Parthenogenetic activation (PA) of
oocytes
Oocytes were activated with two pulses of 120 V/mm
of DC for 60 µs in 280 mM mannitol solution containing 0.01
mM CaCl2 and 0.05 mM MgCl2. For PA, oocytes
that reached MII stage after 42 h of IVM were activated. Following
electrical activation, PA embryos were treated with 2 mM
6-dimethylaminopurine, 0.4 µg/ml demecolcine and 5
µg/ml of cytochalasin B in PZM3 for 4 h. PA embryos were
then washed three times in fresh IVC medium, transferred into
30-µl microdrops of IVC medium in mineral oil and then
cultured at 39°C in a humidified atmosphere of 5% O2, 5%
CO2 and 90% N2 for seven days. The control
system used PZM3 for IVC, while the novel system used PZM5 with 2
µM RES, 10 ng/ml pGM-CSF and 10 µM β-ME.
Embryo evaluation and total cell
count
Embryos were evaluated for cleavage under a
stereomicroscope on day two. Blastocyst formation on day seven
after PA or IVF was classified according to the degree of expansion
and hatching status: Early blastocyst (small blastocyst with a
blastocoel equal to or less than half of the embryo volume),
expanded blastocyst (large blastocyst with a blastocoel greater
than half of the embryo volume or blastocyst with a blastocoel
completely filling the embryo) and hatched blastocyst (hatching or
already hatched blastocyst). To determine the total number of
blastocysts on day seven, all blastocysts were collected, washed in
phosphate-buffered saline (PBS) containing 1% (w/v) bovine serum
albumin (BSA) and stained with 10 µg/ml of Hoechst-33342 for
5 min. After washing again in PBS-BSA, embryos were fixed in PBS
containing 4% paraformaldehyde. Then, the blastocysts were mounted
on glass slides in a drop of glycerol, covered gently with a cover
slip and observed under a fluorescence microscope (Nikon Corp.,
Tokyo, Japan). This study was approved by the committee on the
ethics committee on the ethics of animal experiments (Chungbuk
National Unviersity, Cheongju, Republic of Korea; Permit no.
CBNUA-584-13-01).
Gene expression analysis of blastocysts
and putative pESCs by reverse transcription quantitative polymerase
chain reaction (RT-qPCR)
Total RNA was extracted from blastocysts using
TRIzol reagent (Invitrogen Life Technologies), according to the
manufacturer’s instructions. Complementary DNA (cDNA) was prepared
by subjecting 0.8 µg of total RNA to reverse transcription
using Moloney murine leukemia virus (MMlV) reverse transcriptase
(Invitrogen Life Technologies) and random primers (9-mers; Takara
Bio Inc., Otsu, Japan). Then, quantitative real-time PCR was
performed on MX3000P machine (Stratagene-Agilent Technologies,
Waldbronn, Germany) with 1 µl cDNA template added to 10
µl 2X SYBR Premix Ex Taq (Takara Bio Inc.) containing
specific primers. The reactions were performed over 40 cycles, and
the cycling parameters were as follows: Denaturation at 95°C for 30
sec, annealing at 57°C for 30 sec and extension at 72°C for 30 sec.
All primer sequences are presented in Table I. The expression levels of each
target gene were quantified relative to those of GAPDH. Relative
quantification was based on a comparison of threshold cycle (Ct) at
constant fluorescence intensity. Relative mRNA expression (R) was
calculated using the equation, R=2-[ΔCt sample-ΔCt control]. To
determine a normalized arbitrary value for each gene, every
obtained value was normalized to that of GAPDH. Experiments were
repeated at least three times.
 | Table IPrimer sequences for gene expression
analysis. |
Table I
Primer sequences for gene expression
analysis.
