Introduction
With the increased use of electronic appliances in
daily life and in industrial applications, the electromagnetic
radiation from electronic appliances has become a major source of
environmental pollution (1). In
addition, the use of the electromagnetic pulse bomb (EPB) has
raised health concerns, including the health protection of
employees working with EPB and electromagnetic pollution in areas
exposed to EPB (2). Pulsed
electromagnetic waves (PEMW) are nanosecond and sub-nanosecond
high-voltage pulses (3). It has
been observed that PEMW have various effects on biological systems
(4–12). The effects of PEMW on biological
systems are determined by the amplitude or field strength and the
pulse width and the effects of PEMW on cells are nonlinear,
transient and unstable (13). The
endocrine system is one of the most EMW-sensitive systems (5,6,12,14,15)
and, as one of the most important endocrine organs, the pituitary
gland is essential for physiological functions in humans. However,
the effect of PEMW on the pituitary gland remains to be
elucidated.
The pituitary gland is important in the growth,
development and normal physiological functions of the body due to
its secretion of several hormones (16). There are five types of endocrine
cell, including somatotrophs, lactotrophs, corticotrophs,
gonadotrophs and thyrotrophs, which secrete seven hormones
regulating their targets, the thyroid gland, adrenal glands and
gonads (16). Therefore, damage to
the pituitary can affect the normal functions of the body, thus
leading to pathological abnormalities. However, morphological
changes, particularly ultrastructural changes, in the pituitary
following PEMW exposure remain to be elucidated.
In the present study, the ultrastructural changes
and the expression of HSP70 was investigated in the adenohypophysis
(the anterior pituitary) from rats receiving 1×104,
1×105 and 3×105 pulses of PEMW with a field
strength of 100 kV/m.
Materials and methods
Animals and irradiation
All animal experimental procedures were approved by
the Committee for Animal Experiments at The Fourth Military Medical
University (Xi’an, China). Six-week-old male Sprague Dawley rats
(weighing 200±20 g) were used in the present study. All animals
were obtained from the Animal Center at the Fourth Military Medical
University. The rats were housed at 20–25°C and 40–60% relative
humidity and were maintained with food and water ad libitum
for 1 week prior to PEMW exposure.
The rats were randomly divided into four groups:
PEMW sham exposure group and groups receiving 1×104,
1×105 and 3×105 pulses of PEMW exposure. Each
group contained 24 rats. The 24 rats in each group were sacrificed
12, 24, 48 and 96 h (n=6 in each) after PEMW exposure. The rats
were placed into a plexiglass cage (Wuxi Jiarunfeng Science and
Technology Co., Ltd, Jiangsu, China), where the rats were
comfortably housed. The exposure cage was placed seven meters from
the radiation source. Whole body radiation of 1×104,
1×105 and 3×105 pulses of PEMW were delivered
with a field strength of 100 kV/m. For the PEMW sham exposure
group, the rats were treated under the same conditions, but without
PEMF exposure.
Tissue preparation
The rats were anesthetized with 1% sodium
pentobarbital (50 mg/kg; Sigma-Aldrich, Shanghai, China) 12, 24, 48
and 96 h after PEMW exposure and were transcardially perfused with
ice-cold 4% paraformaldehyde for 1 h. For electron microscopy, the
pituitary tissues were excised and cut into 1 mm3
blocks. The blocks were fixed with 3% glutaraldehyde and post-fixed
with 1% osmium tetroxide, followed by washing in 0.1 mol/l
phosphate-buffered saline (PBS; pH 7.6). The blocks were dehydrated
using graded concentrations of acetone, filtered with acetone and
embedding medium (Sigma-Aldrich) and then embedded in epoxy resin.
Ultrathin sections (70 nm) were obtained using a Leica Ultracut UCT
microtome (Leica, Mannheim, Germany). The sections were viewed
using a JEOL JEM 1400 transmission electron microscope equipped
with a Gatan ORIUS™ TEM CCD camera (Nippon Gatan, Tokyo, Japan).
For immunocytochemistry, the pituitary tissues were excised and
soaked in 20% sucrose in PBS at 4°C overnight. Sections (15
μm) were obtained using a Leica CM3050S cryostat (Leica) and
were stored at −20°C for subsequent immunocytochemical
analysis.
