Introduction
Oral squamous cell carcinoma (OSCC) is the most
common oral and maxillofacial malignancy, which is associated with
high levels of incidence and mortality (1). Recently, the survival of patients
with OSCC has improved, due to improvements in diagnostic and
therapeutic strategies (2);
however, OSCC remains a serious global health issue and therefore
requires further study.
Acylglycerol kinase (AGK) is a multisubstrate lipid
kinase (3). Overexpression of AGK
has previously been shown to promote the proliferation of prostate
cancer cells, suggesting that AGK may act as a potent oncogene
(4,5). However, the underlying mechanisms of
the effects of AGK on cell proliferation and cell cycle progression
of OSCC remain to be elucidated. The cell cycle is a highly
evolutionarily conserved mechanism. which facilitates the growth
and proliferation of cells. Among the cyclin classes, the
cyclin-dependent kinase (CDK) inhibitor p21 and cyclin D1 are two
proteins that have been demonstrated to negatively and positively
control cell cycle progression and cell proliferation, respectively
(6,7). Cyclin D1 exerts its effects by
binding the CDK subunits 4 and 6, resulting in the formation of a
complex, which induces successive phosphorylation of the Rb protein
(8).
The present study aimed to elucidate whether AGK was
overexpressed in OSCC, and whether AGK expression was correlated
with cell proliferation and cell cycle progression. To determine
how AGK influences OSCC cell proliferation and cell cycle
progression, the expression levels of proteins regulating cell
cycle transition, including p21 and cyclin D1, were
investigated.
Patients and methods
OSCC patient samples
Four patients, including two males and two females,
between 42 and 78 years old with OSCC at clinical stage III or IV
undergoing surgical resection at Linyi People’s Hospital (Linyi,
China) between January 1 and July 1, 2013 were recruited, and
written informed consent was obtained from all patients. Tumor
tissue and adjacent non-tumor tissue samples were obtained from
resected tumors and adjacent non-tumor esophageal tissue,
respectively. The tissue was confirmed by pathological review. The
present study was approved by the Ethics Committee of Linyi
People’s Hospital.
OSCC cell culture and transfection
The SCC-9, SCC-25, TSCCa and Tca-8113 OSCC cell
lines, in addition to tongue epithelial cells (TEC) were obtained
from the American Type Culture Collection (Manassas, VA, USA) and
maintained in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) (HyClone; Thermo
Fisher Scientific, Waltham, MA, USA), at 37°C in an atmosphere
containing 5% CO2 and 95% air.
The TSCCa cells were transfected with lentiviral
vectors encoding short hairpin (sh)RNA targeting human AGK for AGK
knockdown [AGK/RNA interference (RNAi)], or with a scrambled shRNA
as a control (Scramble) (Sigma-Aldrich, St. Louis, MO, USA). The
multiplicity of infection was 10, and the cells were cultured for
72 h post-transfection, and were grown to 80% confluency in 60-mm
dishes. TSCCa cells were seeded in six-well plates at a density of
3×105 cells/well in DMEM at 37°C overnight. A total of
10 ng AGK plasmid (ExAGK) or empty plasmid (ExControl)
(Sigma-Aldrich) was transfected into the cells using
Lipofectamine® 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA). The cells were cultured for 48 h
post-transfection in DMEM containing with 10% FBS, at 37°C in an
atmosphere containing 5% CO2.
Western blot analysis
Nuclear or total protein was extracted from the OSCC
cells and tissue samples using radioimmuno-precipitation assay
buffer (Cell Signaling Technology, Inc., Danvers, MA, USA). For
immunoblotting, equal quantities of proteins (20 µg) were
separated by 5–8% SDS-PAGE (Beyotime Institute of Biotechnology,
Haimen, China) and were electrophoretically transferred onto
nitrocellulose membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked in Tris-buffered saline-0.5% Tween 20 (TBST;
Beyotime Institute of Biotechnology) supplemented with 5% milk
(Beyotime Institute of Biotechnology) for 2 h at room temperature,
prior to incubation with primary antibodies (Beyotime Institute of
Biotechnology) overnight at 4°C. The following rabbit antibodies
were used: Polyclonal anti-AGK (HPA020959; Sigma-Aldrich),
polyclonal anti-cyclin D1 (#2922; Cell Signaling Technology, Inc.),
polyclonal anti-p21 (SAB4500065; Sigma-Aldrich), polyclonal
anti-retinoblastoma (Rb) (#9969; Cell Signaling Technology, Inc.)
and monoclonal anti-phosphorylated (p)-Rb (#8180; Cell Signaling
Technology, Inc.). All antibodies were used at dilutions of 1:1,000
unless stated. To control sample loading, the membranes were
incubated with an anti-β-actin antibody (1:5,000; Sigma-Aldrich).