| Gene | Primer sequences
(5′-3′) | Product size
(bp) | GenBank accession
number |
|---|
| CDX2 | F:
GGAACCTGTGCGAGTGGATG | 168 | CK_458871 |
| R:
GCTCGGCCTTTCTCCGAATG | | |
| BAX | F:
TGCCTCAGGATGCATCTACC | 199 | XM_003127290 |
| R:
AAGTAGAAAAGCGCGACCAC | | |
| BAK | F:
GCGGAAAACGCCTATGAGTA | 189 | XM_001928147 |
| R:
GCAGTGATGCAGCATGAAGT | | |
| Bcl-2 | F:
AGGGCATTCAGTGACCTGAC | 193 | NM_214285 |
| R:
CGATCCGACTCACCAATACC | | |
| Sirt1 | F:
GGACAGTTCCAGCCATCTCT | 200 | NM_001145750 |
| R:
CCTCGTACAGCTTCACAGTCA | | |
| OCT4 | F:
GCGGACAAGTATCGAGAACC | 200 | NM_001113060 |
| R:
CCTCAAAATCCTCTCGTTGC | | |
| Nanog | F:
AGCAACCAAACCTGGAACAG | 209 | NM_001129971 |
| R:
TGGGTACCGCAGTACTTTGA | | |
| SOX2 | F:
CTGCAGTACAACTCCATGACCA | 216 | NM_001123197 |
| R:
CATGCTGATCATGTCCCGTA | | |
| NESTIN | F:
GCCCACAATAGATTGGTATTT | 201 | TC295480 |
| R:
AGCATCTTTACAGCGACAGTC | | |
| BLBP | F:
TTGGTGATGTGGTTGCTGTT | 174 | TC241634 |
| R:
TCACATTTTCCACCTCCACA | | |
| C.actin | F:
CAGGTATTGCTGATCGCATGCA | 201 | TC270296 |
| R: ATTTGCGG
GGACGATGGA | | |
| PECAM | F: CATTTCCAA
AGTCAGCAGCA | 171 | TC238302 |
| R:
ATCATCATGCCTCCCTTCTG | | |
| GATA6 | F: CAGGAA ACG AAA
ACCTAAGAGCAT | 201 | TC238300 |
| R:
TTCTCGGGATTAGCGCTCTC | | |
| SOX17 | F:
CGCACGGAGTTTGAACAATA | 167 | TC248504 |
| R:
CAGACGTCGGGGTAGTTACAG | | |
| GAPDH | F:
GTCGGTTGTGGATCTGACCT | 207 | NM_001206359 |
| R:
TTGACGAAGTGGTCGTTGAG | | |
The cDNA from pESCs was amplified in a 20-µl
PCR reaction containing 10 pmol forward and reverse primers (iNtRON
Biotechnology, SungNam., Korea), 2 units Taq polymerase (iNtRON
Biotechnology), 10X PCR buffer (iNtRON Biotechnology), 5 pmol
desoxyribonucleotide triphosphate mixtures (iNtRON Biotechnology)
and template (cDNA), on a PTC-100 thermal cycler (MJ Research,
Inc., Watertown, MA, USA). The PCR reactions were performed for 30
cycles with the following conditions: Denaturation for 30 sec at
95°C, annealing for 30 sec at 57°C and extension for 30 sec at
72°C. The reaction products were analyzed on a 1.5% agarose gel
(Pronadisa, Laboratorios Conda SA, Madrid, Spain) pre-stained with
ethidium (Sigma-Aldrich Chemical Company, St. Louis, MO, USA). The
gels were scanned by a Gel Doc 2000 apparatus (Bio-Rad
Laboratories, Hercules, CA, USA) and compared to a 100-bp ladder of
DNA (iNtRON Biotechnology).
Preparation of mouse embryonic
fibroblasts as the feeder cell layer
The feeder cell layer was prepared from ICR mice
(DBL, Eumseong, Republic of Korea) at pregnancy day 13, when the
mice were sacrificed and the fetuses recovered. Fetal heads,
internal organs and legs were removed, after which the remaining
tissues were minced in PBS and centrifuged at 357 × g for 3 min at
least twice until mouse embryonic fibroblasts (MEFs) were obtained.
MEFs were cultured in Dulbecco’s modified Eagle’s medium (DMEM;
Gibco-BRL, Carlsbad., CA., USA) with 10% fetal bovine serum
(Gibco-BRL), 1% non-essential amino acids (Gibco-BRL), 1% glutamine
(Gibco-BRL), 0.1 mM β-mercaptoethanol (Gibco-BRL) and 1%
antibiotics-antimycotics (Gibco-BRL) (growth medium) at 37°C under
5% CO2 in air. MEFs were passaged 2–3 times and then
inactivated with 10 µg/ml mitomycin C (Roche, Basel,
Switzerland) for 2–2.5 h for use as culture pig blastocysts.
Inactivated MEFs were plated at a density of 5×105
cells/ml in a four-well dish coated with 0.5% gelatin in growth
medium.
Derivation and culture of putative pESC
lines
Stem cell culture medium consisted of low-glucose
DMEM/F10 (Gibco-BRL) containing 1% non-essential amino acids, 1%
glutamine, 0.1 mM β-mercaptoethanol and 1% antibiotics-antimycotics
with 4 ng/ml basic fibroblast growth factor (Invitrogen Life
Technologies) and 10% FBS. Inactive feeder cells were replaced by
stem cell culture medium 2 h prior to blastocyst plating.
Blastocysts were removed from the zona pellucida using 0.5%
protease. For plating, blastocysts were washed three times in stem
cell culture medium and plated onto the feeder cell layer. The
plating efficiency of primary cultures was determined by scoring
the number of attached colonies after 48 h. Medium was changed
daily, and cells were passaged manually every 7–10 days under phase
contrast microscopy (DMI 4000B, Leica Microsystems, Wetzlar,
Germany).
AP activity detection and in vitro
differentiation
After removal of the medium, pESCs were washed with
PBS three times and then fixed with PBS containing 4%
paraformaldehyde for 5 min at room temperature. AP activity was
then assayed with nitroblue
tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate solution (Roche).