Immunocytochemistry
Sections (15 μm) were washed in 0.1 M PBS and
treated with 0.3% hydrogen peroxide in 100% methanol for 30 min at
room temperature, followed by washing with PBS. The sections were
blocked using 5% bovine serum albumin for 20 min at room
temperature and then incubated with primary antibodies against
HSP70 (rabbit anti-rat antibodies; 1:200; Abcam, Shanghai, China)
overnight at 4°C. The primary antibody was then washed three times
using PBS for 5 min and biotinalyted goat anti-rabbit
immunoglobulin G (Vector Laboratories, Inc., Burlingame, CA, USA)
was applied for 20 min at room temperature and was then reacted
with horseradish peroxidase conjugated streptavidin (Vector
Laboratories, Inc.) for 2 h. The sections were then developed using
diaminobenzidine (Sigma-Aldrich) and were mounted onto gelatinized
glass slides, which were dehydrated using graded ethanol solutions
prior to mounting under coverslips. Sections, in which the primary
antibodies were omitted, were used as negative controls.
Five sections were selected from each
rat
The distal section of the pituitary gland was
divided into four regions (Fig.
1), as previously reported (17). Since region A, adjacent to the
neurohypophysis, was easily located under a light microscope, three
fields were randomly selected, without overlaps, in region A at low
magnification (×10) for each section. At high magnification (×40),
the immunopositive cells in region A were counted and the
percentage of positive cells and intensity of immunoreactivity were
analyzed using Leica Quantimet quantitative analysis software
(570C; Leica).
Statistical analysis
All statistical analyses were performed using SPSS
11.0 (SPSS, Inc., Chicago, IL, USA). The values are expressed as
the mean± standard deviation. One-way analysis of variance was used
to compare the differences among the groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Ultrastructural changes induced by
PEMW
PEMW did not induce any clear morphological changes
in the pituitary cells, stromal cells or their surrounding tissues
when examined under a light microscope. Therefore, electron
microscopy was used in the present study to investigate the
ultrastructure of pituitary glands in rats treated with PEMW. Based
on the characteristic cellular morphology and the distinguished
size and morphology of secretory granules in the adenohypophysis,
the somatotrophs, lactotrophs, corticotrophs, gonadotrophs and
thyrotrophs were distinguished from each other using electron
microscopy. In the PEMW sham exposure group, no changes in the
intracellular organelles or secretory granules in these cells were
identified in the adenohypophysis. By contrast, PEMW exposure
induced ultrastructural damage in the adenohypophysis from rats
following 3×105 pulse PEMW exposure (Fig. 2). After 12 h exposure,
intercellular gaps between the pituitary cells and their
surrounding tissues were increased and capillary congestion was
evident. Swollen mitochondria with enlarged and broken cristae were
found in the somatotrophs (Fig.
2A) and vacuolated mitochondria with broken cristae were
