Introduction
Human hepatocellular carcinoma (HCC) is one of the
most common causes of cancer-related mortality worldwide and its
poor prognosis mainly depends on the clinicopathological
characteristics regarding invasion and metastasis. HCC frequently
shows early intrahepatic metastases as well as blood vessel
invasion followed by extrahepatic metastases at later stages. As
with any other malignancy, the propensity for local invasion and
distant metastasis of HCC is based on its ability to degrade the
surrounding extracellular matrix (ECM) and invade the basement
membrane (BM) (1).
Matrix metalloproteinases (MMPs) are a family of 24
secreted Zn2+-dependent endopeptidases and are
identified to be involved in the proteolysis of the ECM and
establishment of metastatic deposits. The natural inhibitors of
MMPs, termed tissue inhibitors of metalloproteinase (TIMPs),
counterbalance the activity of MMPs in vivo and may have
direct effects on cell proliferation (2).
Gene therapy, a modern molecular medicine strategy,
holds great promise for the treatment of HCC and has the potential
to revolutionize cancer treatment. However, liver gene therapy
remains in the developmental stage and efficient and innocuous
liver-directed gene transfer vectors are therefore urgently
required. To achieve efficient cytosolic delivery of therapeutics,
various nanomaterials have been developed that consider the diverse
physicochemical nature of therapeutics (macromolecules to small
molecules; water soluble to water insoluble) and various
membrane-associated and intracellular barriers that these systems
have to overcome to efficiently deliver and retain therapeutics in
the cytoplasmic compartment. Biodegradable particles formulated
from poly-DL-lactide-poly(ethylene glycol) (PELA), hydrophobic
polylactic acid (PLA) and polylactide-co-glycolide (PLGA), have
been extensively investigated for sustained and targeted/localized
delivery of various agents, including plasmid DNA, proteins,
peptides, drugs, enzymes, antibodies or nucleotides, and are able
to be directed to a specific organ, tissue or tumor (3,4).
In the present study, the polymer microsphere was
prepared by encapsulating the recombinant TIMP-1 adenovirus in
biodegradable PELA instead of the traditional vectors. Its
biomedical characteristics were discussed in regard to its
application in gene therapy of liver cancer.
Materials and methods
Experimental animals, cell lines and
culture method
HepG2 cells (Cell Bank of the Chinese Academy of
Sciences, Shanghai, China) were used as a poorly differentiated
human HCC cell line and the cells were maintained in RPMI 1640
(HyClone, Rockford, IL, USA). Low-passage cells were used in all
experiments. Male Wistar rats (age, 10–12 weeks; weight, 250–300 g)
were provided by the Medical Experimental Animal Centre of Luzhou
Medical College (Luzhou, China). The study was performed in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health. The
animal use protocol was reviewed and approved by the Institutional
Animal Care and Use Committee (IACUC) of Luzhou Medical College.
All other chemicals and solvents were of reagent grade or
better.
Preparation of rAd-microspheres
The previously described method for recombinant
adenovirus rAdTIMP-1 construction was used and the ideal virus
titer ranging from 1.0×1010−1011 efu/ml was
obtained (5). PELA (Chengdu
Institution of Organic Chemistry, Chinese Academy of Science,
Chengdu, China) were used to prepare the polymer microsphere. The
target microspheres were prepared by solvent extraction based on
the formation of a modified double-emulsion water-1/oil/water-2
(W1/O/W2) system as reported previously
(6). Briefly, inner aqueous phase
(W1) was prepared with adenovirus aqueous solution, Oil
phase (O) was prepared with 20% PELA solution (200 g/l) dissolved
in methylene chloride, and external aqueous phase (W2)
was prepared with 2.0% polyvinyl alcohol aqueous solution (20 g/l).
The adenovirus aqueous solution was added to PELA methylene
chloride solution and stirred (890 × g) for 1 h. Subsequently, it
was added to the PVA aqueous solution and stirred (890 × g) for 4
h. The organic solvent was removed with the solvent extraction
method (50 g/l isopropyl alcohol solution). The solution was then
centrifuged (4,360 × g) for 8 min, washed with double-distilled
water three times and finally freeze-dried to acquire a powder of
rAd-microspheres.
Physicochemical characteristics and virus
release curve
rAd-microspheres powder (2 mg) was dispersed in
distilled water with ultrasonication for 30 min, and the average
particle size, standard deviation and distribution curves were
determined using a laser diffraction particle size analyzer
(Mastersizer 2000, Malvern Instruments Ltd., Malvern, UK). The
rAd-microspheres were hydrated and then dried. Their surface
morphology and dispersed state were observed using a scanning
electron microscope (SEM; Amray, Drogheda, Ireland).
The virus titer in the remaining liquid following
encapsulation was determined and compared with the antecedent
titer. The encapsulation efficiency and virus loading rate were
calculated as previously described (6). To measure the viral release from
rAd-microsphere preparations, the virus titers at 0, 24, 48, 72,
96, 120, 144, 168, 192, 216 and 240 h were determined by assessing
their fluorescence, and virus release curves were plotted.
Toxicity testing
A total of 20 Wistar rats were randomly divided into
an experimental group and a control group, which were administered
a single intraperitoneal injection of blank microspheres suspension
(3×1011 efu/ml) or normal saline (Chengdu Bao Yikang
Medicines and Health Products Co., Ltd, Chengdu, China),
respectively. Rats were fed separately under a 12-h light/dark
cycle in order to observe the general condition and survival period
over three months. All rats were sacrificed by cervical
dislocation.
Transfection efficiency and growth
curve
HepG2 cells in the logarithmic growth phase were
conventionally digested using 0.25% trypsin (Fuzhou Maixin
Biotechnology Development Co., Ltd, Fuzhou, China) and incubated
overnight in a 24-well culture plate (1×105 cells/well).
Thereafter, 0.01, 0.1, 1, 10 or 100 mg rAd-microspheres, were added
into the 24-well culture plates. The culture medium was removed at
48 and 120 h, respectively. Thereafter, the number of green
fluorescent cells (containing green fluorescent protein) and total
cells in inverted culture plates were counted under a BX61
fluorescent microscope (Olympus Corp., Tokyo, Japan). The ratio
between them indicated the transfection efficiency.
The HepG2 cells transfected with rAd-microspheres
and blank microspheres as well as the control group were inoculated
onto 24-well plates (1×105 cells/well). For each group,
cells were digested and suspended for analysis daily. The number of
cells was counted and the mean was recorded for six consecutive
days. The cell growth curve was plotted as cell growth versus
time.
Gelatin zymography
Analysis of TIMP-1 in rAd-microspheres-transfected
HepG2 cells was performed on SDS-polyacrylamide gels impregnated
with 0.1% gelatin (w/v) and 10% polyacrylamide (w/v) as described
previously (7). Culture
supernatants were grown in 100-mm2 tissue culture plates
in Dulbecco’s modified Eagle’s medium (DMEM; HyClone)/Ham’s F-12
containing 10% fetal calf serum (FCS) until they reached 80%
confluence. Cells were washed and placed in serum-free medium, and
the conditioned medium was collected following 48 h. Four parts of
medium containing equal quantities of protein were mixed with one
part of sample buffer prior to electrophoresis. Gels were run at a
constant current and then washed twice for 30 min in 50 mmol/l
Tris-HCl, pH 7.5, with 2.5% Triton X-100 and incubated overnight at
37°C in 50 mmol/l Tris-HCl, pH 7.6. Gels were stained with
Coomassie Brilliant Blue R-250 and then destained.
Western blot analysis
Posttransfection (72 h), 5 µl of the
supernatant of the cultured cells was collected, subjected to 12%
SDS-PAGE, and electrically transferred to nylon membranes.
Nonspecific binding was blocked with Tris-buffered saline (TBS)
containing 5% (w/v) skimmed milk for 2 h at room temperature, and
then filters were stained with an affinity-purified mouse
anti-human TIMP-1 polyclonal antibody (1:1,000, Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) for 2 h at room
temperature followed by incubation with a goat anti-mouse
immunoglobulin G secondary antibody (1:2,000; Santa Cruz
Biotechnology, Inc.) for 1 h. Following further washing with PBS,
the blot was incubated in 3,3′-diaminobenzidine (DAB) and captured
on photographic film (5).
Matrigel matrix invasion assay
The cell invasion assay was performed by the
invasion of cells through Matrigel™-coated Millicell Chamber
(Millipore, Billerica, MA, USA) inserts according to a procedure
described previously (8). Briefly,
5-mm diameter polycarbonate filters (pore size, 8 µm) were
coated with Matrigel, dried, and reconstituted at 37°C with RPMI
1640 prior to use. The lower chambers were filled with supernatant
of NIH3T3, cultured in serum-free DMEM for 24 h, as a chemotactic
factor. HepG2 cells were divided into three groups: Cells treated
with rAd-microspheres and normal cells served as experimental as
well as control group, respectively; PBS-treated cells acted as the
blank control group and the filter was without Matrigel. The cells
were pre-transfected 48 h prior to the assay, and then added to the
upper chamber at 1×105 cells per chamber in RPMI 1640
containing 5% FCS. Following 12 h of incubation at 37°C, the
suspended media in the lower chamber were removed, fixed and
stained. The cells that passed through the filter into the lower
chamber were stained with Hema-3 and counted under a phase contrast
microscope (five random fields per chamber). Each invasion
experiment was performed in duplicate and repeated at least
twice.
Statistical analysis
SPSS 11.5 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. One-way analysis of variance was
used for comparisons among multiple groups by randomization. The
t-test was used for comparison between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Physicochemical properties and virus
release curve
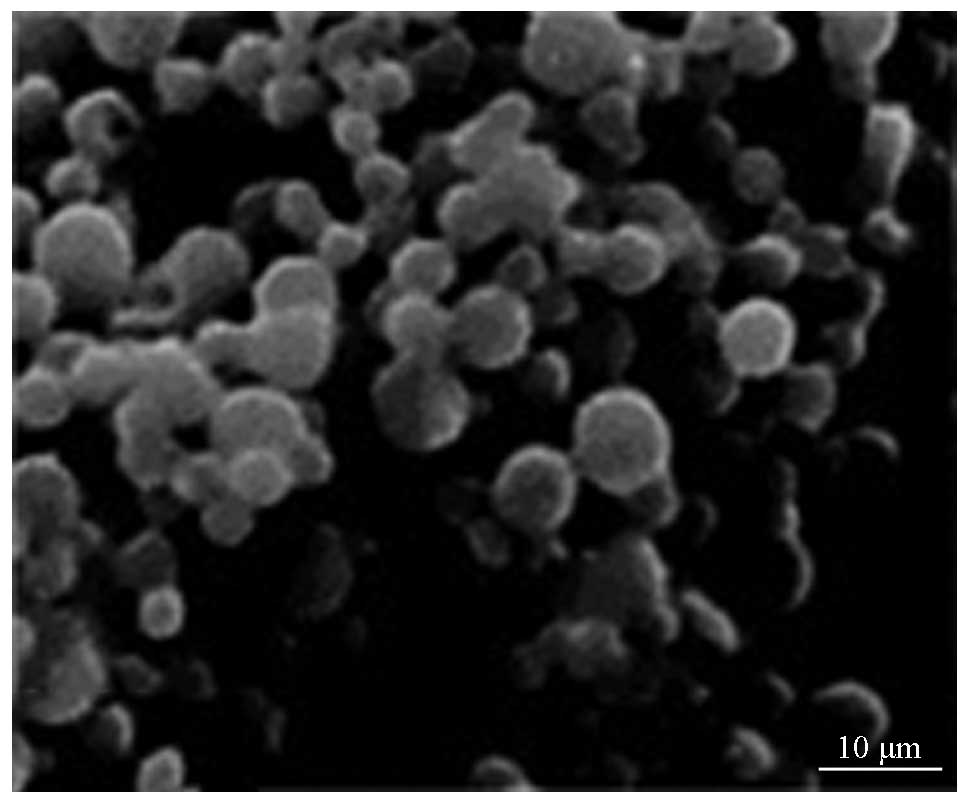
Fig. 1 shows the
SEM images of rAd-microspheres prepared by a double emulsion
(W1/O/W2) based on the solvent evaporation
method. The resulting microsphere was smooth and spherical with a
uniform size, regular shape, good dispersion between microspheres
and no apparent evidence of collapse. As shown in Fig. 2, the mean particle size has a
normal distribution as assessed using a laser diffraction particle
size analyzer: Of all particles, 50% were 1.965 µm, <10%
were 1.250 µm and >40% were 3.320 µm in size. The
mean spacing of microspheres was 1.360 µm.
The virus titer of the stock solution prior to
encapsulation was 3.59×1011 efu/ml. With additional 5 ml
virus stock solution during encapsulation, the virus titer of the
residual liquid was 1.4×109efu/ml following
encapsulation, with residual liquid of 500 ml. PELA (1,000 mg) was
added during encapsulation. Therefore, the encapsulation efficiency
was 60.0% and the virus loading rate was 10.5×108/mg.
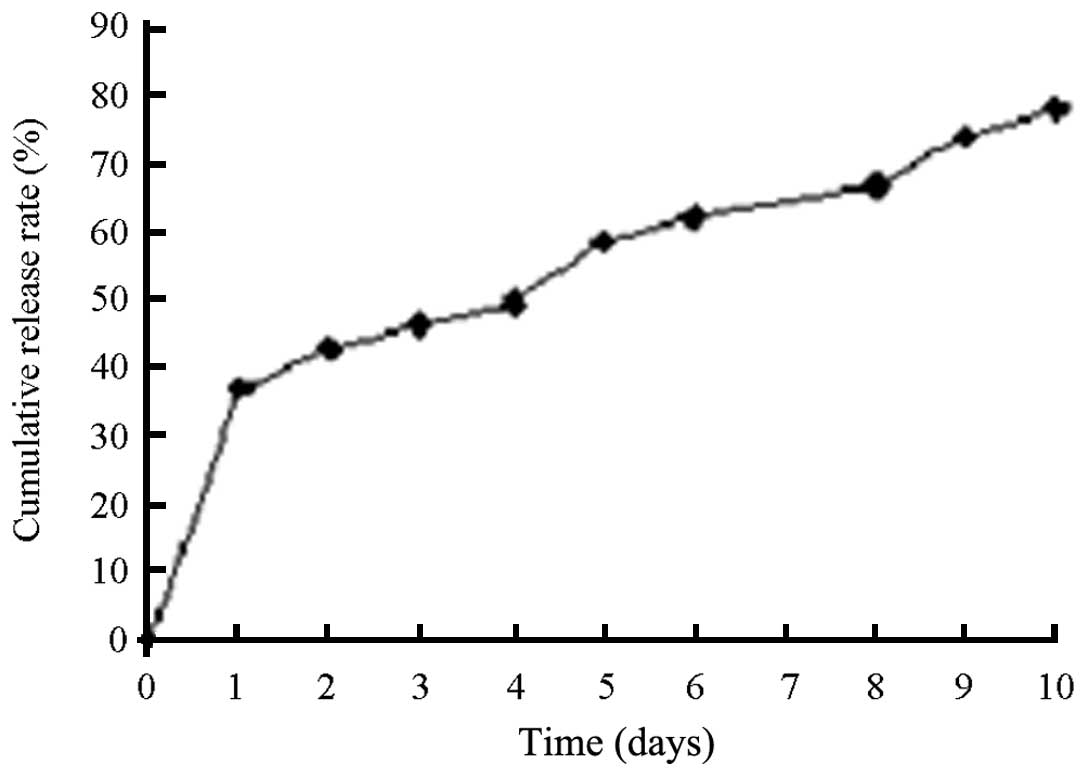
The viable virus release of rAd-microspheres in the DMEM medium at
37°C was almost 60% within 120 h, with a total release time >240
h (Fig. 3). A toxicity test of
blank microspheres demonstrated that the microspheres were nontoxic
with few side effects in accordance with the requirements for
microspheres for in vivo application (6).
From the aforementioned results, the conclusion was
drawn that rAd-microspheres may function as a suitable
gene-delivery system with appropriate particle size, particle-size
distribution and high gene-loading efficiency.
Transfection efficiency and inhibition on
in vitro growth of HepG2 cells
With the viral multiplication, the transfection
efficiency of HepG2 cells was also increased. When the quantity of
virus was >10 mg, the transfection efficiency was able to reach
>90%, indicating the enhanced gene transfection activity.
Fig. 4 shows the
release profiles over a six-day period. The cells in the three
groups, days 1–3, were at the stationary phase. With increasing
time, the cell counts in the rAd-microspheres group were
significantly lower than those in the blank micro-sphere and
control groups, suggesting that the rAd-microspheres carrying
TIMP-1 were able to inhibit the proliferation of the HepG2 cells
(P<0.05, data not shown).
Toxicity of the microspheres in vivo
A total of 20 Wistar rats were injected with either
a blank microspheres suspension (3×1011 efu/ml) or
normal saline in order to determine the toxicity of the
microspheres in vivo. Rats were kept under good general
condition, with normal activities but poor mental state, decreased
appetite and depilation. Following observation for 4 weeks, all the
animals were still alive and there were no significant difference
observed between the experimental and control groups. This
indicated that the microspheres were non-toxic as they had no
observable side effects under these conditions in vivo (data
not shown).
TIMP-1 activity determined by gelatin
zymography
Previous studies by our group have reported in
vivo overexpression of endogenous TIMP family members inhibits
tumor-induced, MMP-dependent matrix proteolysis under pathological
conditions (5,9,10).
The functional activity of TIMP-1 encapsulated in the
rAd-microspheres was detected using the gelatin zymography assay on
HepG2 cells. As shown in Fig. 5,
the expression and activity of TIMP-1 were low in
rAd-micro-spheres-transfected HepG2 cells, which indicated that
TIMP-1 overexpression was able to downregulate the activity of MMPs
by inhibiting its cleavage activation without suppressing its
expression in HCC cells.