To confirm the in vitro differentiation of putative pESCs,
cells were harvested and washed to remove feeder cells. Putative
pESCs were transferred into DMEM supplemented with 10% FBS without
bFGF for 14 days of suspension.
Statistical analysis
Statistical analyses were performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). Percentages and rates (e.g.,
cleavage, blastocyst formation and number of nuclei) were compared
by one-way analysis of variance followed by Duncan’s multiple range
test. All values are expressed as the mean ± standard error of the
mean.
Results
Effect of the novel system on blastocyst
quality
In the first set of experiments, the novel system
was evaluated regarding subsequent embryonic development following
PA and IVF. The blastocyst formation rates were significantly
greater (P<0.05) in the novel system (54.5±1.8%) compared to
those in the control system (43.4±1.2%) in PA (Table II). The total numbers of
blastocysts also tended to be greater (P<0.05) in the novel
system (55.1±2.0) compared to those in the control system
(45.6±2.0). In the IVF experiment, the blastocyst formation rate
and total number of blastocysts were significantly greater
(P<0.06) in the novel system (36.9±3.3% and 78.9±6.8,
respectively) compared to those in the control system (26.2±2.9%
and 58.5±7.2, respectively) (Table
III). Regarding the cleavage pattern, there were significantly
more 6- to 8-cell PA embryos and fewer 2- to 3-cell PA embryos in
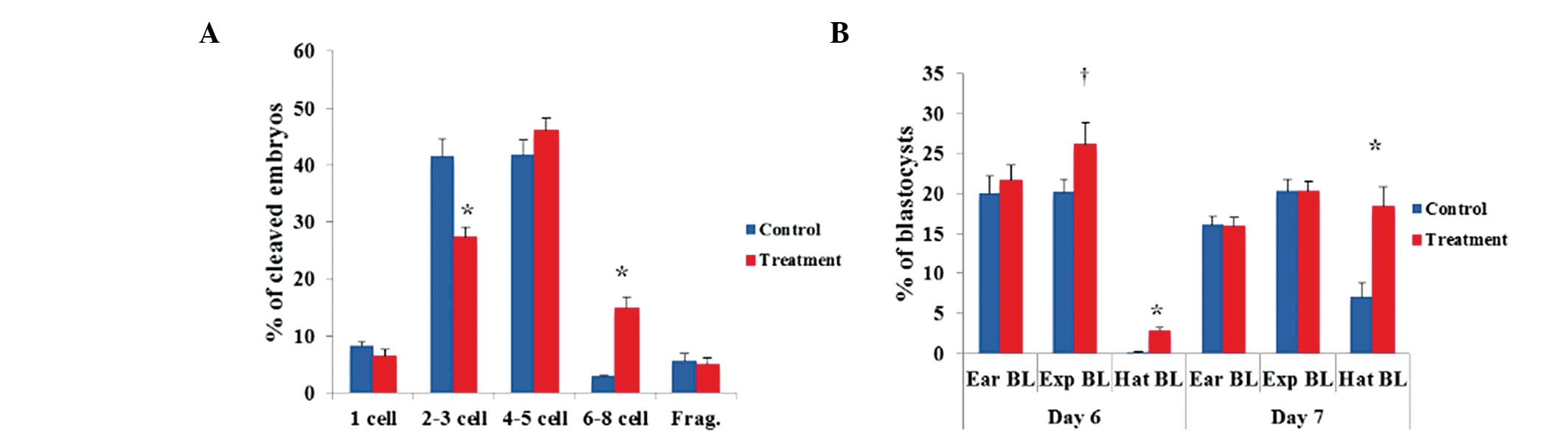
the novel system compared to the control system (Fig. 1A). However, no significant
differences were observed in the cleavage patterns of 4- to 5-cell
PA embryos. Regarding the blastocyst formation patterns on days 6
and 7, the number of hatched PA blastocysts was significantly
(P<0.05) greater in the novel system than that in the control
system (Fig. 1B). However, there
were no significant differences in the numbers of early and
expanding PA blastocysts. In the IVF experiment, there were
significantly more 6- to 8-cell IVF embryos produced by the novel
system compared to the control system (Fig. 2A). The hatched IVF blastocyst
formation rates on day 7 were significantly higher in the novel
system than those in the control system (P<0.05) (Fig. 2B).
 | Table IIEffects of the novel system on
embryonic development in porcine embryos following parthenogenetic
activation. |
Table II
Effects of the novel system on
embryonic development in porcine embryos following parthenogenetic
activation.
| Groups | Embryos cultured
(n) | Embryos developed
to
| Total cell number
in blastocysts, n (N)a |
|---|
| ≥2 cells, n
(%) | Blastocysts, n
(%) |
|---|
| Control | 649 | 562
(86.2±0.8)b | 280
(43.4±1.2)b | 45.6±2.0 (34)b |
| Novel system | 561 | 499
(88.5±0.6)c | 313
(54.5±1.8)c | 55.1±2.0
(58)c |
 | Table IIIEffects of the novel system on
embryonic development after in vitro fertilization. |
Table III
Effects of the novel system on
embryonic development after in vitro fertilization.