The membranes were subsequently washed with TBST and incubated with
a horseradish peroxidase-conjugated anti-rabbit secondary antibody
(MFCD00162786; Sigma-Aldrich) for 2 h at room temperature.
Immunocomplexes were visualized using the ECL Advance western
blotting detection kit (GE Healthcare Bio-Sciences, Pittsburgh, PA,
USA), according to the manufacturer’s instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the OSCC cells using
TRIzol (Qiagen China Co., Ltd., Shanghai, China). cDNA synthesis
was performed according to the manufacturer’s instructions using
the QuantiNova SYBR Green PCR kit (Qiagen China Co., Ltd.). qPCR
was performed using the SYBR PCR kit (Qiagen China Co., Ltd) on a
7500 Sequence Detection system (Applied Biosystems Life
Technologies, Foster City, CA, USA). The PCR reaction conditions
for all assays were as follows: 95°C for 30 sec, followed by 40
cycles of amplification (95°C for 5 sec, 59°C for 30 sec and 72°C
for 30 sec). Target RNA expression was normalized against β-actin
mRNA expression. The primers were synthesized by GeneCopoeia Co.
(Rockville, MD, USA). The primer sequences were as follows: AGK
forward, 5′-CGA AGG CTT GCG TCC TAC TG-3′ and reverse, 5′-TGG TGG
ACA GCT GCA CAT CT-3′ (4); cyclin
D1 forward, 5′-AAC TAC CTG GAC GCT TCC T-3′ and reverse, 5′-CCA CTT
GAG CTT GTT CAC CA-3′; p21 forward, 5′-CAT GGG TTC TGA CGG ACA T-3′
and reverse, 5′-AGT CAG TTC CTT GTG GAG CC-3′; and β-actin forward,
5′-CCA TGT ACG TTG CTA TCC AGG-3′ and reverse, 5′-TCT CCT TAA TGT
CAC GCA CGA-3′. Expression data were normalized to the geometric
mean of β-actin to control the variability in expression levels and
were calculated as 2-[(Ct of AGK, CyclinD1 and p21) – (Ct of
β-actin)].
MTT and colony formation assays
Cell growth was assessed by MTT assay. The TSCCa
cells were seeded in 96-well plates in DMEM supplemented with 10%
FBS at ~2×103 cells/well. For quantification of cell
viability, the cultures were stained following 1, 2, 3, 4 and 5
days of incubation. Briefly, 20 µl 5 mg/ml MTT solution
(Sigma-Aldrich) was added to each well and incubated at 37°C for 4
h. The medium was then removed from each well and the resulting MTT
formazan crystals were solubilized in 150 µl dimethyl
sulfoxide (Sigma-Aldrich). The absorbance was measured at a
wavelength of 490 nm using a Multiskan plate reader (Thermo Fisher
Scientific).
For the colony formation assay, TSCCa cells were
plated in three 6-cm cell culture dishes (1×103
cells/dish) and incubated for 12 days in medium supplemented with
10% FBS at 37°C in an atmosphere containing 5% CO2. The
plates were then washed with phosphate-buffered saline (PBS) and
stained with Giemsa (Beyotime Institute of Biotechnology). The
number of colonies containing >50 cells was manually counted in
10 fields using a Motic AE30 inverted fluorescence microscope
(Microscope Systems Limited, Glasgow, UK) at magnification,
x100.
Flow cytometric analysis
Flow cytometric analysis was performed as previously
described (8). Briefly, 48 h
post-transfection, the TSCCa cells were harvested, washed with PBS
and fixed with 70% ethanol. The fixed cells were subsequently
treated with 20 µg/ml RNase A and 50 µg/ml propidium
iodide (Sigma-Aldrich) for 30 min. The stained cells were
immediately analyzed using a FACScan flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
All statistical analyses were performed using SPSS
version 19.0 (IBM, Armonk, NY, USA). Continuous data were measured
using Student’s t-test, and differences in measurements were
compared using one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
AGK expression is upregulated in OSCC
cell lines and human OSCC tissues
To determine whether AGK contributed to the
pathogenesis of OSCC, the present study examined the expression
levels of AGK in OSCC cell lines and human OSCC tissue samples. The
protein and mRNA expression levels of AGK were differentially
upregulated in all four OSCC cell lines, as compared with normal
TECs, and in all four OSCC patient tissue samples, as compared with
the paired adjacent non-tumor tissue samples (Fig. 1). These results indicated that AGK
may be overexpressed in OSCC. AGK expression levels were lower in
the TSCCa cells than those in the SCC-9 and Tca-8113 cells, but
were higher than those in the SCC-25 cells. Therefore, TSCCa
appeared to be the best-fit cellular model for evaluating the up-
and downregulation of the expression levels of AGK.
