Introduction
The benefits of aerobic exercise training (AET) for
patients with myocardial infarction (MI) are well-documented in the
literature (1–3). Continuous moderate training (CMT) and
high-intensity interval training (HIT) are the two forms of AET,
which have been most studied in previous research, and a growing
body of evidence has demonstrated that HIT is superior to CMT in
terms of enhancing exercise capability (4–6).
Cardiac functional recovery is another focus in the process of
cardiac rehabilitation for patients with MI (7). However, the effects of CMT and HIT on
cardiac functional recovery have not been well documented. Skeletal
muscle and cardiopulmonary adaption to aerobic training are the
major reasons for the improvement in exercise capacity in MI
patients following cardiac rehabilitation, although other elements
may also contribute (8,9). Moreira et al (10) reported that skeletal muscle
adaption to exercise training was notably similar between CMT and
HIT. Based on the above, it was suggested that a comparison of the
benefits of CMT and HIT on cardiac functional recovery may provide
evidence for further exploration of the precise mechanisms of
metabolism underlying the superior outcomes produced by HIT.
In addition, apoptosis and oxidative stress are
involved in the development of multiple cardiovascular diseases,
including MI, and mounting evidence has shown that alleviation of
oxidative injury and myocardial apoptosis may exert
cardioprotective effects and improve recovery of cardiac function
(11,12). Although previous reports have
documented that AET may attenuate cardiac apoptosis and oxidative
stress in the post-MI heart (13,14),
the effects of distinct exercise protocols on these factors have
rarely been directly compared.
Altering energy metabolism by decreasing fatty acid
oxidation or increasing glucose oxidation may improve cardiac
recovery following an ischemic insult (15,16).
Abnormal glucose metabolism was also found to be associated with
reduced left ventricular contractile reserve and exercise
intolerance in patients with chronic heart failure (17). It has been confirmed that AET is
able to decrease the insulin sensitivity and increase the glucose
uptake of the skeletal muscles of animals and humans (18–20).
Furthermore, it was reported that exercise training is able to
effectively prevent the depression in myocardial glucose metabolism
observed in the diabetic rat (20). Based on these results, it was
hypothesized that aerobic exercise may influence glucose and fatty
acid metabolism in the infarcted myocardium, and that this may
contribute to the recovery of cardiac function induced by AET.
In the present study, the effects of two AET
protocols (HIT and CMT) on cardiac functional recovery and exercise
capability were compared. Furthermore, the impact of HIT and CMT on
potentially associated mechanisms, including apoptosis, oxidative
stress and glucolipid metabolism, were investigated. The
phosphatidylinositol-3-kinase/serine/threonine kinase (PI3K/Akt),
p38 mitogen-activated protein kinase (p38mapk) and adenosine
monophosphate activated protein kinase (AMPK) signaling pathways
are widely agreed to be pivotal signal pathways, which have key
regulatory roles in cellular apoptosis (21), oxidative stress (22) and fatty acid metabolism (23), respectively. Alterations in their
expression levels following various AET protocols were also
evaluated in the present study.
Materials and methods
Animal models
Female Sprague Dawley rats (n=40) aged 8–10 weeks
were provided by the Animal Center of China Academy of Military
Medical Science (Beijing, China) and were randomly divided into
groups (10/group). The rats were maintained at 20–24°C with 40–60%
humidity under a 12/12 h light/dark cycle, receiving standard chow
and water ad libitum. Studies commenced following one week
of rat acclimatization. MI was induced as previously described by
Pfeffer et al (24). The
rats were fully anaesthetized by intraperitoneal injection of 10
ml/kg 4% amobarbitalum (AstraZeneca, Shanghai, China) and
artificially ventilated. The heart was exposed by a left
thoracotomy between the fourth and fifth ribs. For the animals in
which MI was induced, a 6–0 mononylon suture was passed under the
main left descending coronary artery at the point between 1 and 2
mm distal to the edge of the left atrium, and the left coronary
artery was ligated. Age-matched rats in the sham group underwent
the same procedure without ligation of left descending coronary
artery. Subsequently the thorax was closed, the skin was sutured
and the pneumothorax was drained by a continuous aspiration system.
The study was conducted according to the Guide for the Care and Use
for Laboratory Animals (version 1; 1983; National Institutes of
Health, Beijing, China) and approved by the Ethics Committee of
Peking University People’s Hospital (Beijing, China).
Aerobic exercise protocols
AET was performed on a WI-78059 treadmill
specifically designed for small animals (Yiyan Technology, Jinan,
China). Animals were adapted to treadmill exercise for two weeks,
gradually increasing from 10 to 30 min/day. An eight-week exercise
protocol began, following this adaptation period. The rats in the
exercise groups were subjected to either CMT or HIT five days per
week. CMT was performed at a constant running speed corresponding
to 50–60% of maximal oxygen uptake (VO2max)
throughout the training session (50 min/day). HIT included training
sessions which comprised 4 min running at 85–90%
VO2max followed by 3 min running at 50–60%
VO2max, repeated seven times (49 min training in
total). Prior to and following the exercise period, there was a 5
min warm-up and cool-down at 40% VO2max. Rats in
the sham and MI groups were not subjected to any additional
exercise. Running intensity for each protocol was based on a
previous report outlining the association between running speed and
VO2max in an identical rat model of MI (25,26).
Echocardiography
Echocardiographic evaluation was performed prior to
and following the exercise training, using the Vevo770 ultrasound
system (Visualsonics Inc., Toronto, ON, Canada). Animals were
anesthetized by intraperito-neal injection of 4% amobarbitalum (10
ml/kg) and placed in a supine position on the examination table.
The probe frequency was set at 17.5 MHz, sampling frequency was in
M-mode at 1000/s and the scanning speed was 50–100 mm/s. The probes
were placed on the precordium and the detection was conducted from
the section of the ventricular bands. The left ventricular
end-diastolic diameters (LVEDD) and left ventricular end-systolic
diameters (LVESD) were measured and the left ventricular
end-diastolic volume (LVEDV), left ventricular end-systolic volume
(LVESV), left ventricular ejection fraction (LVEF) and left
ventricular fraction shortening (LVFS) were calculated based on
these measurements, using the following formulae:
LVEDV=(7.0×LVEDD3)/(2.4+LVEDD);
LVESV=(7.0×LVESD3)/(2.4+LVESD);
LVEF=(LVEDV-LVESV)/LVEDV×100%; LVFS=(LVEDD-LVESD)/LVEDD×100%.
Exercise capability and aerobic capacity
measurement
Exercise capability was measured prior to and
following the exercise training. The method was similar to that
previously described by Moreira et al (10). Briefly, the rats ran on a graded
treadmill at 15° inclination at an initial speed of 6 m/min. The
speed was subsequently increased by 3 m/min every 3 min until the
rats were unable to run. The total distance run by each rat was
considered to indicate the exercise capability.
Tissue preparation
Rats were sacrificed by decapitation once the
eight-week exercise protocol ended, and the hearts were harvested
as quickly as possible. The atriums and right ventricles were
trimmed off and the left ventricles were stored in liquid nitrogen
(Chaoyang Gas Plant, Beijing, China) for future evaluation. For
measurement of the activity of carnitine palmitoyl transferase-1
(CPT-1) and the rate of adenosine triphosphate (ATP) synthesis,
fresh heart tissues were used and the experiments were completed
within 4 h of the harvest of the heart.
Measurement of biomarkers of oxidative
stress
The concentrations of malondialdehyde (MDA),
glutathione peroxidase (GPx) and superoxide dismutase (SOD) in the
heart homogenate were determined by ELISA assay. The experiment was
performed using a commercially available kit (ELISA kit for the
Measurement of Oxidative Stress in Rat) according to the
manufacturer’s instructions (Jiancheng Bioengineering Institute,
Nanjing, China). Briefly, heart tissues were collected and lysed
with cell lysis buffer (in ELISA kit). Then cell lysates were
centrifuged at 1600 × g for 10 min at 4°C and the supernatants were
collected for the detection of MDA, GPx and SOD. Following
incubation with the reagents included in the respective kits, the
absorbance values at 450 nm, 412 nm and 532 nm were measured using
a spectrophotometer (721D; Pudong Shanghai Physical Optical
Instrument Factory, Shanghai, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The messenger (m)RNA levels of CPT-1 and
phosphofructokinase (PKF-1) were evaluated by RT-qPCR. Cardiac
tissues were homogenized in TRIzol agent (Invitrogen Life
Technologies, Carlsbad, CA, USA) and total RNA was extracted and
reverse-transcribed into cDNA using the PrimeScript RT reagent kit
(Takara Biotechnology, Co., Ltd., Dalian, China) with an Oligo dT
primer. All samples were run in triplicate in a total reaction
volume of 25 µl, which was comprised of 12.5 µl SYBR
Premix Ex TaqII, 2 µl primer, 2 µl templates
(1 µg cDNA) and 8.5 µl nuclease-free water. RT-qPCR
was performed using the SYBR Premix Ex TaqII (Takara
Biotechnology, Co., Ltd.) and two-step Real Time PCR system
(Thermal Cycle Dice; Takara Bio, Inc., Otsu, Japan) in triplicate
wells. The cycling parameters were as follows: Activation at 95°C
for 30 sec, 40 cycles of denaturation at 95°C for 5 sec, and then
annealing and extension at 60°C for 30 sec. The rat GAPDH gene was
used as a reference. The primers used in the present study are
presented in Table I. The
normalized fold-changes of the target gene mRNA expression were
expressed as 2−ΔΔCt.
 | Table IPrimer sequences for CPT-1, PFK-1 and
GAPDH. |
Table I
Primer sequences for CPT-1, PFK-1 and
GAPDH.
| Gene name | Primer
sequence |
|---|
| CPT-1 F |
5′-AAGAACACGAGCCAACAAGC-3′ |
| CPT-1 R |
5′-TACCATACCCAGTGCCATCA-3′ |
| PFK-1 F |
5′-GATGCCCAAGGTATGAATGC-3′ |
| PFK-1 R |
5′-CTCCCTGATGTGCTCTCCAC-3′ |
| GAPDH F |
5′-CCCTTCATTGACCTCAACTACATG-3′ |
| GAPDH R |
5′-CTTCTCCATGGTGGTGAAGAC-3′ |
Measurement of CPT-1 and PFK-1
activity
At the time of sacrifice, mitochondria were isolated
from the myocardium by differential centrifugation. Briefly, 200 mg
fresh ventricle tissue was homogenized with ice-cold lysis buffer
and the homogenate was centrifuged at 800 × g for 5 min at 4°C. The
supernate was collected and added to another tube containing medium
buffer and then centrifuged at 15,000 × g for 10 min at 4°C. The
sediment was kept, resuspended in wash buffer and centrifuged at
15,000 × g for 10 min at 4°C. The supernate was removed and finally
the mitochondria of the ventricle tissue were obtained. The kit
used in this protocol was provided by Beyotime Institute of
Biotechnology (Haimen, China).
The CPT-1 activity of the myocardium was measured as
previously reported (27).
Briefly, assays were performed in 1 ml reaction medium containing
220 mM sucrose, 40 mM KCl, 10 mM Tris and 1 mM ethylene glycol
tetraacetic acid (EGTA), as well as mitochondrial inhibitors
rotenone (1 pg/ml), antimycin A (0.5 pg/ml) and oligomycin (1
pg/ml) at pH 7.0, at 30°C. Reactions were initiated by the addition
of 0.60 mg mitochondrial protein and were stopped with 2 ml
butanol-saturated 0.73 M HCl 10 min later. The product was
extracted into butanol (1 ml) and following centrifugation (10,000
× g; 10 min; 4°C and washing with 2 ml butanol-saturated 0.5 M
sodium phosphate (pH 7.0), 0.5 ml of the butanol layer was used for
final determination. The reaction product was measured every 5 min
until the palmitoyl-CoA concentration was low (10–20 µM), at
which point the reaction product was measured every 2 min. All the
chemicals used in this protocol were provided by Sigma-Aldrich
Shanghai Trading Co. Ltd (Shanghai, China).
The PFK-1 activity of the myocardium was also
measured. In brief, assays were conducted in reaction medium
containing 50 mol/l Hepes buffer, 10 mol/l HCl, 6.5 mol/l
MgCl2, 1 mol/l NH4Cl, 5 mol/l
KH2PO4, 0.1 mol/l adenosine monophosphate,
0.3 mol/l NADH, 0.5 U/ml aldolase, 0.5 U/ml glutamate
dehydrogenase, 5 U/ml, 0.1 mol/l fructose 6-phosphate, 0.3 mol/l
glucose 6-phosphate and 1.5-mol/l ATP (pH 7.0). The activity of
PFK-1 was assayed by monitoring the oxidation of NADH at 340 nm
using a spectrophotometer (721D; Pudong Shanghai Physical Optical
Instrument Factory) consecutively when triosephosphate isomerase
and glycerophosphate were added into the reaction medium. All the
chemicals used in this protocol were provided by Sigma-Aldrich
Shanghai Trading Co. Ltd.
Measurement of the rate of ATP
synthesis
The rate of ATP synthesis was modulated by glucose
6-phosphate accumulation using an adenosine diphosphate
(ADP)-regenerating system based on hexokinase plus glucose and ATP
which was described previously (28). The reaction medium (120 mM KCl, 5
mM KH2PO4, 1 mM EGTA, 2 mM MgCl2,
3 mM Hepes; pH 7.4) was supplemented with 5 mM succinate, 5
µM rotenone, 20 mM glucose and 125 µM ATP to initiate
the reaction. Glucose 6-phosphate formation was monitored at 37°C
from NADPH content by spectrophotometry at 340 nm (721D; Pudong
Shanghai Physical Optical Instrument Factory). All the chemicals
used in this protocol were provided by Sigma-Aldrich Shanghai
Trading Co. Ltd.
Western blot
Total protein was exacted from left ventricle
myocardium lysates following centrifugation at 14,000 × g for 10
min at 4°C. Proteins were separated by 12% SDS-PAGE and transferred
onto a nitrocellulose membrane (Beyotime Institute of
Biotechnology). The membrane was then blocked with 5% non-fat dry
milk and incubated in solution containing primary antibodies
overnight. The following primary antibodies were used in the
present study: Anti-bax antibody (rabbit/rat; polyclonal; 1:1,000;
#2772; Cell Signaling Technology, Inc., Danvers, MA, USA),
anti-bcl-2 antibody (rabbit/rat; polyclonal IgG; 1:100; sc-492;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-PI3K
antibody (rabbit/rat; polyclonal IgG; 1:100; sc-67306; Santa Cruz
Biotechnology, Inc.), anti-phosphor-ylated-AKTser473
antibody (rabbit/rat; polyclonal IgG; 1:100; sc-33437; Santa Cruz
Biotechnology, Inc.), anti-p38mapk antibody (rabbit/rat;
polyclonal; 1:1,000; sc-728; Santa Cruz Biotechnology, Inc.),
anti-phosphorylated-AMPKThr172 antibody (rabbit/rat;
monoclonal; 1:1,000; #5759; Cell Signaling Technology, Inc.) and
anti-β-actin antibody (rabbit/rat; polyclonal; 1:1,000; sc-130657;
Santa Cruz Biotechnology, Inc.). The membrane was subsequently
incubated with the corresponding secondary antibody (goat/rabbit;
polyclonal; 1:1,000; A0208; Beyotime Institute of Biotechnology)
for 30 min the next day. Blots were imaged using the Bio-Rad gel
imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and
the densities of bands were quantified using Quantity One software,
version 4.5 (Bio-Rad Laboratories, Inc.).
Statistical analysis
All results are presented as the mean ± standard
deviation. Differences among grouped data were analyzed using a
one-way analysis of variance followed by Dunnett’s post-hoc test or
the Mann-Whitney Rank sum test. P<0.05 was considered to
indicate a statistically significant difference. All data was
analyzed with SPSS 19.0 software (IBM SPSS, Armonk, NY, USA).
Results
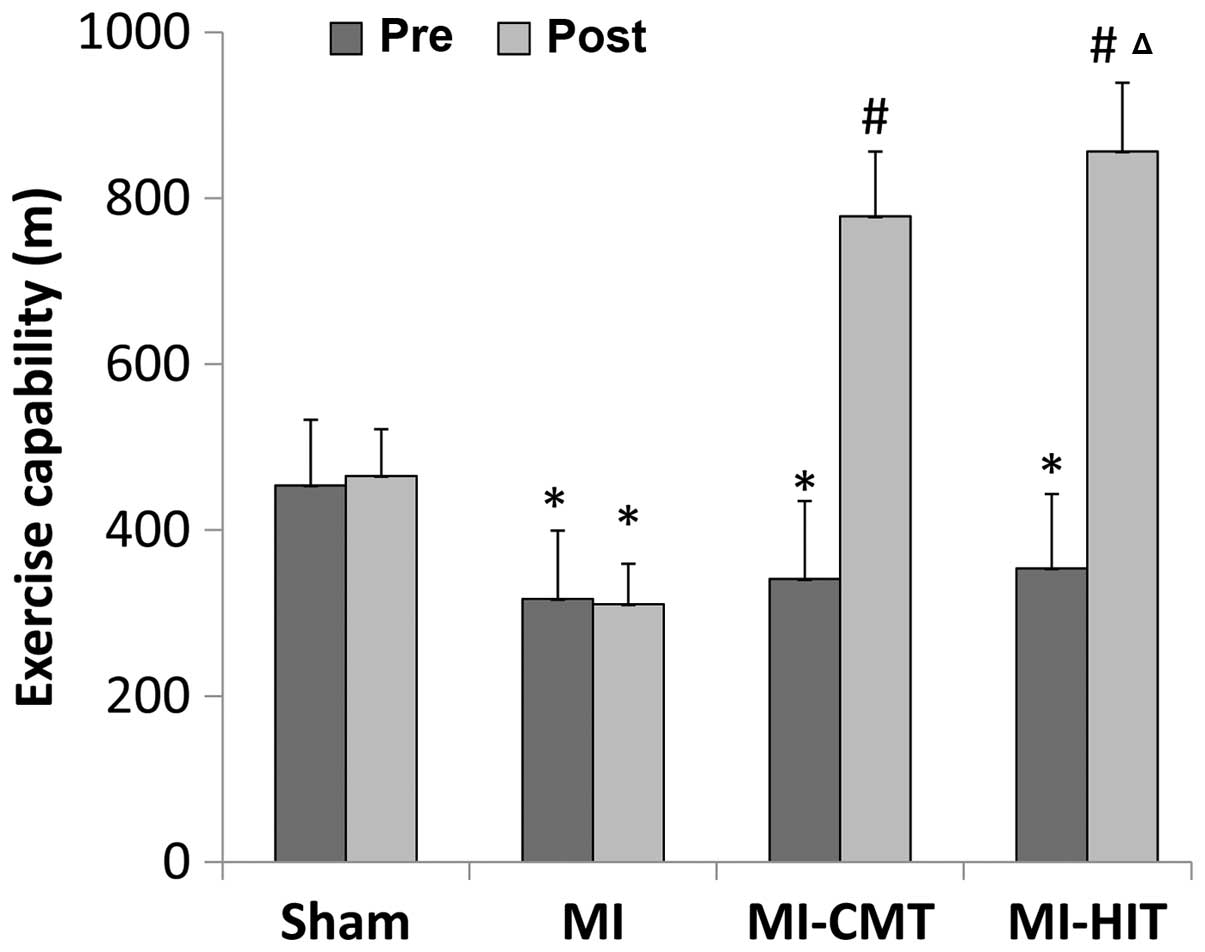
HIT is superior to CMT with regard to
enhancing exercise capability
As indicated in Fig.
1, the baseline exercise capability of the rats in the
MI-treated groups (MI, MI+CMT and MI+HIT) was significantly
inferior to that of the sham group (P<0.05) and no significant
differences were observed among the various MI-treated groups.
Following the training period, CMT markedly enhanced the exercise
capability of rats compared with that of the MI group, in which
rats remained sedentary for the majority of the time. However, HIT
was able to improve exercise capability to a greater extent and
rats ran ~100 m more than those in the CMT group (856.5±82.6 vs.
778.12±78.1; P<0.05).
HIT improves post-MI cardiac function
more than CMT
LVEF and FS in the MI-treated groups were
significantly decreased compared with those of the sham group, and
no significant differences were observed among the various
MI-treated groups prior to exercise training (Table II). When exercise training ended,
LVEF and FS in the two exercise training groups were significantly
increased compared with those of the MI group. In particular, LVEF
and FS were better restored in the HIT group than the CMT group
(change of LVEF: 20.4±0.52% vs. 10.4±0.39%, P<0.05; change of
FS: 5.7±0.70% vs. 10.3±0.55%, P<0.05).
 | Table IIEchocardiographic parameters of rats
pre- and post-aerobic exercise training. |
Table II
Echocardiographic parameters of rats
pre- and post-aerobic exercise training.
| Parameter | Sham (n=10)
| MI (n=10)
| MI+CMT (n=10)
| MI+HIT (n=10)
|
|---|
| Pre | Post | Change | Pre | Post | Change | Pre | Post | Change | Pre | Post | Change |
|---|
| LVEF (%) | 91.9±1.21 | 90.2±2.04 | −1.7±0.75 | 42.3±0.99a | 45.2±1.01a | 2.9±0.45a | 43.8±0.62a | 54.2±1.07a,b | 10.4±0.39a,b | 42.3±1.08a | 62.7±1.23a,b,c | 20.4±0.52a,b,c |
| FS (%) | 41.4±1.33 | 40.3±2.07 | −1.1±0.78 | 23.5±0.84a | 24.8±2.11a | 1.3±0.72a | 23.4±1.57a | 29.1±1.53a,b | 5.7±0.70a,b | 24.1±1.12a | 34.4±1.32a,b,c | 10.3±0.55a,b,c |
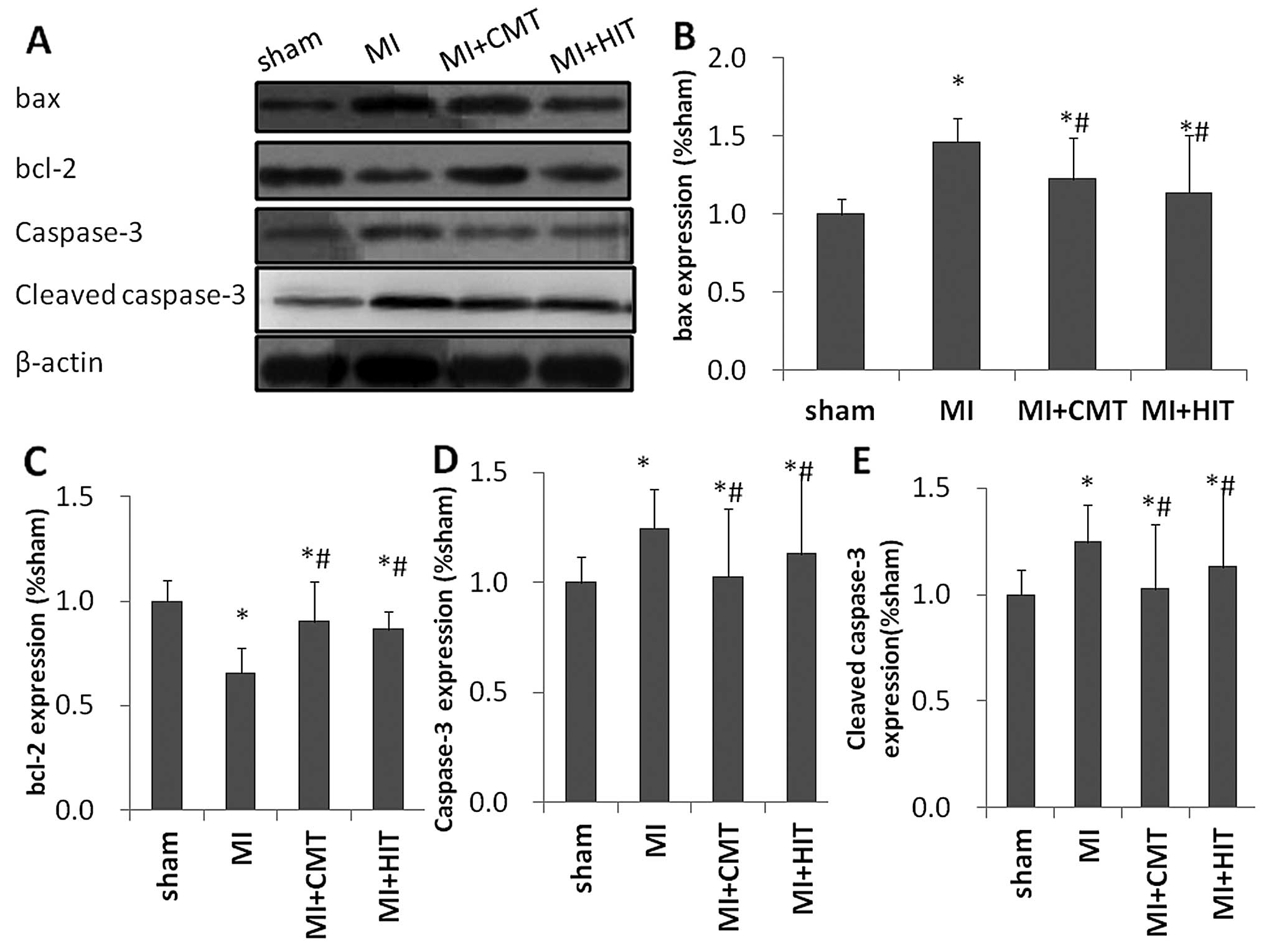
CMT and HIT attenuate apoptosis of the
infarcted myocardium
As shown in Fig. 2,
expression levels of pro-apoptotic proteins B cell lymphoma 2
(bcl-2)-associated protein X (bax) and caspase-3 were markedly
decreased by CMT and HIT, compared with those in the MI group.
Expression levels of bcl-2, the classic anti-apoptotic marker,
exhibited a significant reduction in the MI group compared with
those of the sham group (P<0.05); and a significant increase in
the CMT and HIT groups compared with those of the MI group
(P<0.05). Notably, the expression levels of bax, bcl-2 and
caspase-3 were altered approximately equally in the two exercise
groups and no significant differences were detected between the two
groups.
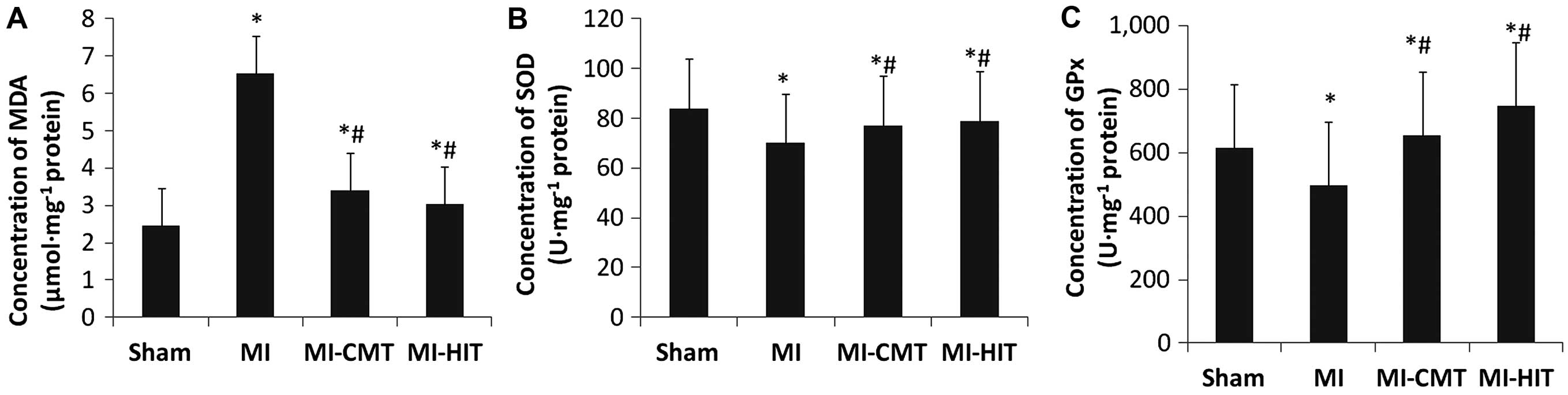
CMT and HIT ameliorate oxidative stress
of the infracted myocardium
As shown in Fig.
3A, the two forms of AET used in the present study markedly
decreased the expression of MDA (µmol/mg protein) compared
with that of the MI group (CMT: 3.41±0.13 vs. 6.53±0.21, P<0.05;
HIT: 3.03±0.17 vs. 6.53±0.21, P<0.05). Although CMT and HIT
significantly increased the concentration of SOD (U/mg protein),
the net effect was minor (CMT: 77.1±14.9 vs. 70.2±15.1, P<0.05;
HIT: 79.0±13.2 vs. 70.2±15.1, P<0.05; Fig. 3B). By contrast, the increase in GPx
(U/mg protein) concentration induced by AET was more marked,
accounting for 31.3 and 40.9% greater increases than that of the MI
group (P<0.05; Fig. 3C).
Notably, HIT was significantly superior to CMT in elevating the
concentration of GPx; although this effect was not observed in the
expression of MDA or SOD.
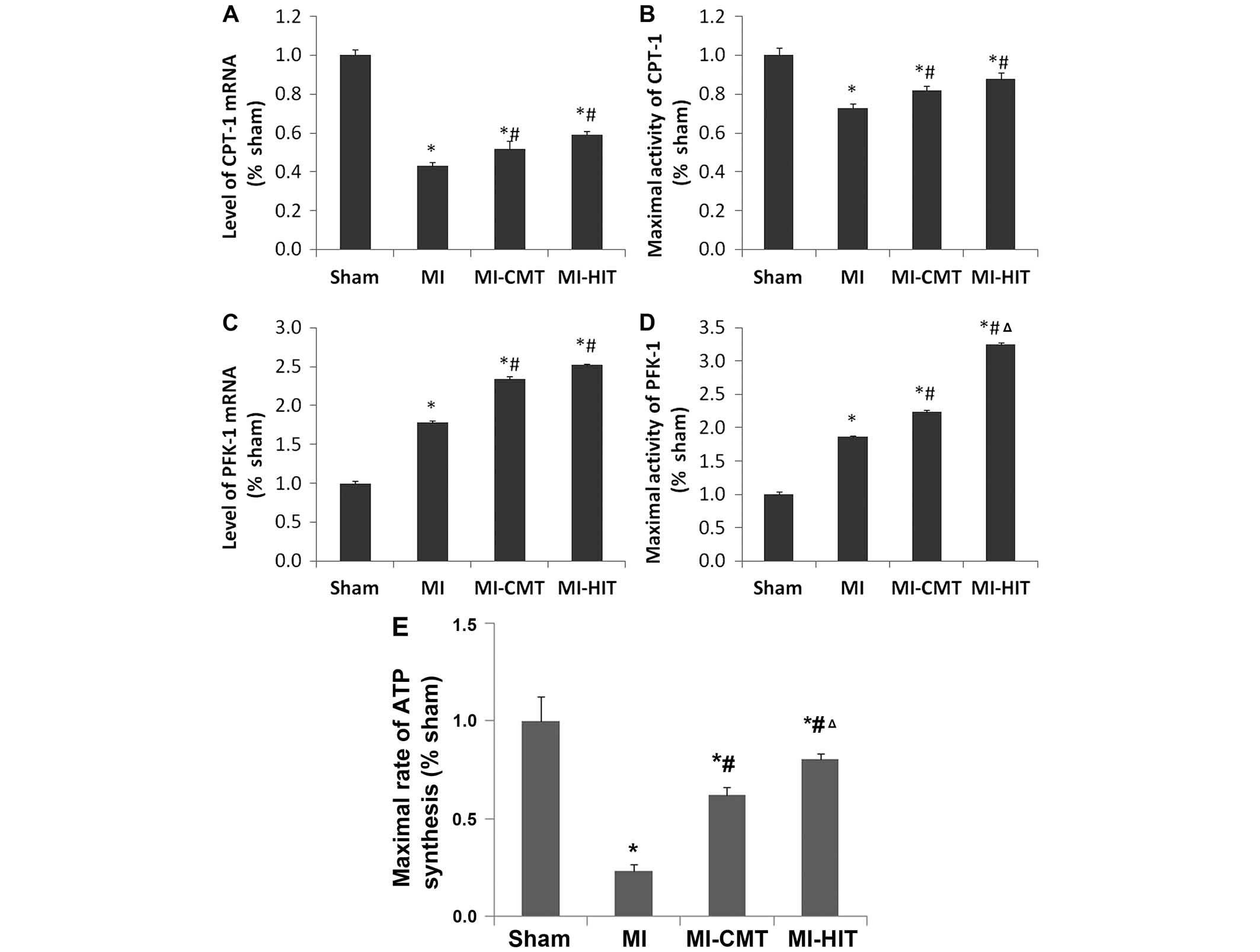
CMT and HIT influence CPT-1, PFK-1 and
ATP synthesis of the infarcted myocardium
MI induced a significant down-regulation in the mRNA
expression and maximal activity of CPT-1 (Fig. 4A and B). AET exerted a small but
significant effect on CPT-1 expression, and mRNA expression and
maximal activity were enhanced. No significant differences were
observed between the two exercise groups. By contrast, the effects
of aerobic exercise on mRNA expression and maximal activity of
PFK-1 were markedly greater, and the effects of HIT on PFK-1
maximal activity were greater than those of CMT (Fig. 4C and D). HIT induced a greater
increase in the maximal activity of PFK-1 than CMT (3.25±0.03 vs.
2.24±0.02, P<0.05). In addition, aerobic training, and
particularly HIT (P<0.05), significantly increased the maximal
rate of ATP synthesis compared with that of the MI group (0.62±0.04
and 0.80±0.03 vs. 0.23±0.03; P<0.05; Fig. 4E).
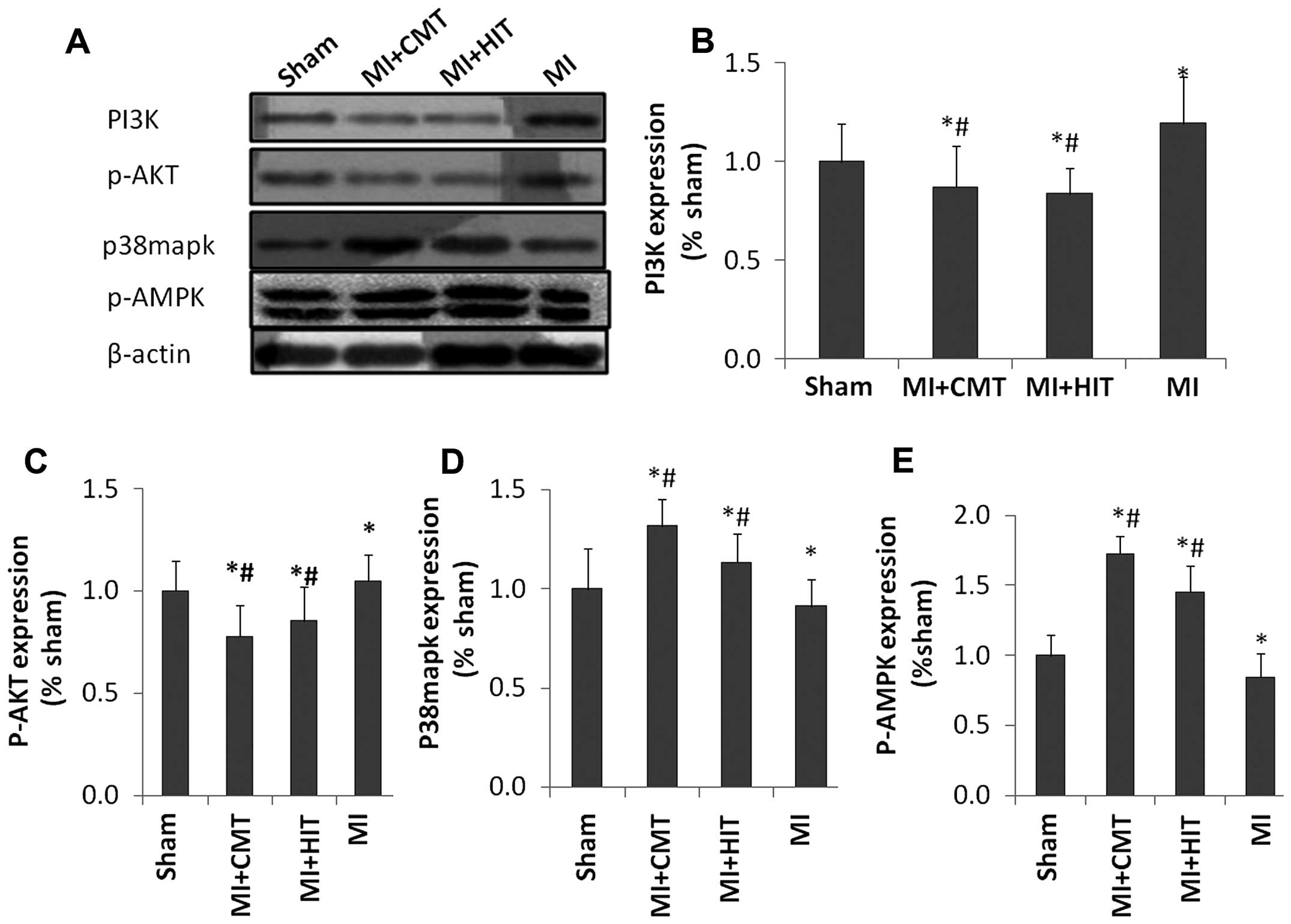
CMT and HIT influence the expression of
associated signaling pathway proteins
As shown in Fig.
5A–C, the levels of PI3K and p-AKT were significantly enhanced
in the MI group, compared with those of the sham group; but CMT and
HIT effectively attenuated this effect (P<0.05). The two forms
of aerobic training also abated expression of p38mapk protein
compared with that of the MI group (P<0.05; P<0.05; Fig. 5A and D). In addition, as indicated
in Fig. 5A and E, AET, and HIT in
particular, effectively enhanced the expression of p-AMPK, which
was attenuated in the MI group (P<0.05; P<0.05).
Discussion
The modifications exerted by distinct forms of AET
on post-MI cardiac functional recovery has previously been rarely
reported. In the present study, the effects of two forms of AET,
CMT and HIT, on exercise capability and the recovery of cardiac
function in a post-MI rat model were compared. In addition, the
effects of AET on associated pathophysiological processes and
signaling pathways that may contribute to the recovery of cardiac
function, including apoptosis, oxidative stress and glucolipid
metabolism, were investigated. The results indicated that HIT was
superior to CMT with regard to enhancing exercise capability and
cardiac functional recovery in post-MI rats. In addition, the
results demonstrated that HIT was superior to CMT in terms of
alleviating oxidative stress, ameliorating glycolipid metabolism
and enhancing ATP production. However, with regard to attenuating
apoptosis, no significant differences were observed.
Increasing evidence has demonstrated that CMT may
significantly attenuate apoptosis in the rat heart, as indicated by
changes in the levels of the apoptosis index, cleaved caspase-3
levels and caspase-3 activity (13,15,29).
The results of the present study regarding the effect of CMT on
apoptosis in the post-MI heart did not contradict these results.
However, the effect of HIT on apoptosis of the post-MI myocardium
had previously remained to be elucidated. By controlling the total
exercise time and distance between the CMT and HIT group, the
results of the present study revealed that HIT exerted a similar
level of influence on the levels of pro-apoptotic and
anti-apoptotic proteins as CMT.
A growing body of evidence has demonstrated that
aerobic exercise may alleviate oxidative stress in a post-MI rat
model. One previous study demonstrated that only high-intensity
exercise and moderate-intensity exercise of long duration were able
to effectively upregulate SOD activity in the ventricular
myocardium, while short durations of moderate-intensity exercise
failed to achieve this effect (30). Another study investigating HIT
indicated that oxidative stress marker superoxide anion and MDA
levels were decreased by AET (31). However, notably, there was
significant diversity amongst the training protocols utilized in
previous reports and the adaptation of oxidative stress to AET
depended upon the type, duration and intensity of exercise
(30–34). As such, according to these previous
reports, direct comparisons of distinct forms of exercise training
on oxidative stress are difficult and to the best of our knowledge
has not been done before. In the present study, particular
attention was paid to controlling the total training time and
distance between the various exercise groups during the training
protocol, which was designed to ameliorate potential interference
factors to the greatest extent. The results indicated that CMT and
HIT were able to attenuate oxidative stress in the post-MI heart,
which was consistent with other reports (31). Additionally, it was demonstrated
that HIT exerted an equal influence on the levels of MDA and SOD,
while it induced a greater change in the levels of GPx, in
comparison to that of CMT.
The effects of AET on glucose metabolism of the
myocardium following ischemic insult have rarely been reported
previously and the results have been contradictory. Broderick et
al (35) indicated that
exercise training was able to restore decreased myocardial
glycolysis in diabetes rats and enhance myocardial glycolysis
during ischemia. However, in another study, glycolysis was found to
be 25–30% lower prior to and following ischemia in the heart of
exercise-trained rats than that of the control in a rat model of
I/R (36). An accompanying
improvement in cardiac function was also observed in these two
studies. In the present study, glycolysis of cardio-myocytes in the
post-MI rat model was significantly enhanced following AET. The
models used in these studies possessed varying basic metabolic
hypotypes, which may account for the discrepancies observed. The
potential mechanisms underlying this effect remain elusive;
however, it was hypothesized that this may be associated with the
concentration of glucose transporter-4 which may determine the rate
of glucose metabolism to a large extent (37) and is potentially increased
following AET (38). As to the
effect of AET on myocardial fatty acid oxidation, the results of
the present study indicated that aerobic exercise significantly
increased the mRNA expression and activity of CPT-1, and the
expression of p-AMPK. CPT-1 is the rate-limiting enzyme for fatty
acid β-oxidation and is regulated by the AMPK/ACC signaling pathway
(39). Burelle et al
(36) reported that exercise
training increased myocardial palmitate oxidation by 50–65%,
compared with that of the sedentary rats prior to and following
ischemia. Conversely, another study in a normal heart model found
that fatty acid oxidation was not influenced by exercise (40). The results of the present study
were consistent with those of Burelle et al (36), and indicated that exercise may
exert a differential effect on fatty acid oxidation in the normal
and ischemic myocardium. Additionally, the results of the present
study revealed that glycolysis was enhanced to a greater extent
than fatty acid oxidation, which would increase the proportion of
glucose metabolism in total ATP production and alleviate the damage
that induced by excessive fatty acid oxidation following ischemic
insult in cardiomyocytes.
Improvements in exercise capability following
regular and effective exercise training should mainly be attributed
to the overall functional enhancement of the motor and
cardiopulmonary systems. Moreira et al (10) utilized a similar exercise protocol
to that used in the present study and found that skeletal muscle
adaptation to HIT and CMT was analogous. Therefore, the results of
the present study may in part explain the fact that HIT frequently
results in a more favorable increase in exercise capability than
CMT for MI patients. In addition, improvements of additional
systems and elements may also contribute to the improvement in
exercise capacity.
Although HIT has been utilized in the field of
medical rehabilitation for decades, the standardized exercise
protocol for optimal clinic outcome has remained under debate. The
diversity of regimens applied in previous studies also makes it
difficult to perform a crosswise comparative analysis among them.
Therefore, a series of experiments with standardized protocols
performed under strict control are required for the effective
determination of the most favorable exercise regimens.
In conclusion, the present study demonstrated that
HIT was superior to CMT in improving cardiac function and exercise
capability in a post-MI rat model. