Introduction
Radiotherapy is an important modality used for the
treatment of nasopharyngeal carcinoma (NPC). The prognosis of
5-year survival for patients with NPC who have received irradiation
treatment has not improved, at least in part, due to the resistance
of NPC cells to radiation (1,2).
Various mechanisms regulating the radiosensitivity of tumor cells
have been suggested, including the induction of DNA damage by
reactive oxygen species (ROS) (3)
and resistance, mediated by the epithelial-mesenchymal transition
(EMT) (4).
MicroRNAs (miRNAs) are endogenous, non-coding RNA
molecules, which suppress the expression of target genes by binding
to 3′ untranslated regions. miRNAs provide suitable therapeutic
targets, which regulate a number of complex biological processes
(5). Previous studies have
demonstrated the effects of ionizing radiation on the expression
levels of miRNAs, and the radiosensitivity of tumor cells has been
demonstrated to be regulated by miRNAs in vitro (6,7). In
addition, the expression levels of miRNAs have been reported to be
altered by ROS in cells, which have been exposed to ionizing
radiation (8). Although the
overexpression of miR-9 and let-7 g have been observed to decrease
the surviving fraction of γ-irradiated cells and increased the
sensitivity to ionizing radiation in H1299 cells (9), our previous study demonstrated that
inhibition of the expression of miR-9 promoted the ultraviolet
(UV)-induced production of ROS, DNA damage and apoptosis in NPC
cells (10,11). To examine the mechanism underlying
radiosensitivity in NPC, the present study investigated whether
miR-9 suppresses the sensitivity of NPC cells to UV through
UV-induced ROS damage.
miRNAs regulate the progression of cancer by
targeting various messenger RNAs with cancer-associated functions.
The EMT has been implicated in the increased resistance to
radiotherapy and downregulation of the expression of E-cadherin
associated with EMT, and has also been demonstrated to promote
resistance to radiation therapy in human tumor cells in
vitro (4,5). It has been suggested that the
decreased expression of E-cadherin is associated with advanced
disease and poor survival rates in patients with NPC, and that
altered expression levels of E-cadherin may affect the prognosis of
patients with NPC (12,13). miR-9 directly targets E-cadherin
mRNA and thereby regulates cell motility and the invasiveness of
breast cancer cells (14). Whether
miR-9 modulates the sensitivity of NPC cells to UV radiation by
regulating E-cadherin remains to be elucidated.
The present study investigated changes in the
expression levels of miR-9 in different NPC cell lines following
exposure to UV radiation, to determine the mechanism underlying the
effects of miR-9 on radiosensitivity, examine the potential role of
E-cadherin, and improve current understanding of the responsiveness
of NPC cells to UV radiation.
Materials and methods
Cell lines and chemicals
The CNE1, CNE2 and C666 NPC cell lines were
cultured, as described previously (15). CNE1 and CNE2 cells are derived from
well- and poorly-differentiated squamous carcinoma, and C666 is
derived from undifferentiated carcinoma (15). The NPC cell lines were maintained
in Roswell Park Memorial Institute-1640 medium (HyClone
Laboratories, Inc., Logan, UT, USA), supplemented with 10% fetal
bovine serum (HyClone Laboratories, Inc.) at 37°C in a humidified
incubator containing 5% CO2. The cell lines were
provided by Professor Musheng Zeng (State Key Laboratory of
Oncology in Southern China Cancer Institute, Sun Yat-sen
University, Guangzhou, China). Once the cells reached 50%
confluence (3×105 cells), they were irradiated with UV,
at a wavelength of 254 nm, for 45 min in an ESCO biosafety cabinet
(Esco Micro Pte Ltd., Singapore). The
2′,7′-dichlorodihydrofluorescein diacetate and N-acetyl-L-cysteine
(NAC) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
To quantify the expression levels of miR-9, the
total RNA was extracted from the cells using TRIzol reagent (Omega
Bio-Tek, Norcross, GA, USA). The total RNA (1 µg) was
reverse transcribed using Moloney Murine Leukemia Virus Reverse
Transcriptase (Promega Corporation, Madison, WI, USA) and the
products were used for qPCR analysis using SYBR Green PCR Master
mix (Toyobo Life Science, Osaka, Japan), according to the
manufacturer’s instructions. ABI9700 and ABI7500 systems (Applied
Biosystems Life Technologies, Foster City, CA, USA) were used to
conduct PCR and qPCR, respectively. The forward primers for the
miR-9 and U6 internal control were
5′-ACACTCCAGCTGGGTCTTTG-GTTATCTAGCTG-3′ and
5′-CTCGCTTCGGCAGCACA-3′, respectively. The expression levels of
miR-9 were measured in the various NPC cell lines at numerous
time-points (6, 12 and 24 h) following radiation exposure. The
expression levels of miR-9 in the cancer cells without radiation
were considered the control. The primers were designed by the Land
Co., Ltd., (Guangzhou, China). The PCR conditions were set as
follows: 95°C for 5 min, followed by 40 cycles of 95°C for 15 sec,
65°C for 15 sec and 72°C for 32 sec, and a final extension step at
72°C for 5 min. The 2−∆∆Ct method was used to compare
the miR-9 levels between different nasopharyngeal cells.