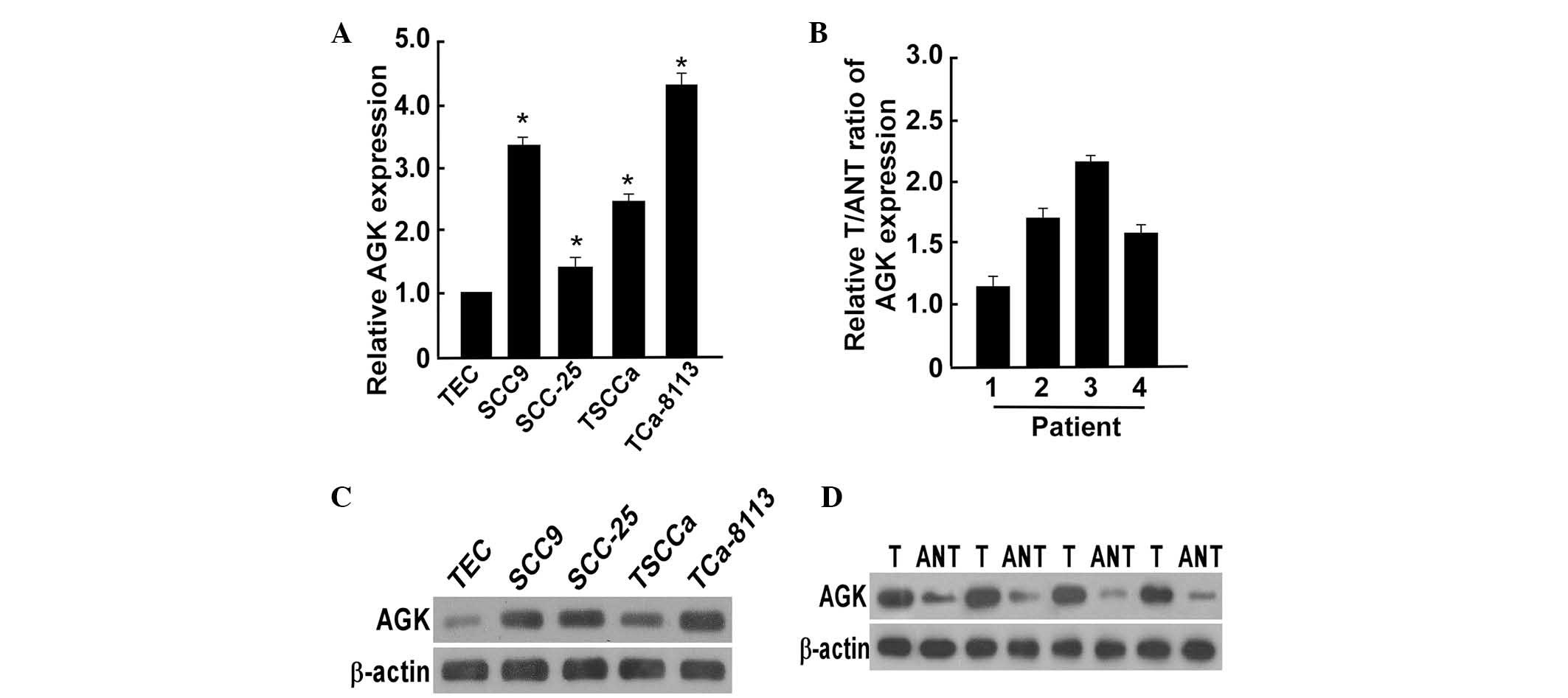 | Figure 1AGK expression levels in OSCC cell
lines and human OSCC tissue. (A) mRNA expression levels of AGK in
TEC and four OSCC cell lines, as determined by PCR. (B) mRNA
expression levels of AGK in four paired primary OSCC tissues and
the matched ANT from the same patient, as determined by PCR. (C)
Western blotting was performed to detect the protein expression
levels of AGK in TEC cells and four OSCC cell lines. β-actin was
used as a loading control. (D) Western blotting was performed to
detect the protein expression levels of AGK in four paired primary
OSCC tissues and matched ANT from the same patient. β-actin was
used as a loading control. Values are expressed as the mean ±
standard deviation. *P<0.05, OSCC cell lines vs. TEC.
