Introduction
Lung cancer is the first and second leading cause of
cancer-related mortality in males and females, respectively,
worldwide. In numerous regions of the world, the number of cases
and deaths associated with lung cancer is on the rise (1), however, the etiological factors of
the disease remain unknown. Early-stage lung cancer is curable and
may be alleviated by radiation, chemotherapy, targeted therapies
and surgical resection. However, lung cancer is diagnosed at
advanced stages in the majority of patients (2); therefore, studies investigating the
etiology of lung cancer and its carcinogenic mechanisms are a
primary focus in lung cancer studies.
Air pollution, primarily from fossil fuel
combustion, is an important factor for the development of cancer.
The combustion of diesel fuel is a likely source of air pollution
that affects cancer risk on a large scale (3). Diesel exhaust particles (DEPs), a
component of the diesel exhaust product, was classified as a
‘probable’ carcinogen by the International Agency for Research on
Cancer (IARC) in 1989 (4). This
classification was confirmed by a series of human and animal
studies suggesting that DEPs have an important role in
tumorigenesis in a variety of cancer types, including
gastrointestinal, cervical, breast and particularly, lung cancer
(4–8). However, the mechanism by which DEPs
cause cancer remains unclear.
MicroRNAs (miRNAs) are single-stranded RNA 20–30
nucleotides in length that function as negative regulators to
silence or suppress gene expression. Aberrant miRNA expression has
been implicated in several cellular processes and the pathogenic
pathways of a number of diseases (9). Initial studies in ovarian, breast,
bladder and lung cancer patients demonstrated that the levels of
specific miRNAs in cells are important with regard to the
occurrence, development and treatment of the types of cancer
(10–13).
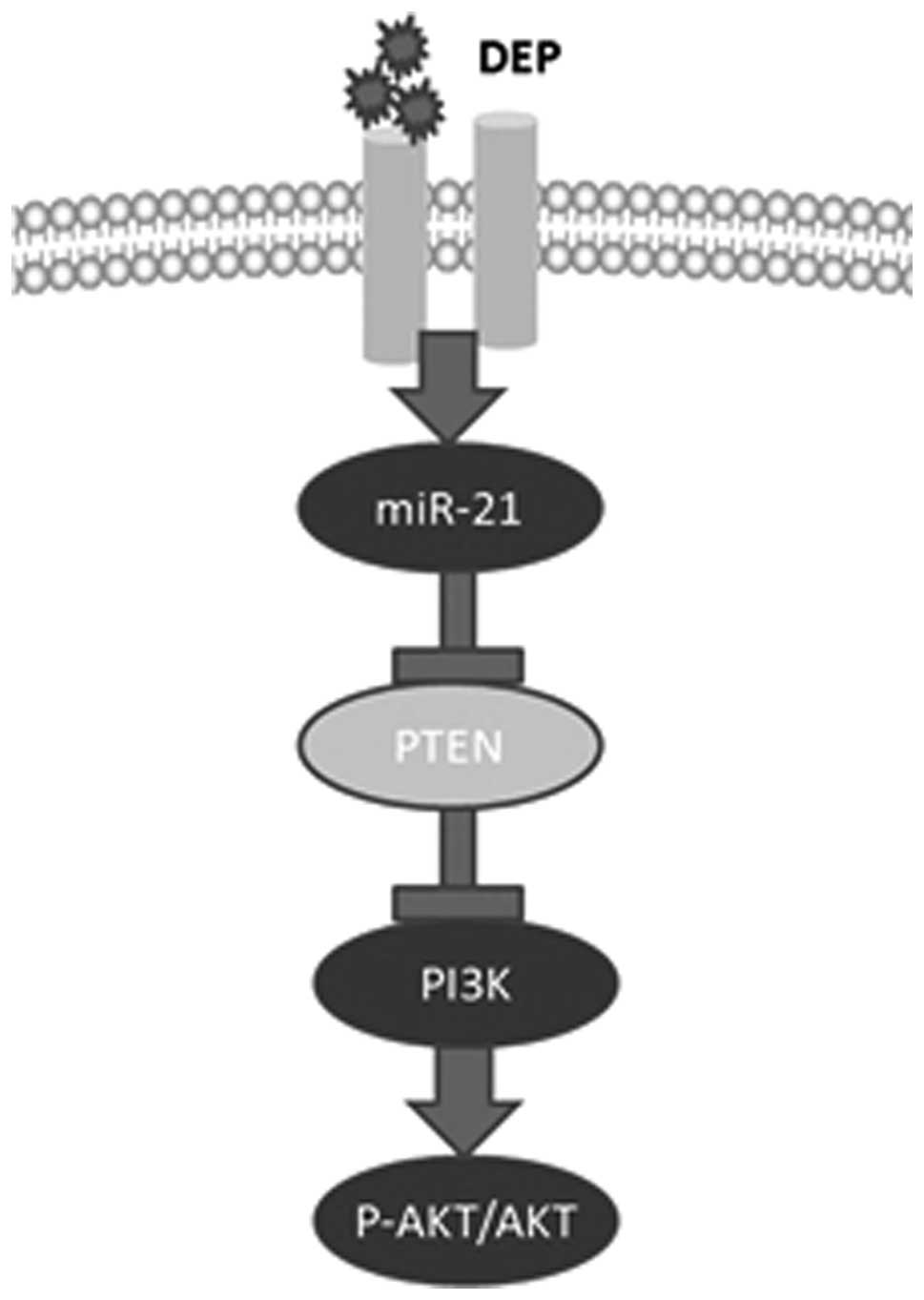
Previous studies demonstrated that DEPs activate a
variety of carcinogenic pathways (14). Additional studies have indicated
that DEPs increase or decrease the expression of a variety of
microRNAs (15). However, this
finding is not a definitive result. In the present study, microRNAs
were combined with carcinogenic pathways to examine the potential
carcinogenic mechanism of DEPs in normal human bronchial epithelial
(HBE) cells. It was confirmed that DEPs upregulate the expression
of miR-21. DEPs activate phosphatase and tensin homolog
(PTEN)/phosphatidylinositol 3′-kinase (PI3K)/AKT signaling through
miR-21. The present study may provide a new mechanism for lung
carcinogenesis and it is expected to serve as a reference for
investigating lung cancer etiology.