| Groups | Embryos cultured
(n) | Embryos developed
to
| Total cell number
in blastocysts, n (N)a |
|---|
| ≥2 cells, n
(%) | Blastocysts, n
(%) |
|---|
| Control | 375 | 267 (70.9±1.4) | 94
(26.2±2.9)b | 58.5±7.2 (11)b |
| Novel system | 237 | 172 (73.4±3.6) | 87
(36.9±3.3)c | 78.9±6.8 (18)c |
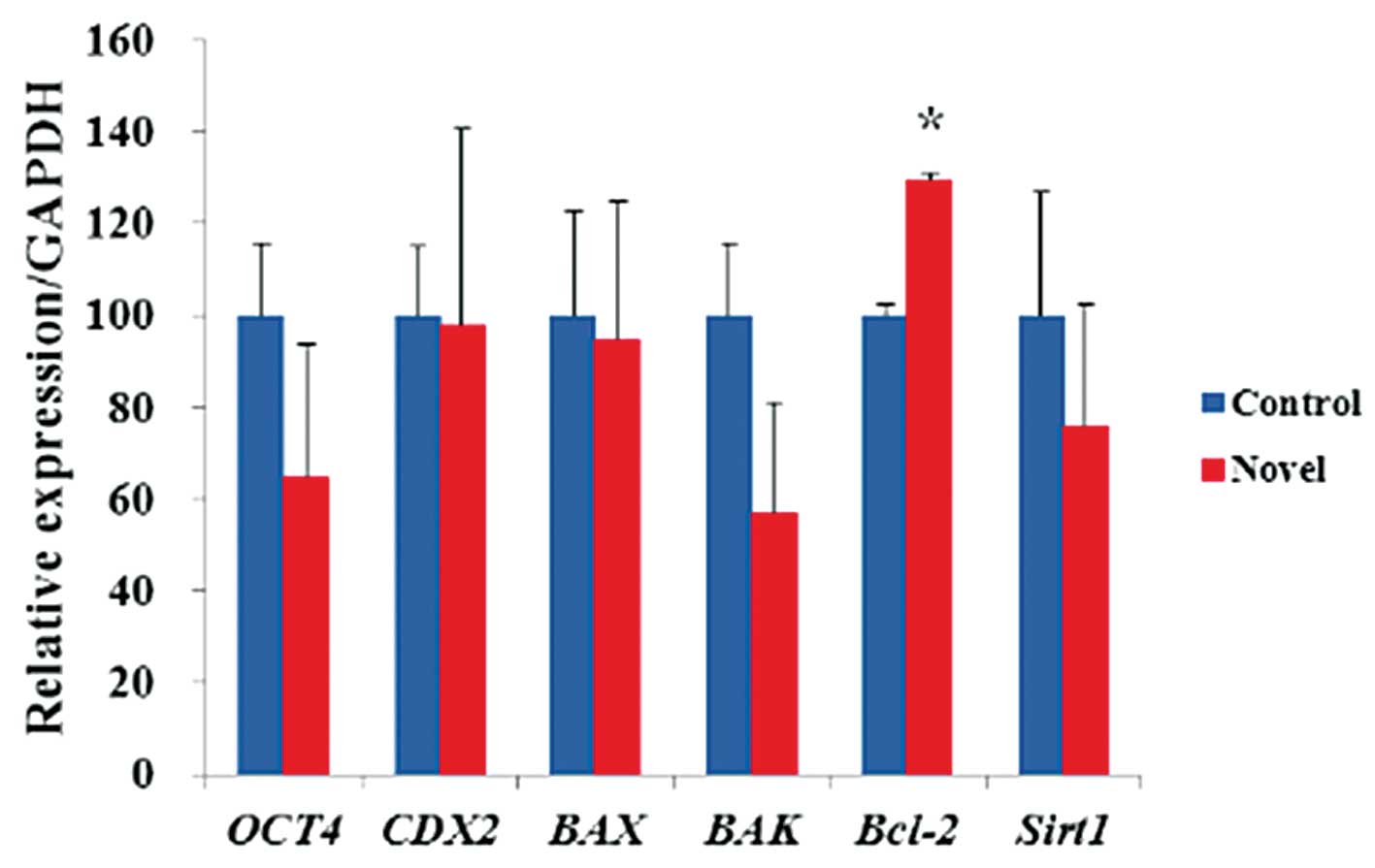
To examine the expression of pluripotency- and
apoptosis-associated genes, the mRNA expression levels of
Oct4, Cdx2, Bax, Bak, Bcl-2 and
Sirt1 in blastocysts from each system were examined
(Fig. 3). The blastocysts derived
from the novel system showed significantly (P<0.067) higher mRNA
expression levels of Bcl-2 compared to those from the
control system. No significant differences were observed for the
other transcripts.