Western blotting and incubation with
antibodies
The cells were washed twice in phosphate-buffered
saline and dissolved in radioimmunoprecipitation assay buffer
(Beyotime Institute of Biotechnology, Shanghai, China) for 15 min.
The cell lysates were then collected and centrifuged at 12,000 × g
for 15 min at 4°C. Protein concentrations were determined using
KeyGen BCA Protein Quantitation assay (cat. no. KGPBCA; Nanjing
KeyGen Biotech Co. Ltd., Nanjing, China). The protein samples (100
µg) were subjected to 7% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, transferred onto
nitrocellulose membranes (EMD Millipore, Billerica, MA, USA),
blocked with 5% fat-free milk (Dingguo Biotechnology Co., Ltd.,
Beijing, China) and incubated with the following primary
antibodies: Polyclonal rabbit anti-human E-cadherin (1:100, cat.
no. BA0474; Boster Biotechnology Co., Ltd., Wuhan, China) and
polyclonal rabbit anti-human GAPDH (1:500; cat. no. sc-25778; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA), at 4°C overnight.
Protein detection was performed using horseradish
peroxidase-conjugated goat anti-rabbit secondary antibodies
(1:2,000; cat. no. KGAA35; Nanjing KeyGen Biotech Co., Ltd.,
Nanjing, China) and enhanced chemiluminescence reagents (Nanjing
KeyGen Biotech Co., Ltd.).
RNA oligoribonucleotides and cell
transfection
An RNA duplex with a forward sequence of
5′-UCUUUGGUUAUCUA GCUGUAUGA-3′ was used to mimic endogenous mature
miR-9 molecules. The small interfering RNA (siRNA) targeting human
E-cadherin mRNA was designed, as previously described, using the
sequence of 5′-CAGACAAAGACCAG-GACUATT-3′ (16). The control RNA duplex (negative
control) for the miR-9 mimics and the siRNA was not homologous to
any known gene sequences. All RNA oligoribonucleotides were
purchased from Genepharma (Shanghai, China). Once the cells reached
~60% confluence, they were transiently transfected using
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA), according to the manufacturer’s instructions.
Colony formation assay
Following transfection and incubation for 48 h, the
cells were irradiated with UV for 45 min. The cells were
subsequently trypsinized (0.25% trypsin-EDTA; Invitrogen Life
Technologies), collected and cultured in a fresh six-well plate at
a density of 100 cells per well. Following 10 days incubation, the
cells were fixed with methanol (Dingguo Biotechnology Co., Ltd.)
for 10 min and stained with 0.1% crystal violet (Dingguo
Biotechnology Co., Ltd.) for 15 min. The number of colonies were
counted under an inverted microscope (CKX41; Olympus Corporation,
Tokyo, Japan) and the data were evaluated by statistical analyses
(SPSS version 13.0; SPSS Inc., Chicago, IL, USA).
Apoptosis assay
The levels of UV-induced apoptosis were evaluated 48
h after transfection and UV irradiation for 45 min. Annexin
V-fluorescein isothiocyanate conjugate (5 µl) and propidium
iodide solution (5 µl) were added to the cell suspension for
the apoptosis assay, according to the manufacturer’s instructions
(Nanjing KeyGen Biotech Co., Ltd.). The stained cells were analyzed
using a flow cytometer (FACSCalibur; BD Biosciences, Franklin
Lakes, NJ, USA).
Alkaline comet assay
To detect DNA damage in individual cells following
exposure to UV radiation, DNA damage was assessed using a
single-cell gel electrophoresis assay. Alkaline conditions were
generated with 1 mmol/l EDTA and 300 mmol/l NaOH for
electrophoresis. A total of 48 h post-transfection and irradiation
with UV (45 min), the cells were embedded in agarose, and lysis and
electrophoresis were performed under alkaline conditions according
to the manufacturer’s protocol (Nanjing KeyGen Biotech Co., Ltd.).
The fluorescence images were captured using a fluorescence
microscope system (IX71; Olympus Corporation). The olive tail
moment was measured from the comet images to reflect the DNA
damage, as described previously (17).
Intracellular ROS assay
The determine the levels of intracellular ROS in the
cells, 20 µM 2′, 7′-dichlorodihydrofluorescin diacetate
(Sigma-Aldrich) was added to the culture medium 48 h after
transfection, and incubated at 37°C for 20 min. The cells were
subsequently irradiated with UV for 45 min and the levels of
fluorescence were measured using a Synergy HT microplate reader
(BioTek Instruments Inc., Winooski, VT, USA) at a filter pair
excitation of 485/20 and an emission of 528/20 (8).