frequently observed in the corticotrophs (Fig. 2B). Similar ultrastructural
abnormalities in the mitochondria, were also observed in other
types of pituitary cell, although to a lesser extent.
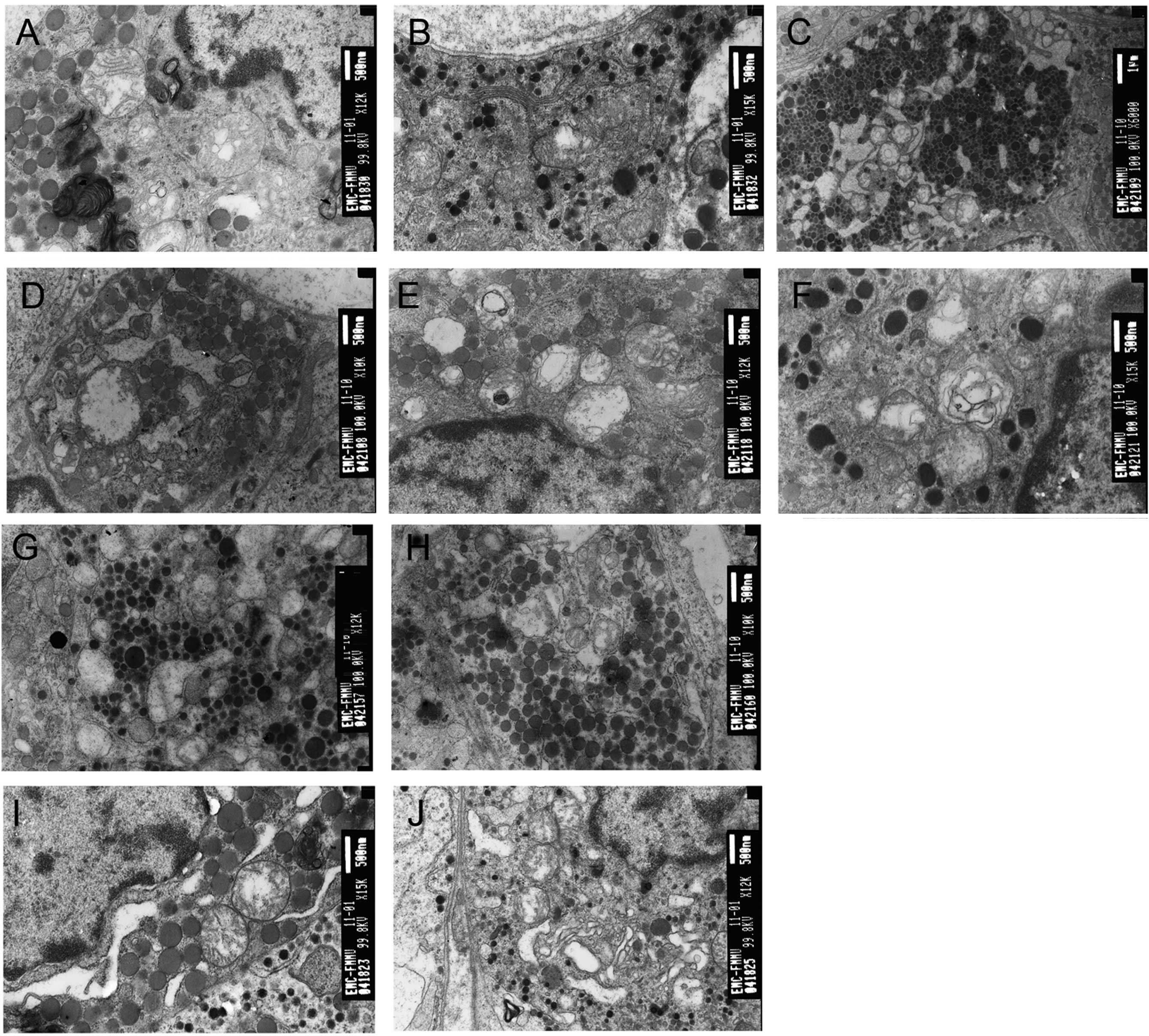 | Figure 2Pulsed electromagnetic wave (PEMW)
induces ultrastructural damage in the adenohypophysis. (A) Electron
micrograph of a somatotroph 12 h after 3×105 pulses of
PEMW exposure, exhibiting swollen mitochondria with enlarged and
broken cristae, vacuolated mitochondria, myelin-like structure in
the mitochondria and chromatin aggregation at the periphery of the
nuclear membrane (magnification, ×12,000). (B) Electron micrograph
of a corticotroph 12 h after 3×105 pulses of PEMW
exposure exhibiting vacuolated mitochondria with enlarged and
broken cristae (magnification, ×15,000). (C) Electron micrograph of
a lactotroph 24 h after 3×105 pulses of PEMW exposure
exhibiting severe vacuolation in the mitochondria with broken and
fused membrane and the enlargement of ER (magnification, ×6,000).
(D) Electron micrograph of a gonadotroph 24 h after
3×105 pulses of PEMW exposure exhibiting cell edema,
vacuolation in the mitochondria with a dissolved membrane and
enlargement of the ER (magnification, ×10,000). (E) Electron
micrograph of a somatotroph 48 h after 3×105 pulses of
PEMW exposure exhibiting myelin-like structure in the mitochondria,
broken cristae in certain mitochondria and bulky heterochromatin
accumulated close to the nuclear membrane (magnification, ×12,000).
(F) Electron micrograph of a lactotroph 48 h after 3×105
pulses of PEMW exposure exhibiting myelin-like structure in the
mitochondria (magnification, ×15,000). (G) Electron micrograph of a
lactotroph 24 h after 1×104 pulses of PEMW exposure
exhibiting complete depletion of secretory granules, partial
depletion of secretory granules and enlargement of ER
(magnification, ×12,000). (H) Electron micrograph of a somatotroph
24 h after 1×104 pulses of PEMW exposure exhibiting
enlargement of ER and vacuolation in the mitochondria
(magnification, ×10,000). (I) Electron micrograph of a somatotroph
24 h after 1×105 pulses of PEMW exposure exhibiting
enlargement of ER, vacuolation in the mitochondria and chromatin
aggregation at the periphery of the nuclear membrane
(magnification, ×15,000). (J) Electron micrograph of a thyrotroph
24 h after 1×105 pulses of PEMW exposure exhibiting
enlargement of ER, increased Golgi vesicles and vacuolation in the
mitochondria (×12,000). ER, endoplasmic reticulum. |
After 24 h PEMW exposure, there was frequent
depletion of secretory granules in the cells. Vacuolated
mitochondria with abnormal membranous structure and enlarged rough
endoplasmic reticulum (RER) were observed in the lactotrophs
(Fig. 2C). Swollen mitochondria,
vacuolated mitochondria with a myelin-like structure, which had the
appearance of a concentric circle, were also found in mitochondria
in the gonadotrophs (Fig. 2D). In
addition, enlarged Golgi complexes were present in the somatotrophs
and vacuolated mitochondria with broken cristae were observed in
the corticotrophs and thyrotrophs.