Effect of the novel system on the
attachment and outgrowth of blastocysts
As it is difficult to isolate ICM cells from intact
porcine blastocysts produced in vitro, whole blastocysts
were plated directly onto mouse embryonic fibroblasts. Following
plating, the attachment rates were higher in blastocysts produced
by the novel system (28.8±3.9% in PA and 35.0±29.2% in IVF)
compared to those in the control system (17.2±2.4% in PA and
13.9±3.9% in IVF) (Table IV).
Furthermore, the colonization rate was higher in the novel system
(13.1±11.7% in PA and 17.8±14.2% in IVF) (Table V). Two putative pESC lines were
derived from PA blastocysts using this novel system and one
putative pESC line using the control system. The association
between blastocyst quality and attachment efficiency was also
analyzed. The numbers of expanding and hatched blastocysts
(55.4±12.9% and 78.4±7.9%, respectively) were significantly greater
than those of early blastocysts (5.0±5.0%) (Table VI).
 | Table IVEffects of the novel system on the
attachment rate of porcine blastocysts. |
Table IV
Effects of the novel system on the
attachment rate of porcine blastocysts.
| IVP | Group | Blastocysts
attached, n (%)
| Average (mean ±
SEM) |
|---|
| Trial no. 1 | Trial no. 2 | Trial no. 3 |
|---|
| PA | Control | 2/10 (20.0) | 4/21 (19.0) | 2/16 (12.5) | 17.2±2.4 |
| Novel system | 5/14 (35.7) | 6/21 (28.6) | 6/27 (22.2) | 28.8±3.9a |
| IVF | Control | 3/15 (20.0) | 1/21 (7.7) | – | 13.9±8.7 |
| Novel system | 10/18 (55.6) | 1/7 (14.3) | – | 35.0±29.2 |
 | Table VEffects of the novel system on the
colonization rate of porcine blastocysts. |
Table V
Effects of the novel system on the
colonization rate of porcine blastocysts.
| IVP | Group | No. (%) of
blastocyst colonized
| Average (mean ±
SEM) |
|---|
| Trial no. 1 | Trial no. 2 |
|---|
| PA | Control | 0/10 (0) | 1/21 (4.8) | 2.4±3.4 |
| Novel system | 4/14 (21.4) | 1/21 (4.8) | 13.1±11.7 |
| IVF | Control | 0/15 (0) | 1/7 (14.3) | 7.2±10.1 |
| Novel system | 5/18 (27.8) | 1/12 (7.7) | 17.8±14.2 |
 | Table VIAssociation between the quality and
attachment efficiency of in vitro fertilization
blastocysts. |
Table VI
Association between the quality and
attachment efficiency of in vitro fertilization
blastocysts.
| Group | Blastocysts
attached, n (%)
| Average (mean ±
SEM) |
|---|
| Trial no. 1 | Trial no. 2 | Trial no. 3 | Trial no. 4 | Trial no. 5 |
|---|
| Early
blastocysts | 0/6 (0) | 0/3 (0) | 0/5 (0) | 1/5 (20.0) | – | 5.0±5.0a |
| Expanding
blastocysts | 1/2 (50.0) | 1/2 (50.0) | 2/5 (20.0) | 4/7 (57.1) | 4/4 (100) | 55.4±12.9b |
| Hatched
blastocysts | 2/3 (66.7) | 2/3 (66.7) | 1/1 (100) | 4/5 (80.0) | – | 78.4±7.9b |
Generation and characterization of
porcine pluripotent cell lines
Putative pESC lines were derived from PA embryos
created by the novel and control systems. For characterization,
various parameters were compared between putative pESC lines from
PA embryos derived by the two systems, including typical
morphology, AP activity analysis, EB formation and gene expression
analysis (OCT4, SOX2, Nanog, NESTIN, BLBP, C.actin, SOX17,
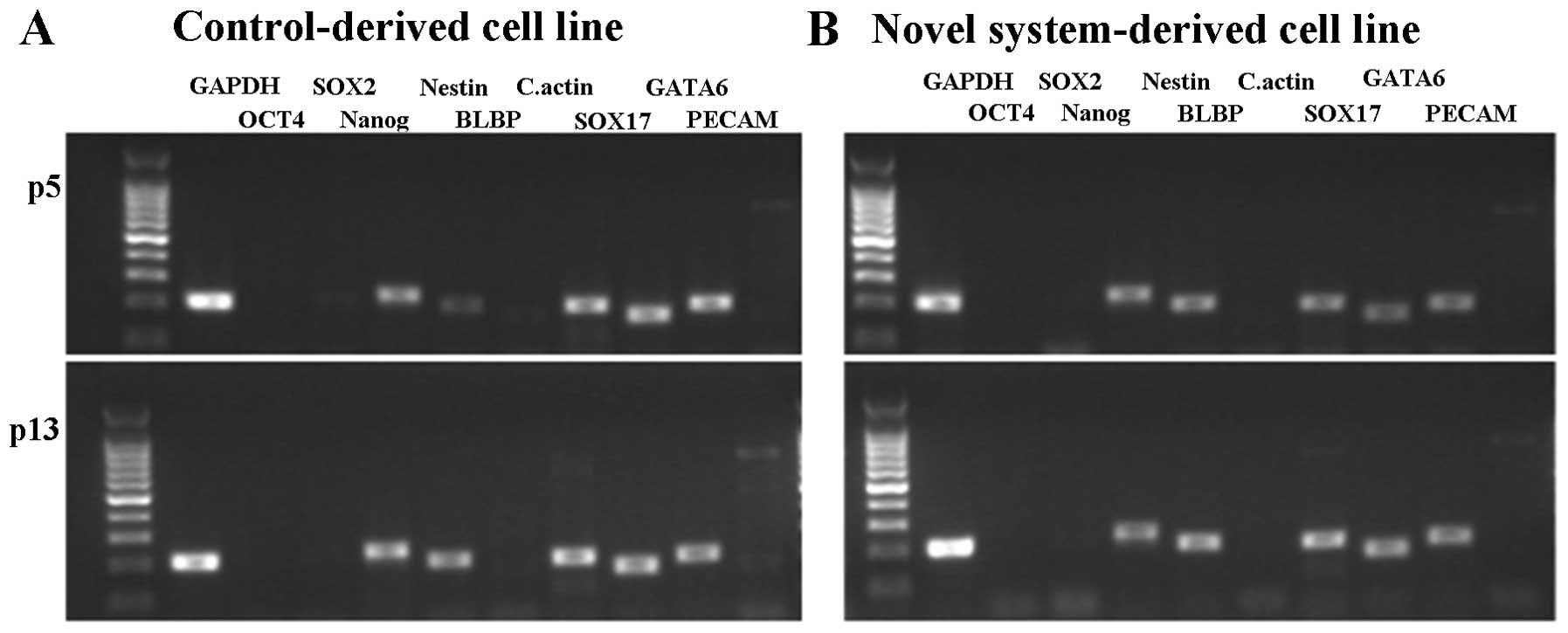