AGK, acylglycerol kinase; OSCC, oral squamous cell carcinoma; mRNA,
messenger RNA; TEC, tongue epithelial cells; PCR, polymerase chain
reaction; ANT, adjacent non-tumor tissues; T, primary OSCC
tissues. |
AGK overexpression in OSCC cell lines
promotes cell proliferation
To determine whether AGK promoted the proliferation
of OSCC cells, plasmid transfection and shRNA knockdown were
performed in TSCCa cells. Transfection with the AGK plasmid
significantly upregulated the protein and mRNA expression levels of
AGK, whereas transfection with the AGK shRNA decreased AGK protein
and mRNA expression levels in the TSCCa cells (Fig. 2A and B). The proliferation rate of
the TSCCa cells was determined using an MTT assay. AGK upregulation
promoted cell growth, whereas a significant decrease in
proliferation was observed in the TSCCa cells transfected with the
AGK shRNA, as compared with the negative control (P<0.05;
Fig. 2C). Consistent with the
results of the MTT assay, the colony formation assay demonstrated
that AGK overexpression in TSCCa cells induced an increase in foci
numbers, whereas AGK knockdown decreased the colony forming ability
of the cells (P<0.05; Fig.
2D).
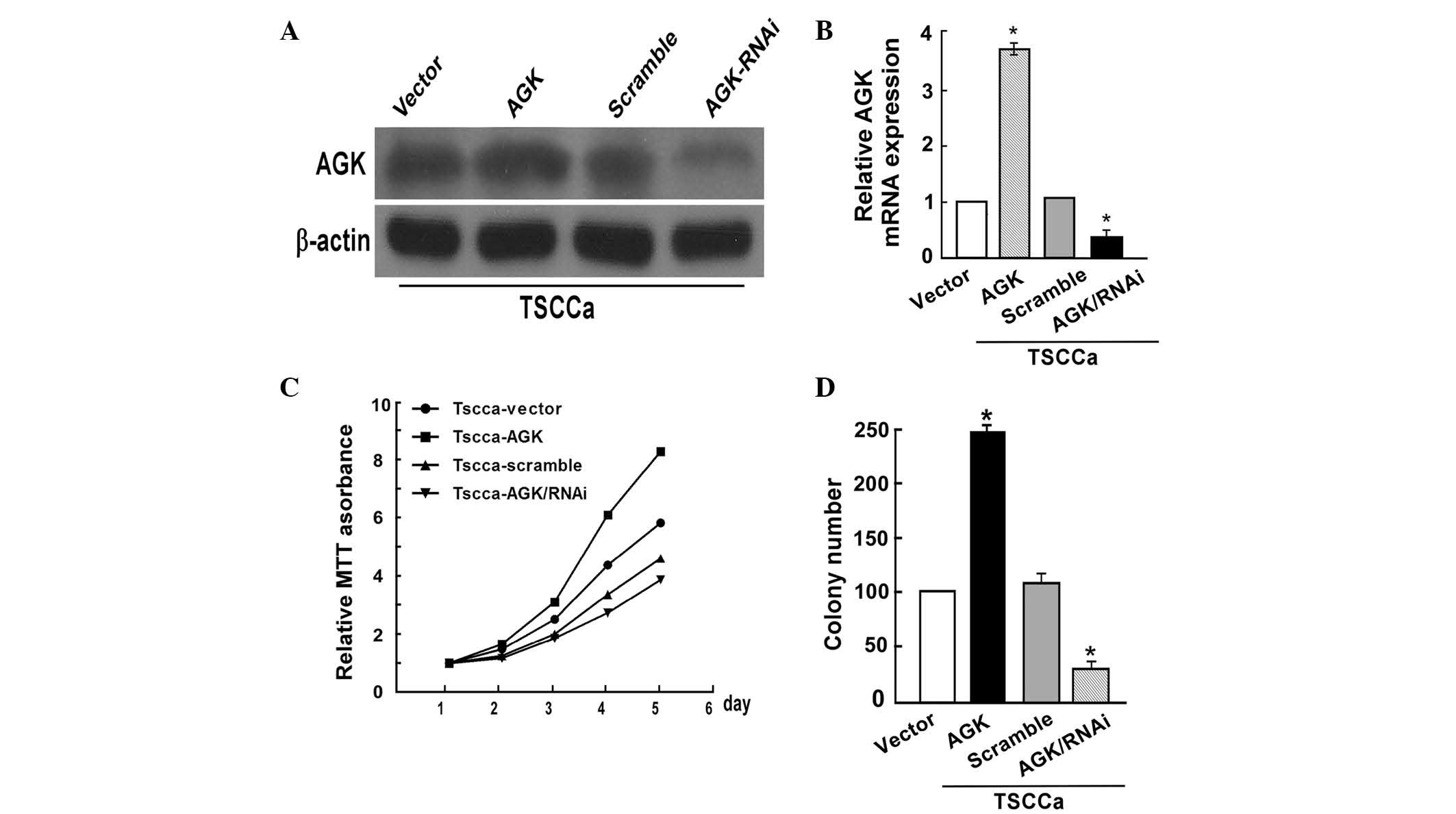 | Figure 2AGK overexpression promotes oral
squamous cell carcinoma cell proliferation, whereas AGK knockdown
inhibits cell proliferation. (A) Validation of AGK protein
expression levels post-transfection by western blot analysis. (B)
Validation of post-transfection AGK mRNA expression levels by
polymerase chain reaction. (C) AGK overexpression enhanced the rate
of proliferation, whereas AGK knockdown decreased the rate of
proliferation, as determined by MTT assay (Day 5, P<0.05). (D)
AGK overexpression markedly increased colony number, whereas AGK
knockdown decreased colony number, as determined by colony
formation assay. Values are expressed as the mean ± standard
deviation. *P<0.05, AGK vs. vector or AGK/RNAi vs.
