Introduction
A number of promising strategies to treat cancer
have been developed over the past few decades (1–4).
Among these approaches, immunotherapy provides safe, efficient and
long-lasting effects (3). Cancer
immunotherapy uses the immune system of the patient to attack
malignant tumor cells (5). To
achieve this, three approaches have been developed: Cancer
vaccine-based immunization of the patient, therapeutic
antibody-dependent recruitment of the patient’s immune system and
cell-based activating immune cells (3,5).
Recently, cell-based immunotherapy, for example, using dendritic
cell (DC)-induced tumor antigen-specific cytotoxic T lymphocytes
(CTLs), has become an attractive approach in cancer therapy
(6). The success of DC-based
immunotherapy depends on the identification of a tumor-specific
antigen.
Survivin, a tumor-specific antigen, is highly
expressed in the majority of malignant cancer types, including
lung, pancreas, colon and prostate cancer. Survivin is crucial for
the survival and proliferation of tumor cells; however, its
expression is limited in normal tissues (7). Overexpression of survivin in tumors
is associated with poor prognosis and is correlated with resistance
to several types of anticancer therapy (8–10).
Due to these findings, survivin has become an attractive candidate
for tumor-specific therapy, and approaches that target survivin
have been developed in recent years. For example,
adenovirus-mediated transfer of small interfering (si)RNA against
survivin has been demonstrated to induce tumor cell apoptosis and
reduce tumor growth (11). The
survivin peptide-pulse approach and the transduction of survivin
into DCs have also been investigated for DC-based immunotherapy in
human urological cancer cells (12,13).
Although these approaches have achieved marked progress in
survivin-specific immunotherapy in certain types of cancer cells,
optimization of these approaches is required to achieve efficient
therapy. Furthermore, the efficiency of DC-based immunotherapy
requires examination in other types of cancer cells, such as lung
cancer cells, to expand the application of this treatment.
In the present study, an adenovirus system was used
to overexpress the survivin gene in DCs derived from peripheral
blood mononuclear cells (PBMCs). Survivin-overexpressing DCs were
treated with interleukin 4 (IL-4)/granulocyte macrophage
colony-stimulating factor (GM-CSF) and a combination of
proinflammatory cytokines. Subsequent to treatment, the
survivin-specific CTLs that were induced by survivin-overexpressing
DCs were examined.
Materials and methods
Reagents and antibodies
Reagents were obtained from the following sources:
RPMI-1640 medium, Dulbecco’s modified Eagle’s medium (DMEM) and
fetal bovine serum (FBS) were HyClone™ products (GE Healthcare,
Logan, UT, USA); GM-CSF, IL-4 and ELISA kits for tumor necrosis
factor (TNF)-α (cat. no. BMS223/4) and IL-12 (cat. no. BMS616) were
bought from eBioscience, Inc. (San Diego, CA, USA);
lipopolysaccharide (LPS) and fluorescein isothiocyanate
(FITC)-dextran (cat. no. 46945-100MG-F) were obtained from
Sigma-Aldrich (St. Louis, MO, USA); and control siRNA and survivin
siRNA were provided by Life Technologies (Carlsbad, CA, USA).
Anti-CD14 (ab45870), anti-CD16 (ab94773), anti-CD86 (ab53004),
anti-CD80 (ab64116), and major histocompatibility complex (MHC) I
(ab52992) and MHC II (ab116378) antibodies were purchased from
Abcam (Cambridge, MA, USA); and survivin (product no. 2803) and
actin (product no. 8456) antibodies were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA).
Cell culture
All DCs and DC-derived cells were cultured in
RPMI-1640 with 10% FBS. A549, 293, MDA-MB-231, HCT116 and H460
cells were purchased from the American Type Culture Collection
(Manassas, VA, USA) and cultured in DMEM with 10% FBS. All cells
were maintained in an incubator with 5% CO2 at 37°C.
Generation of DCs
DCs were obtained from the fresh heparinized
peripheral blood of healthy donors, as previously described
(12,14). Briefly, PBMCs were isolated from
the blood of the healthy volunteers by density gradient
centrifugation with Ficoll-Paque (GE Healthcare), and were then
cultured in RPMI-1640 complete medium supplied with 20 ng/ml GM-CSF
and 10 ng/ml IL-4, for seven days. Subsequently,
fluorescence-activated cell sorting (FACS) was employed to examine
the expression of CD14 and CD16 in these cells. For combined
proinflammatory cytokine stimulation, the DCs were incubated with
GM-CSF and IL-4 for two days, followed by treatment with 10 ng/ml
TNF-α (cat. no. 210-TA-005; R&D Systems, Minneapolis, MI, USA)
for another two days. The DCs were then incubated with 100 ng/ml
LPS for 48 h.