GATA6 and PECAM). All of the cell lines showed similar
primary colonies with typical morphology and AP activity, as shown
in Fig. 4A–D. The differentiation
potentials of these cell lines into EBs were subsequently assessed.
As shown in Fig. 4E and F, the two
cell lines showed the potential to undergo EB formation following
five days of culture. When EBs were cultured onto plates coated
with 0.1% gelatin, a variety of differentiated cells were observed
after 2 weeks (data not shown). The expression of Nanog was
confirmed in the two cell lines, whereas OCT4 and
SOX2 were weakly expressed or not expressed at all (Fig. 5). The three genes known to be
involved in differentiation (ectoderm markers NESTIN and
BLBP; mesoderm marker C.actin; endoderm markers
SOX17 and GATA6) were expressed in all cell
lines.
Discussion
Blastocyst quality is one of the most important
factors in implantation as well as in pregnancy as is indicated by
the presence of distinct and large ICMs in high-quality blastocysts
vs. poor-quality blastocysts (25,26).
For the establishment of ESCs from the ICM of expanded and hatched
blastocysts in an in vitro system, initial blastocyst
quality is crucial. The present study established a novel system
using a modified culture medium treated with resveratrol, pGM-CSF
and β-ME, which improved blastocyst quality and subsequently
enhanced the colonization rates of putative pESCs.
Several studies have attempted to enhance the
quality of blastocysts in an in vitro system in IVM as well
as IVC. According to a previous study by our group, treatment with
2.0 µM resveratrol during IVM improved the developmental
potentials of PA and IVF porcine embryos by increasing
intracellular glutathione levels, decreasing reactive oxygen
species (ROS) and regulating gene expression during oocyte
maturation due to the anti-oxidative effect of resveratrol
(27). Treatment with pGM-CSF in
the IVC medium also improved the developmental potential of porcine
IVF embryos (28). In addition,
the supplementation of IVC medium with β-ME was able to increase
the developmental competence of porcine PA embryos (29). β-ME was also able to increase
intracellular glutathione levels and reduce damage to the embryo
caused by ROS, thus improving embryo development. The present study
confirmed the positive combination effect of the above regents,
showing that the novel system, by adding RES during IVM and RES,
pGM-CSF, β-ME during IVC, produced more blastocysts with higher
quality in PA and IVF embryos.
Furthermore, the novel system produced embryos with
significantly higher expression levels of Bcl-2 mRNA when
compared with the control system. Bcl-2 is well known to
inhibit apoptosis in oocytes and embryos, while Bax a is
pro-apoptotic gene (30). During
the development of the early embryo, apoptosis can cause embryonic
damage, including embryo arrest and developmental failure (31). In in vitro conditions,
environmental stresses such as oxidative stress can also lead to
apoptosis (32,33). Therefore, the expression levels of
anti-apoptotic genes suggested that the DNA damage occurring under
in vitro conditions was significantly reduced in the novel
system.