After 48 h PEMW exposure, marked infiltration of
inflammatory cells and severe cell swelling were identified in the
pituitary tissue. Bulky heterochromatins were accumulated close to
the nuclear membrane in the somatotrophs (Fig. 2E) and myelin-like structures in the
mitochondria and enlarged Golgi complexes were observed in the
lactotrophs (Fig. 2F). In
addition, enlarged and dilated RER were present in the
gonadotrophs. Cell degeneration, pyknosis and increased
intracellular gaps were also found in the thyrotrophs and more
vacuolated mitochondria were found in the corticotrophs.
After 96 h PEMW exposure, interstitial edema and
vascular congestion remained, however the morphology of the cells
in the adenohypophysis had recovered almost to normal.
The present study also investigated ultrastructural
changes in the adenohypophysis 24 h after 1×104,
1×105 and 3×105 pulses of PEMW. After 24 h
following the 1×104 pulses of PEMW exposure, mild injury
to the vascular endothelia and platelet aggregation was observed. A
depletion in secretory granules and enlarged RER were observed in a
number of lactotrophs (Fig. 2G)
and mild enlargement of RER and mitochondrial vacuolation had
occurred in the somatotrophs (Fig.
2H). After 24 h following the 1×105 pulses of PEMW
exposure, mitochondrial swelling and vacuolation was more prevalent
in these pituitary cells. Clearly enlarged RER and vacuolated
mitochondria were observed in the somatotrophs and thyrotrophs.
Accumulation of heterochromatins close to the nuclear membrane was
observed in the somatotrophs (Fig.
2I) and increased Golgi vesicles were identified in the
thyrotrophs (Fig. 2J). In
addition, enlarged RER and vacuolated mitochondria were also found
in the somatotrophs and thyrotrophs. After 24 h following the
3×105 pulses of PEMW exposure, more severe
ultrastructural damage was observed in the pituitary cells. Marked
inflammatory cell infiltration and severe cell swelling were
identified and mitochondrial swelling and vacuolation was observed
throughout the cells. Disruption of the mitochondrial membrane was
observed in the lactotrophs (Fig.
2C) and in the gonadotrophs (Fig.
2D).
Expression of HSP70 following PEMW
exposure
The present study further investigated the
expression of the HSP70 protein 12, 24, 48 and 96 h after
3×105 pulses of PEMW exposure using immunocytochemistry.
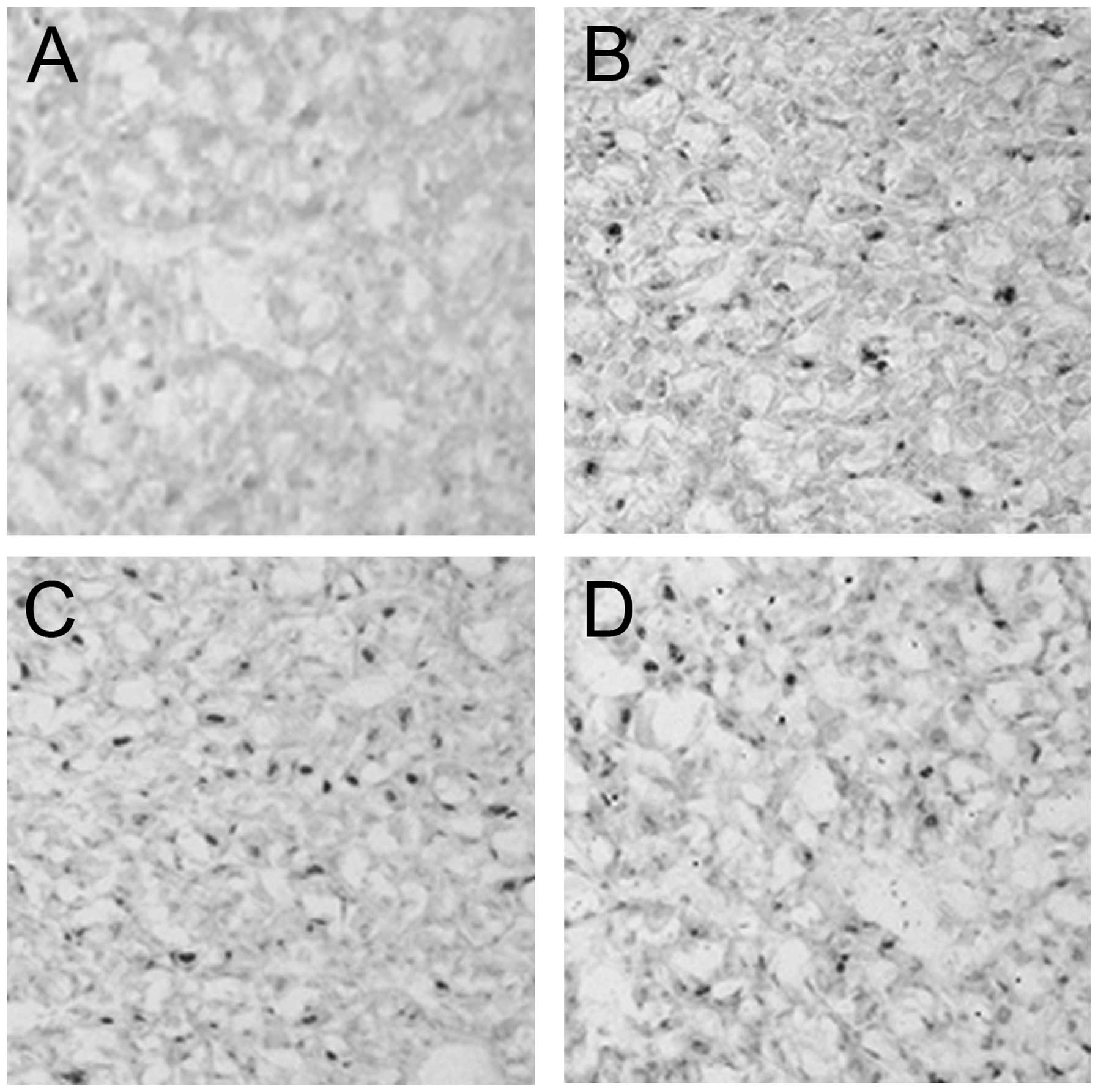
In the sham exposure group, HSP70 immunopositive cells were
diffusely distributed with a weak brownish staining in the
cytoplasm and little nuclear immunoreactivity was observed