scramble. AGK, acylglycerol kinase; mRNA, messenger RNA; RNAi, RNA
interference. |
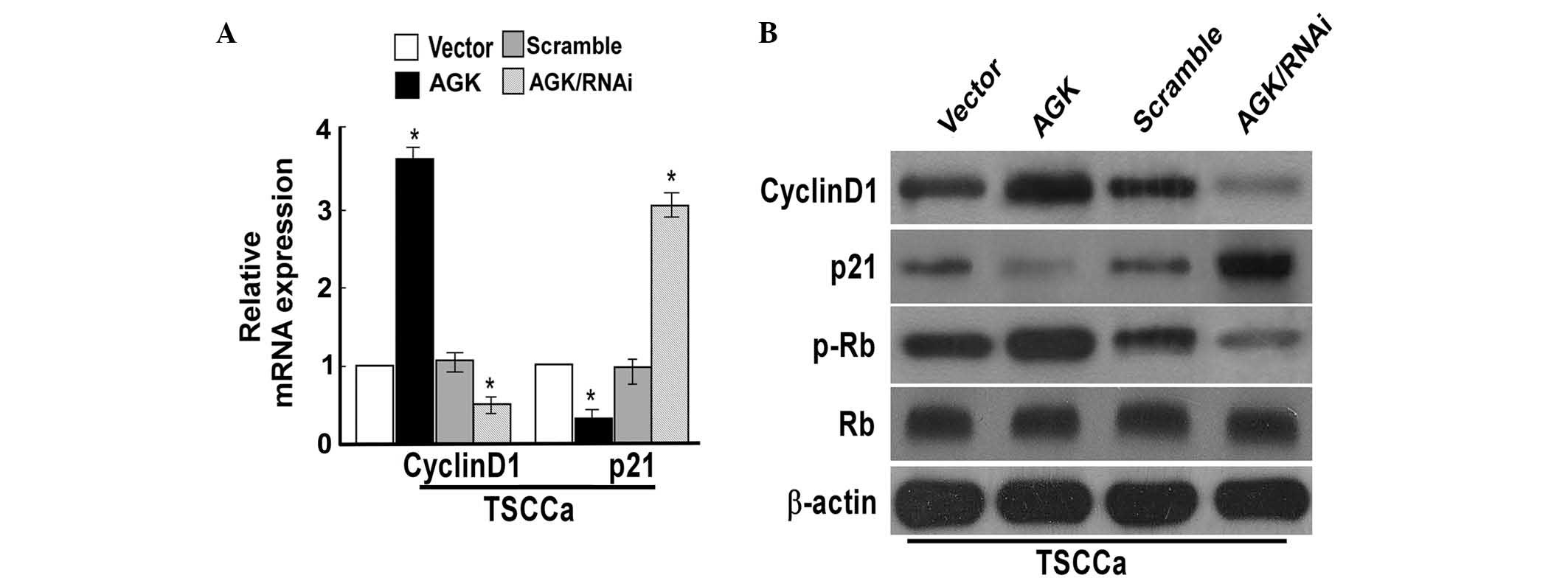
AGK regulates cell cycle progression, and
expression of cyclin D1 and p21 in OSCC cells
To investigate the mechanisms underlying cell
proliferation, the effects of AGK on cell cycle progression, and
the expression of cell cycle-associated proteins cyclin D1, p21, Rb
and p-Rb were evaluated. CDK inhibitor p21 and cyclin D1 are two
proteins targeted by AGK, which negatively and positively modulate
cell cycle progression and proliferation, respectively (6). Cyclin D1 exerts its effects by
binding the CDK subunits 4 and 6, resulting in the formation of a
complex, which induces successive phosphorylation of the Rb protein
(9). The results of the present
study demonstrated that the overexpression of AGK resulted in
upregulation of the protein and mRNA expression levels of cyclin
D1, and increased the expression levels of p-Rb; while p21
expression levels were downregulated (Fig. 3). Conversely, knockdown of AGK
resulted in reduced mRNA and protein expression levels of cyclin D1