Glutathione assay
The levels of total glutathione and reduced
glutathione were detected using a GSH and GSSG Assay kit (Beyotime
Institute of Biotechnology) via the benzoic acid method, following
UV exposure for 45 min. The cells were treated according to the
manufacturer’s instructions and measurements were recorded using a
microplate reader (Synergy HT, BioTek Instruments Inc.) at 412 nm
(18).
NAC exerts its protective effect predominantly as a
glutathione precursor in various models with disorders associated
with oxidative stress, by directly scavenging ROS (19). To further define the role of
glutathione in the modulation of radio-sensitivity by miR-9, the
cells were pre-treated with 1 mM NAC for 4 h prior to intracellular
ROS analysis.
Statistical analysis
Unless otherwise stated, the data are expressed as
the mean ± standard deviation of at least three independent
experiments. Statistical analyses were conducted using SPSS version
13.0. Significance was determined using Student’s t-test and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Kinetics of the expression of miR-9
following exposure to UV
The present study examined whether miR-9 regulated
the sensitivity of NPC cells to UV-induced damage, and investigated
the potential mechanisms underlying the radiosensitivity of NPC
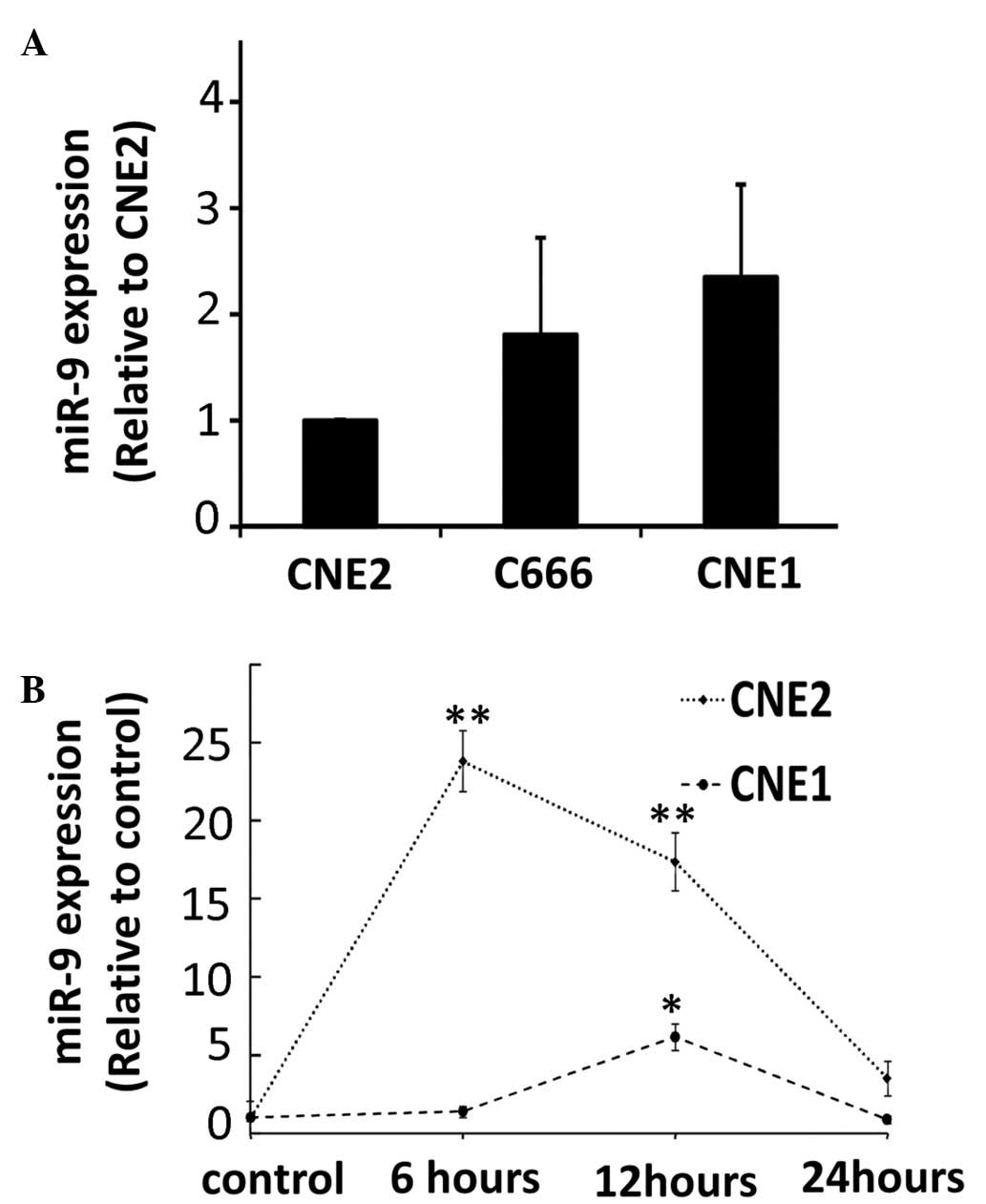
cells. The results demonstrated no significant changes in the
expression levels of miR-9 in the different NPC cell lines
(Fig. 1A). The expression levels
of miR-9 were measured in different NPC cell lines at several
time-points following exposure to radiation, ranging between 1 and
24 h. Increased expression levels of miR-9 were observed in the
well- and poorly-differentiated tumor cells in response to UV
exposure (Fig. 1B). The induction
of miR-9 following UV radiation was transient and was particularly
marked in the CNE2 cells.
miR-9 suppresses the radiosensitivity of
CNE2 cells to UV
The previously reported significant upregulation in
the expression of miR-9 in CNE2 cells following exposure to UV
radiation prompted the present study to investigate the role of
miR-9 in radiosensitivity. The colony forming capacities of the
cells were assessed following exposure to UV radiation in the cells
transfected with the miR-9 mimics. The results demonstrated that
the CNE2 cells transfected with the miR-9 mimic exhibited an
increased number of colonies following UV radiation compared with
the control cells (P<0.01; Fig.
2A), suggesting that miR-9 inhibited the radiosensitivity of
CNE2 cells. By contrast, no miR-9-induced increase in the
clonogenic potential of the CNE1 cells was observed.
Effects of miR-9 on UV-induced apoptosis
and DNA damage
Ionizing radiation causes severe cellular damage by
disrupting the DNA integrity indirectly by forming intracellular
free radicals (3). To determine
whether miR-9 regulates cellular damage in response to UV, the
present study examined the extent of apoptosis in cells
overexpressing miR-9. The results demonstrated that the CNE2 cells
transfected with the miR-9 mimics exhibited decreased apoptosis
compared with the control cells (P<0.01; Fig. 2B and C). In addition, by performing
a single-cell gel electrophoresis assay under alkaline conditions,
the extent of DNA damage following irradiation in the CNE2 cells
transfected with the miR-9 mimics was found to be lower compared
with that observed in the control cells (P<0.001; Fig. 2D). miR-9 failed to protect the CNE1
cells from irradiation-induced apoptosis and did not affect the DNA
damage response, consistent with its inability to alter the
clonogenic potential in these cells.
ROS levels in tumor cells following
exposure to UV radiation
Radiation induces severe cellular damage through the
generation of ROS, which are controlled by free radical scavengers,
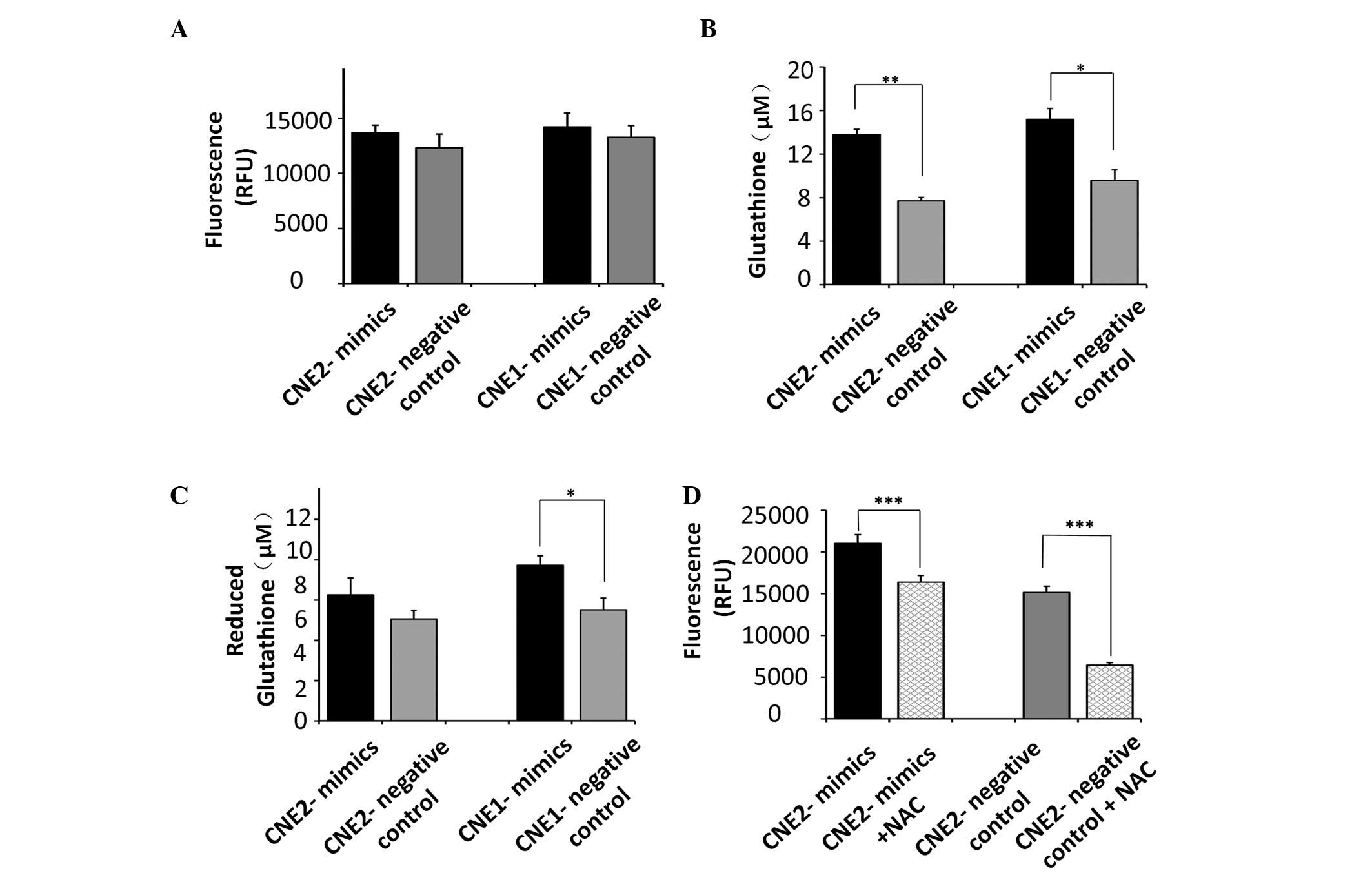
including glutathione. No significant increase was observed in the
production of ROS in tumor cells transfected with the miR-9 mimics
