Introduction
Osteoporosis associated with estrogen deficiency is
among the most common diseases in post-menopausal women (1). After menopause, women undergo a sharp
decline in bone mass, losing ~10–15% of bone over a period of 5–10
years (2). Hormone replacement
therapy (HRT) is able to effectively prevent post-menopausal
osteoporosis and reduce the incidence of fractures (3). However, HRT also increases the risk
of breast and endometrial cancer, in addition to other undesirable
side effects (4). Since
phytoestrogens extracted from traditional Chinese medicines are
different from hormones used in hormone replacement therapy, they
are potentially important in the prevention of postmenopausal
osteoporosis.
Phytoestrogens are divided into three classes:
Isoflavones, coumestans and lignans. Puerarin [7-hydroxy-3-(4-hydro
xyphenyl)-1-benzopyran-4-one-8-(β-D-glucopyranoside] (Fig. 1), the main isoflavone glycoside
found in the root of Pueraria lobata (Willd.) Ohwi, has been
used for various medicinal purposes in Traditional Chinese Medicine
for thousands of years (5).
Phytoestrogens, including isoflavones, are molecules of plant
origin, and are structurally associated with the mammalian estrogen
17β-estradiol (6,7). Modern pharmacological research has
demonstrated that puerarin has key effects on prevention and
treatment of cardiovascular diseases, osteoporosis, diabetes and
obesity, menopausal symptoms, renal diseases and various cancers
(8,9). Interestingly, puerarin has estrogenic
activity (10,11). Studies have shown that Radix
Puerariae prevents bone loss by growth hormone release in
ovariectomized rats (9,12). Puerarin has also been reported to
have a stimulatory effect on bone formation and activation of the
PI3K/Akt pathway, regulating cell proliferation in rat calvaria
osteoblasts (13). However, the
detailed mechanisms underlying the anabolic effects of puerarin on
osteoblasts have remained elusive.
Further studies have indicated that estrogen 2 (E2)
stimulation of osteoblast proliferation and differentiation is
mediated via the nitric oxide (NO)/NO synthase (NOS) pathway
(14–16). NO release in osteoblastic cells
increases cGMP formation by binding guanylate cyclase (GC), and the
resulting cGMP signal regulates osteoblastic proliferation and
differentiation (17,18). It was also revealed that the
GC/cGMP system has a key role in spasmolytic signaling mechanisms.
Previous studies showed that the phytoestrogen genistein stimulates
osteoblastic differentiation via NO/cGMP in bone marrow-derived
mesenchymal stem cells (BMSCs) of mice in primary culture (19). Thus, puerarin may also act on bone
cells through the NO/cGMP pathway.
In the present study, the in vitro effect of
puerarin on proliferation and osteoblastic maturation of hBMSCs was
investigated. Furthermore, the effects of puerarin on osteoblastic
bone formation and NO/cGMP production were assessed. To elucidate
the underlying mechanism, estrogen activity and the NO pathway were
blocked by ICI182780 or Nx-nitro-L-arginine methyl ester (L-NAME),
respectively. The present study suggested that puerarin stimulates
osteoblastic bone formation, likely through activation of the
NO/cGMP signaling pathway.
Materials and methods
Reagents
Puerarin (99% purity), pronase E, ascorbic acid,
β-glycerophosphate, p-nitrophenol, diethanolamine,
p-nitrophenol phosphate (p-NPP), L-NAME,
3-isobutyl-L-methylxanthine (IBMX) and dextran-charcoal were all
purchased from Sigma-Aldrich (St. Louis, MO, USA). Alpha minimum
essential medium (α-MEM), fetal bovine serum (FBS),
penicillin-streptomycin solution, SDS and TRIzol reagent were
obtained from GIBCO-BRL (Invitrogen Life Technologies, Grand
Island, NY, USA). ICI182780 was purchased from Tocris Cookson Ltd.
(Avonmouth, Bristol, UK). Tissue culture plastic dishes and flasks
were purchased from Corning-Costar Co. (Corning, NY, USA). A
nitrate/nitrite colorimetric assay kit was purchased from
Sino-American Biotechnology Company (Beijing, China). Molecular
biology reagents and enzymes, including Tris-HCl buffer, 0.1% (w/v)
alizarin red S and alkaline phosphatase were purchased from
Boehringer Ingelheim (Ingelheim, Germany); Bio-Rad Protein Assay
kit; 0.1 N NaOH/0.1% SDS; 2.5% glutaraldehyde and 70% ethanol were
purchased from Bio-Rad Laboratories, Inc., Hercules, CA, USA; A
cyclic GMP immunoassay kit was purchased from R&D systems, Inc.
(Minneapolis, MN, USA). All other chemicals were of analytical
grade and purchased from Shanghai Biotech Co., Ltd. (Shanghai,
China).
Cell culture
Primary hBMSCs were obtained from ribs discarded at
the time of open thoracotomy in patients without metabolic bone
disease using a protocol approved by the First Hospital of Lanzhou
University Ethics Committee. The patients consisted of 14 males and
6 females whose mean age was 38.2±2.3 years (range, 18–57 years),
and written informed consent was obtained from each patient.
Primary hBMSCs were isolated and cultured in DMEM/F12 medium
(GIBCO-BRL) containing 10% FBS, 100 U/ml penicillin and 100
µg/ml streptomycin. Cells were maintained at 37°C in a
humidified 5% CO2 incubator and identified as
osteoblast-lineage by positive staining with alizarin red and
alkaline phosphatase. Confluent (85–95%) hBMSCs were washed twice
with PBS prior to the experiments. Cells were then treated with a
series of concentrations of puerarin (2.5–100 µM, dissolved
in PBS) or 10−8 mM 17β-estradiol (Sigma-Aldrich) in
fresh medium containing 10% FBS for the indicated times.
