Introduction
A disintegrin and metalloproteinases (ADAMs) are a
family of transmembrane proteins, anchored to the cell membrane
surface (1), which are implicated
in a variety of physiological and pathological processes, including
sperm-egg binding, neuronal development and myotube formation
(2). In addition, ADAMs have been
observed to be overexpressed in a variety of types of tumor
(3).
Human ADAM15 is unique among the ADAM family,
containing the integrin binding motif Arg-Gly-Asp (RGD) sequence in
its disintegrin domain (4). ADAM15
is important in cell adhesion, extracellular matrix degradation,
intracellular signal transduction and pathological changes in
tumors. Previous studies have revealed that the disintegrin domain
of ADAM15 interacts with integrins αvβ3, α5β1 and α9β1, and
inhibits platelet aggregation and cell migration (3,5,6). The
downregulation or overexpression of ADAM15 alters cell-cell
interactions and cell behavior (6–8). In
addition, ADAM15 is overexpressed in several types of solid tumor
and is closely associated with tumor occurrence and development of
(9,10).
Angiogenesis is essential in the process of tumor
growth. Certain proteolytic fragments of the extracellular matrix,
including endostatin and angiostatin have been found to inhibit
tumor angiogenesis (11,12). Previous studies have observed that
ADAM15 is overexpressed in the vascular endothelial cells of tumors
(13,14). In a previous investigation using a
mouse model of retinopathy of prematurity, neovascularization in
ADAM15−/− mice was markedly reduced compared with
wild-type mice. In addition, the tumor sizes in the
ADAM15−/− mice were significantly smaller than those in
the wild-type mice (15).
Trochon-Joseph et al (10)
found that the recombinant disintegrin domain of ADAM15 inhibits
in vitro angiogenesis and the growth of breast and lung
cancer metastases in melanoma.
In our previous investigations, the recombinant
human disintegrin domain of ADAM15 (rhddADAM15) was expressed in
Escherichia coli, and the inhibitory activity of rhddADAM15
on Bel-7402 liver cancer cells was evaluated (16,17).
The present study aimed to assess the antitumor and anti-angiogenic
activities of rhddADAM15 in vivo, using a zebrafish model.
The human umbilical vein cell line EAhy926, which is an adherent
cell line and was established by fusing primary human umbilical
vein cells with a thioguanine-resistant clone of A549, was also
used. The mechanism underlying the inhibition of proliferation of
the Bel-7402 cells was investigated, and the inhibitory effects of
rhddADAM15 on vascular endothelial cells were evaluated, to
determine whether rhddADAM15 affects angiogenesis in zebrafish.
Materials and methods
Cell culture
Human breast cancer MCF-7, mouse melanoma B16, human
cervical cancer Hela, human pancreatic cancer 8988, human liver
cancer Bel-7402 and human liver cancer HEPG-2 cells were all
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China), and doxorubicin resistant breast cancer
MCF-7/ADM and taxol resistant breast cancer MCF-7/PTX cells were
produced in our laboratory. All of the above cells were grown in
RPMI 1640 essential medium (Gibco Life Technologies, Carlsbad, CA,
USA), supplemented with 10% fetal bovine serum (Gibco Life
Technologies) and penicillin-streptomycin (100 U/ml and 100
µg/ml, respectively; Gibco Life Technologies). The cells
were incubated in 5% CO2 at 37°C. Technical support for
the zebrafish study was provided by Hunter Biotechnology, Inc.
(Hangzhou, China) and was approved by the ethics committee of the
Association for Assessment and Accreditation of Laboratory Animal
Care International (certificate no. 001458) and Science and
Technology Department of Zhejiang Province, China (certificate no.
SYXK20120171).
Cell proliferation assay
A sulforhodamine B assay (SRB; Sigma-Aldrich, St.
Louis, MO, USA) was used to assess cell proliferation. The cells
were seeded into a 96-well plate (Corning Life Sciences, Shanghai,
China) at a density of 6–7×103 cells/well and cultured
for 24 h at 37°C. Subsequently, the medium was removed and replaced
with 100 µl medium containing rhddADAM15 (produced in our
laboratory; 0, 1.0, 1.5, 2.0, 4.0, 5.0, 8.0 and 10.0
µmol/l). Following incubation for 24 h, the medium was
removed and the cells were fixed by adding 100 µl cold 10%
trichloroacetic acid (Sigma-Aldrich) and incubated for 60 min at
4°C. The fixed cells were then washed in water and stained with 100
µl 0.4% (w/v) SRB at 37°C. After 30 min, the unbound SRB was
washed off with 1% acetic acid (Sinopharm Chemical Reagent Co.,
Ltd., Shanghai, China), and the stained cells were solubilized with
100 µl 10 mM Tris-base (Sinopharm Chemical Reagent Co.,
Ltd.). The absorbance of the stained cells in the wells was
measured at 540 nm (Abs540 nm) using a Multiskan MK2
microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). The
inhibitory rate of cell proliferation was calculated using the
following formula: Inhibitory rate = (Abs540nm, control
- Abs540nm, rhddADAM15) / (Abs540nm, control
- Abs540nm, blank) × 100%.
Cell migration assay
A wound-healing assay was used to assess cell
migration. The cells were seeded into a 24-well plate at a density
of 1–5×105/well, and were incubated for 24 h at 37°C. A
‘wound’ was formed by manually scraping the monolayer in the middle
of each well with a pipette tip. The floating cells were washed off
with phosphate-buffered saline (PBS) and the first set of images of
each well were captured. The location of each image was marked on
the bottom of each well. Fresh medium, or medium with rhddADAM15
(0, 1.0, 1.5, 2.0, 4.0 and 8.0 µmol/l), was added and the
cells were incubated for a further 24 h. Subsequently, a second set
of images were captured at the marked locations. The area of the
wound (A), indicating the ability of the cells to migrate, was
measured using ImageJ software, version 1.4.3.67 (National
Institutes of Health, Bethesda, MD, USA). The inhibitory rate of
cell migration was calculated using the following formula: Rate of
migration (MR) = (A0 h-A24 h) / A0
h × 100%. The rate of inhibition of migration was calculated
as: MRcontrol - MRrhddADAM15.
Western blot analysis
Following treatment with rhddADAM15, the cells were
collected, lysed (Beyotime Institute of Biotechnology, Nantong,
China) and clarified by centrifugation (5418; Eppendorf, Hamburg,
Germany) for 10 min at 4°C, 12,391 × g. Subsequently, the samples
were incubated with an equal volume of 2X sodium dodecyl sulfate
(SDS; Sinopharm Chemical Reagent Co., Ltd.) sample buffer and
heated for 5 min at 95°C. SDS-PAGE was then performed, following
which the proteins were transferred onto nitrocellulose membranes
(Pall Life Sciences, Ann Arbor, MI, USA). The membranes were
blocked with 5% bovine serum albumin in Tris-buffered saline-0.1%
Tween 20 for 1 h at room temperature. The blots were then incubated
with the following antibodies for ~2 h at 4°C: Rabbit polyclonal
phosphorylated Src Y416 (#2101; 1:1,000; Cell Signaling Technology,
Boston, MA, USA), rabbit polyclonal phosphorylated Src Y527 (#2105;
1:1,000; Cell Signaling Technology), mouse monoclonal total Src
(ab16885; 1:100; Abcam, Cambridge, UK) and mouse monoclonal β-actin
(AA128; 1:1,000; Beyotime Institute of Biotechnology). The blots
were then incubated with horseradish peroxidase-conjugated goat
anti-rabbit (A0208) and goat anti-mouse (A0216) IgG secondary
antibodies (Beyotime Institute of Biotechnology) for 1 h at 37°C.
The blots were visualized using enhanced chemiluminescence reagents
(Beyotime Institute of Biotechnology). β-actin was used as the
internal reference.
Tube formation assay
Matrigel basement membrane matrix (Matrigel; BD
Biosciences, Franklin Lakes, NJ, USA) was thawed and 96-well plates
and tips were precooled at 4°C overnight. Subsequently, the plates
were coated with 60 µl Matrigel and incubated for 30 min at
37°C. The EAhy926 cells were seeded at a density of
6–8×104/well with different concentrations of rhddADAM15
(0, 1.0, 2.0, 4.0, 6.0 and 8.0 µmol/l). After 6 h incubation
at 37°C, the tube forming structures were observed and images were
captured under a microscope (CKX41; Olympus, Tokyo, Japan). At
least three images were captured randomly in each group and the
number of formed tubes were counted.
Analysis of apoptosis
An Annexin V-Propidium Iodide (PI) Apoptosis
Detection kit (Beyotime Institute of Biotechnology) was used to
detect the apoptosis induced by rhddADAM15. The EAhy926 cells were
seeded at a density of 3–5×105/well in 6-well plates and
cultured overnight, followed by treatment with different
concentrations of rhddADAM15 (0, 4.0 and 6.0 µmol/l) for 6 h
at 37°C. The cells were then harvested and washed twice with PBS,
followed by resuspension in binding buffer and staining with
annexin V to detect early apoptotic cells for 10 min. The annexin V
was removed via centrifugation at 1,000 × g for 5 min at room
temperature, and the cells were washed and stained with PI, to
detect early and late apoptotic cells, for 5 min at room
temperature. The percentage of apoptotic cells was determined using
flow cytometry (BD Biosciences) and the data were processed using
Cell Quest software, version 6.0 (BD Biosciences).
Cell cycle analysis
PI staining was used to analyze the cell cycle. The
cells were seeded at a density of 3–5×105/well in a
6-well plate and cultured overnight, followed by treatment with or
without rhddADAM15 for 24 h at 37°C. Subsequently, the cells were
harvested, washed twice with PBS and fixed with 70% cold ethanol
for at least 2 h at 4°C. The cells were then washed and incubated
with 50 µg/ml RNase (Sigma-Aldrich) and stained using 10
µg/ml PI (Beyotime Institute of Biotechnology) at 37°C for
30 min. The cell cycle was then evaluated by flow cytometry and the
data were analyzed using Modfit software (Verity Software House,
Topsham, ME, USA).
Angiogenesis model in zebrafish
The fli1a: enhanced green fluorescent protein (EGFP)
transgenic zebrafish used in the present study were raised and
maintained according to the guidelines of the ethics committees
mentioned at Wenzhou Medical University (Wenzhou, China), and the
zebrafish embryos were generated by natural pair-wise mating in a
light:dark cycle of 14:10 h/day (18). The zebrafish were fed with
Artemia and cultured in water containing sea salt (200 mg/l)
at ~pH 7.2 and 28°C in an experimental tank system made of
polycarbonate. The present study was approved by the Association
for Assessment and Accreditation of Laboratory Animal Care (001458)
and the Science and Technology Department of Zhejiang Province,
China (SYXK20120171). The subintestinal vessels (SIVs) and
intersegmental vessels (ISVs) of the fli1a:EGFP transgenic
zebrafish embryos were observed to evaluate the anti-angiogenic
activity of rhddADAM15. To assess the anti-SIV and anti-ISV
angiogenic activity of rhddADAM15, fli1a:EGFP transgenic
zebrafish embryos were used at 48 h post-fertilization (hpf) and 23
hpf, respectively. At least 10 zebrafish embryos, allocated
randomly, were used in each experimental group, including a control
group. Either rhddADAM15, positive control (endostatin; Simcere
Pharmaceutical, Nanjing, China) or PBS was microinjected into the
yolk-sac of the fli1a:EGFP transgenic zebrafish embryos.
Images of the zebrafish embryos in each group were captured at 0,
6, 12 and 24 h post-injection, to evaluate the time course of
rhddADAM15 onset and to assess the area (AS) of the SIVs and the
pairs (P) of the ISVs, which are indicative of the angiogenic
ability of zebrafish. The inhibitory rates of angiogenesis of the
SIVs or ISVs were calculated using the following formulae:
Inhibitory rate of SIVs (%) = (1 - ASrhddADAM15 /
AScontrol) × 100% and, inhibition rate of ISVs (%) = (1
- PrhddADAM15 / Pcontrol) × 100%.
Statistical analysis
The results are presented as the mean ± standard
error of the mean of triplicates from at least three independent
experiments. Statistical differences were determined using
Dunnett’s t-test with GraphPad Prism software, version 5.01
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
rhddADAM15 inhibits the proliferation and
migration of tumor cell lines in vitro
The SRB assay revealed that rhddADAM15 inhibited the
proliferation of several tumor cell lines, with a half maximal
inhibitory concentration (IC50) range between 1.04 and
5.77 µM (Table I). The most
sensitive cells were the Bel-7402 human liver cancer cells, with an
IC50 of 1.04 µM. rhddADAM15 also inhibited the
migration of the eight tumor cell lines, which was determined using
a wound-healing assay, the most sensitive of which were the
Bel-7402 cells, with an IC50 of 2.09 µM (Table I).
 | Table IInhibitory effect of recombinant
human disintegrin domain A disintegrin and metalloproteinase 15 on
the proliferation and migration of tumor cell lines. |
Table I
Inhibitory effect of recombinant
human disintegrin domain A disintegrin and metalloproteinase 15 on
the proliferation and migration of tumor cell lines.
| Cell line | IC50 of
proliferation (µmol/l) | IC50 of
migration (µmol/l) |
|---|
| MCF-7 human breast
cancer | 2.90 | 1.95 |
| MCF-7/ADM
doxorubicin resistant breast cancer | 5.30 | 2.19 |
| MCF-7/PTX taxol
resistant breast cancer | 5.77 | 3.86 |
| B16 mouse
melanoma | 3.60 | 1.64 |
| HeLa human cervical
cancer | 2.44 | 0.61 |
| 8988 human
pancreatic cancer | 5.76 | 4.73 |
| Bel-7402 human
liver cancer | 1.14 | 2.09 |
| HEPG-2 human liver
cancer | 4.73 | 1.98 |
rhddADAM15 reduces Src activation in
Bel-7402 cells
It has been demonstrated that rhddADAM15 inhibits
the proliferation and migration of Bel-7402 cells in vitro
and in vivo, and induces apoptosis and partial
G2/M phase arrest (17). To investigate the mechanism
underlying the inhibition of proliferation, the present study
investigated whether rhddADAM15 affected the mitogen-activated
protein kinase (MAPK) signaling pathway, one of the most important
pathways associated with cell proliferation and migration. U0125,
an inhibitor of extracellular signal regulated kinase (Erk)1/2, and
epidermal growth factor (EGF), an agonist of MAPK, were used in the
present study. The concentration of rhddADAM15, EGF and U0125 were
1.0, 0.8 and 10 nM, respectively. As shown in Fig. 1A, rhddADAM15 inhibited the
proliferation of the Bel-7402 cells, with an inhibitory rate of
41.9% (column 1). Similarly, U0125 inhibited the proliferation of
the Bel-7402 cells, and the inhibitory effect was increased when
combined with rhddADAM15 (columns 2 and 3). By contrast, EGF
increased the proliferative effect (column 4) and weakened the
inhibitory effect, partially induced by rhddADAM15 or U025 (columns
5–7). Src is an integrator of divergent signals and channels
phosphorylation signals through the Ras/Raf/ERK1/2 signaling
pathway and, in certain cells, the phosphatidylinositol
3-kinase/AKT signaling pathway (19). To investigate whether rhddADAM15
affected the activation of Src, the phosphorylation levels of Src
at Tyrosine 416 (Y416) and Tyrosine 529 (Y529) of the Bel-7402
cells were evaluated, following treatment with rhddADAM15. As shown
in Fig. 1B, no significant
difference was observed in the total level of Src in the Bel-7402
cells treated with or without rhddADAM15. Following treatment with
rhddADAM15, a significant reduction in the phosphorylation levels
of Y416 was detected in the Bel-7402 cells, compared with the
control cells. By contrast, the phosphorylation levels of Y527 was
increased (Fig. 1B). The
dephosphorylation of Src Y529 is a requirement for Src activation.
The present data revealed that rhddADAM15 inhibited the
phosphorylation of Y416 and dephosphorylation of Y529, therefore,
rhddADAM15 downregulated the activation of Src.
rhddADAM15 inhibits the proliferation,
migration and tube formation of EAhy926 cells
To investigate the effect of rhddADAM15 on
angiogenesis, its activity on the proliferation, migration and tube
formation of EAhy926 cells was evaluated. rhddADAM15 inhibited the
proliferation and migration of EAhy926 cells in a dose-dependent
manner, and the IC50 values were 3.78 and 3.18
µM, respectively (Fig. 2A).
The EAhy926 cells formed tube structures on the Matrigel following
6 h incubation (Fig. 2B). Although
the cells remained adhered to each other, few tubes were identified
in each image, and the number of tubes formed was 79% lower
following rhddADAM15 treatment at a concentration of 4 µM
(Fig. 2B). This result
demonstrated that rhddADAM15 inhibited the formation of tube
structures.
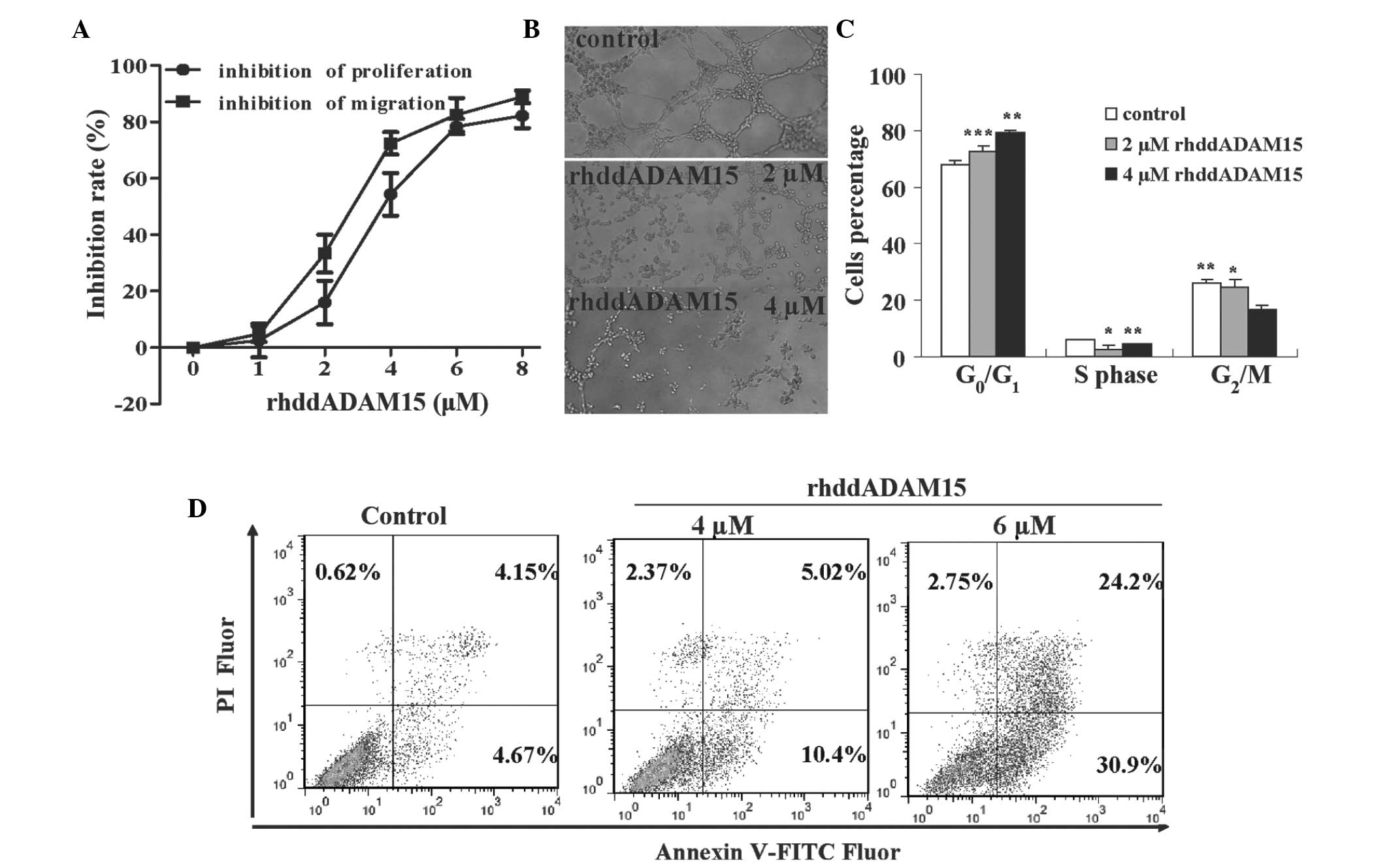 | Figure 2rhddADAM15 inhibits the
proliferation, migration and tube formation, and induces cell cycle
arrest and apoptosis of Eahy926 cells. (A) Inhibitory effect of
rhddADAM15 on the proliferation and migration of EAhy926 cells. (B)
Representative images of tube formation in the EAhy926 cells
following rhddADAM15 treatment. Cells were analyzed under a
fluorescence microscope (magnification, ×40). (C) Assessment of
EAhy926 cell cycle following rhd-dADAM15 treatment. (D) Assessment
of apoptosis in EAhy926 cells treated with rhddADAM15. In the
four-quadrant plots, normal cells are in the lower-left, necrotic
cells are in the upper-right, early apoptotic cells are in the
lower-right, and late apoptotic cells in are the upper-right. Data
are expressed as the mean ± standard error of the mean (n=3;
*P<0.0, **P<0.01 and
***P<0.001, vs. untreated control). rhdd, recombinant
human disintegrin domain; ADAM15, A disintegrin and
metalloproteinase 15; FITC, fluorescein isothiocyanate; PI,
propidium iodide. |
The cell cycle and apoptotic rates were analyzed to
determine how rhddADAM15 inhibited the proliferation of EAhy926
cells. The number of cells in the G0/G1 phase
increased and the number in the G2 and S phase
decreased, indicating that a proportion of the cells were arrested
at G0/G1 (Fig.
2C). The annexin V-PI double-staining method was used to
determine the percentage of apoptotic cells after 6 h rhddADAM15
treatment. rhddADAM15 induced apoptosis in the EAhy926 cells in a
dose-dependent manner, with a maximum inhibitory rate of 55.1±2.3%
at 6 µM rhddADAM15 (Fig.
2D), which was a 7-fold increase compared with the controls
(~8.8%), suggesting that rhddADAM15 markedly induced apoptosis in
the EAhy926 cells.
rhddADAM15 inhibits angiogenesis in
zebrafish
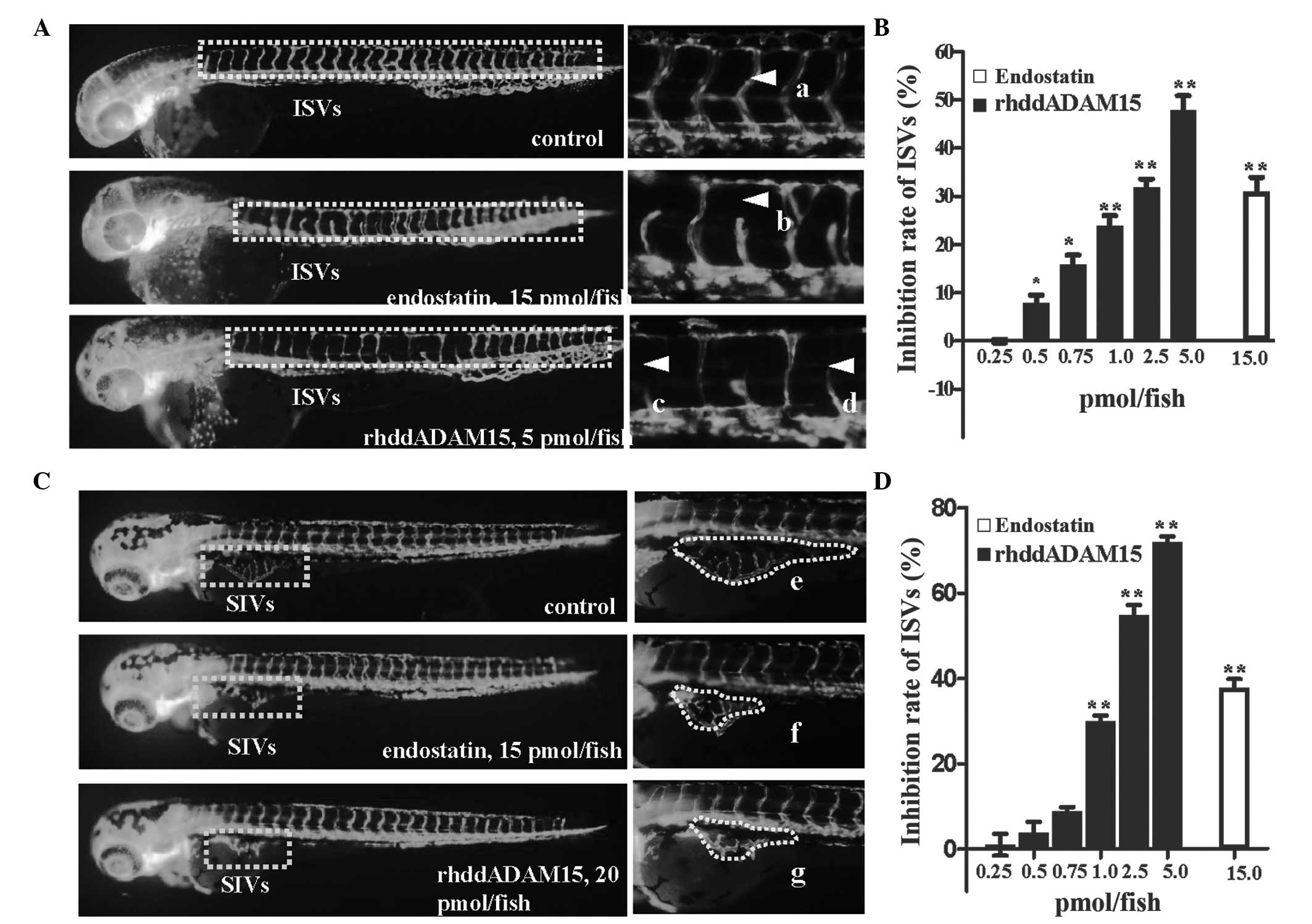
rhddADAM15-induced anti-angiogenic activity was
evaluated in the ISVs and SIVs of zebrafish embryos. A total of 28
pairs of well-arranged ISVs in zebrafish embryos were observed in
the fluorescent-labeled vascular endothelial cells (Fig. 3A). Following treatment with
rhddADAM15 or endostatin for 24 h, the zebrafish embryos exhibited
a decreased in ISV formation (Fig.
3A–D). Compared with the negative control, the zebrafish
embryos treated with rhddADAM15 exhibited a thinning or absence of
ISVs, in a dose-dependent manner, with a maximum inhibitory rate of
48±2.92% at 5.0 pmol/fish rhddADAM15.
The anti-angiogenic activity of rhddADAM15 on SIVs
was also assessed, which formed a regular basket-like network
(Fig. 3C and E). Following
treatment with rhddADAM15 or endostatin, the SIVs were irregular
and the area was reduced in a dose-dependent manner (Fig. 3C, F and G). The maximum inhibitory
rate was 72±1.26% at 20.0 pmol/fish rhddADAM15 and 40±1.36% at 20
pmol/fish with endostatin. In addition, as shown in Fig. 3C, the ISVs, which had formed in
these zebrafish did not exhibit any significant pathological
changes, indicating that rhddADAM15 or endostatin inhibited the
process of angiogenesis, not the vessels that formed. These results
demonstrated that rhddADAM15 caused marked inhibition of the
angiogenesis of ISVs and SIVs in zebrafish, with a more marked
effect compared with endostatin.
Discussion
The interaction between ECM and integrins is
important in tumor progression. The disintegrin domain of human
ADAM15, which contains an RGD motif, is an important functional
domain and has been revealed to mediate cell-cell and cell-matrix
interactions (20,21). Previous studies have demonstrated
that ADAM15 is overexpressed in tumor cells and vascular
endothelial cells in multiple types of tumor. However, the function
of ADAM15 in tumor progression remains to be elucidated, as do the
potential anti-angiogenic activities of rhddADAM15 in vivo,
as an exogenous therapeutic protein.
The present study demonstrated that, as an exogenous
protein, rhddADAM15 inhibited the proliferation and migration of
several tumor cell lines in vitro. It was observed in our
previous study that rhddADAM15 induces apoptosis via the caspase 8
and caspase 9 pathways (17).
Since rhddADAM15 is considered to interact with integrins (5,6,22),
the signaling pathways associated with integrins and cell
proliferation were investigated. The results revealed that
rhddADAM15 downregulates the activity of c-Src and regulates the
phosphorylation of cyclin-dependent kinase 2 (data not shown).
However, the specific target remains to be elucidated.
As anti-angiogenic therapies have the advantage of
exhibiting broad-spectrum effects with a low risk for metastasis,
there has been increasing attention on the identification of
anti-angiogenic drug treatments that control tumor growth.
Currently, the majority of anti-angiogenic drug treatments target
vascular endothelial growth factor (VEGF), and several VEGF
inhibitors have been approved for clinical use in the treatment of
cancer and eye diseases (23,24).
However, these agents require optimization, and the development of
novel targets is required for several reasons: Certain patients do
not respond to the treatment, drug resistance may occur during the
course of the treatment and importantly, a previous study revealed
that tumor angiogenesis can occasionally become VEGF-independent
(25). Certain integrins are
involved in angiogenic processes, and cyclic RGD peptides have been
observed to inhibit angiogenesis, leading to tumor regression via
the targeting of integrins (14,26–29).
The disintegrin domain of human ADAM15 contains the
RGD integrin binding motif. In addition, ADAM15 has been found to
be upregulated on the angiogenic endothelial cell surface (13). Therefore, the anti-angiogenic
activity of rhddADAM15 was further evaluated in the present study.
Although the in vitro activity of rhddADAM15 against
angiogenesis has been evaluated (10), investigation of angio-genesis in
vivo faces several challenges. The use of zebrafish offers an
intuitive and effective model in the investigation of in
vivo angiogenesis and tumor formation (30–32)
for advanced drug screening, owing to their small size, short life
cycle and transparency. In the present study, rhddADAM15 inhibited
the proliferation, migration and tube formation of EAhy926 cells,
which was in agreement with a previous study by Trochon-Joseph
et al (10). In addition,
the present study demonstrated that rhddADAM15 induced apoptosis
and G0/G1 phase arrest in the EAhy926 cells,
which was responsible for the inhibition of proliferation. In
addition, rhddADAM15 in the zebrafish exhibited superior
anti-angiogenic activity compared with endostatin.
ADAM15 is a multi-domain and multifunctional
protein, and several molecular pathways have been suggested to
explain its effects. The key mechanisms of rhddADAM15 are
considered to be associated with its integrin-binding domain
(10). Crociani et al
(33) identified a novel
angiogenic pathway in colorectal cancer, which is triggered by β1
integrin-mediated adhesion and leads to the secretion of VEGF-A. In
addition, Lucena et al (34) found that the r-mojastin 1 and
r-viridistatin 2 recombinant disintegrins, which bind to αvβ3 and
αvβ5, have anti-angiogenic activities. Although the precise
mechanisms underlying the anti-angiogenic activity of rhddADAM15
requires further investigation, the results of the present study
demonstrated the anti-angiogenic activity of rhddADAM15 in
vivo for the first time, to the best of our knowledge, and
revealed that, as a recombinant exogenous protein, rhddADAM15 is
effective against angiogenesis in vitro and in vivo
and may be a potential anticancer agent.
Acknowledgments
The authors would like to thank Dr Li Chunqi
(Wenzhou Medical University, China) for providing the
fli1a:EGFP transgenic zebrafish and Dr IC Bruce for reading
the manuscript. The present study was supported by the China
National Natural Science Foundation (grant no. 30772586).
References
|
1
|
Wolfsberg TG and White JM: ADAMs in
fertilization and development. Dev Biol. 180:389–401. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seals DF and Courtneidge SA: The ADAMs
family of metallo-proteases: multidomain proteins with multiple
functions. Genes Dev. 17:7–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arribas J, Bech-Serra JJ and
Santiago-Josefat B: ADAMs, cell migration and cancer. Cancer
Metastasis Rev. 25:57–68. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krätzschmar J, Lum L and Blobel CP:
Metargidin, a membrane-anchored metalloprotease-disintegrin protein
with an RGD integrin binding sequence. J Biol Chem. 271:4593–4596.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang XP, Kamata T, Yokoyama K,
Puzon-McLaughlin W and Takada Y: Specific interaction of the
recombinant disintegrin-like domain of MDC-15 (metargidin, ADAM-15)
with integrin alphavbeta3. J Biol Chem. 273:7345–7350. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eto K, Puzon-McLaughlin W, Sheppard D,
Sehara-Fujisawa A, Zhang XP and Takada Y: RGD-independent binding
of integrin alpha9beta1 to the ADAM-12 and -15 disintegrin domains
mediates cell-cell interaction. J Biol Chem. 275:34922–34930. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nath D, Slocombe PM, Stephens PE, et al:
Interaction of metargidin (ADAM-15) with alphavbeta3 and
alpha5beta1 integrins on different haemopoietic cells. J Cell Sci.
112:579–587. 1999.PubMed/NCBI
|
|
8
|
Herren B, Garton KJ, Coats S, Bowen-Pope
DF, Ross R and Raines EW: ADAM15 overexpression in NIH3T3 cells
enhances cell-cell interactions. Exp Cell Res. 271:152–160. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moss ML and Bartsch JW: Therapeutic
benefits from targeting of ADAM family members. Biochemistry.
43:7227–7235. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trochon-Joseph V, Martel-Renoir D, Mir LM,
et al: Evidence of antiangiogenic and antimetastatic activities of
the recombinant disintegrin domain of metargidin. Cancer Res.