(Fig. 3A). However, our previous
study demonstrated that NPC cells with silenced expression of miR-9
produced lower levels of glutathione (11). Since miR-9 protected the CNE2 cells
from UV radiation (Fig. 2), the
effect of miR-9 on glutathione, which is important in protecting
tumor cells from ROS following exposure to UV radiation, was
assessed. The cells transfected with the miR-9 mimics exhibited
significant increases in the total glutathione levels compared with
the control CNE2 (P<0.01) and CNE1 cells (P<0.05; Fig. 3B). In addition, the cells
transfected with the miR-9 mimics produced more reduced glutathione
compared with the control CNE1 cells (P<0.05). However, miR-9
had no significant effect on the levels of reduced glutathione in
the CNE2 cells (Fig. 3C).
The analyses revealed significant variation in the
total gluta-thione concentration regulated by miR-9 in NPC cells.
Since NAC exerts its protective effect predominantly as a
glutathione precursor in various models of oxidative stress, the
present study used NAC to pharmacologically increase cellular ROS
defenses, to elucidate the contribution of glutathione, regulated
by miR-9, in UV-induced radiosensitivity. The results demonstrated
that the levels of ROS following exposure to UV radiation were
inhibited by the antioxidant, NAC, in the CNE2 cells (P<0.001;
Fig. 3D), which indicated a role
for glutathione in resistance against UV exposure in CNE2
cells.
miR-9 does not regulate radiosensitivity
through modulation of the expression of E-cadherin
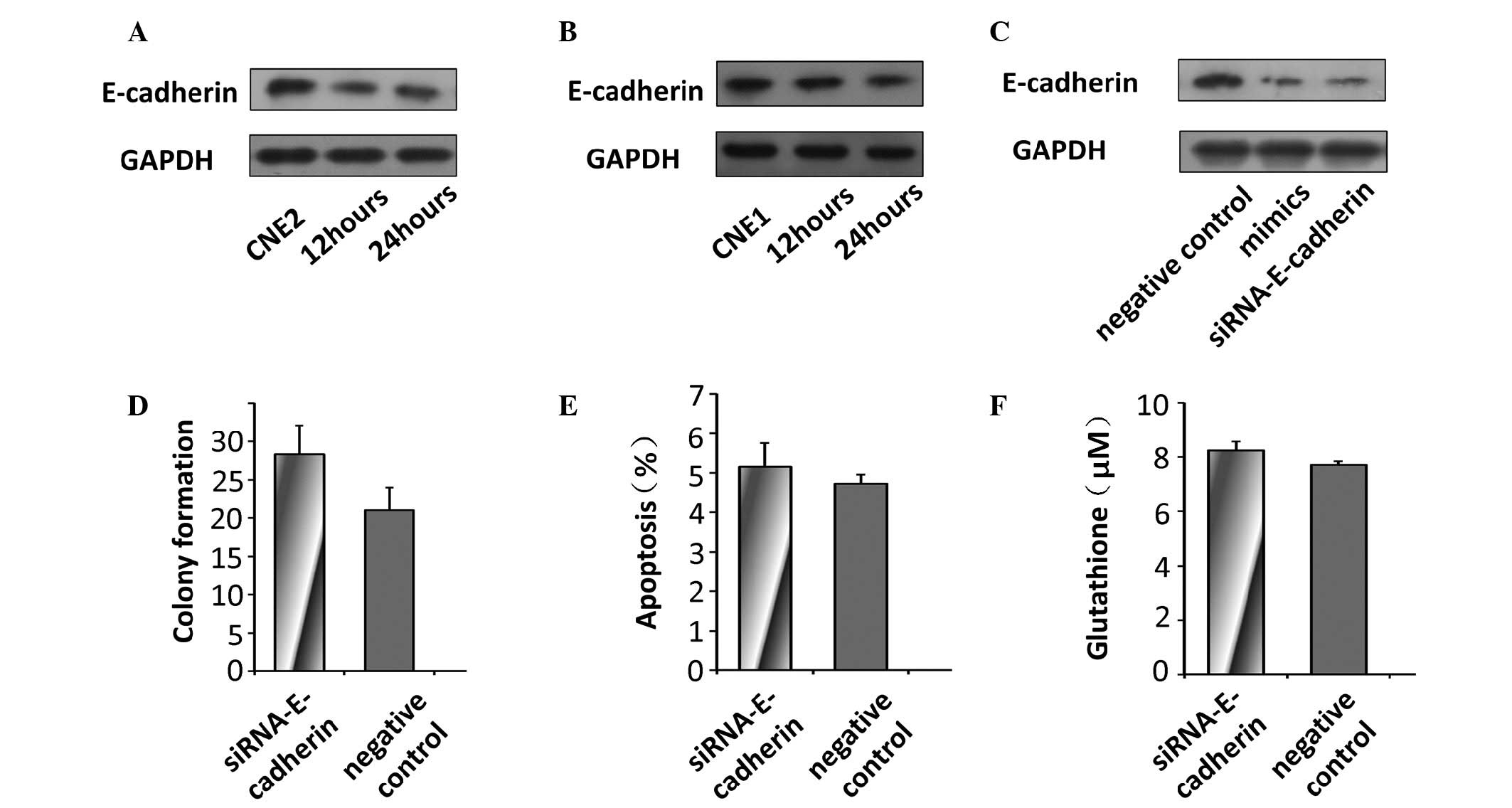
Decreased expression levels of E-cadherin were
observed in the NPC cells 24 and 48 h following radiation exposure
(Fig. 4A and B), which suggested
that the expression of E-cadherin may regulate the
radiosensi-tivity of NPC cells to UV. In addition, the levels of
E-cadherin were decreased in the CNE2 cells transfected with the
miR-9 mimics and, as expected, in the cells transfected with
E-cadherin-specific siRNA (Fig.
4C). To determine whether miR-9 regulated radiosensitivity by
targeting the expression of E-cadherin in the CNE2 cells, the