and p-Rb, whereas the expression levels of p21 were increased
(Fig. 3). These results suggested
that AGK influenced p21 and cyclin D1 expression in way that means
increases in AGK expression may be able to accelerate cell cycle
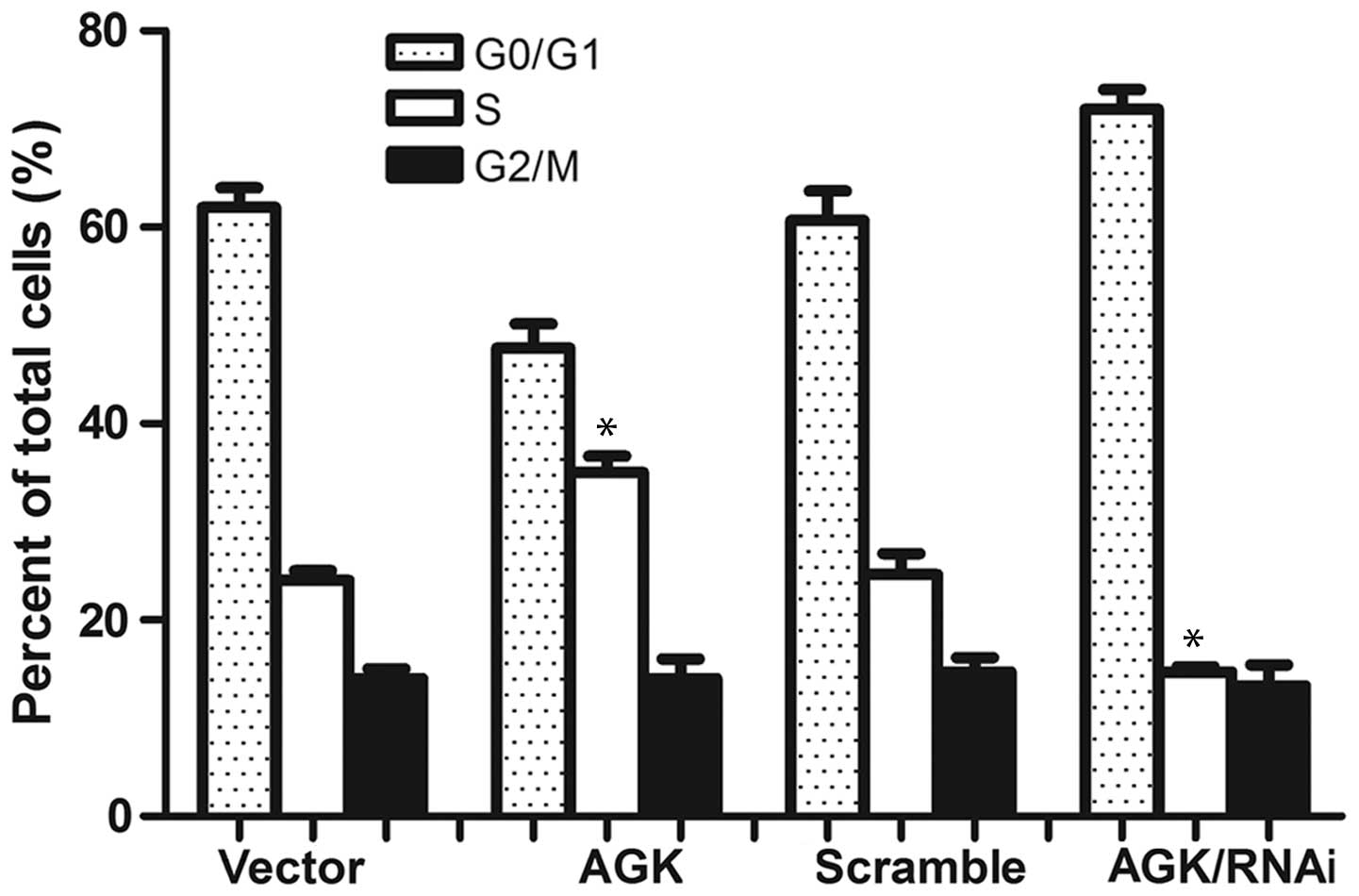
progression and cell proliferation. In order to evaluate this
hypothesis, the present study aimed to analyze cell cycle
distribution. The percentage of cells in the
G0/G1 phase was demonstrated to be decreased
in those cells overexpressing AGK; whereas it was increased in the
AGK knockdown cells. Conversely, the percentage of cells in the S
phase was increased in the cells transfected with AGK plasmid and
decreased in the cells transfected with AGK shRNA (Fig. 4). These results suggested that AGK
expression may promote malignant cancer growth by regulating the
expression of cyclin D1 and p21, during the G1-S phase
transition.
Discussion
The effects of AGK have been described in numerous
types of cell; however, the role of AGK in cancer remains poorly
understood and most relevant studies have been conducted in cell
lines (4,10,11).