MTT assay
Cell proliferation was determined by a colorimetric
assay based on the ability of viable cells to metabolize MTT
(Jiancheng Bioengineering Institute, Nanjing, China). MTT is a
yellow tetrazolium salt which is reduced by the mitochondria of
metabolically active cells to form a blue formazan dye precipitate
that can be extracted using an organic solvent. Cells were seeded
at a density of 2×103 cells/well in 96-well plates in
DMEM/F12 medium with 20% FBS. After 48 h, confluent (85–95%) cells
were cultured with various concentrations of puerarin or
17β-estradiol in serum-free DMEM/F12 medium for 48 h, respectively.
Three hours prior to the end of the cell incubation period, 10
µl 0.5% MTT solution was added to each culture well.
Following an additional 3-h incubation at 37°C, the medium was
removed and the formazan crystals were dissolved in 0.2 ml dimethyl
sulfoxide for 30 min at 37°C. The optical density (OD) of each well
was measured at 570 nm using a microplate reader (Spectra Max M2e
Microplate Reader; Molecular Devices Corporation, Sunnyvale, CA,
USA). Values are expressed as the percentage of the OD of the
control cells.
Measurement of NO production
hBMSCs were grown in the differentiation medium
[1.0% Triton X-100, 2 mM MgCl2, DEA 10 ml (Sino-American
Biotechnology Company) and 8 mM PNPP (Sigma-Aldrich)] and treated
as indicated for 12 days. The culture media were collected, and
nitrite production in the conditioned media (CM) was measured using
a nitrite chromometry assay kit in a modified Griess assay.
Briefly, 100 µl CM or nitrite standards (0–100 µM)
were mixed with 100 µl of Griess reagent (Sino-American
Biotechnology Company). Absorbance was then measured at 530 nm
against a blank prepared with distilled water, and the release of
NO into the culture medium was evaluated as the nitrite
concentration, which was determined from a standard curve.
Alkaline phosphatase (ALP) assay
ALP is known to be associated with bone metabolism
and differentiation of osteoblasts. ALP activity is one of the most
common indicators of osteoblast differentiation and osteogenic
properties. Following treatment with puerarin or 17β-estradiol
(10−8 M) for 48 h, hBMSCs were washed twice with PBS and
lysed in 10 mM Tris-HCl buffer (pH 7.6) containing 2 mM
MgCl2 and 0.1% Triton X-100 on ice for 30 min, then
centrifuged at 18,000 × g for 10 min (at 4°C). The clear
supernatant was stored frozen at −20°C until use. Intracellular ALP
was determined using an ALP kit (Jiancheng Bioengineering
Institute, Nanjing, China). Briefly, 20 ml of the diluted cell
lysates were incubated in 96-well plates with 180 m 0.1 mM
NaHCO3-Na2CO3 buffer (pH 10.0)
containing 1.0% Triton X-100, 2 mM MgCl2 and 8 mM p-NPP
for 30 min at 37°C. The absorbance of p-nitrophenol liberated in
the reactive solution was read at 450 nm. 25 ml of the diluted cell
lysates was measured at 550 nm for total protein content using a
bicinchoninic acid protein assay kit (Shanghai Biotech Co.,
Ltd.).
Mineral nodule formation assay
To examine the effect of puerarin on nodule
formation, hBMSCs were incubated in 24-well plates for 24 h and
treated with puerarin or 17β-estradiol (10−8 M) for 14
days. Cultures were fed every two days by replacing them with fresh
medium (containing 10% FBS) and reagents. Cells were rinsed with
PBS, fixed in 2.5% glutaraldehyde for 10 min and washed three times
in 70% ethanol. Following drying for 20 min, cells were stained
with 01.% (w/v) alizarin red S for 30 min. Mineral nodules with a
short diameter >100 µm in 10 fields were counted using a
light microscope (Olympus BX5001; Olympus Optical Co. Ltd., Tokyo,
Japan). Calcium deposition in mineralized nodules was assessed by a
modification of the Wada procedure (20). The cultures were decalcified with
0.6 N HCl for 24 h. The calcium content in the HCl supernatant was
determined by the o-cresolphthalein complexone method
(21). After decalcification, the
cultures were washed with PBS and solubilized with 0.1 N NaOH/0.1%
SDS. Total protein content was measured with a Bio-Rad protein
assay kit. The calcium content of the cell layer was normalized to
the protein content.
Measurement of the accumulation of
cGMP
Cells were grown in differentiation medium and
treated with puerarin or 17β-estradiol (10−8 M) in the
presence or absence of L-NAME (6×10−3 M) or ICI182780
(10−7 M) for eight days. Following being washed with
phenol-free α-MEM, the cells were incubated with phenol-free α-MEM
supplemented with 5×10−4 M IBMX, a diesterase inhibitor,
at 37°C for 15 min. The reagents used for treatment were then added
to the media as above and the cells were incubated for another
hour. After incubation, the amount of cGMP in each sample was
measured using a cGMP ELISA kit from R&D Systems.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from each six-culture well
at the end of the incubation period (nodule formation) using RNA
simple Total RNA kit [Tiangen Biotech (Beijing) Co., Beijing,
China]. Reverse transcription of total RNA to cDNA was performed
with SuperScript III First Strand Synthesis System for RT-PCR
(Invitrogen Life Technologies, Carlsbad, CA, USA) in a MyCycler
Thermal Cycler (Bio-Rad Laboratories, Inc.) following the
manufacturer’s instructions.
qPCR was performed using a LightCycler 480 SYBR
Green I Master Mix (Roche Diagnostics GmbH, Mannheim, Germany) in a
LightCycler 480 system (Roche Diagnostics). The PCR reaction was
performed in a 20-µl volume in a LightCycler 480 96-well
plate and the cycling protocol was as follows: 95°C for 5 min,
followed by 45 PCR cycles of 95°C for 5 sec, 60°C for 15 sec and
72°C for 20 sec. Dissociation curves were run after amplification
to identify the specific PCR products. LightCycler 480 software,
version 1.5 was employed to perform the relative quantification for
the expression of target genes.
Specific primers for Runt-related transcription
factor 2 (Runx2), osterix and osteocalcin mRNA were as follows:
Runx2 mRNA forward, 5′-TGC TTC ATT CGC CTC ACAAA-3′ and reverse,
5′-TTG CAG TCT TCC TGG AGA AAGTT-3′; osterix mRNA forward, 5′-CCT
CTG CGG GAC TCA ACAAC-3′ and reverse, 5′-TAA AGG GGG CTG GAT AAG
CAT-3′; osteocalcin mRNA forward, 5′-GGA CTG TGA CGA GTT GGCTG-3′
and reverse, 5′-CCG TAG AAG CGC CGA TAGG-3′. β-actin was used as
the reference: β-actin mRNA forward, 5′-CGG TCA GGT CAT CAC
TATCG-3′ and reverse, 5′-TTC CAT ACC CAG GAA GGAAG-3′.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 5 software (GraphPad Software, Inc., La Jolla, CA,
USA). All values were expressed as the mean ± standard deviation
and statistically analyzed by one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Puerarin (10 µM) stimulates hBMSC
proliferation
To evaluate the effect of puerarin on cell
viability, an MTT assay was performed. hBMSCs were treated with
puerarin for 48 h at various concentrations and the results are
presented in Fig. 2. Puerarin at
lower concentrations significantly increased cell growth of hBMSCs,
with increases of 54.6±1.6% at 10 µM and 39.1±1.3% at 25
µM, which were similar to those of 17β-estradiol (45.1±1.1%)
(Fig. 2A). However, the
concentration of 100 µM had an obvious inhibition on
osteoblastic growth, and cell viability was reduced to 79.1±2.3%
(P<0.05) of that of the control group, suggesting that its
higher concentration may be cytotoxic in hBMSCs, as confirmed by
light microscopy following trypan blue staining (20% cell death;
results not shown). As 10 µM was the puerarin concentration
which was most effective on osteoblastic differentiation and
proliferation of hBMSC cultures, it was selected to be used in the
subsequent experiments. The puerarin-induced increase in
proliferation was completely abrogated in the presence of the
estrogen inhibitor ICI182780 (10−7 M) or the NOS
inhibitor L-NAME (6×10−3 M). ICI182780 or L-NAME alone
did not have any detectable effects (Fig. 2B).
Puerarin (10 µM) enhances osteoblastic
differentiation of hBMSCs
To determine whether puerarin altered ALP activity,
a marker for the differentiation of hBMSCs, cellular ALP activity
was measured following incubation with different concentrations of
puerarin (Fig. 3). Puerarin had