Overexpression and knockdown of the
survivin gene
The recombinant adenoviral vector pAdTrack harboring
survivin, which was packaged and purchased from Yrbio, Inc.
(Changsha, China), was transfected into the 293 cells (packaging
cells) to generate a virus. DCs were infected with control or
survivin-loading virus at multiplicity of infection (MOI) of 10,
25, 50 or 100. Subsequently, the cells were centrifuged at 1,000 ×
g at 37°C for 1 h. Survivin expression levels were examined after
48 h using western blot analysis. For survivin knockdown, A549
cells were transfected with control siRNA or survivin-specific
siRNA using Lipofectamine® 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA). The effects of survivin knockdown
were verified by western blotting.
Western blot analysis
Total cell lysates were extracted using lysis buffer
containing 2% sodium dodecyl sulfate, 10% glycerol, 10 mm Tris pH
6.8 and 100 mm dithiothreitol. The samples were boiled for 10 min
and then subjected to immunoblotting as described previously
(15).
Generation of CD40L stable line
To establish stable lines expressing CD40L in 293
cells, human CD40L gene was cloned into a p3xFLAG-CMV™-10 vector
(cat no. E4401; Sigma-Aldrich) using the polymerase chain reaction
through the BamHI restriction enzyme site. PCR reactions
were performed using Extensor Long PCR ReddyMix Master mix with
buffer 1 (Thermo Fisher Scientific, Waltham, MA, USA; cat. no.
AB-0794/B) according to the manufacturer’s instructions. For each
25 µl reaction, the CD40L gene fragment was obtained by
mixing the following reagents: 10X Extensor buffer 1 (1.25
µl); dNTP mix, 2 mM each (3.125 µl); forward primer
5′ CGCGGATCCATACAACCAAACT 3′ (0.5 µl, final: 200 nM),
reverse primer 5′ TGAGTTTGAGACTCCTAGGCGC 3′ (0.5 µl, final:
200 nM), extensor PCR enzyme mix (0.125 µl). The primers
were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China).
cDNA was prepared using TRIzol® reagent (Invitrogen Life
Technologies) according to the manufacturer’s instructions template
(5 µl, 100 ng), water (to 25 µl). PCR cycling
conditions were as follows: Initial denaturation at 94°C for 2 min,
followed by 25 cycles of denaturation at 94°C for 10 sec, annealing
at 60°C for 30 sec and extension at 68°C for 2 min, and a final
extension at 68°C for 7 min.
The 293 cells were cultured to 80% confluence
in 60-mm dishes prior to transfection with 3 µg CD40L
plasmid or empty vector for 48 h using Lipofectamine®
2000 (Invitrogen Life Technologies). Subsequently, 800 µg/ml
Geneticin (G418, cat no. A1720-1G; Sigma-Aldrich) was added to the
culture medium to select clones expressing the neo gene in
CD40L-plasmid- or empty-vector-transfected 293 cells for two weeks.
The medium was replaced and fresh G418 was added every two days
until the cells reached 80% confluence. The best 2–3 clones (as
determined by high expression of CD40L) were transferred to T-75
culture flasks (Corning Inc., Corning, NY, USA) for scale-up
production. The stable cell lines were used for the LPS stimulation
experiments, as described for the DCs.
ELISA
The supernatants of the DC cultures under the
different conditions were collected. TNF-α and IL-12 concentrations
were measured with the respective specific ELISA kits according to
standard procedures.
Generation of CTLs and CTL assay
PBMCs from fresh heparinized peripheral blood were
separated by density gradient centrifugation with Ficoll-Paque. The
cells were cultured in RPMI-1640 medium for 2 h. The adherent
fraction of PBMCs was used for the generation of DCs as described
above. The nonadherent fraction was collected for CTL generation.
DCs with or without survivin expression were seeded in 24-well
plates (2×105 cells/well) and incubated with the
corresponding nonadherent PBMCs at a ratio of 1:10 for seven days.