(Fig. 3A). After12 h following the
3×105 pulses of PEMW exposure, the percentage of HSP70
immunopositive cells was significantly increased (P<0.01) and
the intensity of the immunoreactivity was increased significantly
(P<0.05) compared with the PEMW sham exposure group (Table I). The majority of the
immunoreactivity was observed in the nuclei, which exhibited dark
staining (Fig. 3B). The nuclear
immunoreactivity started to decrease 24 h after 3×105
pulses of PEMW exposure and remained at a significantly higher
level 48 h after 3×105 pulses of PEMW exposure (Fig. 3C). The percentage of HSP70
immunopositive cells were significantly higher and the intensity of
the immunoreactivity was increased significantly 24 and 48 h after
3×105 pulses of PEMW exposure compared with the sham
exposure group (Table I). After 96
h following 3×105 pulses of PEMW exposure, the
percentage of immunopositive cells decreased significantly, however
it remained higher compared with the that in the sham control group
(Fig. 3D). The percentage of HSP70
immunopositive cells were significantly lower 24, 48 and 96 h after
3×105 pulses of PEMW exposure compared with that
observed 12 h after 3×105 pulses of PEMW exposure
(P<0.01; Table I).
 | Table IHeat shock protein 70 immunoreactivity
following 3×105 pulses of pulsed electromagnetic wave
exposure. |
Table I
Heat shock protein 70 immunoreactivity
following 3×105 pulses of pulsed electromagnetic wave
exposure.
| Time (h) following
exposure | Immunopositive cells
(%) | Immunoreactivity
intensity |
|---|
| 0 | 0.02±0.00 | 164.67±1.42 |
| 12 | 0.49±0.15a | 184.50±21.74a |
| 24 |
0.16±0.08ab | 175.90±5.76 |
| 48 |
0.14±0.04ab | 169.77±4.01 |
| 96 | 0.08±0.04b | 173.57±1.72 |
Discussion
In the present study, ultrastructural changes in the
adenohypophysis from rats receiving 1×104,
1×105 and 3×105 pulses of PEMW, were
investigated using transmission electron microscopy. The results
revealed that PEMW induced adenohypophysal damage in the
mitochondria, endoplasmic reticulum (ER) and chromatin. PEMW with a
higher number of pulses produced more severe damage in the
adenohypophysis compared with the PEWM with a lower pulse. In
addition, the expression of HSP70 was increased significantly
following PEMW exposure and reached its peak 12 h following PEMW
exposure. Since the HSP70 protein is upregulated in response to
stress stimuli (18), the findings
of the present study suggests that PEMW-induced cellular damage may
be associated with the cellular response to the stress induced by
PEMW.
