Introduction
At present, tissue engineered bones can be prepared,
according to the specific requirements of the host, through the
implantation of tissues and materials with the desirable
physiological functions required to fix and repair bone defects
(1). This technique has attracted
significant focus, as it is a minimally invasive, easily performed
procedure that maintains the blood supply to the defective area.
Currently, there are two main types of scaffolds, natural
bio-derived materials (e.g. collagen, coral and bio-derived bones),
and synthetic materials (e.g. bioceramics and polymer materials).
As only one type of material is incapable of meeting the
requirements of bone tissue repair, composite scaffold materials
are preferred (2). However,
histocompatibility remains problematic (3,4).
Therefore, a novel injectable chitosan-β-tricalcium phosphate
composite was used in the present study to act as a carrier for
mesenchymal stem cells (MSCs). The compatibility of the MSCs with
the composite scaffold and the subsequent effects on cellular
proliferation were analyzed. The results of this study may provide
valuable evidence for the preparation of analogous injectable
tissue engineered bones.
Materials and methods
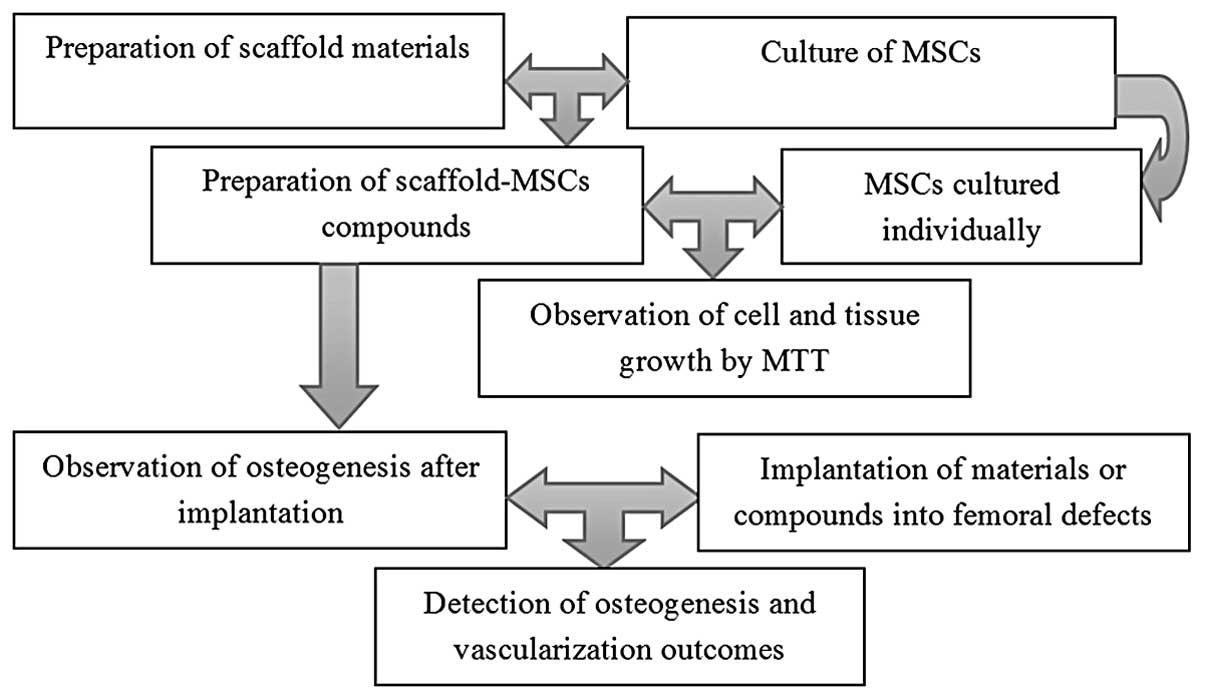
General information
MSCs (China Infrastructure of Cell Line Resources,
Beijing, China) were divided into two groups: An experimental study
group that was cultured with injectable chitosan-β-tricalcium
phosphate composite and a control group that was cultured with
β-tricalcium phosphate composite only. The growth of the cells and
tissues was verified by MTT assay to determine the effects of the
composite. In addition, 10 12-month-old beagles were implanted with
composite scaffolds and subsequently observed for the effects on
osteogenesis and vascularization. The dogs were subsequently
divided into Groups A and B (n=5/group). Group A was subjected to
15-mm defects on the femur and was then was implanted with
scaffolds carrying MSCs. Group B was implanted with the composite
scaffold materials alone, without MSCs. The two groups were
observed on the 2nd, 4th, 8th and 12th weeks post-implantation
(Fig. 1).
Sampling site and criteria
The experiments were performed in the Experimental
Center of Hukou County Hospital of Traditional Chinese Medicine
(Hukou, China). Healthy 12-month-old beagles weighing 8–10 kg were
purchased from the Experimental Animal Center of Shandong
University (Jinan, China). The dogs were free of infections or
genetic diseases, and without history of limb bone fracture. The
present study was approved by the Ethics Committee of The
Saint-Petersburg State I.P. Pavlov Medical University (St.
Petersburg, Russia).
Cell culture
MSCs were cultured under sterile conditions, in
Dulbecco’s modified Eagle’s medium containing high-glucose levels
as a carbon source and serum in 10–20% CO2. MSCs were
extracted from the animals by puncture under anesthesia. Bone
marrow (10 ml) was collected using a 16-gauge bone needle, and then
filtered and cultured at 37°C in 10–20% CO2. The culture
medium was refreshed every three days until the end of the primary
culture. Pancreatin enzyme was subsequently added to the culture
medium in order to separate adhesive cells that were selectively
cultured in α-minimal essential medium supplemented with 10% fetal
bovine serum, with alendronate sodium used as an inhibitor
(Gibco-BRL, Gaithersburg, MD, USA). This new culture medium was
also changed every three days (5–7).
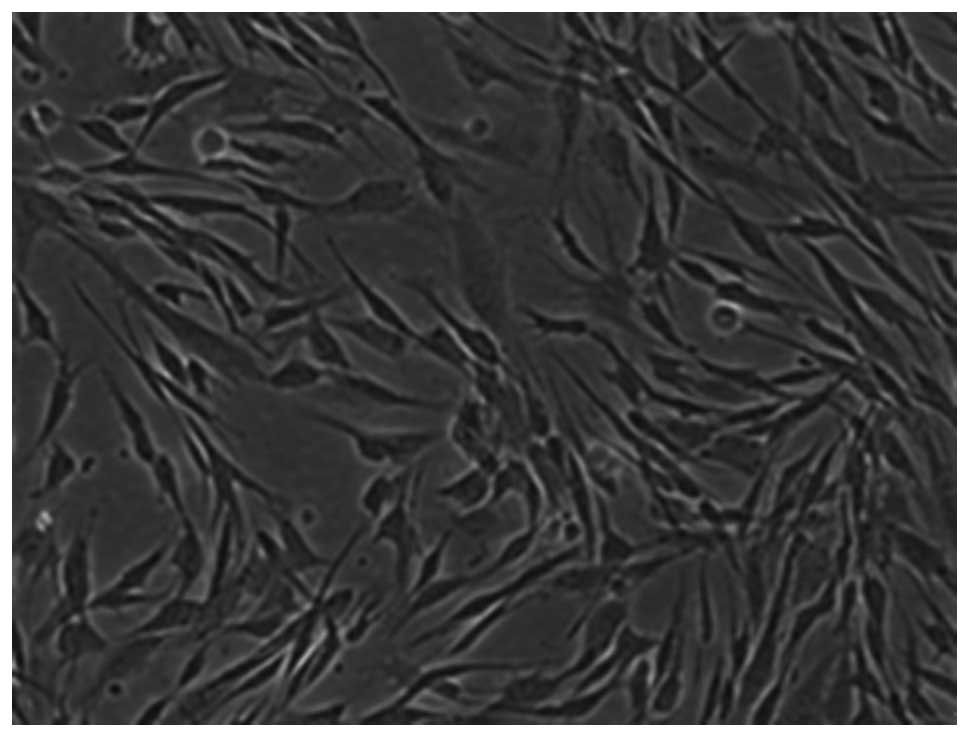
After three weeks of culture, the osteogenic differentiation of
MSCs (third generation) was determined by positive alkaline
phosphatase staining (Fig. 2).
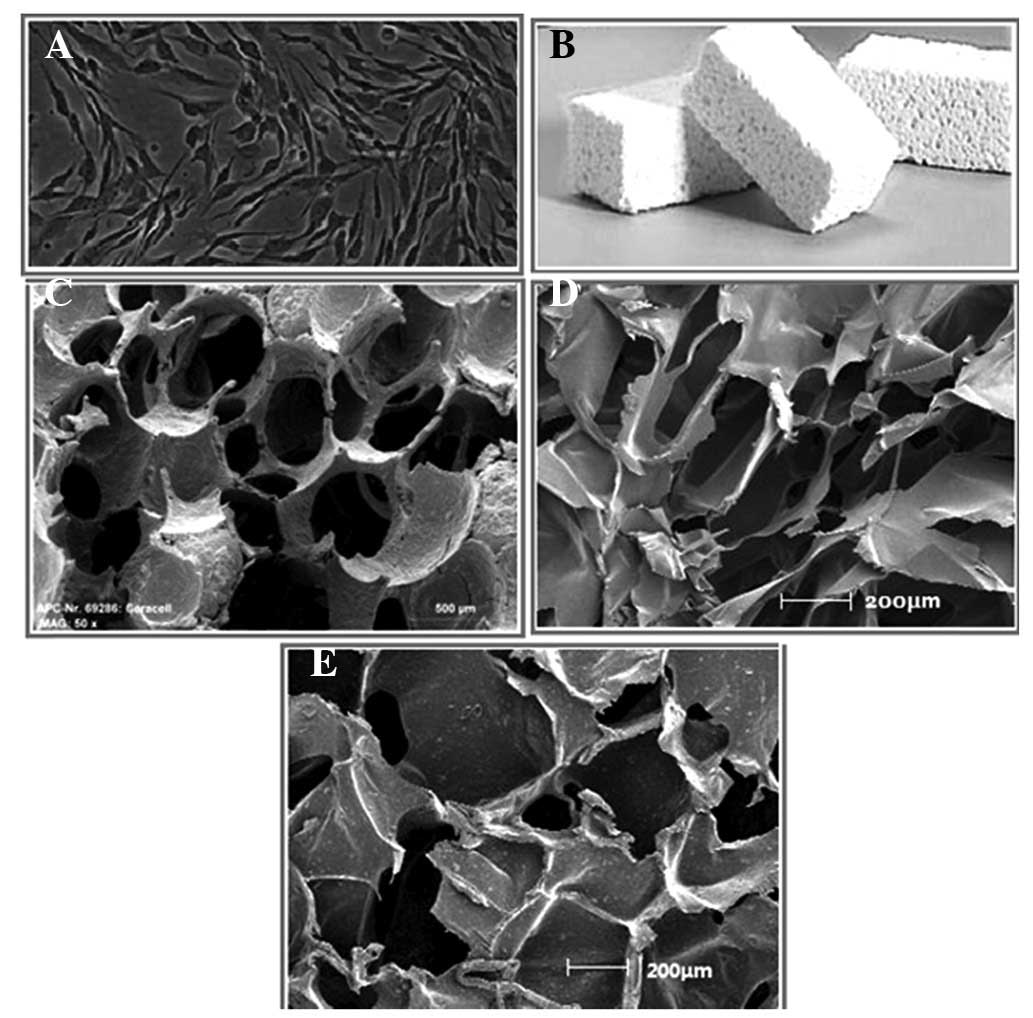
Material preparation
Scaffold materials were prepared under sterile
conditions at 37°C in an atmosphere containing 5% CO2,
using chitosan and β-tricalcium phosphate as the liquid and solid
material components, respectively (8). The mixture transformed from a liquid
to solid state, and was then cut into 48-mm3 sections
using a sterile knife. The third-generation MSCs, culture medium
and incised scaffold materials were transferred to 24-well plates
(Fig. 3).
Construction of scaffolds
The composite material was cut into 12×15 mm pieces,
into which were injected third-generation MSCs. The compounds were
then cultured under sterile conditions in CO2 at 37°C
for 4–6 h, and were subsequently covered with culture medium.
MTT assay
Culture medium was discarded from the culture
plates, which were then washed with phosphate-buffered saline. The
cultures were subsequently stained with MTT and universal buffer,
and cultured in 5% CO2 at 37°C for 5 h. The MTT and
buffer were discarded and 0.1 ml acidified isopropanol was added to
the plates and mixed by agitation for 15 min. Finally, the
absorbance of the products was measured at 570 nm.
Scaffold implantation
The beagles were anesthetized by intravenous
administration of 0.03 ml/kg 1% sodium pentobarbital, and the
mucoperiosteum was separated following a skin incision 15 mm from
the posterior limb. Femoral defects, ~15 mm in length on one side
of the femur, were prepared by an ultrasonic bone knife, followed
by hemostasis. The tissue engineered bone scaffold materials were
implanted, followed by suturing of the soft tissues, embedding and
hemostasis. The wounds were rinsed with normal saline, sutured by
layered closure and bandaged. Following the surgical procedure, the
dogs were intramuscularly injected with 400,000 units penicillin
for three consecutive days (qd). Under normal conditions,
osteogenesis and vascularization would occur within 12 weeks.
Cluster of differentiation 31 (CD31)
immunohistochemical staining
Histological analysis was performed using Masson’s
trichrome staining (Sigma-Aldrich, St. Louis, MO, USA). Sections
were fixed at 60°C heat for 10 min and deparaffinized through
ethanol hydration. The sections were subsequently sterilized with
peroxidase and rinsed in buffer, prior to undergoing separation
using trypsin and blocking with bovine serum albumin. The sections
were then exposed to diluted anti-dog CD31 primary (1:100) and
anti-goat secondary antibodies (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), in order to perform double-antibody sandwich
enzyme-linked immunosorbent assay. Upon clear color development,
the reaction was terminated using distilled water. The sections
were then double-stained with hematoxylin, prior to being
differentiated, recovered, dehydrated, resealed and visualized
under an inverted microscope. Areas of vascularization were
stained.
Vascular casting method
Dogs were anesthetized by intravenous administration
of 0.03 ml/kg 1% sodium pentobarbital. Skin incisions were then
made through the subcutaneous tissues to expose the femoral
arteries and veins for centrifugal intubation and fixation. Benzoyl
peroxide phthalocyanine (5 g), dimethylaniline (3 ml), dibutyl
phthalate (35 ml) and red oil paint (all Life Technologies,
Carlsbad, CA, USA) were then added to 300 ml pre-polymerized methyl
methacrylate. Following the initiation of polymerization, blood
vessels were infused until casting agents outflowed from the veins,
which were then ligated. The infusion was continued until the
peripheral skin was completely stained red (250 ml casting agent
was used on each side); at this point, the infusion was terminated
and the arteries were ligated. The infused methyl methacrylate
monomers underwent complete polymerization following cryogenic
storage of the blood vessels for 24–48 h while maintaining
intravascular pressure, and the samples were preserved and fixed in
10% formaldehyde solution for 2–3 weeks.
Statistical analysis
All data were analyzed by SPSS 10.0 (SPSS Inc.,
Chicago, IL, USA). Groups were compared by a two-sample t-test to
detect significant differences between the means of two samples and
their totals. P<0.05 was used to indicate a statistically
significant difference. An independent t-test was conducted using
the following formula:
Where S1 and S2 are two sample variances, and n1 and n2 are two
sample sizes.
Results
MTT assay
The absorbances at 570 nm were measured on the 2nd,
4th, 6th and 8th days. The absorbances were lower in the
experimental group than those in the control group (Table I). These results suggest that the
experimental group had a higher proliferative capacity than the
control group.
 | Table IComparison between the MTT assay
results. |
Table I
Comparison between the MTT assay
results.
| Day | Absorbancea
| t-test | P-value |
|---|
| Group A | Group B |
|---|
| 2nd | 0.118±0.032 | 0.123±0.031 | 0.550 | 0.384 |
| 4th | 0.367±0.064 | 0.412±0.039 | 2.941 | 0.247 |
| 6th | 0.847±0.062 | 0.911±0.089 | 2.890 | 0.239 |
| 8th | 1.128±0.084 | 1.203±0.069 | 3.380 | 0.084 |
Osteogenesis outcomes following
implantation
The scaffold-implanted areas were sampled 2, 4, 8
and 12 weeks after surgical intervention and the sections were
subjected to Masson’s trichrome staining. In the first two weeks,
the degree of osteogenesis between the two groups was equivalent.
After four weeks, Group A had significantly larger areas of newborn
tissues as compared with Group B (Table II).
 | Table IIComparison between bone areas. |
Table II
Comparison between bone areas.
| Week | Bone area
(mm2)
| t-test | P-value |
|---|
| Group A | Group B |
|---|
| 2nd |
3.5±0.8 |
3.2±0.4 | 1.64 | 0.213 |
| 4th | 17.6±1.6 |
6.8±1.2 | 26.45 | 0.004 |
| 8th | 50.4±6.3 | 18.4±4.1 | 20.85 | 0.007 |
| 12th | 80.7±8.2 | 51.2±6.1 | 14.14 | 0.009 |
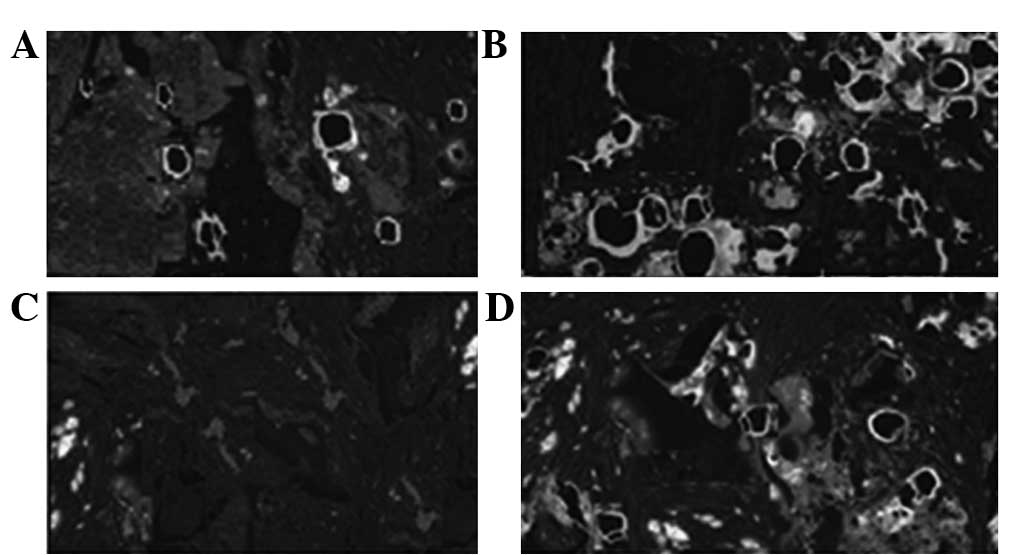
Vascularization outcomes following
implantation
Sections were sampled at the sites of implantation
2, 4, 8 and 12 weeks after surgical intervention, and were
subjected to CD31 staining to observe the number of new blood
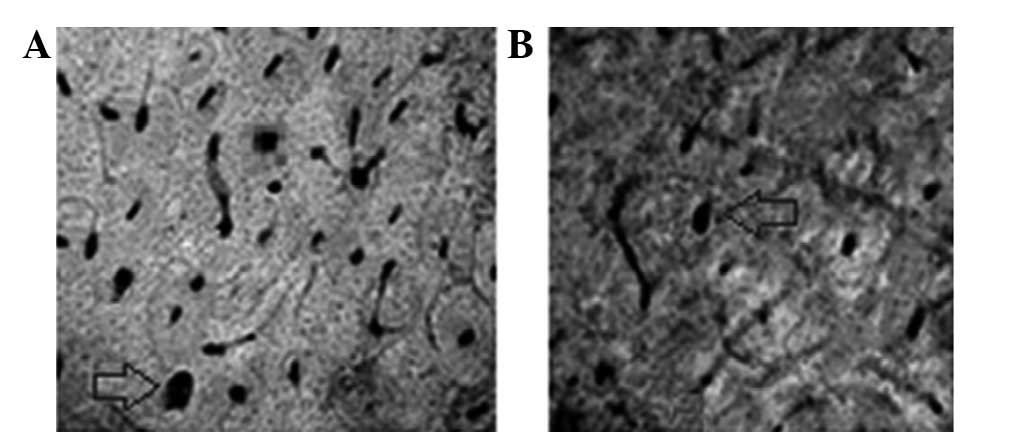
vessels. As shown in Figs. 4 and
5, Group A had a significantly
higher number of new blood vessels than Group B.
The sections showed clear microvessels and blood
vessels, which were reticulated along Haversian and Volkmann’s
canals. Nutrient vessels had extended into the endostea and
periostea. In addition, Haversian canals were observed in the
middle and internal and external lamellae, with blood vessels
reticulating along Haversian and Volkmann’s canals (Table III). Blood vessels were observed
to have extended and grown well, and the differences between Groups
A and B became more apparent with increased time.
 | Table IIIComparison between the number of blood
vessels in the cross-sections. |
Table III
Comparison between the number of blood
vessels in the cross-sections.
| Week | Blood Vessels (n)
| t-test | P-value |
|---|
| Group A | Group B |
|---|
| 2nd | 83.2±6.4 | 26.4±4.6 | 35.31 | 0.004 |
| 4th | 254.8±8.4 | 84.1±6.5 | 78.73 | 0.002 |
| 8th | 342.3±24.7 | 126.1±16.4 | 35.72 | 0.004 |
| 12th | 512.4±36.6 | 156.4±24.6 | 39.55 | 0.004 |
Discussion
The injection of tissue engineered bones is a highly
efficient intervention with minimal associated trauma. In the
procedure, seed cells are carried in the liquid state, which then
solidifies at the sites of bone defect following injection
(9,10).
Previous studies have endeavored to improve the
histocompatibility of tissue engineering bones. Currently, only one
type of material fails to satisfy the requirements of bone tissue
repair, therefore composite scaffold materials are required
(11,12). Compared with previous studies, the
present study presents the vascularization of repaired limb bone
defects more clearly and directly. The findings suggest that
chitosan-β-tricalcium phosphate composite is a suitable injectable
tissue engineering bone material when stem cells are implanted
simultaneously.
The currently available biomaterials are gels. These
produce satisfactory osteogenesis but are low in stability and
highly brittle, and thus perform poorly in repairing weight-bearing
bones, such as femurs. At present, eligible tissue engineered bones
are generally made from both organic or inorganic compounds, using
citric acid as the crosslinking agent, thus fusing tissue cells
well due to high strength and low toxicity (13–15).
To maintain the survival of tissue engineered bones
in vivo, the vascularization and nerve generating capacities
of the seeded cells should also be enhanced to accelerate bone
recovery (16). The growth of
seeded cells following implantation is associated with blood
microcirculation. In cases of severe bone defect, the deep tissues
may succumb to ischemic necrosis. It is therefore essential to
reconstruct the microcirculation to elevate the survival rate of
seed cells. Periosteal tissue is abundant in nerves, of which a
number enters the bone marrow together with nourishing blood
vessels (17–20). The femur is supplied by the direct
branches of blood vessels surrounding bone tissues as well as the
periosteal vasoganglion. The vasoganglion forms with both skeletal
muscles with arteries adhering to the bones and the periosteal
branches adjoining to the arteries, and nutrients further penetrate
into the tissues through pores in the bone.
Nervous tissue, which is widely distributed
throughout bone tissue, controls the proliferation of osteoblasts
and facilitates healing at the sites of defect upon bone fracture.
Under normal conditions, nervous tissues regulate the metabolism of
bone cells and therefore the nerves are considered to dominate the
process of bone repair. Neurotransmitters secreted by nerve cells
can upregulate or downregulate microcirculation and stimulate cell
secretion. Peripheral nerves and the central nervous system
cooperate in regulating the repair of defects (21,22).
To date, liquid compounds have only been applicable
to filling, rather than repairing, defects. Despite normal growth
in liquid gels, it remains to be determined whether MSCs can grow
in solid compounds (23).
Reconstructing blood vessels and nervous tissues in defects not
only recovers the blood supply and self-regulation of bones, but is
also required for postoperative treatment and effective recovery.
This study has therefore initiated a novel approach for the
surgical repair of bone defects.
References
|
1
|
Dimitriou R, Jones E, McGonagle D and
Giannoudis PV: Bone regeneration: current concepts and future
directions. BMC Med. 9:662011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bose S, Roy M and Bandyopadhyay A: Recent
advances in bone tissue engineering scaffolds. Trends Biotechnol.
30:546–554. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muschler GF, Nakamoto C and Griffith LG:
Engineering principles of clinical cell-based tissue engineering. J
Bone Joint Surg Am. 86-A:1541–1558. 2004.PubMed/NCBI
|
|
4
|
Muschler GF, Raut VP, Patterson TE, Wenke
JC and Hollinger JO: The design and use of animal models for
translational research in bone tissue engineering and regenerative
medicine. Tissue Eng Part B Rev. 16:123–145. 2010. View Article : Google Scholar
|
|
5
|
Tasso R, Fais F, Reverberi D, Tortelli F
and Cancedda R: The recruitment of two consecutive and different
waves of host stem/progenitor cells during the development of
tissue-engineered bone in a murine model. Biomaterials.
31:2121–2129. 2010. View Article : Google Scholar
|
|
6
|
Yeatts AB and Fisher JP: Bone tissue
engineering bioreactors: dynamic culture and the influence of shear
stress. Bone. 48:171–181. 2011. View Article : Google Scholar
|
|
7
|
Yilgor P, Tuzlakoglu K, Reis RL, Hasirci N
and Hasirci V: Incorporation of a sequential BMP-2/BMP-7 delivery
system into chitosan-based scaffolds for bone tissue engineering.
Biomaterials. 30:3551–3559. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mouriño V and Boccaccini AR: Bone tissue
engineering therapeutics: controlled drug delivery in
three-dimensional scaffolds. J R Soc Interface. 7:209–227. 2010.
View Article : Google Scholar :
|
|
9
|
Holzwarth JM and Ma PX: Biomimetic
nanofibrous scaffolds for bone tissue engineering. Biomaterials.
32:9622–9629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamada Y, Nakamura S, Ito K, et al:
Injectable bone tissue engineering using expanded mesenchymal stem
cells. Stem Cells. 31:572–580. 2013. View Article : Google Scholar
|
|
11
|
Bi ZG, Han XG, Fu CJ, Cao Y and Yang CL:
Reconstruction of large limb bone defects with a double-barrel free
vascularized fibular graft. Chin Med J (Engl). 121:2424–2428.
2008.
|
|
12
|
Noaman HH: Management of upper limb bone
defects using free vascularized osteoseptocutaneous fibular bone
graft. Ann Plast Surg. 71:503–509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang Q, Reddy N and Yang Y:
Cytocompatible cross-linking of electrospun zein fibers for the
development of water-stable tissue engineering scaffolds. Acta
Biomater. 6:4042–4051. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gyawali D, Nair P, Zhang Y, et al: Citric
acid-derived in situ crosslinkable biodegradable polymers for cell
delivery. Biomaterials. 31:9092–9105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuo YC and Yeh CF: Effect of
surface-modified collagen on the adhesion, biocompatibility and
differentiation of bone marrow stromal cells in
poly(lactide-co-glycolide)/chitosan scaffolds. Colloids Surf B
Biointerfaces. 82:624–631. 2011. View Article : Google Scholar
|
|
16
|
Amini AR, Laurencin CT and Nukavarapu SP:
Differential analysis of peripheral blood- and bone marrow-derived
endo-thelial progenitor cells for enhanced vascularization in bone
tissue engineering. J Orthop Res. 30:1507–1515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun X, Su J, Bao J, et al: Cytokine
combination therapy prediction for bone remodeling in tissue
engineering based on the intracellular signaling pathway.
Biomaterials. 33:8265–8276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao J, Luo C, Han W, Tu M, Zeng R, Cha Z
and Zhou C: Fabrication and properties of
poly(L-lactide)/hydroxyapatite/chitosan fiber ternary composite
scaffolds for bone tissue engineering. J Polymer Eng. 32:283–289.
2012. View Article : Google Scholar
|
|
19
|
Pan L, Pei X, He R, Wan Q and Wang J:
Multiwall carbon nanotubes/polycaprolactone composites for bone
tissue engineering application. Colloids Surf B Biointerfaces.
93:226–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Higuita-Castro N, Gallego-Perez D,
Pelaez-Vargas A, et al: Reinforced Portland cement porous scaffolds
for load-bearing bone tissue engineering applications. J Biomed
Mater Res B Appl Biomater. 100B:501–507. 2012. View Article : Google Scholar
|
|
21
|
Ji Y, Xu GP, Zhang ZP, Xia JJ, Yan JL and
Pan SH: BMP-2/PLGA delayed-release microspheres composite graft,
selection of bone participate diameters, and prevention of aseptic
inflammation for bone tissue engineering. Ann Biomed Eng.
38:632–639. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clarke SA, Walsh P, Maggs CA and Buchanan
F: Designs from the deep: marine organisms for bone tissue
engineering. Biotechnol Adv. 29:610–617. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alge DL, Bennett J, Treasure T,
Voytik-Harbin S, Goebel WS and Chu TM: Poly(propylene fumarate)
reinforced dicalcium phosphate dihydrate cement composites for bone
tissue engineering. J Biomed Mater Res A. 100:1792–1802. 2012.
View Article : Google Scholar : PubMed/NCBI
|