Overexpression of AGK has previously been shown to constitutively
activate janus kinase 2/signal transducer and activator of
transcription 3, consequently augmenting the tumorigenicity of
esophageal squamous cell carcinoma cells (12). In addition, the expression of AGK
has been shown to be associated with the development and
progression of prostate cancer (13). The present study examined AGK
expression levels in normal TECs, OSCC cell lines and human OSCC
tissue samples. The expression levels of AGK were differentially
upregulated in the OSCC cell lines, compared with those of the
TECs. Furthermore, AGK was overexpressed in the tumor samples from
patients with OSCC, compared with those of adjacent normal tissues.
These results suggested that AGK may be involved with malignant
processes in OSCC. The present study identified AGK as a
significant factor in the modulation of tumor cell proliferation in
OSCC.
To gain insight into the biological roles of AGK in
OSCC tumorigenesis, AGK was upregulated and knocked down in the
TSCCa cell line, and MTT and colony formation assays were
subsequently used to detect cell proliferation. The results of the
present study demonstrated that upregulation of AGK increased the
rate of cell proliferation and colony formation, whereas AGK
knockdown inhibited the rate of proliferation and colony formation.
However, the underlying mechanisms remain to be elucidated. The
majority of G1-S regulators have significant roles in
tumor progression (14–17). Cyclin D1 is a vital protein that
positively regulates cell cycle progression through the
G1 to S phase transition (18,19).
Aberrant cyclin D1 expression has been shown to contribute to the
loss of normal cell cycle control (20), and cyclin D1 degradation is
sufficient to induce G0/G1 cell cycle arrest
in cancer cells (21–23). The transition to S phase is induced
by the activation of the cyclin D/CDK complex, which phosphorylates
Rb, a well-characterized regulator of cell proliferation (9,24,25).
In addition, this complex activates cyclin E/CDK 2 by sequestering
the CDK inhibitor p21 (7,26). p21 inhibits cell cycle progression
at checkpoint G1, where it results in sustained
G1 blockade (26,27).
To elucidate the potential underlying mechanisms of the effects of
AGK, the expression levels of numerous cell cycle-associated
molecules, including cyclin D1 and p21, and cell cycle distribution
were investigated. Overexpression of AGK increased the expression
levels of cyclin D1 and p-Rb, whereas AGK knockdown reduced the
expression levels of cyclin D1 and p-Rb. In addition, AGK
overexpression resulted in a significant decrease in p21 expression
levels, whereas knockdown of AGK increased the expression levels of
p21. Flow cytometric analysis revealed that TSCCa cells transfected
with an AGK plasmid entered S phase earlier, whereas knockdown of
AGK arrested cell cycle progression at the
G0/G1 boundary. Therefore, it was
hypothesized that knockdown of AGK bound to the cyclin D1-CDK4/6
complex induced a reduction in cyclin D1 expression, which may
disrupt the interaction between CDK4/6 and cyclin D1, resulting in
cyclin D1 proteasome-dependent degradation, suppression of Rb
phosphorylation and G0/G1 cell cycle
arrest.
To the best of our knowledge, few studies have
identified the specific molecules and their roles in the molecular
mechanisms underlying OSCC tumor progression. The results of the
present study provide evidence for the role of AGK as a potential
tumor promoter. AGK was demonstrated to be upregulated in OSCC
cells and tissues, and AGK upregulation promoted cell
proliferation, cell cycle progression and cyclin D1 and p21
expression, whereas AGK knockdown had the opposite effect. These
results identify AGK as a potential key molecule involved in OSCC,
and provide a basis for the elucidation of the functional
importance of AGK in the progression of OSCC.
Acknowledgments
The present study was supported by Linyi People’s
Hospital (Linyi, China). All authors designed the study and
performed the experiments together; Chunhai Gao and Wei Zhang
analyzed the data and wrote the paper, and all authors approved the
final manuscript.
References
|
1
|
Jiang Q, Yu YC, Ding XJ, Luo Y and Ruan H:
Bioinformatics analysis reveals significant genes and pathways to
target for oral squamous cell carcinoma. Asian Pac J Cancer Prev.
15:2273–2278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rigual NR, Shafirstein G, Frustino J, et
al: Adjuvant intraoperative photodynamic therapy in head and neck
cancer. JAMA Otolaryngol Head Neck Surg. 139:706–711. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Epand RM, Shulga YV, Timmons HC, et al:
Substrate chirality and specificity of diacylglycerol kinases and
the multisubstrate lipid kinase. Biochemistry. 46:14225–14231.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bektas M, Payne SG, Liu H, Goparaju S,
Milstien S and Spiegel S: A novel acylglycerol kinase that produces
lysophosphatidic acid modulates cross talk with EGFR in prostate
cancer cells. J Cell Biol. 169:801–811. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spiegel S and Milstien S: Critical role of
acylglycerol kinase in epidermal growth factor-induced mitogenesis
of prostate cancer cells. Biochem Soc Trans. 33:1362–1365. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sherr CJ and Roberts JM: CDK inhibitors:
Positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yong ST and Wang XF: A novel,
non-apoptotic role for Scythe/BAT3: A functional switch between the
pro- and anti-proliferative roles of p21 during the cell cycle.