marked effects on ALP activity, which was increased by 34.65±1.4%
and 24.5±1.2% (P<0.05) following incubation with 10 and 25
µM puerarin, while it was suppressed by 16.1±0.8%
(P<0.05) following incubation with 100 µM puerarin.
However, ALP activity was unaffected by 17β-estradiol. It is
clearly shown that a dose of 10 µM puerarin had the maximum
effect on ALP activity (Fig. 3A).
A previous study by our group identified a time-dependent increase
in ALP activity and calcium deposition of hBMSCs in culture, which
reached the highest levels after 8 and 12 days, respectively.
Therefore, the present study determined these two parameters after
8 or 12 days of incubation with puerarin. As shown in Fig. 3A, puerarin (2.5–100 µM)
caused a dose-dependent increase in ALP activity at day eight, and
the highest effect was obtained at a dose of 10 µM. Again,
this elevation was clearly eliminated in the presence of the
estrogen antagonist ICI182780 or the NOS inhibitor L-NAME (Fig. 3B). Treatment with ICI182780 or
L-NAME alone had no effect on ALP activity.
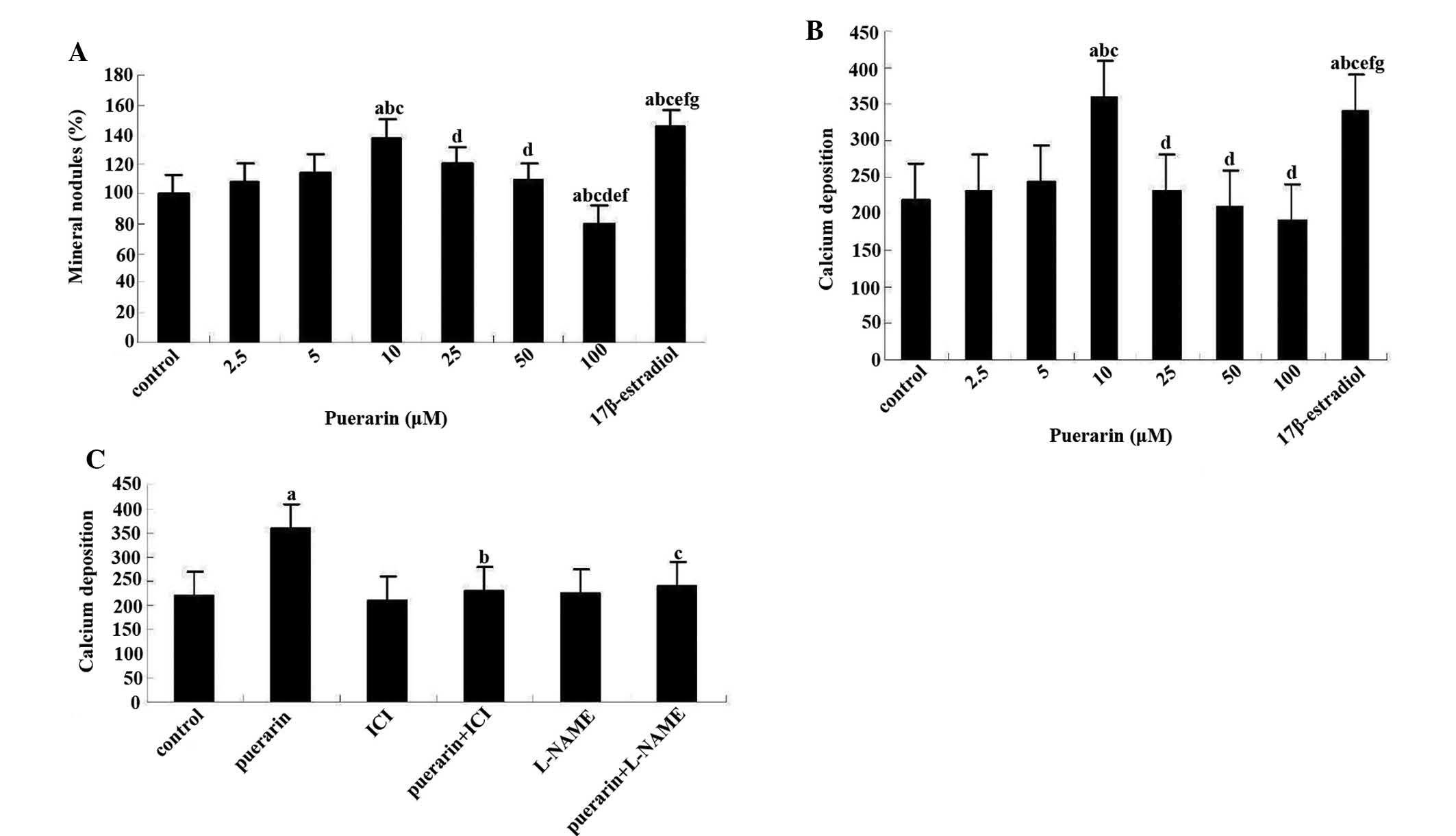
Puerarin (10 µM) increases mineralized
nodule formation and calcium deposition in hBMSCs
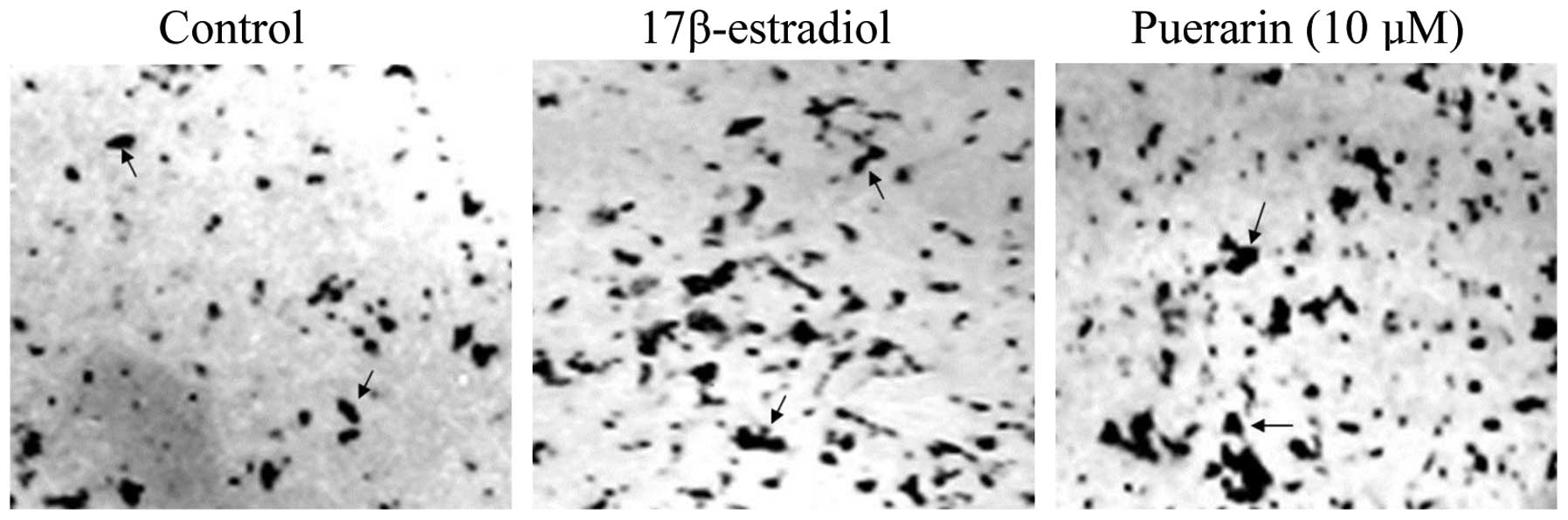
The formation of mineralized nodules was observed in
the present study. Alizarin red S staining indicated that mineral
nodules were formed after 12 days of culture. Collagen I fibers and
needle-like dense deposits, indicative of crystalline apatite
structures, were obvious in these nodules (Fig. 4). Only a concentration of 10
µM puerarin increased the number of mineral nodules formed,
causing a 41±2.1% increase (P<0.05) in the number of nodules
(Fig. 5A). This effect was
slightly weaker than that of 17β-estradiol. By contrast, puerarin
at higher concentrations markedly decreased bone nodule formation,
with decreases of 17±0.3% (P<0.05) at 50 µM and 21±0.7%
(P<0.05) at 100 µM. In another experiment, assessment of
calcium deposition showed that, as expected, 10 µM puerarin
caused the largest amount of calcium deposition (Fig. 5B). Furthermore, co-administration
of puerarin (10 µM) with ICI182780 or L-NAME abolished the
increase in calcium deposition in the cultures following 12 days,
while treatment with ICI182780 or L-NAME alone had no effect on
calcium deposition (Fig. 5C).
Puerarin (10 µM) enhances mRNA expression
of Runx2, osterix and osteocalcin in hBMSCs
The effect of puerarin on the expression of
Runx2/core-binding factor alpha 1 (CBFA1), osterix and osteocalcin
were evaluated by RT-qPCR analysis in RNA preparations from hBMSC
cultures at day eight (Table I).
Puerarin (10 µM) significantly increased mRNA levels of
Runx2/CBFA1 as well as its downstream genes osterix and osteocalcin
as compared with those in the control. ICI182780 (10−7
M) or L-NAME (6×10−3 M) reversed the puerarin-mediated
upregulation of Runx2/Cbfa1, osterix and osteocalcin. In addition,
ICI182780 (10−7 M) or L-NAME (6×10−3 M) alone
had no effect on the expression of these genes, whereas E2
(10−8 M) served as a positive control and produced
similar results to those of puerarin. These results were consistent
with the previously observed NO/GMP signaling involved in puerarin-
or E2-induced osteoblastic differentiation (22,23).
 | Table IEffect of estrogen receptor
antagonist ICI182780 and nitrogen oxide synthase inhibitor L-NAME
on the puerarin-induced mRNA expression of Runx2, osterix and
osteocalcin of human bone marrow stromal cells by quantitative
polymerase chain reaction. |
Table I
Effect of estrogen receptor
antagonist ICI182780 and nitrogen oxide synthase inhibitor L-NAME
on the puerarin-induced mRNA expression of Runx2, osterix and
osteocalcin of human bone marrow stromal cells by quantitative
polymerase chain reaction.
| Group | Runx2 | Osterix | Osteocalcin |
|---|
| Control | 1.0±0.18 | 1.0±0.16 | 1.0±0.21 |
| Puerarin (10
µM) | 3.7±0.24a | 4.8±0.19a | 4.3±0.21a |
| ICI182780 | 1.0±0.24 | 1.1±0.18 | 0.9±0.24 |
| Puerarin +
ICI182780 | 1.1±0.22b | 1.2±0.19b | 1.0±0.16b |
| L-NAME | 0.9±0.13 | 0.8±0.25 | 1.1±0.24 |
| Puerarin +
L-NAME | 1.2±0.17b | 1.3±0.24b | 1.0±0.21b |
| E2 | 4.1±0.22a | 3.9±0.24a | 3.1±0.24a |
| E2 +
ICI182780 | 1.2±0.21c | 0.9±0.14c | 1.3±0.24c |
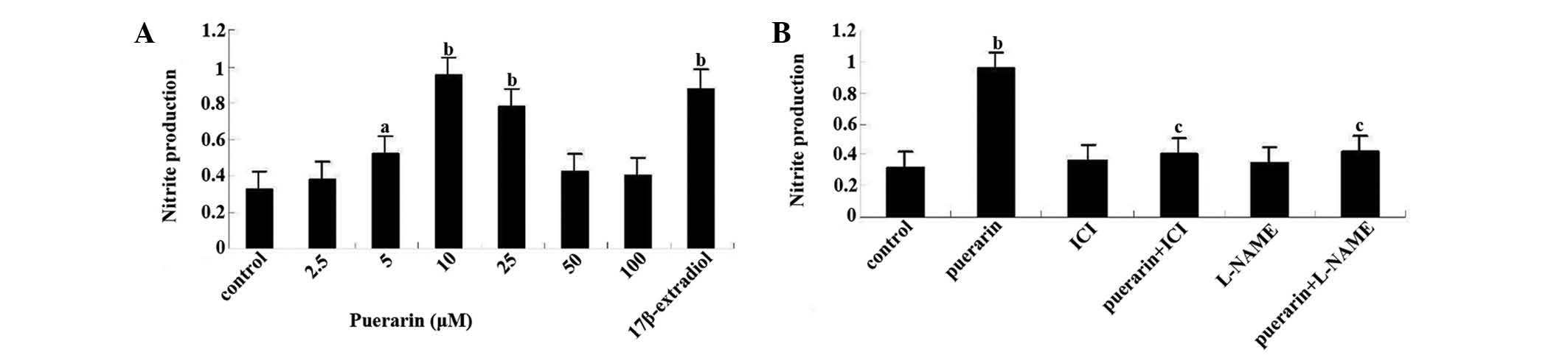
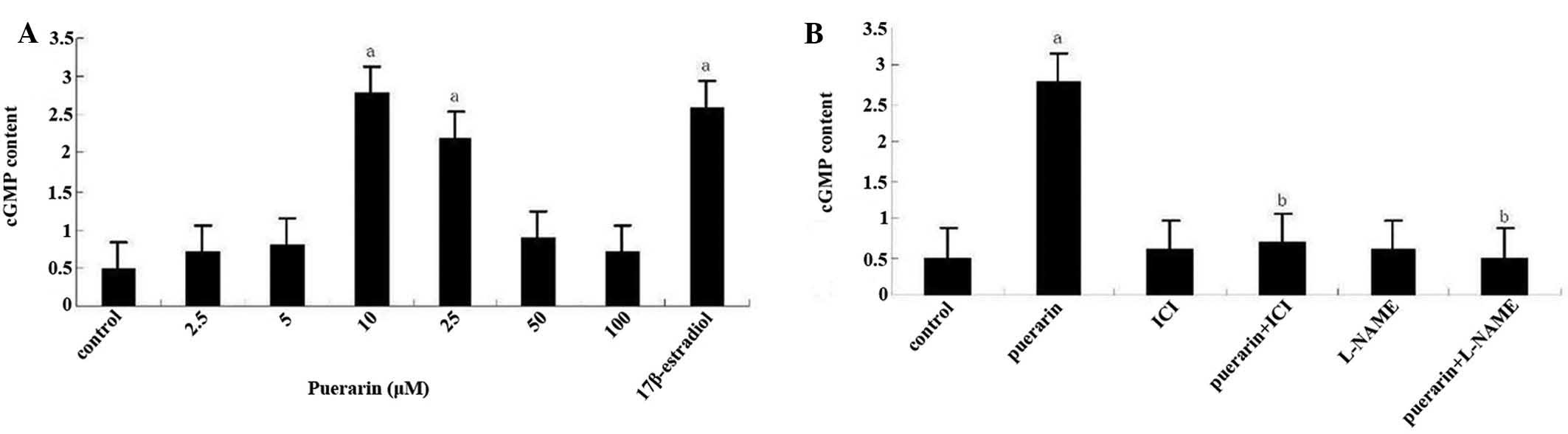
Puerarin (10 µM) increases the production
of NO and cGMP in hBMSC cultures
Puerarin at concentrations of 2.5–10 µM
dose-dependently enhanced NO levels in the culture media of BMSCs
following 12 days, while these increases in NO production compared
with the control faded with increasing doses of puerarin (>10
µM) (Fig. 6A). This effect
was abolished in the presence of ICI182780 or L-NAME (Fig. 6B). Assessment of cGMP formation in
hBMSC cultures showed that only 10 and 25 µM puerarin caused
significant increases in cGMP levels, which were up to three-fold
those in the 5 µM puerarin group or the control group
(Fig. 7A). This stimulating effect
was completely inhibited by co-incubation with ICI182780 or L-NAME.
Again, treatment with ICI182780 or L-NAME alone did not affect the
cGMP content in the culture media (Fig. 7B).
Discussion
Estrogen has long been thought to only affect
osteoclasts (24); however, it was
recently shown that estrogen can also stimulate osteoblasts
proliferation (25). Turners
(26) hypothesized that estrogen
can promote bone formation mainly by increasing the number of
osteoblasts, which coincides with its effects on osteoporosis
integrally. It is even thought that estrogen may act on the
increased inducible NOS (iNOS) gene to gradually release NO and
then regulate osteoblast activity (27,28).
Puerarin is a naturally occurring polyphenol that structurally
resembles E2 and possesses estrogenic activity (29), suggesting that it may exert similar
functions to those of E2 on NO synthesis and osteoblastic
metabolism (30,31).
The present study found that puerarin promotes
osteoblastic anabolism in hBMSCs through the NO/cGMP/type II cGMP
dependent protein kinase (PKG II) signaling pathway. It was
demonstrated that puerarin dose-dependently elevated NO production
as well as cGMP content in hBMSC cultures, which was associated
with the stimulatory effects of puerarin on proliferation and
osteoblastic differentiation of hBMSCs. Moreover, the
puerarin-induced increase in NO levels was abolished by L-NAME, a
non-selective NOS inhibitor, suggesting that puerarin has
NO-releasing effects. This is consistent with the demonstrated
protective effects of puerarin in the cardiovascular system
(32).
NO is important for bone remodeling, as evidenced by
in vitro and in vivo studies. NO synthesis in
osteoblasts incubated with puerarin was mediated, in part, through
the activation of endothelial NO synthase (eNOS) and increased iNOS
expression (33,34). NO produced in osteoblasts incubated
with puerarin was shown to activate soluble guanylate cyclase,
increasing intracellular cGMP, which activates soluble type I and
membrane-bound type II PKG; other cGMP targets include
phosphodiesterases and cyclic nucleotide-gated ion channels
(35). PKG II-deficient mice are
dwarfs due to a block in chondrocyte differentiation in bone growth
plates, while PKG I-deficient mice have no obvious skeletal
abnormalities (36). Pre-clinical
and clinical studies supported osteogenic functions of NO, although
optimal dosing of NO and the potential for NO-induced oxidative
stress may be problematic (37).
The present study identified that puerarin
dose-dependently elevated NO production as well as cGMP/PKG II
content in hBMSC cultures. However, puerarin at higher
concentrations (100 µM) remarkably decreased the NO/cGMP/PKG
II content in cultured hBMSCs. The mechanisms underlying these
processes require further study. Of note, L-NAME did not have any
significant effect on control levels of nitrite production,
although previous studies have shown an association between NO
production, osteoblast differentiation and bone production.
In the present study, NO production and the anabolic
effect of puerarin on hBMSCs were simultaneously blocked by
ICI182780, an estrogen receptor (ER) antagonist, suggesting that
puerarin exerts its function through ERs and that the NOS-NO
pathway may include downstream effectors of the ER. These findings
are consistent with other studies showing the stimulatory effects
of puerarin on the osteoblastic cell line MC3T3-E1, which were also
blocked by tamoxifen, another anti-estrogen reagent (38).
The present study demonstrated that puerarin, the
major active component of the Traditional Chinese Medicine Radix
Puerariae, potently induced osteoblastic differentiation markers,
including ALP, Runx2, osterix and osteocalcin, and mineralization
in hBMSCs. Upregulation of ALP, an enzyme serving as a marker of
osteoblastic differentiation, occurs at the middle stage of
differentiation. Puerarin, at concentrations <10 µM,
significantly increased ALP activity in a dose-dependent manner.
Runx2, which belongs to the Runx family, is a key transcriptional
modulator of osteoblast differentiation (39). Runx2 has been shown to induce ALP
activity, expression of bone matrix protein genes and
mineralization in immature mesenchymal cells and osteoblastic cells
in vitro (40). Osterix and
osteocalcin (also known as the bone gla protein), which induce
mesenchymal cells to differentiate into osteoblasts, are
traditionally considered markers of osteoblast activity, as they
are produced in osteoblasts and are associated with high bone
turnover and increased bone mineral density (BMD) in a variety of
clinical settings (41,42). In the present study, puerarin (10
µM) significantly increased mRNA expression of Runx2/CBFA1
as well as that of its downstream genes osterix and osteocalcin.