Materials and methods
Cell culture
HBE cells were purchased from American Type Culture
Collection (ATCC; Rockville, MD, USA). Lung adenocarcinoma cells
(A549) were obtained from the Laboratory of Neuro-oncology, Tianjin
Neurological Institute (Tianjin, China). The cells were cultured in
RPMI-1640 supplemented with 10% fetal calf serum (FCS), 1%
penicillin-streptomycin and 1% glutamine. All of the cells were
cultured in six-well plates in a 37°C humidified incubator supplied
with 5% CO2.
DEP treatment
The DEPs were a gift from the State Key Laboratory
of Internal Combustion Engine Fuel Science of Tianjin University
(Tianjin, China). DEPs (100 µg/ml) were freshly prepared and
suspended in 100 ml RPMI-1640 with 10% FCS, 1%
penicillin-streptomycin and 1% glutamine by sonication. The
suspended particles were then stored at 4°C in the dark. The cells
were cultured in six-well plates and then treated with 3 ml
RPMI-1640 in the experimental group.
Oligonucleotides and cell transfection
with the 2′-O-methyl (2′-O-Me-) miR-21
The inhibitors were chemically synthesized by
Shanghai GenePharma (Shanghai, China). The 2′-O-Me oligonucleotides
were composed entirely of 2′-O-methyl bases and contained the
following sequences: 5′-GTCCAC TCT TGT CCT CAA TG-3′; scrambled
sequences were 5′-AAG GCA AGC UGA CCC UGA AGU-3′. The
oligonucleotides were purified using a high-pressure liquid
chromatography system, dissolved in diethylpyrocarbonate (DEPC)
water and then frozen at −20°C. Oligonucleotides (50 nm/l) were
transfected into HBE cells at 70% confluence using Oligofectamine
according to the manufacturer’s instructions (Invitrogen Life
Technologies, Carlsbad, CA, USA).
RNA extraction and quantitative
polymerase chain reaction (qPCR)
Total RNA was extracted using the TRIzol reagent
(Invitrogen Life Technologies) according to the standard
procedures. A NanoDrop spectrophotometer (Gene Co., Ltd., Hong
Kong, China) was used to detect the total RNA concentration. Total
RNA (1 µg) was used to synthesize cDNA by reverse
transcription with MMLV reverse transcriptase (Promega Corporation,
Madison, WI, USA), according to the manufacturer’s instructions.
qPCR analysis was performed to determine the miR-21 expression in
HBE cells, A549 and DEP-treated HBE cells 48 h following
transfection with the miR-21 inhibitor or scrambled negative
control. The expression of u6 was used as an internal control. A
total of 40 cycles of qPCR were performed, consisting of 95°C for 3
min, 95°C for 15 sec and 60°C for 30 sec. RT and PCR primers were
purchased from GenePharma, 5S was used for normalization. The
relative quantification was conducted using amplification
efficiencies derived from cDNA standard curves. The data are
expressed as the fold change (2−ΔΔCt) and initially
analyzed using Opticon Monitor Analysis Software V2.02 software (MJ
Research Inc., Waltham, MA, USA).
Protein extraction and western
blotting
Following treatment with DEPs and DEPs in
combination with the miR-21 anti-sense oligonucleotide (ASODN) for
48 h, the adherent cells in the six-well plate were rinsed with
phosphate-buffered saline (PBS), and the cell extracts were
prepared by suspension in ice-cold lysis buffer. Floating cells
from the same well were collected, lysed and mixed with SDS loading
buffer (100 mM Tris-Cl, pH 6.8; 200 mM DTT; 4% SDS; 0.2% bro-P-40
lysis buffer [20 mM Tris, pH 8.0; 137 mM NaCl; 1% Nonidet P-40; 10%
glycerol; 1 mM CaCl2; 1 mM MgCl2; 1 mM phenylmethylsulfonyl
fluoride; 1 mM sodium fluoride; 1 mM sodium orthovanadate; and a
protease inhibitor mixture]). Homogenates were clarified by
centrifugation at 12,000 × g for 15 min at 4°C, and protein
concentrations were determined using a bicinchoninic acid protein
assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA). The next
40 mg of lysates was subjected to SDS-PAGE on 8% SDS-acrylamide
gels. The separate proteins were transferred onto PVDF membranes
(Millipore, Bedford, MA, USA) and incubated with primary antibodies
against AKT (1:1,000 dilution; rabbit polyclonal; Signalway
Antibody, College Park, MD, USA), PI3K (1:1,000 dilution; mouse
monoclonal; Cell Signaling Technology, Inc., Beverly, MA, USA),
PTEN, caspase 3, PCNA, cyclin D1, Bcl-2 (1:100 dilution; mouse
polyclonal; Zhongshan Goldenbridge Biotechnology Co., Ltd.,
Beijing, China) and GAPDH (1:100 dilution; rabbit polyclonal;
Zhongshan Goldenbridge Biotechnology Co., Ltd.). The membranes were
then incubated with an HRP-conjugated secondary antibody (Zymed
Laboratories Inc., San Francisco, CA, USA). Specific proteins were
detected using a Super Signal protein detection kit (Pierce
Biotechnology, Inc.). The membrane was reprobed with an antibody
against GAPDH (1:100 dilution; rabbit polyclonal; Zhongshan
Goldenbridge Biotechnology Co., Ltd.) using the procedures
described above.