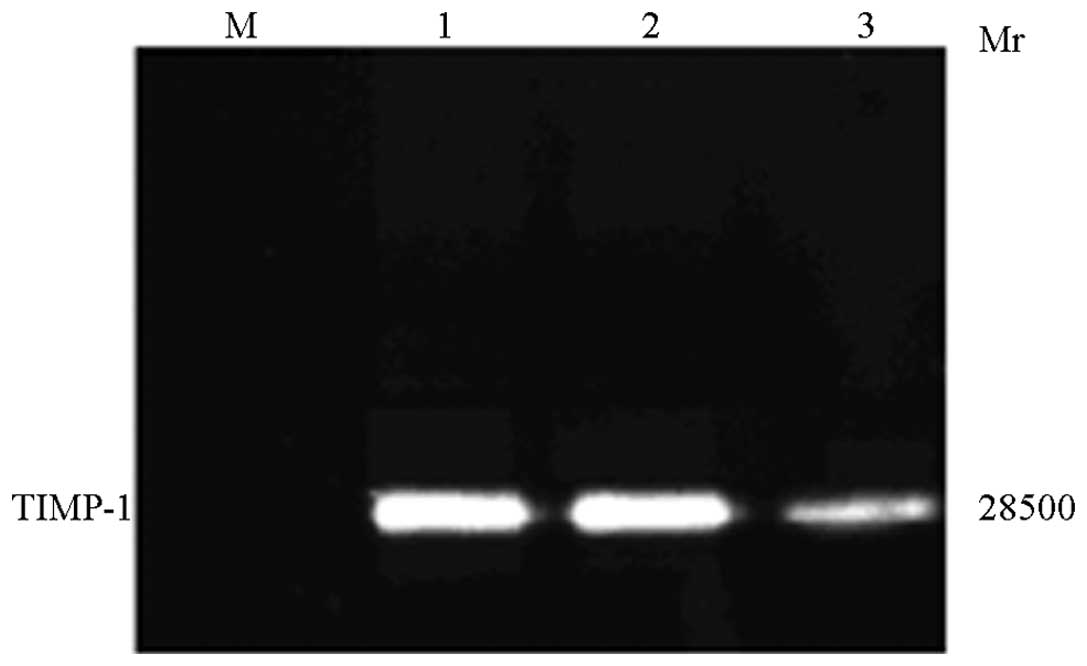
TIMP-1 western blot analysis
HepG2 cells were transfected with rAd-microspheres
for 72 h, and the supernatant was collected. The TIMP-1 protein was
subjected to SDS-PAGE and transferred to the membrane for western
blot analysis. A major band exhibiting a molecular weight of 28.5
kDa was specifically detected in rAd-microspheres-transfected HepG2
cells, but not in the control, indicating that normal HepG2 cells
are not able to express TIMP-1 protein and that, following
trans-fection, rAd-microspheres-transfected HepG2 cells are able to
express TIMP-1 protein, and even secrete it (Fig. 6).
Matrigel matrix invasion assay
Matrigel-coated Millicell chambers were used in a
standard test to investigate whether transfection by
rAd-microspheres suppresses the invasion of HepG2 cells. The
results demonstrated that the percentage of cells invading the
Matrigel-coated filter in experimental group
(rAd-microspheres-transfected cells) and control group (normal
cells) was 12.4±3.5% and 36.5±4.3%, respectively, as compared with
the PBS-treated HepG2 cells crossing through the blank filter set
as 100%. This demonstrated that virally induced TIMP-1 secretion
markedly inhibited HCC cell invasive migration (Fig. 7). Furthermore, the results of the
present study confirm that increased expression of TIMP-1 by
rAd-microspheres is responsible for the inhibitory effect on cell
motility and invasiveness of HCC cells.
Discussion
HCC is the sixth most common type of cancer
worldwide in terms of incidence, accounting for approximately
630,000 newly diagnosed cases per year. In addition, HCC is the
third leading cause of cancer-relaetd mortality. Around 80% of HCC
cases occur in developing countries, with the areas of high
incidence being sub-Saharan Africa and Eastern and Southeast Asia,
particularly China. However, the incidence of HCC is low in
numerous developed countries, as well as in Latin America and
South-central Asia (11,12).
HCC has a high degree of malignancy, a high
metastasis rate and unfavorable prognosis. Generally, metastasis of
HCC involves multi-step processes and various cytophysiological
changes, including local invasion, entering the lymphatic and blood
vascular system, surviving in the bloodstream,
extra-vascularization from the microvessels and colonization at the
secondary site. The key step in these processes may be the
degradation of the ECM causing destruction of the BM. MMPs and
TIMPs are two groups of functionally antagonistic proteases. A
previous study suggested that perturbing the balance of MMPs and
TIMPs may lead to direct inhibition of MMPs and increase of TIMPs
in cancer and may be a particularly attractive target for
therapeutic intervention in tumor invasion and metastasis (13). Therefore, perturbing the MMP-TIMP
balance may cause degradation of ECM and destruction of BM
following tumor metastasis. TIMPs have been observed to be
synthesized by the same cell secreting MMPs, which may specifically
close the catalytic active site and have an important role in ECM
remodeling as well as the invasion and metastasis of tumors. At
present, four TIMPs have been identified, TIMP-1–4; however, only
TIMP-1 and -2 were found to be expressed in the liver. TIMP-1 is a
secreted glucoprotein with a relative molecular weight of 28.5 kD.
Its activity was able to be inhibited by forming complexes with
almost all collagenases at ratios of 1:1. Upregulation of TIMP-1
was shown to suppress the tumor invasion and metastasis in various
types of human cancer, which may be correlated with its inhibition
of MMP-2/9 (14).
The use of recombinant adenoviral vectors as an
alternative delivery method for genes or combinations of genes to
tumor cells is being investigated (6,15–17).
Since adenoviruses are able to efficiently enter replicating and
quiescent cells, it may be used as a prospective mediator for
macromolecular transport into cells. Furthermore, recombinant
adenoviruses have numerous advantages, including transfection
ability, high titer, efficient multicopy, no insertional
mutagenesis and no genetic toxicity. In addition, it was not
possible to integrate the free vector into the host DNA.
Recombinant adenoviruses were able to infect a wider range of
hosts, particularly cells in copy division phase. However, the
relatively short expression time may evoke an immune response in
the host upon repeated application (18). The two aims to improve the
effectiveness of adenoviral-mediated gene transfer for HCC therapy
are to increase the efficiency of gene transfer and to reduce the
requirement for frequent re-dosing regimens. Theoretically, the
medical microspheres produced in the present study,
rAd-microspheres, PELA encapsulated recombinant TIMP-1 adenovirus,
with enhanced transfection efficiency and sustained release
capability, were expected to achieve these requirements.
Pharmaceutical research has led to the
identification of numerous reagents compatible with controlled
delivery of drugs enterically and systemically. These include
particles, nanoparticles, microemulsions, submicron emulsions and
liposomes (19–23). Therapeutics may require efficient
cyto-solic delivery if the receptors for those drugs are located in
the cytosol or their site of action is an intracellular organelle
that requires transport through the cytosolic compartment.
Biodegradable microspheres formulated from biodegradable PELA,
hydrophobic PLA and PLGA have been successfully used to deliver
drugs at a controlled rate to target specific organs including the
liver (3,24).
PELA is a type of degradable polymer, obtained by
polymerization of PLA and hydrophilic polyvinyl alcohol (PEG). It
is hydrophilic and nontoxic, with no immunogenicity but high
encapsulation efficiency, and it was also able to improve the
stability and adjustability of the encapsulation contents. It is a
focus of recent research of materials and has been applied in
encapsulating albumin, DNA and vaccines (24,25).
Ren et al (26)
investigated the partial characteristics of a microsphere vaccine
prepared by encapsulation of recombinant outer membrane protein K
(OmpK) of Vibrio harveyi with PELA in crucian carp
inoculated orally. The study indicated the feasibility of PELA as a
system for oral vaccine delivery to fish. Ruan et al
(27) investigated the effect of
different polymers used for the preparation of human serum albumin
(HSA)-loaded microparticles and suggested that the HSA
encapsulation efficiency value of PELA microparticles was ~10%
higher than that of PLGA microparticles. Yang et al
(28) incorporated bovine serum
albumin (BSA) into porous PLGA scaffolding containing microspheres
of PELA and observed that the microsphere-incorporated scaffold
prolonged BSA release, and the cumulative release on day 10 reached
85% of total encapsulated BSA. Wei et al (29) compared PELA microspheres with
narrow size distribution for sustained release of recombinant human
growth hormone (rhGH) with PLA and PLGA microspheres to determine
the difference in encapsulation efficiency, initial burst release,
high burst levels and integrity of rhGH, and concluded that PELA
was an effective polymer for rhGH encapsulation and stabilization.
Compared with the commonly used PLA and PLGA, PELA microspheres
showed potential as delivery systems for macromolecular drugs
(including protein and peptide drugs), which may be due to the
amphiphilic structure of the block copolymer. PELA microspheres
undergo slow degradation by hydrolysis of ester linkages to yield
lactic and glycolic acid. Additionally, it is able to control the
rate of release of entrapped antigens and therefore, offers
potential for the development of single-dose drugs. Accordingly,
previous studies performed by our group demonstrated the
feasibility of encapsulating recombinant adenovirus into PELA
micro-spheres with retention of virus viability (6).
Among the microspheres described in this study,
>90% were <3 µm in size, with a mean particle size of
1.965 µm. They exhibited good dispersion, with a mean
spacing of 1.360 µm and a virus loading rate of
10.5×108/mg. A previous study (30) has shown that decreased sphere size
results in improvements in the encapsulation yield. However, to the
best of our knowledge, no studies to date have been performed to
optimize the encapsulation of live viral vectors. Given the
relatively large size of the adenovirus (~100 nm), and
consideration of mechanical forces upon encapsulation, gentle
methods for encapsulation were used, which resulted in a large
sphere size (>10–20 µm). This size may be advantageous
when delivering the antigenic adenovirus. A study that directly
compared the immune response to antigens in 1–10- versus
10–110-µm spheres demonstrated a 20-fold reduction in
immunogenicity when encapsulated in larger particles (31).
The present study suggests that the viral release in
the initial 24 h was faster than that after 24 h, and the
cumulative release percentage was close to 60% within 120 h. As a
previous study suggested (32),
the release is able to be characterized by at least two phases. The
first phase, usually comprising the initial 24 h, is a rapid
release of the compound as a result of diffusion from the surface
of the microspheres. The second phase is a relatively slow release
with the erosion of the polymer through hydrolysis. This solves the
problem of a rapid in vivo clearance of the drugs, which
hinders drugs from acting efficiently over a long period of
time.
Previously, acute toxicological experiments of blank
microspheres showed that the microsphere vectors themselves were
nontoxic with no side effects in accordance with the requirements
of in vivo applications (6). In the present study, the HCC cell
line HepG2 was transfected with rAd-microspheres, which displayed
the highest transfection efficiency (~90%) compared with other
microspheres. The MTT experiment and cell growth curve confirmed
that rAd-microspheres carrying TIMP-1 were able to inhibit the
in vitro proliferation of HepG2 cells. Based on the
efficient, high-loading, sustained-release rAd-microspheres, the
TIMP-1 gene was stably expressed in HCC cells over a long time, and
the biological activities of HCC cells were inhibited through
upregulation of TIMP-1 expression and downregulation of the
activity of MMPs (6,33). The expression of the TIMP-1 protein
was steadily detected by western blot analysis, and the
overexpression of TIMP-1 was able to downregulate the activity of
MMPs by effectively inhibiting gelatinase degradation as indicated
by the results of the gelatin zymography assay. The cell invasion
assay confirmed migration in vitro, and the invasion
capacity was markedly inhibited by transfection with
rAd-microspheres, which is consistent with the concept that the
inhibition of invasion by TIMPs is mediated via the prevention of
tissue-remodeling.
In conclusion, the present study revealed that
PELA-encapsulated adenoviral-mediated TIMP-1 gene transfer is
efficient for the treatment of HCC and may pave the way for
application in prospective in vivo trials and further
comprehensive therapy of liver cancer. In addition, different
polymers should be probed in regard to their ability to perform
sustained release of recombinant viral vectors. Furthermore, other
methods including hydrogels, or self-diffusion and self-regulated
systems may be applicable. The formulation of viral vectors for
gene delivery may improve their applicability for the treatment of
HCC, and may have wide-spread application in human disease
(34–36).
Acknowledgments
The present study was supported by the Sichuan
Provincial Education Department Foundation (grant no. 2006B108) and
the Sichuan Provincial Health Department Foundation (grant no.
090210).
References
|
1
|
Hernandez-Gea V, Toffanin S, Friedman SL
and Llovet JM: Role of the microenvironment in the pathogenesis and
treatment of hepatocellular carcinoma. Gastroenterology.
144:512–527. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen JS, Huang XH, Wang Q, et al: Sonic
hedgehog signaling pathway induces cell migration and invasion
through focal adhesion kinase/AKT signaling-mediated activation of
matrix metalloproteinase (MMP)-2 and MMP-9 in liver cancer.
Carcinogenesis. 34:10–19. 2013. View Article : Google Scholar
|
|
3
|
Giri TK, Choudhary C, Ajazuddin, Alexander
A, Badwaik H and Tripathi DK: Prospects of pharmaceuticals and
biophar-maceuticals loaded microparticles prepared by double
emulsion technique for controlled delivery. Saudi Pharm J.
21:125–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chintale AG, Kadam VS, Maske KS, Raut DB,
Kale SV and Rai SD: Recent advances in microsphere drug delivery
system: a review. Res J Pharm Technol. 6:307–312. 2013.
|
|
5
|
Xia D, Yan LN, Tong Y, Wang XP, Zhang MM
and Zhao LY: Construction of recombinant adenoviral vector carrying
human tissue inhibitor of metalloproteinase-1 gene and its
expression in vitro. Hepatobiliary Pancreat Dis Int. 4:259–264.
2005.PubMed/NCBI
|
|
6
|
Xia D, Yao H, Liu Q and Xu L: Preparation
of microspheres encapsulating a recombinant TIMP-1 adenovirus and
their inhibition of proliferation of hepatocellular carcinoma
cells. Asian Pac J Cancer Prev. 13:6363–6368. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh A, Maurya OPS, Jagannadhan MV and
Patel A: Matrix metalloproteinases (MMP-2 and MMP-9) activity in
corneal ulcer and ocular surface disorders determined by gelatin
zymography. J Ocul Biol Dis Inform. 5:31–35. 2012. View Article : Google Scholar
|
|
8
|
Jung JS, Ahn JH, Le TK, Kim DH and Kim HS:
Protopanaxatriol ginsenoside Rh1 inhibits the expression of matrix
metalloproteinases and the in vitro invasion/migration of human
astroglioma cells. Neurochem Int. 63:80–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang ZD, Huang C, Li ZF, et al:
Chrysanthemum indicum ethanolic extract inhibits invasion of
hepatocellular carcinoma via regulation of MMP/TIMP balance as
therapeutic target. Oncol Rep. 23:413–421. 2010.PubMed/NCBI
|
|
10
|
Dai ZJ, Wang BF, Lu WF, et al: Total
flavonoids of Scutellaria barbata inhibit invasion of
hepatocarcinoma via MMP/TIMP in vitro. Molecules. 18:934–950. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
EI-Serg HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273. 2012. View Article : Google Scholar
|
|
12
|
Leonardi GC, Candido S, Cervello M, et al:
The tumor micro-environment in hepatocellular carcinoma. Int J
Oncol. 40:1733–1747. 2012.PubMed/NCBI
|
|
13
|
Remacle AG, Shiryaev SA, Radichev IA,
Rozanov DV, Stec B and Strongin AY: Dynamic interdomain
interactions contribute to the inhibition of matrix
metalloproteinases by tissue inhibitors of metalloproteinases. J
Biol Chem. 286:21002–21012. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fukazawa T, Matsuoka J, Yamatsuji T, Maeda
Y, Durbin ML and Naomoto Y: Adenovirus-mediated cancer gene therapy
and virotherapy. Int J Mol Med. 25:3–10. 2010.
|
|
16
|
Liu X, Cao X, Wei R, et al:
Gene-viro-therapy targeting liver cancer by a dual-regulated
oncolytic adenoviral vector harboring IL-24 and TRAIL. Cancer Gene
Therapy. 19:49–57. 2012. View Article : Google Scholar
|
|
17
|
Kim KI, Park JH, Lee YJ, et al: In vivo
bioluminescent imaging of α-fetoprotein-producing hepatocellular
carcinoma in the diethylnitrosamine-treated mouse using recombinant
adenoviral vector. J Gene Med. 14:513–520. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia D, Zhang MM and Yan LN: Recent
advances in liver-directed gene transfer vectors. Hepatobiliary
Pancreat Dis Int. 3:332–336. 2004.PubMed/NCBI
|
|
19
|
Shiba H, Okamoto T, Futagawa Y, et al:
Adenovirus vector-mediated gene transfer using degradable starch
microspheres for hepatocellular carcinoma in rats. J Surg Res.
133:193–196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sailaja G, HogenEsch H, North A, Hays J
and Mittal SK: Encapsulation of recombinant adenovirus into
alginate micro-spheres circumvents vector-specific immune response.
Gene Ther. 9:1722–1729. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bertram JP, Rauch MF, Chang K and Lavik E:
Using polymer chemistry to modulate the delivery of neurotrophic
factors from degradable microspheres: delivery of BDNF. Pharm Res.
27:82–89. 2010. View Article : Google Scholar
|
|
22
|
Blatsios G, Tzimas AS, Mattheolabakis G,
Panagi Z, Avgoustakis K and Gartaganis SP: Development of
biodegradable controlled release scleral systems of triamcinolone
acetonide. Curr Eye Res. 35:916–924. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Celik O and Akbuğa J: Preparation of
superoxide dismutase loaded chitosan microspheres: characterization
and release studies. Eur J Pharm Biopharm. 66:42–47. 2007.
View Article : Google Scholar
|
|
24
|
Madhavan Nampoothiri K, Nair NR and John
RP: An overview of the recent developments in polylactide (PLA)
research. Bioresour Technol. 101:8493–8501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barakat NS and Ahmad AA: Diclofenac sodium
loaded-cellulose acetate butyrate: effect of processing variables
on microparticles properties, drug release kinetics and ulcerogenic
activity. J Microencapsul. 25:31–45. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ren Y, Zhang XJ, Chang OQ, et al: Partial
characteristics of PELA-OmpK microsphere vaccine and its immune
effect in crucian carp inoculated by oral route. Chinese Journal of
Biologicals. 11:231–236. 2011.in Chinese.
|
|
27
|
Ruan G, Feng SS and Li QT: Effects of
material hydrophobicity on physical properties of polymeric
microspheres formed by double emulsion process. J Control Release.
84:151–160. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang YF, Tang GW, Zhang H, et al:
Controlled release of BSA by microsphere-incorporated PLGA
scaffolds under cyclic loading. Mater Sci and Eng, C. 31:350–356.
2011. View Article : Google Scholar
|
|
29
|
Wei Y, Wang YX, Wang W, Ho SV, Wei W and
Ma GH: mPEG-PLA microspheres with narrow size distribution increase
the controlled release effect of recombinant human growth hormone.
J Mater Chem. 21:12691–12699. 2011. View Article : Google Scholar
|
|
30
|
Davidson BL, Hilfinger JM and Beer SJ:
Extended release of adenovirus from polymer microspheres: potential
use in gene therapy for brain tumors. Adv Drug Deliv Rev. 27:59–66.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ahmed AR and Bodmeier R: Preparation of
performed porous PLGA microparticles and antisense oligonucleotides
loading. Eur J Pharm Biopharm. 71:264–270. 2009. View Article : Google Scholar
|
|
32
|
Mok H, Park JW and Park TG:
Microencapsulation of PEGylated adenovirus within PLGA microspheres
for enhanced stability and gene transfection efficiency. Pharm Res.
24:2263–2269. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chaisri W, Hennink WE and Okonogi S:
Preparation and characterization of cephalexin loaded PLGA
microspheres. Curr Drug Deliv. 6:69–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu Q, Leong J, Chua QY, et al: Combined
modality doxorubicin-based chemotherapy and chitosan-mediated p53
gene therapy using double-walled microspheres for treatment of
human hepatocellular carcinoma. Biomaterials. 34:5149–5162. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kerr SH and Kerr DJ: Novel treatments for
hepatocellular cancer. Cancer Letters. 286:114–120. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu AX: Molecularly targeted therapy for
advanced hepatocellular carcinoma in 2012: current status and
future perspectives. Semin Oncol. 39:493–502. 2012. View Article : Google Scholar : PubMed/NCBI
|