expression of E-cadherin was silenced using siRNA to examine the
effect of E-cadherin deficiency on the radiosensitivity of the
tumor cells.
No significant changes in the colony forming
capacity or levels of apoptosis were observed between the control
cells and the cells transfected with siRNA targeting E-cadherin
(Fig. 4D and E). In the cells
transfected with E-cadherin siRNA, no significant difference in the
production of glutathione was observed compared with the control
CNE2 cells (Fig. 4F).
These results demonstrated that miR-9 suppressed the
radiosensitivity of the cells to UV through its effects on
glutathione, rather than its ability to modulate the expression of
E-cadherin.
Discussion
The mechanisms underlying NPC radiosensitivity
remain to be fully elucidated. The formation of ionizing
radiation-induced ROS is associated with radiation-induced
apoptosis and necrosis. The expression levels of several miRNAs are
significantly altered in response to radiation treatment, and
changes in the expression levels of miRNAs, including miR-17, may
be involved in DNA damage and the production of ROS (8,20).
miRNAs are, therefore, suitable candidates for modulating the
radiosensitivity of cancer cells. UV-induced ROS have been reported
to trigger cellular damage, and our previous study revealed that
miR-9 promotes the UV-induced production of ROS (11). The present study investigated the
sensitivity of NPC to UV. The potential role of miRNAs in the
protection of tumor cells from radiosensitivity and the production
of ionizing radiation-induced ROS have not been previously
investigated in NPC cells. The present study demonstrated that CNE2
cells overexpressing miR-9 exhibited less DNA damage and increased
levels of total glutathione. These cells were found to exhibit
decreased apoptosis and increased clonogenic potential in response
to radiation.
miR-9 appears to have varying effects on
proliferation and apoptosis in different types of cancer cell.
miR-9 is overexpressed in caudal type homeobox 2-negative gastric
cancer cells, and knockdown of miR-9 inhibits the proliferation of
human gastric cancer cells (21).
By contrast, the overexpression of miR-9 also suppresses
proliferation of different lines of gastric cancer cells (22,23).
UV-induced production of ROS and DNA damage modulates growth
inhibition and apoptosis by altering the ratios of Bax/Bcl-2 and
other signaling pathways (24).
The data from the present study demonstrated that miR-9 protected
the tumor cells from UV-induced cell damage. In the CNE2 cells,
overexpression of miR-9 increased the clonogenic potential and
suppressed the apoptosis induced by UV. By contrast, acute
knockdown of miR-9 has no effects on the survival of human neural
progenitor cells or tumor cells (25). The production of ROS can also
result in DNA damage due to the accumulation of p53 and the
formation of 8-OH guanine from UV irradiation (10). In addition to exhibiting lower
levels of ROS, certain cancer stem cells develop less DNA damage
following irradiation (3).
As the biological effects of ionizing radiation are
largely mediated by free radicals, the present study examined the
effect of miR-9 on the production of ROS and free radical
scavengers. UV causes cellular damage by producing ROS species,
including O2, O2− and
H2O2 (10).
The present study demonstrated that NPC cells with silenced
expression of miR-9 produced lower levels of glutathione following
exposure to UV radiation. Since the overexpression of miR-9
revealed no increased production of ROS following exposure to UV
radiation, the present study focused on glutathione, a critical
cellular reducing agent, which is implicated in regulating
mutagenic mechanisms, DNA synthesis, growth and multidrug and
radiation resistance (26). In
addition, increased glutathione biosynthesis in a subset of cancer
stem cells has been observed to contribute to tumor
radiosensitivity (3). The results
of the present study revealed changes in the total and reduced
levels of glutathione regulated by miR-9 in the different NPC
cells. The upregulation of the total glutathione was important in
rescuing tumor cells from irradiation-induced apoptosis. The
protective effect of miR-9 was more marked in the CNE2 cells
compared with the CNE1 cells, however, no changes were observed in
reduction of glutathione in the CNE2 cells. These data suggested
that miR-9 regulated the UV-exposure-induced radiation response, at
least in part, through its effect on glutathione. The mechanism
whereby miR-9 regulates glutathione levels remains to be
elucidated. In the present study, no correlation was observed
between miR-9 and glutamate-cysteine ligase, which is critical in
GSH biosynthesis.
miR-9 has been demonstrated to have different
effects on the expression levels of E-cadherin in different types
of cancer cells (27). The present
study demonstrated that miR-9 down-regulated the expression of
E-cadherin in the CNE2 cells, and that miR-9 protected the CNE2
cells following exposure to UV radiation. Although E-cadherin has
been demonstrated to regulate chemoresistance and radioresistance
of cancer cells, silencing of the expression of E-cadherin had no
effect on the response of the NPC cells to UV or on alter the
levels of total glutathione. The expression of E-cadherin is
regulated by ubiquitination and multiple signaling pathways,
therefore, it is likely that several factors are involved in the
downregulation of E-cadherin following irradiation in NPC cells
(28).
The present study demonstrated that the
overexpression of miR-9 suppressed the radiosensitivity of CNE2
cells, as demonstrated by an increased number of colonies, reduced
levels of apoptosis and reduced DNA damage following UV radiation.
In addition, miR-9 was found to exert it effects through
glutathione, rather than through its effect on the expression of
E-cadherin, in the CNE2 cells. Taken together, these findings
suggested that miR-9 may be a potential therapeutic target in NPC
therapy.
Acknowledgments
This study was supported by the Natural Science
Foundation of Guangdong Province (no. 10251008901000023).
References
|
1
|
Preston RJ: Radiation biology: concepts
for radiation protection. Health Phys. 88:545–556. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei WI and Sham JS: Nasopharyngeal
carcinoma. Lancet. 365:2041–2054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie
MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, Joshua B, et al:
Association of reactive oxygen species levels and radioresistance
in cancer stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Wakeman TP, Lathia JD, Hjelmeland
AB, Wang XF, White RR, Rich JN and Sullenger BA: Notch promotes
radioresistance of glioma stem cells. Stem Cells. 28:17–28.
2010.
|
|
5
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ilnytskyy Y, Zemp FJ, Koturbash I and
Kovalchuk O: Altered microRNA expression patterns in irradiated
hematopoietic tissues suggest a sex-specific protective mechanism.
Biochem Biophys Res Commun. 377:41–45. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Josson S, Sung SY, Lao K, Chung LW and
Johnstone PA: Radiation modulation of microRNA in prostate cancer
cell lines. Prostate. 68:1599–1606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Simone NL, Soule BP, Ly D, Saleh AD,
Savage JE, Degraff W, Cook J, Harris CC, Gius D and Mitchell JB:
Ionizing radiation-induced oxidative stress alters miRNA
expression. PLoS One. 4:e63772009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arora H, Qureshi R, Jin S, Park AK and
Park WY: miR-9 and let-7 g enhance the sensitivity to ionizing
radiation by suppression of NFκB1. Exp Mol Med. 43:298–304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ravanat JL, Douki T and Cadet J: Direct
and indirect effects of UV radiation on DNA and its components. J
Photochem Photobiol B. 63:88–102. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng CP, Han L, Hou WJ, Wen YH, Fu R, Ma
RQ and Wen WP: Inhibition of micro RNA-9 expression promotes
UV-induced ROS damage in nasopharyngeal carcinoma cells. Zhonghua
Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 48:668–672. 2013.In Chinese.
PubMed/NCBI
|
|
12
|
Theys J, Jutten B, Habets R, Paesmans K,
Groot AJ, Lambin P, Wouters BG, Lammering G and Vooijs M:
E-Cadherin loss associated with EMT promotes radioresistance in
human tumor cells. Radiother Oncol. 99:392–397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie LQ, Bian LJ, Li Z, Li Y, Li ZX and Li
B: Altered expression of E-cadherin by hepatocyte growth factor and
effect on the prognosis of nasopharyngeal carcinoma. Ann Surg
Oncol. 17:1927–1936. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma L, Young J, Prabhala H, Pan E, Mestdagh
P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S,
et al: miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin
and cancer metastasis. Nat Cell Biol. 12:247–256. 2010.PubMed/NCBI
|
|
15
|
Lu J, He ML, Wang L, Chen Y, Liu X, Dong
Q, Chen YC, Peng Y, Yao KT, Kung HF and Li XP: MiR-26a inhibits
cell growth and tumorigenesis of nasopharyngeal carcinoma through
repression of EZH2. Cancer Res. 71:225–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hayashida Y, Honda K, Idogawa M, Ino Y,
Ono M, Tsuchida A, Aoki T, Hirohashi S and Yamada T: E-cadherin
regulates the association between beta-catenin and actinin-4.
Cancer Res. 65:8836–8845. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Undeğer U, Zorlu AF and Basaran N: Use of
the alkaline comet assay to monitor DNA damage in technicians
exposed to low-dose radiation. J Occup Environ Med. 41:693–698.
1999. View Article : Google Scholar
|
|
18
|
Vandeputte C, Guizon I, Genestie-Denis I,
Vannier B and Lorenzon G: A microtiter plate assay for total
glutathione and glutathione disulfide contents in cultured/isolated
cells: performance study of a new miniaturized protocol. Cell Biol
Toxicol. 10:415–421. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang F, Lau SS and Monks TJ: The
cytoprotective effect of N-acetyl-L-cysteine against ROS-induced
cytotoxicity is independent of its ability to enhance glutathione
synthesis. Toxicol Sci. 120:87–97. 2011. View Article : Google Scholar :
|
|
20
|
Weidhaas JB, Babar I, Nallur SM, Trang P,
Roush S, Boehm M, Gillespie E and Slack FJ: MicroRNAs as potential
agents to alter resistance to cytotoxic anticancer therapy. Cancer
Res. 67:11111–11116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rotkrua P, Akiyama Y, Hashimoto Y, Otsubo
T and Yuasa Y: MiR-9 down-regulates CDX2 expression in gastric
cancer cells. Int J Cancer. 129:2611–2620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsai KW, Liao YL, Wu CW, Hu LY, Li SC,
Chan WC, Ho MR, Lai CH, Kao HW, Fang WL, Huang KH and Lin WC:
Aberrant hypermethylation of miR-9 genes in gastric cancer.
Epigenetics. 6:1189–1197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng L, Qi T, Yang D, Qi M, Li D, Xiang
X, Huang K and Tong Q: microRNA-9 suppresses the proliferation,
invasion and metastasis of gastric cancer cells through targeting
cyclin D1 and Ets1. PLoS One. 8:e557192013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmitt CA and Lowe SW: Apoptosis and
therapy. J Pathol. 187:127–137. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuva-Aydemir Y, Simkin A, Gascon E and Gao
FB: MicroRNA-9: Functional evolution of a conserved small
regulatory RNA. RNA Biol. 8:557–564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Estrela JM, Ortega A and Obrador E:
Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci.
43:143–181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu S, Kumar SM, Lu H, Liu A, Yang R,
Pushparajan A, Guo W and Xu X: MicroRNA-9 up-regulates E-cadherin
through inhibition of NF-κB1-Snail1 pathway in melanoma. J Pathol.
226:61–72. 2012. View Article : Google Scholar
|
|
28
|
Fujita Y, Krause G, Scheffner M, Zechner
D, Leddy HE, Behrens J, Sommer T and Birchmeier W: Hakai, a
c-Cbl-like protein, ubiquitinates and induces endocytosis of the
E-cadherin complex. Nat Cell Biol. 4:222–231. 2002. View Article : Google Scholar : PubMed/NCBI
|