In the present study, the effects of PEMW on
ultrastructural changes in the pituitary were investigated 12, 24,
48 and 96 h after PEMW exposure. The results revealed that cellular
damage, including cellular edema and mitochondrial vacuolation
occurred as early as 12 h after PEMW exposure. More severe cellular
damages, including the depletion of secretory granules, ER and
Golgi complex enlargement, accumulation of heterochromatins close
to the nuclear membrane and myelin-like structure of mitochondria,
occurred 24 h after PEMW exposure, suggesting that PEMW induced
cell degeneration and necrosis. The PEMW-induced cell degeneration
and necrosis was aggravated 48 h after PEMW exposure and an
increase in intercellular gaps also occurred. In addition, the
PEMW-induced cellular damage increased as the pulse time of the
PEMW increased, particularly in the somatotrophs and lactotrophs.
This finding was consistent with those of previous studies that
showed that EMP exposure induced cellular damage in the pituitary
(5,6). However, the degree of cellular damage
differed among these studies, possibly due to differences in the
pulse time and field strength used, suggesting that PEMW-induced
cellular damage may be associated with the pulse time and the field
strength of PEMW.
The occurrence and development of cellular damage
can be caused by several mechanisms, including deficiency in
intracellular adenosine triphosphate (ATP), damage of the cell
membrane, increase in free intracellular calcium and irreversible
mitochondrial damage (19).
Several studies using atomic force microscopy have demonstrated
that electromagnetic pulses induce cell membrane perforation in
cultured pituitary cells, hypothalamic neurons and hippocampal
neurons (20–22). In addition, it has been observed
that high power microwaves induce an increase in free intracellular
calcium and a decrease in the mitochondrial membrane potential in
hypothalamic neurons 6 h after exposure (9). Therefore, the PEMW-induced
ultrastructural changes that were observed in the adenohypophysis
in the present study may have been caused by cell membrane
perforation, an increase in free intracellular calcium and a
decrease in mitochondrial membrane potential, thus leading to a
change in the selectivity and permeability of the mitochondrial
membrane. Mitochondria are the sites of ATP synthesis in the cell
and mitochondrial dysfunction results in a deficiency in
intracellular ATP, which can lead to the malfunction of ion pumps
in the cell membrane, cellular edema, enlargement of ER and
eventual cell death (23).
The upregulation of HSP is a main feature of the
stress response, which is conserved in prokaryotes and eukaryotes,
as a protection from harmful stimuli (18). It has been demonstrated that pulsed
microwaves, a harmful environmental stimuli, induce a stress
response in rats and increase the synthesis of HSP in
Escherichia coli cells (24,25).
HSP70, a member of the HSP superfamily, is abundantly expressed in
the majority of species and is markedly induced by cellular stress
(26). HSP70 is important in the
maintenance of protein stability, improvement of cellular tolerance
to stress stimuli and maintenance of normal physiological functions
in the cell (27,28). There are two isoforms of the HSP70
protein, the constitutive isoform and the stress-inducible isoform
(29). The constitutive isoform of
HSP70 is expressed mainly in the cytoplasm in the absence of stress
and can be moderately upregulated during stress. By contrast, the
stress-inducible isoform of HSP70 is generally not expressed in
unstressed cells and is highly inducible under conditions of
stress. In addition, the HSP70 protein translocates from the
cytoplasm to the nucleus in response to stress and translocates
from the nucleus to the cytoplasm in the absence of stress, in a
temperature independent manner (30).
The present study also examined the effects of PEMW
on the expression of HSP70 in the pituitary 12, 24, 48 and 96 h
after PEMW exposure. In the PEMW sham exposure group, weak brownish
staining was observed in the cytoplasm and minimal nuclear
immunoreactivity was observed in the HSP70 immunopositive cells,
suggesting that the constitutive isoform of HSP70 is expressed
mainly in the absence of stress in the adenohypophysis. By
contrast, 12 h after 3×105 pulses of PEMW exposure, the
percentage of HSP70 immunopositive cells were significantly
increased and the immunoreactivity, with dark brown staining, was
predominantly observed in the nucleus, suggesting that the
stress-inducible isoform of HSP70 was expressed mainly in the
pituitary following PEMW exposure. The nuclear immunoreactivity
began to decline 24 h after PEMW exposure, suggesting that the
synthesis of the inducible isoform of HSP70 had decreased. The
expression of HSP70 remained higher compared with the control 96 h
after PEMW exposure, although to a lesser degree. The PEMW-induced
increase in the expression of HSP70 occurred earlier than the
PEMW-induced ultrastructural changes in the pituitary, suggesting
that PEMW-induced cellular damage may be associated with the
cellular stress induced by PEMW. It has been observed that
radiofrequency radiation and pulsed microwaves induce mitochondrial
DNA damage and cell injury through oxidative stress (31,32).
Therefore, the HSP70-mediated stress response may have contributed
to the PEMW-induced ultrastructural damage in the pituitary.
In conclusion, the present study demonstrated that
PEMW induced ultrastructural damage and the overexpression of HSP70
in the nuclei in the adenohypophysis. Somatotrophs and lactotrophs
were the most sensitive cells to PEMW exposure and the mitochondria
and RER were observed as the organelles most sensitive to the PEMW
exposure. The PEMW-induced adenohypophyseal damage was
time-dependent and was determined by the pulse of PEMW. HSP70 may
have contributed to the PEMW-induced adenohypophyseal damage.
Acknowledgments
This study was supported by the Program for
Changjiang Scholars and Innovative Research Team in University and
the Research Fund of National Natural Science Foundation (nos.
30570447, 30901215 and 81172636, respectively).
References
|
1
|
Dämvik M and Johansson O: Health risk
assessment of electromagnetic fields: a conflict between the
precautionary principle and environmental medicine methodology. Rev
Environ Health. 25:325–333. 2010. View Article : Google Scholar
|
|
2
|
Schwartau W: Information Warfare –
Cyberterroism: Protecting Your Personal Security in the Electronic
Age. Thunder’s Mouth Press; New York, NY: 1996, Available from:
http://www.infowar.com.
|
|
3
|
Minamitani Y, Ohe Y, Ueno T and
Higashiyama Y: Output characteristics of high power pulsed
electromagnetic wave generator for medical applications using water
capacitor and water gap switch. Pulsed Power Conference, 2007 16th
IEEE International. 2:1240–1243. 2007.
|
|
4
|
Ding GR, Li KC, Wang XW, Zhou YC, Qiu LB,
Tan J, Xu SL and Guo GZ: Effect of electromagnetic pulse exposure
on brain micro vascular permeability in rats. Biomed Environ Sci.
22:265–268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang H, Zeng G, Ren D, Jin C, Huang X and
Guo Z: Study on ultrastructural changes of the pituitary gland in
rats exposed to pulsed electromagnetic fields (PEMF). Chin J Radiol
Med Prot. 25:175–177. 2005.
|
|
6
|
Fang HH, Zeng GY, Nie Q, Kang JB, Ren DQ,
Zhou JX and Li YM: Effects on structure and secretion of pituitary
gland in rats after electromagnetic pulse exposure. Zhonghua Yi Xue
Za Zhi. 90:3231–3234. 2010.In Chinese.
|
|
7
|
Li BF, Guo GZ, Ren DQ, Jing L and Zhang
RB: Electromagnetic pulses induce fluctuations in blood pressure in
rats. Int J Radiat Biol. 83:421–429. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li KC, Ma SR, Ding GR, Guo Y and Guo GZ:
Effects of electromagnetic pulse on bone metabolism of mice in
vivo. Biomed Environ Sci. 22:518–521. 2009. View Article : Google Scholar
|
|
9
|
Meng L, Peng RY, Gao YB, Wang SM, Ma JJ,
Hu WH, Wang DW, Su ZT, Dong B and Xu TH: Changes of apoptosis,
mitochondrion membrane potential and Ca2+ of
hypothalamic neurons induced by high power microwave. Zhonghua Lao
Dong Wei Sheng Zhi Ye Bing Za Zhi. 24:739–741. 2006.In Chinese.
|
|
10
|
Wang XW, Ding GR, Shi CH, Zhao T, Zhang J,
Zeng LH and Guo GZ: Effect of electromagnetic pulse exposure on
permeability of blood-testicle barrier in mice. Biomed Environ Sci.
21:218–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang ML, Liu HQ, Guo J, Zhang YL, Zeng LH
and Guo GZ: Effects of electromagnetic pulse on the expression of
GABAA receptor in hypothalamus of male offsprings of rats. Progress
in Modern Biomedicine. 11:1257–1260. 2011.In Chinese.
|
|
12
|
Zhou XG, Zeng GY, Ren DQ, Fang HH, Sun XM
and Huang XF: Study of the ultrastructural changes in the thyroid
gland of rats exposed to pulsed electromagnetic wave. J Radiat Res
Radiat Process. 24:120–124. 2006.
|
|
13
|
Merritt JH, Kiel JL and Hurt WD:
Considerations for human exposure standards for fast-rise-time
high-peak-power electromagnetic pulses. Aviat Space Environ Med.
66:586–589. 1995.PubMed/NCBI
|
|
14
|
Cao XZ, Wang DW, Zhao ML, Gao YB, Cui XM,
Peng RY and Zhang YR: The study on the serum hormone level in
macaques irradiated by electromagnetic pulse (EMP). Chin J Phys Med
Rehabil. 24:679–682. 2002.In Chinese.
|
|
15
|
Cao XZ, Wang DW, Zhao ML, Li CL, Shu T,
Peng RY, Chen HY and Gao YB: A preliminary study on the effect of
99W·CM-2 high powered pulse microwave on serum hormone level in
rats. J Prev Med Chin PLA. 20:8–11. 2002.In Chinese.
|
|
16
|
Nussey S and Whitehead S: Endocrinology:
An Integrated Approach. Oxford: BIOS Scientific Publishers, Oxford;
2001, View Article : Google Scholar
|
|
17
|
Poole MC and Kornegay WD 3rd: Cellular
distribution within the rat adenohypophysis: a morphometric study.
Anat Rec. 204:45–53. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feige U and Polla BS: Hsp70 – a
multi-gene, multi-structure, multi-function family with potential
clinical applications. Experientia. 50:979–986. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Trump BF, Berezesky IK, Chang SH and
Phelps PC: The pathways of cell death: oncosis, apoptosis, and
necrosis. Toxicol Pathol. 25:82–88. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao XZ, Wang DW, Zhao ML and Zhang S: The
atomic force microscope study on the pituitary cell membrane
perforate induced by electromagnetic pulse. Chin J Phys Med
Rehabil. 25:462–464. 2003.
|
|
21
|
Cao XZ, Wang DW, Zhao ML, Zhang S, Zhang
DT, Zhang JG, Liu J and Zhang Y: The atomic force microscope study
on the hypothalamus neuron memberane perforation induced by EMP.
Bull Acad Mil Med Sci. 27:416–418. 2003.
|
|
22
|
Zhao ML, Cao XZ, Wang DW, Zhang S, Liu J,
Zhang Y and Qian X: The Atom force microscopy study on the
hypothalamus neurons membrane perforate induced by EMP. Chin J Med
Phys. 21:147–149. 2004.
|
|
23
|
Ascah A, Khairallah M, Daussin F,
Bourcier-Lucas C, Godin R, Allen BG, Petrof BJ, Des Rosiers C and
Burelle Y: Stress-induced opening of the permeability transition
pore in the dystrophin-deficient heart is attenuated by acute
treatment with sildenafil. Am J Physiol Heart Circ Physiol.
300:H144–H153. 2011. View Article : Google Scholar
|
|
24
|
Del Re B, Bersani F, Mesirca P and Giorgi
G: Synthesis of DnaK and GroEL in Escherichia coli cells exposed to
different magnetic field signals. Bioelectrochemistry. 69:99–103.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Esmekaya MA, Ozer C and Seyhan N: 900 MHz
pulse-modulated radiofrequency radiation induces oxidative stress
on heart, lung, testis and liver tissues. Gen Physiol Biophys.
30:84–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Proctor CJ and Lorimer IA: Modelling the
role of the Hsp70/Hsp90 system in the maintenance of protein
homeostasis. PLoS One. 6:e220382011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gilby KL, Armstrong JN, Currie RW and
Robertson HA: The effects of hypoxia-ischemia on expression of
c-Fos, c-Jun and Hsp70 in the young rat hippocampus. Brain Res Mol
Brain Res. 48:87–96. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lindquist S and Craig EA: The heat-shock
proteins. Annu Rev Genet. 22:631–677. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hageman J, van Waarde MA, Zylicz A,
Walerych D and Kampinga HH: The diverse members of the mammalian
HSP70 machine show distinct chaperone-like activities. Biochem J.
435:127–142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Finka A, Rayees U and Goloubinoff P:
Meta-analysis of heat- and chemically upregulated chaperone genes
in plant and human cells. Cell Stress Chaperones. 16:15–31. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Garaj-Vrhovac V, Gajski G, Pažanin S,
Sarolić A, Domijan AM, Flajs D and Peraica M: Assessment of
cytogenetic damage and oxidative stress in personnel occupationally
exposed to the pulsed microwave radiation of marine radar
equipment. Int J Hyg Environ Health. 214:59–65. 2011. View Article : Google Scholar
|
|
32
|
Xu S, Zhou Z, Zhang L, Yu Z, Zhang W, Wang
Y, Wang X, Li M, Chen Y, Chen C, He M, Zhang G and Zhong M:
Exposure to 1800 MHz radiofrequency radiation induces oxidative
damage to mitochondrial DNA in primary cultured neurons. Brain Res.
1311:189–196. 2010. View Article : Google Scholar
|