64:2062–2069. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O’Reilly MS, Boehm T, Shing Y, et al:
Endostatin: an endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997. View Article : Google Scholar
|
|
12
|
O’Reilly MS, Holmgren L, Shing Y, et al:
Angiostatin: a novel angiogenesis inhibitor that mediates the
suppression of metastases by a Lewis lung carcinoma. Cell.
79:315–328. 1994. View Article : Google Scholar
|
|
13
|
Herren B, Raines EW and Ross R: Expression
of a disintegrin-like protein in cultured human vascular cells and
in vivo. FASEB J. 11:173–180. 1997.PubMed/NCBI
|
|
14
|
Brooks PC, Montgomery AM, Rosenfeld M, et
al: Integrin alpha v beta 3 antagonists promote tumor regression by
inducing apoptosis of angiogenic blood vessels. Cell. 79:1157–1164.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Horiuchi K, Weskamp G, Lum L, et al:
Potential role for ADAM15 in pathological neovascularization in
mice. Mol Cell Biol. 23:5614–5624. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu J, Zhang L, Lei J, et al: Enhancement
of recombinant human ADAM15 disintegrin domain expression level by
releasing the rare codons and amino acids restriction. Appl Biochem
Biotechnol. 157:299–310. 2009. View Article : Google Scholar
|
|
17
|
Hou Y, Chu M, Du FF, et al: Recombinant
disintegrin domain of ADAM15 inhibits the proliferation and
migration of Bel-7402 cells. Biochem Biophys Res Commun.
435:640–645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Halpern ME, Thisse C, Ho RK, et al:
Cell-autonomous shift from axial to paraxial mesodermal development
in zebrafish floating head mutants. Development. 121:4257–4264.
1995.PubMed/NCBI
|
|
19
|
Chang YM, Bai L, Liu S, Yang JC, Kung HJ
and Evans CP: Src family kinase oncogenic potential and pathways in
prostate cancer as revealed by AZD0530. Oncogene. 27:6365–6375.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuefer R, Day KC, Kleer CG, et al: ADAM15
disintegrin is associated with aggressive prostate and breast
cancer disease. Neoplasia. 8:319–329. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bridges LC, Sheppard D and Bowditch RD:
ADAM disintegrin-like domain recognition by the lymphocyte
integrins alpha4beta1 and alpha4beta7. Biochem J. 387:101–108.
2005. View Article : Google Scholar :
|
|
22
|
Beck V, Herold H, Benge A, et al: ADAM15
decreases integrin alphavbeta3/vitronectin-mediated ovarian cancer
cell adhesion and motility in an RGD-dependent fashion. Int J
Biochem Cell Biol. 37:590–603. 2005. View Article : Google Scholar
|
|
23
|
Jain RK, Duda DG, Clark JW and Loeffler
JS: Lessons from phase III clinical trials on anti-VEGF therapy for
cancer. Nat Clin Pract Oncol. 3:24–40. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferrara N: VEGF-A: a critical regulator of
blood vessel growth. Eur Cytokine Netw. 20:158–163. 2009.
|
|
25
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Drake CJ, Cheresh DA and Little CD: An
antagonist of integrin alpha v beta 3 prevents maturation of blood
vessels during embryonic neovascularization. J Cell Sci.
108:2655–2661. 1995.PubMed/NCBI
|
|
27
|
Hammes HP, Brownlee M, Jonczyk A, Sutter A
and Preissner KT: Subcutaneous injection of a cyclic peptide
antagonist of vitro-nectin receptor-type integrins inhibits retinal
neovascularization. Nat Med. 2:529–533. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kerr JS, Wexler RS, Mousa SA, et al: Novel
small molecule alpha v integrin antagonists: comparative
anti-cancer efficacy with known angiogenesis inhibitors. Anticancer
Res. 19:959–968. 1999.PubMed/NCBI
|
|
29
|
Patel SR, Jenkins J, Papadopolous N, et
al: Pilot study of vitaxin - an angiogenesis inhibitor-in patients
with advanced leiomyosarcomas. Cancer. 92:1347–1348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng J, Gu YJ, Wang Y, Cheng SH and Wong
WT: Nanotherapeutics in angiogenesis: synthesis and in vivo
assessment of drug efficacy and biocompatibility in zebrafish
embryos. Int J Nanomedicine. 6:2007–2021. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sofia Vala I, Martins LR, Imaizumi N, et
al: Low doses of ionizing radiation promote tumor growth and
metastasis by enhancing angiogenesis. PLoS One. 5:e112222010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Serbedzija GN, Flynn E and Willett CE:
Zebrafish angiogenesis: a new model for drug screening.
Angiogenesis. 3:353–359. 1999. View Article : Google Scholar
|
|
33
|
Crociani O, Zanieri F, Pillozzi S, et al:
hERG1 channels modulate integrin signaling to trigger angiogenesis
and tumor progression in colorectal cancer. Sci Rep. 3:33082013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lucena SE, Romo K, Suntravat M and Sanchez
EE: Anti-angiogenic activities of two recombinant disintegrins
derived from the Mohave and Prairie rattlesnakes. Toxicon.
78:10–17. 2014. View Article : Google Scholar :
|