In vivo-derived blastocysts have markedly
higher attachment and colony outgrowth rates than their in
vitro-derived counterparts, which show an indistinct ICM in
most of the embryos. Thus, most pESC lines have been established
from in vivo-derived embryos (34). As shown by the results of the
present study, due to the beneficial effects of the novel system,
the embryos demonstrated better attachment rates and primary colony
formation competence than control embryos. In human ESC derivation,
blastocysts with a blastocoel size equal to or larger than half of
the embryo volume had a higher rate of establishing ESC cell lines
(35). In the present study, the
expanding and hatched blastocysts showed a significantly higher
attachment rate than blastocysts at an early stage, which was
expected. As the novel system produced significant more hatched
blastocysts at day 7 compared to the control system, a have a
greater number of opportunities was available to establish putative
pESC cell lines from in vitro-derived porcine embryos.
Putative pESCs from PA embryos derived by the novel
system showed certain pluripotent characteristics, including
alkaline phosphatase activity, embryoid body formation and
Nanog expression. However, the low or absent expression of
Oct4 and Sox2 along with the expression of three germ
layer markers (Nestin, C.actin, Sox17 and
Gata6) showed that these cell lines lost a certain amount of
their pluripotency and may have been partially differentiated. In a
recent study on putative pESC derivation, Oct4 and
Nanog expression were detected (36), and Oct4, Nanog and
Sox2 expression was reported in another study (37). The pluripotent gene expression
profile may have been affected by the different culture conditions.
Therefore, it is presumed that the differences in gene expression
observed in the present study are due to the different culture
conditions and that this reflects the myriad of difficulties faced
in the derivation of porcine ESCs. Thus, further studies are
required.
In conclusion, the novel system using RES during IVM
and RES, β-ME, pGM-CSF during IVC improved the quality of
blastocysts and increased the derivation efficiency of putative
pESC cell lines from in vitro-derived porcine embryos.
Therefore, the present study suggested that this novel system in
combination with techniques for establishing ESCs provides a more
efficient approach to produce bona fide pESC as compared with
traditional systems. The method established in the present study
will help improve in vitro systems for producing porcine
embryos as well as stem cell research on domestic animals.
Acknowledgments
This study was supported, in part, by a grant from
the Cooperative Research Program for Agriculture Science &
Technology Development (no. PJ011288), the Rural Development
Administration and the National Research Foundation of Korea Grant
funded by the Korean Government (nos. NRF-2012R1A1A4A01004885,
NRF-2013R1A2A2A040 08751).
References
|
1
|
Thomson JA, Itskovitz-Eldor J, Shapiro SS,
et al: Embryonic stem cell lines derived from human blastocysts.
Science. 282:1145–1147. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Evans MJ and Kaufman MH: Establishment in
culture of pluripotential cells from mouse embryos. Nature.
292:154–156. 1981. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Handyside A, Hooper Ml, Kaufman MH and
Wilmut I: Towards the isolation of embryonal stem cell lines from
the sheep. Roux’s Arch Dev Biol. 196:185–190. 1987. View Article : Google Scholar
|
|
4
|
Doetschman T, Williams P and Maeda N:
Establishment of hamster blastocyst-derived embryonic stem (ES)
cells. Dev Biol. 127:224–227. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sukoyan MA, Vatolin SY, Golubitsa AN,
Zhelezova AI, Semenova LA and Serov OL: Embryonic stem cells
derived from morulae, inner cell mass and blastocysts of mink:
comparisons of their pluripotencies. Mol Reprod Dev. 36:148–158.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Graves KH and Moreadith RW: Derivation and
characterization of putative pluripotential embryonic stem cells
from preimplantation rabbit embryos. Mol Reprod Dev. 36:424–433.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Talbot NC, Rexroad CE Jr, Pursel VG,
Powell AM and Nel ND: Culturing the epiblast cells of the pig
blastocyst. In Vitro Cell Dev Biol Anim. 29:543–554. 1993.
View Article : Google Scholar
|
|
8
|
First NL, Sims MM, Park SP and Kent-First
MJ: Systems for production of calves from cultured bovine embryonic
cells. Reprod Fertil Dev. 6:553–562. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ouhibi N, Sullivan NF, English J, Colledge
WH, Evans MJ and Clarke NJ: Initial culture behaviour of rat
blastocysts on selected feeder cell lines. Mol Reprod Dev.
40:311–324. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saito S, Ugai H, Sawai K, et al: Isolation
of embryonic stem-like cells from equine blastocysts and their
differentiation in vitro. FEBS Lett. 531:389–396. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li P, Tong C, Mehrian-Shai R, et al:
Germline competent embryonic stem cells derived from rat
blastocysts. Cell. 135:1299–1310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Buehr M, Meek S, Blair K, et al: Capture
of authentic embryonic stem cells from rat blastocysts. Cell.
135:1287–1298. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lunney JK: Advances in swine biomedical
model genomics. Int J Biol Sci. 3:179–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rui R, Qiu Y, Hu Y and Fan B:
Establishment of porcine transgenic embryonic germ cell lines
expressing enhanced green fluorescent protein. Theriogenology.