These cells were collected and restimulated with
survivin-expressing or control DCs. Cytotoxic activity to A549
cells was then analyzed using a CytoTox 96 Non-Radioactive
Cytotoxicity assay kit (Part# TB163, Promega Corporation, Madison,
WI, USA) according to the manufacturer’s instructions.
Flow cytometry and evaluation of antigen
capture ability
The cells were analyzed with the corresponding
antibodies using a BD FACSCalibur™ (BD Biosciences, San Jose, CA,
USA) flow cytometer according to standard procedures. The
antigen-capture ability was determined by the FITC-dextran uptake
as previously described (16). The
cells were incubated with 0.5 mg/ml FITC-dextran for 2 h at 37°C.
Subsequently, after washing with PBS three times, the cells were
analyzed by FACS. The FITC-dextran uptake was quantified as the
mean fluorescence intensity.
Data analysis and presentation
All experiments were repeated at least three times,
independently. The data were analyzed using GraphPad Prism 5
software (GraphPad Software, Inc., La Jolla, CA, USA) and are
presented as the mean ± SD. P<0.05 was considered to indicate a
statistically significant difference.
Results
Generation of survivin-overexpressing
DCs
To confirm the expression of survivin in the
different cancer cell lines, survivin protein levels were examined.
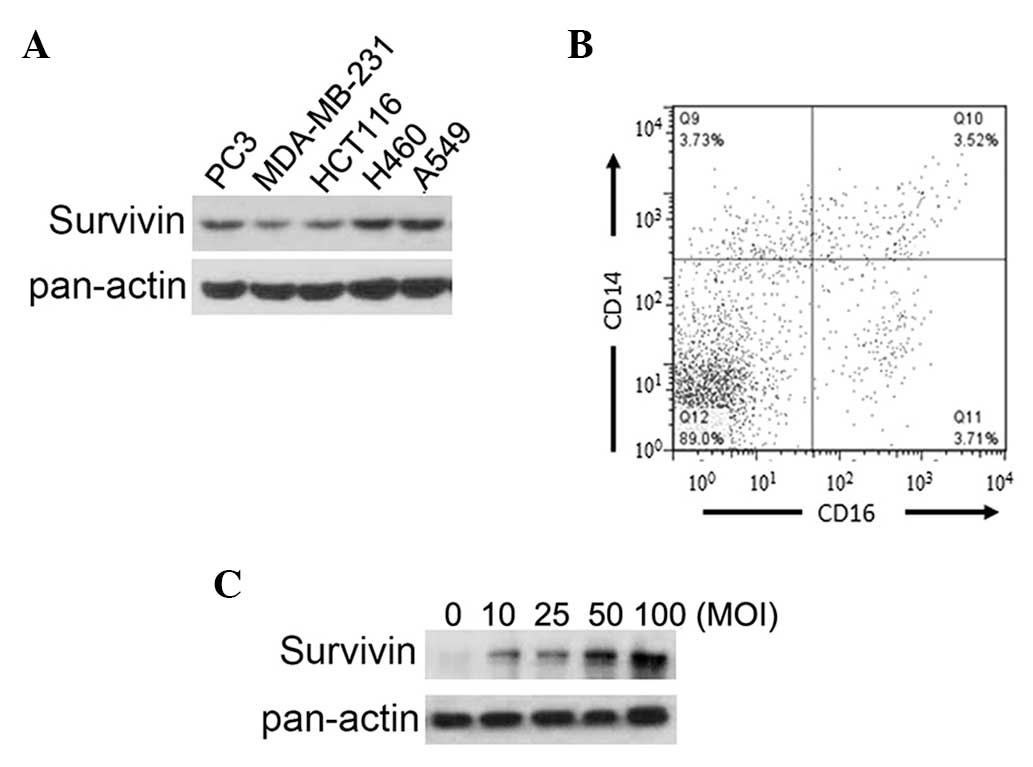
As shown in Fig. 1A, survivin was
highly expressed in all cancer cell lines, particularly in the H460
and A549 lung cancer cells (Fig.
1A). This result suggests that survivin is a potential target
for immunotherapy in this type of cancer. To develop DC-based
immunotherapy, fresh PBMCs were isolated from healthy human donors
and induced to differentiate into DCs. FACS was employed to examine
the differentiation of PBMCs into DCs. The results revealed that
the majority of cells (89%) lost the ability to express CD14 and
CD16 (Fig. 1B), suggesting these
cells were differentiated (17).
To obtain survivin-expressing DCs, the generated DCs were infected
by an adenoviral vector loaded with survivin. Survivin expression
in the DCs was verified by western blotting, which demonstrated
that survivin expression levels were increased when DCs were
infected at a higher MOI (Fig.
1C). An increased MOI elevated the survivin expression levels
in the DCs, and also decreased cell survival (data not shown). To
balance the expression levels and toxic effects of survivin
treatment, 50 MOI was selected for subsequent experiments. In
summary, these results confirm the expression of survivin in
different cancer cell lines and the generation of
survivin-overexpressing DCs.
Sequential treatment with IL-4/GM-CSF and
a combination of proinflammatory cytokines increases the
antigen-presenting and antigen-capture ability of DCs
To achieve maximized efficiency of DC-based
survivin-specific immunotherapy, the generation of
survivin-overexpressing DCs requires optimization. To achieve this
optimization, the antigen-presenting and -capture abilities of
induced DCs first require improvement. GM-CSF and IL-4 were added
to PBMC cultures, and the cultures were incubated for two days.
This was followed by incubation for another two days subsequent to
the administration of a combination of proinflammatory cytokines.
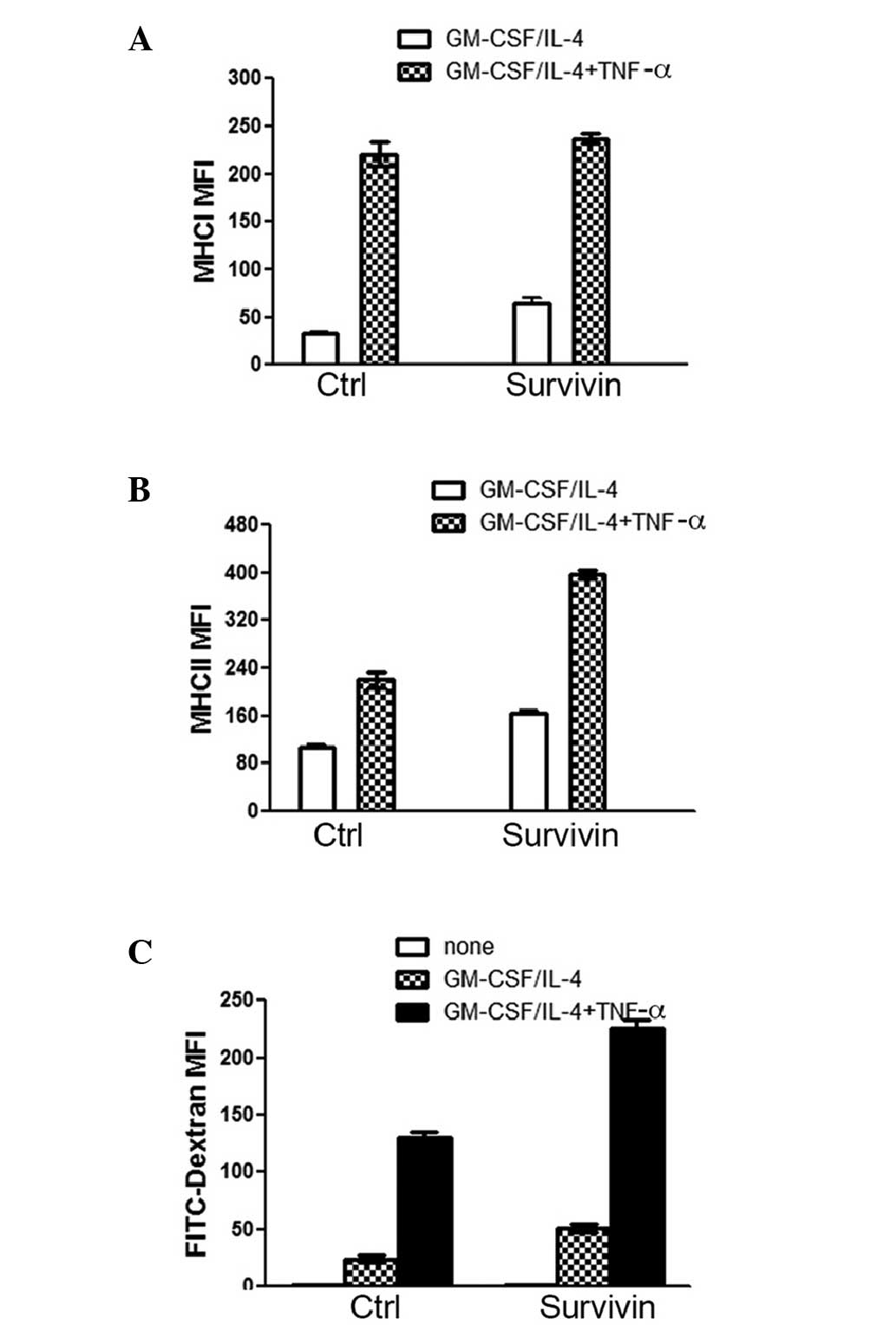
The data revealed that this strategy markedly increased the
expression levels of MHC I and MHC II in DCs with and without
survivin overexpression (Fig. 2A
and B). However, the expression levels of MHC II were increased to
a greater extent in the survivin-over-expressing DCs as compared
with the control DCs. These data indicate that the
antigen-presenting ability of DCs with survivin overexpression was
enhanced by GM-CSF/IL-4 treatment following proinflammatory
cytokine stimulation. Whether the DC antigen capture ability was
affected by the treatments was then analyzed. The FACS results
revealed that the DCs treated with both GM-CSF/IL-4 and
proinflammatory cytokine captured more FITC-dextran than DCs
treated with GM-CSF/IL-4 alone (Fig.
2C). Notably, DCs with survivin expression exhibited a more
marked ability to capture FITC-dextran subsequent to treatment than
the control DCs (Fig. 2C). In
conclusion, treatment with GM-CSF/IL-4 and proinflammatory
cytokines significantly increased the antigen-presenting ability of
the DCs. These treatments also enhanced the antigen-capture ability
of the DCs, particularly for DCs with survivin overexpression.
LPS stimulation increases cytokine
secretion by DCs and sensitizes DCs for the subsequent activation
of T cells
After the DC antigen-presenting and antigen-capture
abilities had been increased, an approach that sensitizes DCs to
signals from T cells was required. In mouse DCs, stimulation with
LPS sensitizes the cells to signals from T cells (18). Whether LPS also enhances the
function of human DCs was thus investigated in the present study.
To examine this hypothesis, LPS at different doses (0, 50, 100, 200
and 500 ng/ml) was added to DCs. The expression levels of CD80 and
CD86, which are required for the activation and survival of T cells
(19), were measured in DCs with
or without LPS treatment. The expression levels of CD80 and CD86
were increased following LPS stimulation, and reached a peak with
100 ng/ml LPS stimulation (Fig.
3). In addition, the expression levels of CD80 and CD86 were
higher in survivin-expressing DCs than in control DCs following LPS
stimulation (Fig. 4A). This result
suggests that survivin-over-expressing DCs exhibit a marked ability
to activate T cells. Survivin expression in DCs also increased the
basal levels of the TNF-α and IL-12 secretion factors (Fig. 4B). To analyze how
survivin-overexpressing DCs respond to T cell signals following
pretreatment with LPS, DCs were treated with LPS for 24 h and then
co-cultured with control 293 cells or CD40L-expressing 293 cells in
the presence of IL-4. The data revealed that the secretion of TNF-α
and IL-12 was markedly increased in LPS-primed cells when the cells
were co-cultured with CD40L-expressing 293 cells, compared with
when the cells were co-cultured with control 293 cells (Fig. 4C). Collectively, the data suggest
that LPS treatment primed survivin-overexpressing DCs, and promoted
the secretion of the TNF-α and IL-12 cytokines in these DCs.
Through this treatment, DCs became more sensitive to activated
T-cell signals.
Survivin-specific CTLs are induced
against lung cancer cells by DCs with survivin overexpression
Subsequent to optimization of the functions of
survivin-overexpressing DCs, the survivin-specific cytotoxicity
against A549 lung cancer cells induced by these treated DCs was
further examined. The DC cytotoxicity to A549 cells was measured
using the CytoTox 96 Non-Radioactive Cytotoxicity assay kit.
Various ratios of effector/target (CTL/A549) cells, as indicated in
Fig. 5, were applied to analyze
the CTL cytotoxicity to A549 cells. As compared with the control
DCs, survivin-overexpressing DCs induced a markedly higher CTL
cytotoxicity to A549 cells (Fig.
5A). To confirm the specificity of survivin-overexpressing
DC-induced CTLs, survivin knockdown was conducted using two
specific siRNAs that target the survivin gene in A549 cells
(Fig. 5B). The results revealed
that the cytotoxic effects of CTLs to A549 cells with
survivin-knockdown were clearly reduced, as compared with the
effects on control A549 cells (Fig.
5C), which demonstrates that the CTLs were survivin-specific.
In conclusion, these data revealed that optimized DCs induced
markedly more effective survivin-specific CTLs against A549 lung
cancer cells.
Discussion
DC-based immunotherapy is an effective approach in
the induction of anticancer immunity (20). Although DC-based immunotherapy has
been investigated for over 10 years, the fundamental problems
concerning optimization of this approach have not been solved
(21). In order to improve the
generation of DCs for immunotherapy, particular strategies have
been developed, including the generation of DCs from different cell
types (22), the culture of cells
with monocyte-conditioned medium (23), or the incubation of DCs with TNF-α
or LPS to stimulate maturation (24,25).
In the present study, DC treatment with a combination of factors
was employed. The cells were first treated with IL-4/GM-CSF,
followed by incubation with a combination of proinflammatory
cytokines during DC maturation. With this treatment, DCs exhibited
increased antigen-presenting and antigen-capture abilities
(Fig. 2). LPS was then used to
sensitize the DCs and enhance the response to T cells (Figs. 3 and 4). Following these optimization
treatments, DCs induced high T-lymphocyte toxicity against A549
lung cancer cells (Fig. 5).
Although the present study provides a novel method for optimizing
the generation of DC-induced CTLs against lung cancer cells ex
vivo, the in vivo efficiency requires analysis in future
studies.
The success of cancer immunotherapy also depends on
the tumor-specific antigen. Survivin, an inhibitor of the apoptosis
protein caspase, is overexpressed in various types of cancer cells,
including lung, pancreas, colon and prostate cancer cells (7), a finding consistent with the result
from the present study, which revealed survivin to be highly
expressed in the corresponding cancer cell lines (Fig. 1A). Survivin is involved in the
inhibition of apoptosis and the regulation of mitosis in cancer
cells (25). Furthermore, the
presence of high survivin expression levels in cancer cells has
been clearly associated with a poor prognosis and resistance to
anticancer agents, radiotherapy and antiandrogen therapy in
prostate cancer cells (8–10). These studies suggest that survivin
is an attractive target for cancer immunotherapy. Therefore,
several strategies that target survivin in cancer cells have been
developed. One strategy is to employ siRNA that targets survivin
(11). Other studies have used
survivin-peptide pulsed- or survivin-transduced DC-based
immunotherapy (12,13). However, the specificity of these
strategies in targeting survivin-expressing cancer cells has not
been examined. In the present study, the specificity of
survivin-overexpressing DC-induced CTLs in lung cancer cells was
analyzed. The results revealed that the CTLs specifically targeted
the A549 lung cancer cells that expressed survivin but not those
cells with survivin-knockdown (Fig.
5B and C). The results clearly indicate that the CTLs induced
by survivin-overexpressing DCs acted specifically against
survivin-expressing cancer cells, which suggests a potential
targeting therapy for cancers with survivin expression.
In conclusion, in the present study, a generation of
DCs transduced with survivin was optimized and the specificity of
survivin-specific CTLs induced by the survivin-overexpressing DCs
against lung cancer cells was verified. Therefore, this study
provides an optimization strategy for survivin-positive
malignancies and promotes the potential application of
survivin-specific CLTs in clinical trials.
Acknowledgments
The authors would like to thank all other members in
the laboratory for technical support and helpful analysis.
References
|
1
|
Lewis LD: Cancer therapeutics revisited;
novel drugs targeting cell signalling pathways, genome wide
association studies and other trials and tribulations. Br J Clin
Pharmacol. 76:317–319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sliwkowski MX and Mellman I: Antibody
therapeutics in cancer. Science. 341:1192–1198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Snook AE and Waldman SA: Advances in
cancer immunotherapy. Discov Med. 15:120–125. 2013.PubMed/NCBI
|
|
4
|
Workman P, Al-Lazikani B and Clarke PA:
Genome-based cancer therapeutics: targets, kinase drug resistance
and future strategies for precision oncology. Curr Opin Pharmacol.
13:486–496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Waldmann TA: Immunotherapy: past, present
and future. Nat Med. 9:269–277. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonaccorsi I, Pezzino G, Morandi B and
Ferlazzo G: Novel perspectives on dendritic cell-based
immunotherapy of cancer. Immunol Lett. 155:6–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodel F, Hoffmann J, Distel L, et al:
Survivin as a radioresistance factor, and prognostic and
therapeutic target for radiotherapy in rectal cancer. Cancer Res.
65:4881–4887. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su L, Wang Y, Xiao M, Lin Y and Yu L:
Up-regulation of survivin in oral squamous cell carcinoma
correlates with poor prognosis and chemoresistance. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 110:484–491. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang M, Latham DE, Delaney MA and
Chakravarti A: Survivin mediates resistance to antiandrogen therapy
in prostate cancer. Oncogene. 24:2474–2482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Uchida H, Tanaka T, Sasaki K, et al:
Adenovirus-mediated transfer of siRNA against survivin induced
apoptosis and attenuated tumor cell growth in vitro and in vivo.
Mol Ther. 10:162–171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kikkawa K, Fujii R, Kuramoto T, et al:
Dendritic cells with transduced survivin gene induce specific
cytotoxic T lymphocytes in human urologic cancer cell lines.
Urology. 74:222–228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmitz M, Diestelkoetter P, Weigle B, et
al: Generation of survivin-specific CD8+ T effector
cells by dendritic cells pulsed with protein or selected peptides.
Cancer Res. 60:4845–4849. 2000.PubMed/NCBI
|
|
14
|
Dauer M, Obermaier B, Herten J, et al:
Mature dendritic cells derived from human monocytes within 48 h: a
novel strategy for dendritic cell differentiation from blood
precursors. J Immunol. 170:4069–4076. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie Z, Chen Y, Li Z, et al: Smad6 promotes
neuronal differentiation in the intermediate zone of the dorsal
neural tube by inhibition of the Wnt/beta-catenin pathway. Proc
Natl Acad Sci USA. 108:12119–12124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dauer M, Obermaier B, Herten J, et al:
Mature dendritic cells derived from human monocytes within 48
hours: a novel strategy for dendritic cell differentiation from
blood precursors. J Immunol. 170:4069–4076. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ziegler-Heitbrock L: The CD14+ CD16+ blood
monocytes: their role in infection and inflammation. J Leukoc Biol.
81:584–592. 2007. View Article : Google Scholar
|
|
18
|
Abdi K, Singh NJ and Matzinger P:
Lipopolysaccharide-activated dendritic cells: “exhausted” or alert
and waiting? J Immunol. 188:5981–5989. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mukherjee S, Maiti PK and Nandi D: Role of
CD80, CD86, and CTLA4 on mouse CD4(+) T lymphocytes in enhancing
cell-cycle progression and survival after activation with PMA and
ionomycin. J Leukoc Biol. 72:921–931. 2002.PubMed/NCBI
|
|
20
|
O’Neill DW, Adams S and Bhardwaj N:
Manipulating dendritic cell biology for the active immunotherapy of
cancer. Blood. 104:2235–2246. 2004. View Article : Google Scholar
|
|
21
|
Zhong H, Shurin MR and Han B: Optimizing
dendritic cell-based immunotherapy for cancer. Expert Rev Vaccines.
6:333–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shurin MR: Preparation of human dendritic
cells for tumor vaccination. Method Mol Biol. 215:437–462.
2003.
|
|
23
|
Jeras M, Bergant M and Repnik U: In vitro
preparation and functional assessment of human monocyte-derived
dendritic cells-potential antigen-specific modulators of in vivo
immune responses. Transpl Immunol. 14:231–244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McIlroy D and Gregoire M: Optimizing
dendritic cell-based anticancer immunotherapy: maturation state
does have clinical impact. Cancer Immunol Immunother. 52:583–591.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li F, Ambrosini G, Chu EY, et al: Control
of apoptosis and mitotic spindle checkpoint by survivin. Nature.
396:580–584. 1998. View
Article : Google Scholar : PubMed/NCBI
|