The increase was slightly weaker than that caused by 17β-estradiol.
Only a puerarin concentration of 10 µM significantly
affected the number of mineral nodules formed, causing a ~38%
increase in the number of nodules. By contrast, at the highest
concentration of 100 µM, puerarin markedly decreased bone
nodule formation. However, the mechanisms underlying these
processes remain elusive.
In conclusion, the results of the present study
indicated that puerarin, at concentrations <10 µM,
stimulated proliferation and osteoblastic differentiation of hBMSCs
in a dose-dependent manner. This effect was mediated by estrogen
receptors acting through the NO/cGMP/PKG II signaling pathway.
However, puerarin at higher concentrations impaired osteoblastic
proliferation and differentiation. The present study therefore
suggested that puerarin may promote osteoblastic formation and
effectively prevent post-menopausal osteoporosis. However, there is
little research in vivo in the present, thus further
experiments in an animal model will provide a foundation for
clinical treatment of osteoporosis.
Acknowledgments
The present study was supported by a grant from the
natural science foundation of Gansu Province in China (grant no.
1308RJZA232).
Abbreviations:
|
NO
|
nitric oxide
|
|
cGMP
|
cyclic guanosine monophosphate
|
|
hBMSC
|
human bone marrow stromal cells
|
|
HRT
|
hormone replacement therapy
|
|
ALP
|
alkaline phosphatase
|
|
L-NAME
|
Nx-nitro-L-arginine methyl ester
|
References
|
1
|
Corina M, Vulpoi C and Brănişteanu D:
Relationship between bone mineral density, weight, and estrogen
levels in pre and postmenopausal women. Rev Med Chir Soc Med Nat
lasi. 116:946–950. 2012.
|
|
2
|
Han KO, Moon IG, Kang YS, et al:
Nonassociation of estrogen receptor genotypes with bone mineral
density and estrogen responsiveness to hormone replacement therapy
in Korean postmenopausal women. J Clin Endocrinol Metab.
82:991–995. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Turner C: Hormone replacement therapy: its
use in the management of acute menopausal symptoms. J Am Acad Nurse
Pract. 6:318–320. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Palacios S, Christiansen C, Sánchez
Borrego R, et al: Recommendations on the management of fragility
fracture risk in women younger than 70 years. Gynecol Endocrinol.
28:770–786. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lees B and Stevenson JC: The prevention of
osteoporosis using sequential low-dose hormone replacement therapy
with estradiol-17 beta and dydrogesterone. Osteoporos Int.
12:251–258. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaya C, Dinçer Cengiz S, Cengiz B and
Akgün G: The long-term effects of low-dose 17beta-estradiol and
dydrogesterone hormone replacement therapy on 24 h ambulatory blood
pressure in hypertensive postmenopausal women: a 1-year randomized,
prospective study. Climacteric. 9:437–445. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Popp AW, Bodmer C, Senn C, et al:
Prevention of postmenopausal bone loss with long-cycle hormone
replacement therapy. Maturitas. 53:191–200. 2006. View Article : Google Scholar
|
|
8
|
Pike MC and Ross RK: Progestins and
menopause: epidemiological studies of risks of endometrial and
breast cancer. Steroids. 65:659–664. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boué SM, Wiese TE, Nehls S, et al:
Evaluation of the estrogenic effects of legume extracts containing
phytoestrogens. J Agric Food Chem. 51:2193–2199. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen L and Lu ZP: Randomized controlled
trial of integrated traditional Chinese and western medicine
treatment for posthepatitic cirrhotic ascites: a systematic review.
Chin J Hepatol. 19:205–209. 2011.In Chinese.
|
|
11
|
Lagari VS and Levis S: Phytoestrogens in
the prevention of postmenopausal bone loss. J Clin Densitom.
16:445–449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Z, Lin SY, Zhou YH, et al: Analysis
of clinical features of traditional Chinese medicine symptoms and
syndromes of 220 patients with chronic aplastic anemia. Chin J
Integr Tradit West Med. 34:43–45. 2014.In Chinese.
|
|
13
|
Boué SM, Burow ME, Wiese TE, et al:
Estrogenic and anti-estrogenic activities of phytoalexins from red
kidney bean (Phaseolus vulgaris L.). J Agric Food Chem. 59:112–120.
2011. View Article : Google Scholar
|
|
14
|
Wang Y, Wang WL, Xie WL, Li LZ, Sun J, Sun
WJ and Gong HY: Puerarin stimulates proliferation and
differentiation and protects against cell death in human
osteoblastic MG-63 cells via ER-dependent MEK/ERK and PI3K/Akt
activation. Phytomedicine. 20:787–796. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Armour KJ, Armour KE, van’t Hof RJ, Reid
DM, Wei XQ, Liew FY and Ralston SH: Activation of the inducible
nitric oxide synthase pathway contributes to inflammation-induced
osteoporosis by suppressing bone formation and causing osteoblast
apoptosis. Arthritis Rheum. 44:2790–2796. 2001. View Article : Google Scholar
|
|
16
|
Samuels A, Perry MJ, Gibson RL, Colley S
and Tobias JH: Role of endothelial nitric oxide synthase in
estrogen-induced osteogenesis. Bone. 29:24–29. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang DH, Hu YS, Du JJ, et al: Ghrelin
stimulates proliferation of human osteoblastic TE85 cells via
NO/cGMP signaling pathway. Endocrine. 35:112–117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
El-Mowafy AM, Alkhalaf M and Jaffal SM:
Nongenomic activation of the GC-A enzyme by resveratrol and
estradiol downstream from membrane estrogen receptors in human
coronary arterial cells. Nutr Metab Cardiovasc Dis. 17:508–516.
2007. View Article : Google Scholar
|
|
19
|
Pan W, Quarles LD, Song LH, et al:
Genistein stimulates the osteoblastic differentiation via NO/cGMP
in bone marrow culture. J Cell Biochem. 94:307–316. 2005.
View Article : Google Scholar
|
|
20
|
Andelman F, Kipervasser S, Maimon S, Fried
I, Parmet Y and Neufeld MY: A revised intracarotid etomidate memory
(Wada) procedure. Acta Neurol Scand. 127:97–102. 2013. View Article : Google Scholar
|
|
21
|
Hokazono E, Osawa S, Nakano T, et al:
Development of a new measurement method for serum calcium with
chlorophosphonazo-III. Ann Clin Biochem. 46:296–301. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Holzer G, Einhorn TA and Majeska RJ:
Estrogen regulation of growth and alkaline phosphatase expression
by cultured human bone marrow stromal cells. J Orthop Res.
20:281–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Almeida M, Martin-Millan M, Ambrogini E,
et al: Estrogens attenuate oxidative stress and the differentiation
and apoptosis of osteoblasts by DNA-binding-independent actions of
the ERalpha. J Bone Miner Res. 25:769–781. 2010.
|
|
24
|
MacIntyre I, Zaidi M, Alam AS, Datta HK,
Moonga BS, Lidbury PS, Hecker M and Vane JR: Osteoclast inhibition:
an action of nitric oxide not mediated by cyclic GMP. Proc Natl
Acad Sci USA. 88:2936–2940. 1991. View Article : Google Scholar
|
|
25
|
Galea GL, Meakin LB, Sugiyama T, et al:
Estrogen receptor α mediates proliferation of osteoblastic cells
stimulated by estrogen and mechanical strain, but their acute
down-regulation of the Wnt antagonist Sost is mediated by estrogen
receptor β. J Biol Chem. 288:9035–9048. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Turner RT, Evans GL and WakIey GK:
Mechanism of action of estrogen on cancellous bone balance in
tibiae of ovariectomized growing rat: Inhibition of indices of
formation and resorption. J Bone Miner Res. 8:359–366. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Q, Sun L, Zhang F, et al: Effects of
17β-estradiol on cell proliferation, cAMP and cGMP content and iNOS
activity in human osteoblast-like osteosarcoma cell line TE85. Chin
Pharmacol Bull. 14:314–317. 1998.
|
|
28
|
Cervellati C, Bonaccorsi G, Cremonini E,
et al: Oxidative stress and bone resorption interplay as a possible
trigger for postmenopausal osteoporosis. Biomed Res Int.
2014:563–569. 2014. View Article : Google Scholar
|
|
29
|
Manolagas SC, O’Brien CA and Almeida M:
The role of estrogen and androgen receptors in bone health and
disease. Nat Rev Endocrinol. 9:699–712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Michihara S, Tanaka T, Uzawa Y, Moriyama T
and Kawamura Y: Puerarin exerted anti-osteoporotic action
independent of estrogen receptor-mediated pathway. J Nutr Sci
Vitaminol (Tokyo). 58:202–209. 2012. View Article : Google Scholar
|
|
31
|
Saha P, Saraswat G, Chakraborty P,
Banerjee S, Pal BC and Kabir SN: Puerarin, a selective oestrogen
receptor modulator, disrupts pregnancy in rats at pre-implantation
stage. Reproduction. 144:633–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deng Y, Ng ES, Yeung JH, et al: Mechanisms
of the cerebral vasodilator actions of isoflavonoids of Gegen on
rat isolated basilar artery. J Ethnopharmacol. 139:294–304. 2012.
View Article : Google Scholar
|
|
33
|
Sheu SY, Tsai CC, Sun JS, Chen MH, Liu MH
and Sun MG: Stimulatory effect of puerarin on bone formation
through co-activation of nitric oxide and bone morphogenetic
protein-2/mitogen-activated protein kinases pathways in mice. Chin
Med J (Engl). 125:3646–3653. 2012.
|
|
34
|
Brown JP, Dempster DW, Ding B, Dent-Acosta
R, San Martin J, Grauer A, Wagman RB and Zanchetta J: Bone
remodeling in postmenopausal women who discontinued denosumab
treatment: off-treatment biopsy study. J Bone Miner Res.
26:2737–2744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rangaswami HI, Marathe N, Zhuang S, et al:
Type II cGMP-dependent protein kinase mediates osteoblast
mechano-transduction. Handb Exp Pharmacol. 191:137–162. 2009.
|
|
36
|
Pfeifer A, Aszódi A, Seidler U, Ruth P,
Hofmann F and Fässler R: Intestinal secretory defects and dwarfism
in mice lacking cGMP-dependent protein kinase II. Science.
274:2082–2086. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wimalawansa SJ: Rationale for using nitric
oxide donor therapy for prevention of bone loss and treatment of
osteoporosis in humans. Ann NY Acad Sci. 1117:283–297. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang F, Zhang R, He F, Wang XX, Zhao S and
Yang G: Osteoblast response to puerarin-loaded porous titanium
surfaces: an in vitro study. J Biomed Mater Res A. 100:1419–1426.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang N, Wang X, Cheng W, et al: Puerarin
promotes osteogenesis and inhibits adipogenesis in vitro. Chin Med.
8:172013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brown JP, Delmas PD, Malaval L, Edouard C,
Chapuy MC and Meunier PJ: Serum bone Gla-protein: a specific marker
for bone formation in postmenopausal osteoporosis. Lancet.
1:1091–1093. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Minisola S, Rosso R, Romagnoli E, et al:
Serum osteocalcin and bone mineral density at various skeletal
sites: a study performed with three different assays. J Lab Clin
Med. 129:422–429. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Koo KT, Lee SW, Lee MH, Kim KH, Jung SH
and Kang YG: Time-dependent expression of osteoblast marker genes
in human primary cells cultured on microgrooved titanium substrata.
Clin Oral Implants Res. 25:714–722. 2014. View Article : Google Scholar
|