65:713–720. 2006. View Article : Google Scholar
|
|
15
|
Evans MJ, Notarianni E, Laurie S and Moor
RM: Derivation and preliminary characterization of pluripotent cell
lines from porcine and bovine blastocysts. Theriogenology.
33:125–128. 1990. View Article : Google Scholar
|
|
16
|
Vackova I, Ungrova A and Lopes F: Putative
embryonic stem cell lines from pig embryos. J Reprod Dev.
53:1137–1149. 2007. View Article : Google Scholar
|
|
17
|
Miyoshi K, Taguchi Y, Sendai Y, Hoshi H
and Sato E: Establishment of a porcine cell line from in
vitro-produced blastocysts and transfer of the cells into
enucleated oocytes. Biol Reprod. 62:1640–1646. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Procházka R, Petlach M, Nagyová E and
Němcová L: Effect of epidermal growth factor-like peptides on pig
cumulus cell expansion, oocyte maturation and acquisition of
developmental competence in vitro: comparison with gonadotropins.
Reproduction. 141:425–435. 2011. View Article : Google Scholar
|
|
19
|
Funahashi H, Cantley TC, Stumpf TT,
Terlouw SL and Day BN: In vitro development of in vitro-matured
porcine oocytes following chemical activation or in vitro
fertilization. Biol Reprod. 50:1072–1077. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Funahashi H, Kim NH, Stumpf TT, Cantley TC
and Day BN: Presence of organic osmolytes in maturation medium
enhances cytoplasmic maturation of porcine oocytes. Biol Reprod.
54:1412–1419. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshida M, Ishigaki K, Nagai T, Chikyu M
and Pursel VG: Glutathione concentration during maturation and
after fertilization in pig oocytes: relevance to the ability of
oocytes to form male pronucleus. Biol Reprod. 49:89–94. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshioka K, Suzuki C and Onishi A: Defined
system for in vitro production of porcine embryos using a single
basic medium. J Reprod Dev. 54:208–213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagashima H, Hiruma K, Saito H, et al:
Production of live piglets following cryopreservation of embryos
derived from in vitro-matured oocytes. Biol Reprod. 76:900–905.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoshioka K, Suzuki C, Tanaka A, Anas IM
and Iwamura S: Birth of piglets derived from porcine zygotes
cultured in a chemically defined medium. Biol Reprod. 66:112–119.
2002. View Article : Google Scholar
|
|
25
|
Dokras A, Sargent IL and Barlow DH: Human
blastocyst grading: an indicator of developmental potential? Hum
Reprod. 8:2119–2127. 1993.PubMed/NCBI
|
|
26
|
Richter KS, Harris DC, Daneshmand ST and
Shapiro BS: Quantitative grading of a human blastocyst: optimal
inner cell mass size and shape. Fertil Steril. 76:1157–1167. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kwak SS, Cheong SA, Jeon Y, et al: The
effects of resveratrol on porcine oocyte in vitro maturation and
subsequent embryonic development after parthenogenetic activation
and in vitro fertilization. Theriogenology. 78:86–101. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kwak SS, Jeung SH, Biswas D, Jeon YB and
Hyun SH: Effects of porcine granulocyte-macrophage
colony-stimulating factor on porcine in vitro-fertilized embryos.
Theriogenology. 77:1186–1197. 2012. View Article : Google Scholar
|
|
29
|
Yuh HS, Yu DH, Shin MJ, et al: The effects
of various antioxidants on the development of parthenogenetic
porcine embryos. In Vitro Cell Dev Biol Anim. 46:148–154. 2010.
View Article : Google Scholar
|
|
30
|
Zinkel S, Gross A and Yang E: BCL2 family
in DNA damage and cell cycle control. Cell Death Differ.
13:1351–1359. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Betts DH and King WA: Genetic regulation
of embryo death and senescence. Theriogenology. 55:171–191. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar
|
|
33
|
Stone JR and Yang S: Hydrogen peroxide: a
signaling messenger. Antiox Redox Signal. 8:243–270. 2006.
View Article : Google Scholar
|
|
34
|
Vackova I, Novakova Z, Krylov V, et al:
Analysis of marker expression in porcine cell lines derived from
blastocysts produced in vitro and in vivo. J Reprod Dev.
57:594–603. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng EH, Chen W, Chang SY, et al:
Blastocoel volume is related to successful establishment of human
embryonic stem cell lines. Reprod Biomed Online. 17:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brevini T, Cillo F and Gandolfi F: 168
Establishment and molecular characterization of pig parthenogenetic
embryonic stem cells. Fertil Dev. 17:235. 2004. View Article : Google Scholar
|
|
37
|
Blomberg LA, Schreier LL and Talbot NC:
Expression analysis of pluripotency factors in the undifferentiated
porcine inner cell mass and epiblast during in vitro culture. Mol
Reprod Dev. 75:450–463. 2008. View Article : Google Scholar
|