PLoS One. 7:e380852012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong S, Li X, Zhao Y, Yang Q and Kong B:
53BP1 suppresses tumor growth and promotes susceptibility to
apoptosis of ovarian cancer cells through modulation of the Akt
pathway. Oncol Rep. 27:1251–1257. 2012.PubMed/NCBI
|
|
9
|
Weinberg RA: The retinoblastoma protein
and cell cycle control. Cell. 81:323–330. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Lin C, Zhao X, et al: Acylglycerol
kinase promotes cell proliferation and tumorigenicity in breast
cancer via suppression of the FOXO1 transcription factor. Mol
Cancer. 13:1062014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cui Y, Lin C, Wu Z, et al: AGK enhances
angiogenesis and inhibits apoptosis via activation of the NF-κB
signaling pathway in hepatocellular carcinoma. Oncotarget.
5:12057–12069. 2014.PubMed/NCBI
|
|
12
|
Chen X, Ying Z, Lin X, et al: Acylglycerol
kinase augments JAK2/STAT3 signaling in esophageal squamous cells.
J Clin Invest. 123:2576–2589. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nouh MA, Wu XX, Okazoe H, et al:
Expression of autotaxin and acylglycerol kinase in prostate cancer:
Association with cancer development and progression. Cancer Sci.
100:1631–1638. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Knudsen KE, Diehl JA, Haiman CA and
Knudsen ES: Cyclin D1: polymorphism, aberrant splicing and cancer
risk. Oncogene. 25:1620–1628. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bali A, O’Brien PM, Edwards LS, Sutherland
RL, Hacker NF and Henshall SM: Cyclin D1, p53 and p21Waf1/Cip1
expression is predictive of poor clinical outcome in serous
epithelial ovarian cancer. Clin Cancer Res. 10:5168–5177. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ratschiller D, Heighway J, Gugger M, et
al: Cyclin D1 overexpression in bronchial epithelia of patients
with lung cancer is associated with smoking and predicts survival.
J Clin Oncol. 21:2085–2093. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Comstock CE, Augello MA, Goodwin JF, et
al: Targeting cell cycle and hormone receptor pathways in cancer.
Oncogene. 32:5481–5491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baldin V, Lukas J, Marcote MJ, Pagano M
and Draetta G: Cyclin D1 is a nuclear protein required for cell
cycle progression in G1. Genes Dev. 7:812–821. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Motokura T and Arnold A: Cyclin D and
oncogenesis. Curr Opin Genet Dev. 3:5–10. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carlos de Vicente J, Herrero-Zapatero A,
Fresno MF and Lόpez-Arranz JS: Expression of cyclin D1 and Ki-67 in
squamous cell carcinoma of the oral cavity: clinicopathological and
prognostic significance. Oral Oncol. 38:301–308. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Masamha CP and Benbrook DM: Cyclin D1
degradation is sufficient to induce G1 cell cycle arrest despite
constitutive expression of cyclin E2 in ovarian cancer cells.
Cancer Res. 69:6565–6572. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seo JH, Jeong ES and Choi YK: Therapeutic
effects of lentivirus-mediated shRNA targeting of cyclin D1 in
human gastric cancer. BMC Cancer. 14:1752014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu J, Zhong D, Fu X, Liu Q, Kang L and
Ding Z: Silencing of Ether à go-go 1 by shRNA inhibits osteosarcoma
growth and cell cycle progression. Int J Mol Sci. 15:5570–5581.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li W, Sun S, Chen Y, Yu H, Chen ZY and Li
H: Disrupting the interaction between retinoblastoma protein and
Raf-1 leads to defects in progenitor cell proliferation and
survival during early inner ear development. PLoS One.
8:e837262013. View Article : Google Scholar
|
|
25
|
Xu C, Wu C, Xia Y, et al: WT1 promotes
cell proliferation in non-small cell lung cancer cell lines through
up-regulating cyclin D1 and p-pRb in vitro and in vivo. PLoS One.
8:e688372013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nojiri S and Joh T: Albumin suppresses
human hepatocellular carcinoma proliferation and the cell cycle.
Int J Mol Sci. 15:5163–5174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rego MA, Harney JA, Mauro M, Shen M and
Howlett NG: Regulation of the activation of the Fanconi anemia
pathway by the p21 cyclin-dependent kinase inhibitor. Oncogene.
31:366–375. 2012. View Article : Google Scholar
|