Cell cycle analysis
For cell cycle analysis using FCM (flow cytometry),
log phase transfected and control cells were harvested, washed with
PBS, fixed with 90% ethanol overnight at 41°C, then incubated with
RNase at 37°C for 30 min. The cell nuclei were stained with
propidium iodide (PI) for an additional 30 min. A total of 104
nuclei were examined using a FACS Calibur flow cytometer
(Becton-Dickinson, Franklin Lakes, NJ, USA). The samples were
analyzed using flow cytometry for the FL-2 area, DNA histograms
were analyzed using Modifit software. The experiments were
performed in triplicate. The results are presented as the
percentage of cells in a given phase. The apoptosis assays were
conducted using annexin staining and TUNEL methods.
Cells apoptosis analysis
To quantify DEP-induced apoptosis, annexin V/PI
staining was performed, and apoptosis was evaluated by flow
cytometry analysis. Following DEP treatment for 48 h, the floating
and attached cells were collected and subjected to annexin V/PI
staining using an annexin V-FITC Apoptosis Detection kit
(BioVision, Palo Alto, CA, USA), according to the manufacturer’s
instructions. The resulting fluorescence was measured by flow
cytometry using a FACS flow cytometer (Becton-Dickinson).
Matrigel invasion assay
The invasive ability of treated and untreated HBE
and A549 cells was assessed using a 24-well transwell chamber and a
reconstituted extracellular matrix membrane (Matrigel, BD Biocoat;
BD Bioscience Franklin Lakes, NJ, USA). The cell invasion chambers
were prepared by placing 100 µl of a 1:5 dilu tion of
Matrigel onto the upper filter and then incubating the cells for
~30 min at 37°C to allow for Matrigel polymerization. The untreated
HBE and A549 cells, as well as the HBE and A549 cells that were
treated with DEPs for 48 h, were removed from the culture flasks
and resuspended at 1×105 cells/ml in serum-free medium.
Then, 0.1 ml of the cell suspension was added to the upper
chambers. The medium supplemented with serum was used as a
chemoattractant in the lower chamber. The chambers were incubated
for 48 h at 37°C in a humid atmosphere of 5% CO2/95%
air. The filters were then fixed in 95% ethanol and stained with
hema toxylin. The upper surfaces of the filters were scraped thrice
with cotton swabs to remove the non-invasive cells. The experi
ments were repeated thrice and the migrated cells were
microscopically counted in five different fields per filter.
Wound healing assay
The HBE and A549 cells were cultured in six well
plates and the clones were grown to confluency. A linear wound was
made by scraping a closed Pasteur pipette across the confluent cell
layer, following 24 h DEP treatment in the experimental group. The
cells were washed twice to remove the detached cells and debris.
Next, the wounds sizes were observed and measured at set times.
Results
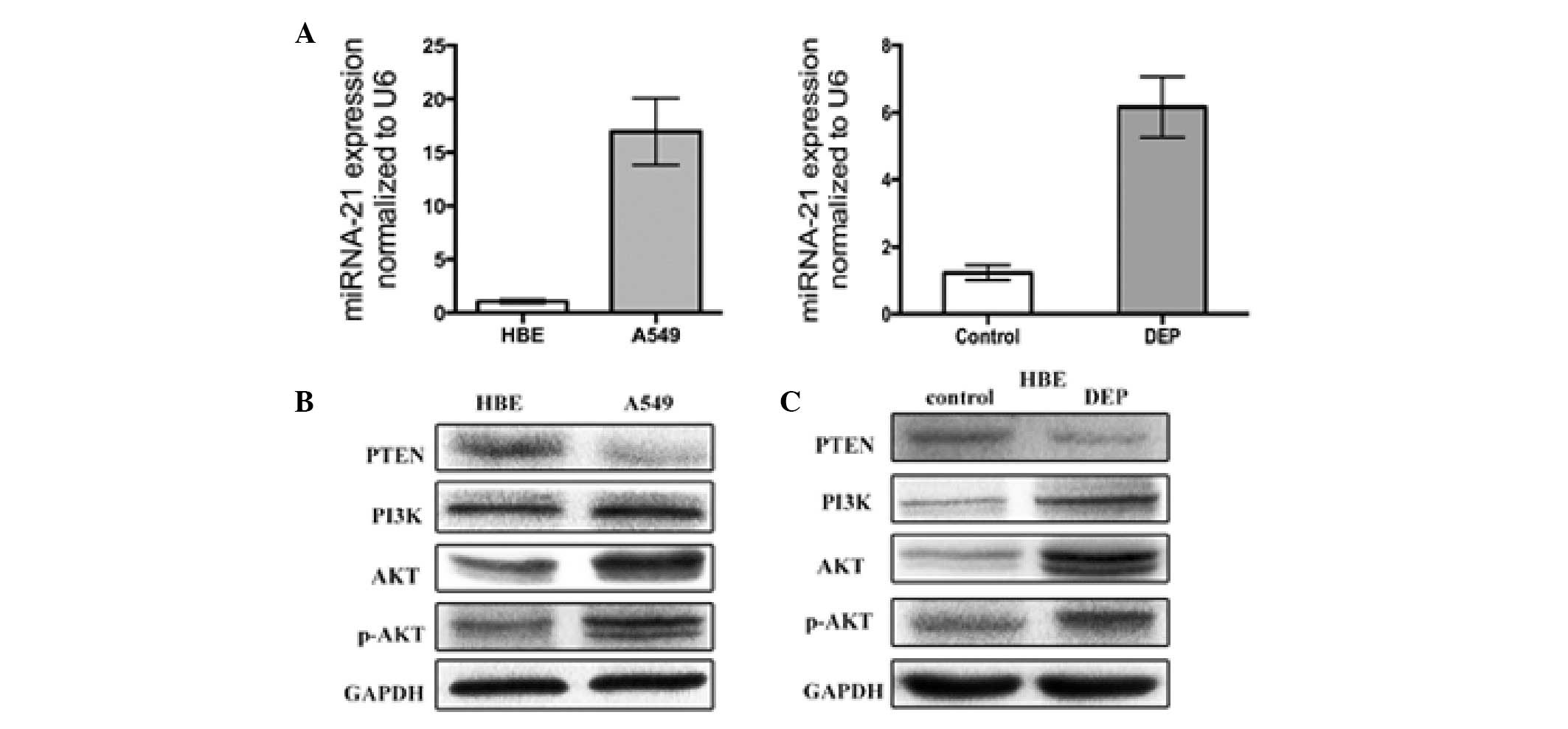
DEPs increase the miRNA-21 expression in
human air way epithelial cells
First, the miR-21 expression in HBE cells, A549 and
treated HBE cells was profiled. The analysis demonstrated that
miR-21 is overexpressed by greater than 16-fold in untreated A549
cells compared with the untreated HBE cells. The miR-21 expression
in the HBE cells treated with DEPs for 48 h was 6-fold higher than
the untreated HBE cells (Fig.
1A).
DEPs activate the PTEN/PI3K/AKT pathway
in HBE cell lines
The effect of DEPs on PTEN/PI3K/AKT activity was
then measured. PTEN, PI3K, phosphorylated AKT (p-AKT) and AKT were
immunoprecipitated from the total cell extracts derived from the
HBE cells treated with 100 µg/ml DEPs. The PTEN, PI3K, p-AKT
and AKT expression in untreated and treated HBE cells were examined
(Fig. 1B). As determined by
western blot analysis, the PTEN expression was significantly
reduced in the untreated HBE cells. However, PI3K, AKT and p-AKT
expression were markedly increased in the HBE cells following
treatment with DEPs for 48 h (Fig.
1C).
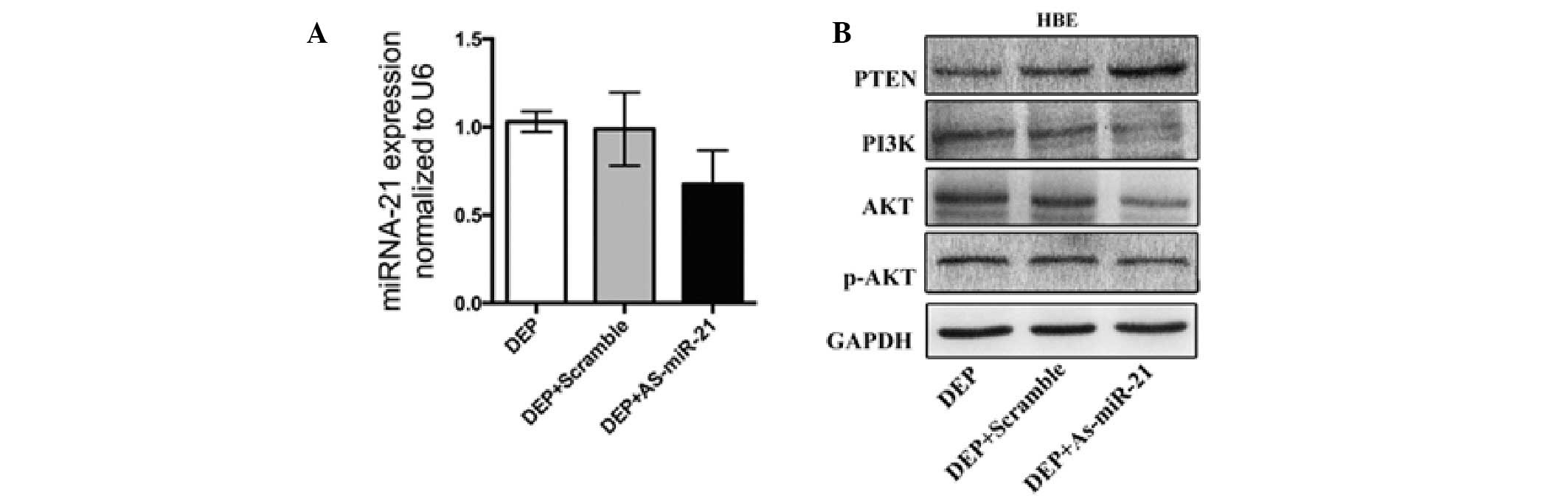
DEPs in combination with miR-21 antisense
oligonucle-otides (As-miR-21) alter DEP-mediated miR-21
expression
To evaluate the effect of the miR-21 inhibitor on
the experimental group treated with DEPs, qPCR was used to compare
the expression of miR21 in HBE cells transfected with the miR-21
inhibitor in the experimental and control groups. The measurements
were performed 48 h following transfection. The data were analyzed
from triplicate samples. DEPs alone increased the miR-21 expression
in the studied cells. However, following transfection of the
DEP-treated group with the miR-21 inhibitor, miR-21 expression was
significantly reduced (Fig.
2A).
As-miR-21 treatment suppresses activation
of the PTEN/PI3K/AKT pathway by DEPs in HBE cell lines
Studies have demonstrated that PTEN is a miR-21
target gene, and PTEN regulates the PI3K/AKT pathway (16). PTEN frequently inhibits PI3K/AKT
pathway activation in lung cancer (15,17).
Therefore, one potential function of spliced miR-21 may be to
upregulate the PTEN tumor suppressor gene. However, the above
analysis demonstrated that increasing the expression of miR-21
suppressed PTEN in HBE cells. Given that the inhibition of gene
expression by microRNAs is well understood, as-miR-21 was
transfected into the experimental group above, and an increase in
the PTEN protein expression and a reduction in the PI3K, p-AKT and
AKT expression in the HBE cells was subsequently observed (Fig. 2B).
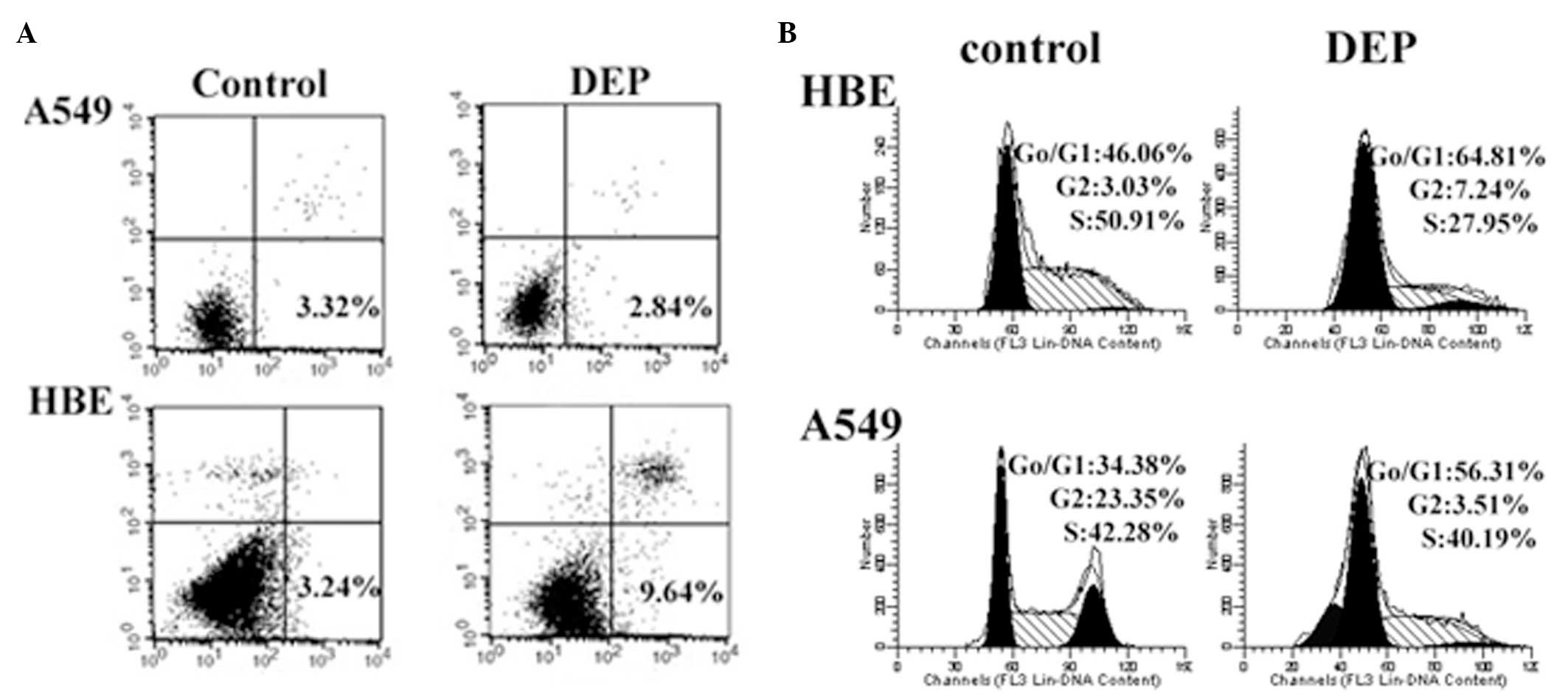
DEPs increase apoptosis in HBE cells but
not A549 cells
To analyze whether increased miR-21 expression in
HBE and A549 cells was able to alter cellular apoptosis,
DEP-induced apoptosis was measured via annexin V-PI staining in HBE
and A549 cells. The cells were exposed to DEPs (100 µg/ml)
for 48 h. As demonstrated in Fig. 3A
and B, a significant increase in early phase apoptotic cells
was observed in the DEP-treated HBE cells (9.64%) compared with the
untreated cells (3.24%) (P<0.05); however, no significant
differences were observed between the treated (2.84%) and untreated
A549 cells (3.32%; P>0.05; Fig.
3A).
DEPs induce G0/G1 arrest in HBE and A549
cells
DEP has been reported to induce growth arrest at the
G1/G0 phase in a wide variety of cells (18–20).
To understand the effects of DEP treatment on cell growth in the
present study, the cell cycle distribution of HBE and A549 cells 48
h following treatment with 100 µg/ml DEPs was measured via
PI staining. The untreated cells served as negative controls. DEPs
induce a significant increase (P<0.05) in the number of S-phase
cells in the A549 cell lines. DEPs markedly enhanced the number of
G0/G1-phase cells in HBE cells (P<0.05; Fig. 3B).
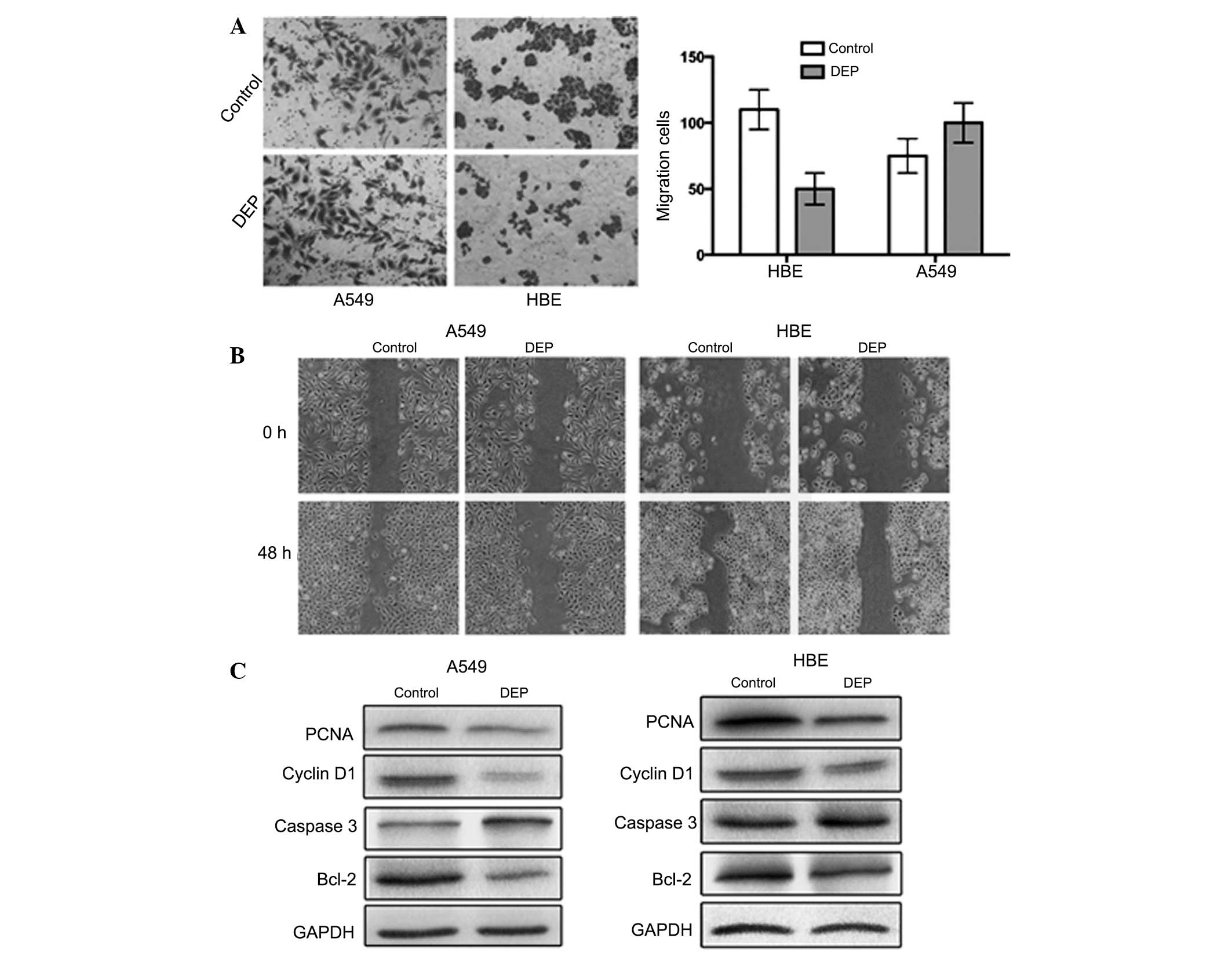
DEPs alter HBE cell migration and
invasion, but have a weak effect on A549 cells
To examine the functional consequences of DEPs in
HBE cells and A549 cell lines, the Transwell assay was used to
determine cell invasiveness and wound healing assays were used to
detect cell motility in the cells treated with DEPs (100
µg/ml). Significant differences in the number of invasive
cells were observed between the control and experimental groups
(Fig. 4A). In addition, evident
differences in the cellular migration remained between the treated
cells and the control samples. A significant portion of HBE cells
invaded the lower chamber (P<0.05), however this phenomenon was
not observed in the A549 cells (P>0.05). Cell motility was
suppressed by DEPs in the experimental group in HBE and A549 cells
(P<0.05), but the effect was less prominent in A549 cells
compared with HBE cells (Fig.
4B).
Discussion
In light of economic development and
industrialization, there is an increasing concern regarding air
pollutants, particularly PM2.5 (21) (particulate matter with an
aerodynamic diameter ≤2.5 µm). Various studies have
indicated that increases in lung cancer mortality are associated
with PM2.5 exposure (22,23). DEPs, an important component of air
pollution, is another research topic that has attracted notable
attention. Lung cancer remains one of the most frequently occurring
malignancies and numerous studies have demonstrated that DEPs may
promote lung carcinogenesis (4,7,8,24).
Therefore, the study of the molecular mechanisms underlying the
effects of DEPs in lung carcinogenesis remains a topic worthy of
investigation.
miR-21 is a unique miRNA highly expressed in a
variety of solid tumor types, including lung, stomach, prostate,
colon, esophageal, pancreatic and cervical cancer. With regard to
lung adenocarcinoma (25–27), miR-21 expression is increased
16-fold compared with normal tissue. Several studies have
demonstrated that miR-21 expression may serve as diagnostic
criteria in lung cancer (28). The
carcinogenic mechanism of DEPs may be associated with increased
expression of the suppressor gene, PTEN, in tumors (29,30).
In the present study, miR-21 oncogenic expression was 16-fold
higher in the A549 cells than in the HBE cells. Following DEP
treatment, miR-21 expression increased 6-fold in the HBE cells;
this expression level was similar in the A549 cells. Therefore, it
was hypothesized that DEPs increase the miR-21 expression in a
process that may be associated with the potential mechanisms of
carcinogenesis.
PTEN, also known as MMAC1 (mutated in multiple
advanced cancers), is a tumor suppressor gene with sequence
homology to tyrosine phosphatases and the cytoskeletal proteins
tensin and auxilin (31). PTEN is
an important miR-21 target gene. Among its numerous activities,
PTEN is involved in the integration of proteins during cell growth
regulation, tumor invasion, angiogenesis and metastasis. Numerous
studies have demonstrated that PTEN is an important signaling
molecule in the PTEN/PI3K/AKT signal transduction pathways
(16). The loss or decrease of
PTEN activity promotes the survival of NSCLC cells (15). However, certain studies suggest
that PTEN tumor suppressor function is exerted through negative
regulation of the PI3K/AKT cell survival pathway (17). Jardim et al (15) treated HBE cells with suspended DEPs
and the total RNA was isolated for the microarray analysis 24 h
following treatment. This study revealed that 197 of 313 detectable
miRNAs (62.9%) were either upregulated or downregulated by
1.5-fold. Furthermore, the expression of PTEN products was
significantly inhibited with the exception of PI3K and AKT, which
exhibited increased expression (15). In the present study, the expression
of PTEN, PI3K, p-AKT and AKT between untreated HBE and A549 cells
was first compared. The PTEN expression in A549 cells was evidently
reduced compared with HBE cells. However, PI3K and p-AKT exhibited
an opposing effect. PTEN expression decreased in the HBE cells
following DEP treatment for 48 h, whereas PI3K and AKT expression
markedly increased. The A549 cells exhibited expression patterns
similar to those observed in the treated HBE cells. When the cells
were treated with DEP in combination with miR-21 inhibitors,
opposite results were obtained. PTEN expression increased, whereas
PI3K and AKT expression decreased. When miR-21 expression was
suppressed by the miR-21 inhibitor, the expression of PTEN, PI3K,
AKT subsequently changed. Considering that PTEN is an important
miR-21 target, it was concluded that DEPs increase miR-21
expression and subsequently activates the PI3K/AKT pathway by
reducing PTEN expression, which may be the mechanism that underlies
the potential carcinogenic effects of DEPs.
Limitless replicative potential and the evasion of
apoptosis are two critical factors in tumorigenesis (32). Numerous studies have demonstrated
that strong, short-term DEP treatment in HBE cells results in
increased apoptosis and decreased proliferation index drop, as well
as cell cycle inhibition at G0/G1 phase (18–20).
In the present study, a similar result was obtained following
treatment of the HBE cells with DEPs (Fig. 4C). Increased caspase 3 expression
and modest Bcl-2, cyclin D1 and proliferation index PCNA expression
in the experimental group was also observed, consistent with the
results of previous studies. In addition, the present study also
assessed the above-mentioned parameters in A549 lung adenocarcinoma
cells and it was identified that the experimental group exhibited
results similar to the HBE cells. However, the apoptosis rate was
not significantly altered in the A549 cells. These findings suggest
that exposure to high concentrations of DEPs in a short time did
not cause evident cancer progression in HBE cell morphology. DEP
exposure also did not cause deteriorating A549 cell morphology.
The present results evidently demonstrate the impact
of DEP exposure on miR-21 expression in HBE cells. DEP-induced
alterations in miR-21 expression may potentially have an important
role in the development and progression of primary lung carcinoma.
Further studies on DEP-induced alterations in PTEN, PI3K and AKT
expression, as well as DEP in combination with a miR-21 antisense
oligonucleotide, may allow for a more comprehensive study of
DEP-induced pathogenic states and underlying molecular mechanisms
of potential carcinogenicity. However, the present study only
examined a single stimulus and its short-term effect on cell
morphology and the PTEN/PI3K/AKT pathway. In addition, the stimulus
concentration was notably greater than the concentration that
people are exposed to in real life. The increased levels of
stimulation may cause a series of destructive changes in cell
morphology in a short period of time, including alterations in
proliferation, invasion and migration. Wu et al found that
DEPs impair cell viability in a dose- and time-dependent manner
(33). However, the concentration
of DEPs in daily life is notably lower than the concentration used
in the experiment. Based on repair capability in the cell, the
concentration of DEPs in real life would not be as damaging to
cells. Using DEPs as a single stimulus, the classic carcinogenic
PTEN/PI3K/AKT pathway was activated. Therefore, it was hypothesized
that long-term exposure to low DEP concentrations may gradually
activate the PTEN/PI3K/AKT pathway, thereby acting as a potential
carcinogen (Fig. 5). Briefly, it
is concluded that DEPs are involved in human lung carcinogenesis
and that one of the potential carcinogenic mechanisms of DEP
involves increased miR-21 expression. Increased miR-21 expression
results in decreased PTEN activity, consequently activating the
classic carcinogenic PI3K/AKT pathway and then promoting
carcinogenesis.
The environmental modulation of gene expression is
the focus of intense study, but how DEPs cause lung cancer remains
unanswered. Overall, further studies are required to focus on
additional miRNAs and signal transduction pathways altered by
diesel exhaust particles or other pollutants involved in lung
carcinogenesis. Therefore, studies investigating the effects of the
environment on cancer remain an important priority.
Acknowledgments
This study was supported by the State Key Laboratory
of Internal Combustion Engine Fuel Science of Tianjin University,
open project 2011 (K2011-04). The authors are grateful to the
members of the Tianjin Laboratory of Neuro-Oncology, Tianjin
Neurological Institute for their technical assistance (Tianjin,
China).
References
|
1
|
Jemal A, Center MM, DeSantis C, et al:
Global patterns of cancer incidence and mortality rates and trends.
Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ramalingam SS, Owonikoko TK, et al: Lung
cancer: New biological insights and recent therapeutic advances. CA
Cancer J Clin. 61:91–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grant WB: Air pollution in relation to
U.S. cancer mortality rates: an ecological study; likely role of
carbonaceous aerosols and polycyclic aromatic hydrocarbons.
Anticancer Res. 29:3537–3545. 2009.PubMed/NCBI
|
|
4
|
Silverman DT, Samanic CM, Lubin JH, et al:
The Diesel Exhaust in Miners study: a nested case-control study of
lung cancer and diesel exhaust. J Natl Cancer Inst. 104:855–868.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Villeneuve PJ, Parent MÉ, Sahni V, et al:
Occupational exposure to diesel and gasoline emissions and lung
cancer in Canadian men. Environ Res. 111:727–735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang CC, Tsai SS, Chiu HF, et al: Traffic
air pollution and lung cancer in females in Taiwan: petrol station
density as an indicator of disease development. J Toxicol Environ
Health A. 72:651–657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laumbach RJ and Kipen HM: Does diesel
exhaust cause lung cancer (yet)? Am J Respir Crit Care Med.
183:843–844. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Attfield MD, Schleiff PL, Lubin JH, et al:
The Diesel Exhaust in Miners study: a cohort mortality study with
emphasis on lung cancer. J Natl Cancer Inst. 104:869–883. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hou L, Wang D and Baccarelli A:
Environmental chemicals and microRNAs. Mutat Res. 714:105–112.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang L, Li N, Wang H, et al: Altered
microRNA expression in cisplatin-resistant ovarian cancer cells and
upregulation of miR-130a associated with
MDR1/P-glycoprotein-mediated drug resistance. Oncol Rep.
28:592–600. 2012.PubMed/NCBI
|
|
11
|
Tao J, Lu Q, Wu D, et al: microRNA-21
modulates cell proliferation and sensitivity to doxorubicin in
bladder cancer cells. Oncol Rep. 25:1721–1729. 2011.PubMed/NCBI
|
|
12
|
Gao W, Lu X, Liu L, et al: MiRNA-21: a
biomarker predictive for platinum-based adjuvant chemotherapy
response in patients with non-small cell lung cancer. Cancer Biol
Ther. 13:330–340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iorio MV, Ferracin M, Liu CG, et al:
MicroRNA gene expression deregulation in human breast cancer.
Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma C, Wang J and Luo J: Activation of
nuclear factor kappa B by diesel exhaust particles in mouse
epidermal cells through phosphatidylinositol 3-kinase/Akt signaling
pathway. Biochem Pharmacol. 67:1975–1983. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jardim MJ, Fry RC, Jaspers I, et al:
Disruption of microRNA expression in human airway cells by diesel
exhaust particles is linked to tumorigenesis-associated pathways.
Environ Health Perspect. 117:1745–1751. 2009.
|
|
16
|
Kandasamy K and Srivastava RK: Role of the
phosphatidylinositol 3′-kinase/PTEN/Akt kinase pathway in tumor
necrosis factor-related apoptosis-inducing ligand-induced apoptosis
in non-small cell lung cancer cells. Cancer Res. 62:4929–4937.
2002.PubMed/NCBI
|
|
17
|
Shu H, Zhang H, Xu C, et al:
Clinicopathological research and expression of PTEN/PI3K/Akt
signaling pathway in non-small cell lung cancer. Zhongguo Fei Ai Za
Zhi. 12:889–892. 2009.In Chinese.
|
|
18
|
Doornaert B, Leblond V, Galiacy S, et al:
Negative impact of DEP exposure on human airway epithelial cell
adhesion, stiffness, and repair. Am J Physiol Lung Cell Mol
Physiol. 284:L119–L132. 2003. View Article : Google Scholar
|
|
19
|
Li N, Wang M, Oberley TD, et al:
Comparison of the pro-oxidative and proinflammatory effects of
organic diesel exhaust particle chemicals in bronchial epithelial
cells and macrophages. J Immunol. 169:4531–4541. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hiura TS, Kaszubowski MP, Li N, et al:
Chemicals in diesel exhaust particles generate reactive oxygen
radicals and induce apoptosis in macrophages. J Immunol.
163:5582–5591. 1999.PubMed/NCBI
|
|
21
|
Chuang KJ, Chuang HC and Lin LY: Indoor
air pollution, nighttime heart rate variability and coffee
consumption among convenient store workers. PLoS One. 8:e633202013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vinikoor-Imler LC, Davis JA and Luben TJ:
An ecologic analysis of county-level PM2.5 concentrations and lung
cancer incidence and mortality. Int J Environ Res Public Health.
8:1865–1871. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hystad P, Demers PA, Johnson KC, et al:
Long-term residential exposure to air pollution and lung cancer
risk. Epidemiology. 24:762–772. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Olsson AC, Gustavsson P, Kromhout H, et
al: Exposure to diesel motor exhaust and lung cancer risk in a
pooled analysis from case-control studies in Europe and Canada. Am
J Respir Crit Care Med. 183:941–948. 2011. View Article : Google Scholar
|
|
25
|
Furuta C, Suzuki AK, Watanabe G, et al:
Nitrophenols isolated from diesel exhaust particles promote the
growth of MCF-7 breast adenocarcinoma cells. Toxicol Appl
Pharmacol. 230:320–326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Møller P, Folkmann JK, Danielsen PH, et
al: Oxidative stress generated damage to DNA by gastrointestinal
exposure to insoluble particles. Curr Mol Med. 12:732–745. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Raaschou-Nielsen O, Andersen ZJ, Hvidberg
M, et al: Air pollution from traffic and cancer incidence: a Danish
cohort study. Environ Health. 10:672011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rabinowits G, Gerçel-Taylor C, Day JM, et
al: Exosomal microRNA: a diagnostic marker for lung cancer. Clin
Lung Cancer. 10:42–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang JG, Wang JJ, Zhao F, et al:
MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes
growth and invasion in non-small cell lung cancer (NSCLC). Clin
Chim Acta. 411:846–852. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang W, Bai W and Zhang W: MiR-21
suppresses the anticancer activities of curcumin by targeting PTEN
gene in human non-small cell lung cancer A549 cells. Clin Transl
Oncol. Nov 29–2013.Epub ahead of print.
|
|
31
|
Hong TM, Yang PC, Peck K, et al: Profiling
the downstream genes of tumor suppressor PTEN in lung cancer cells
by complementary DNA microarray. Am J Respir Cell Mol Biol.
23:355–363. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu Y, Yu T, Gilbertson TA, et al:
Biophysical assessment of single cell cytotoxicity: diesel exhaust
particle-treated human aortic endothelial cells. PLoS One.
7:e368852012. View Article : Google Scholar : PubMed/NCBI
|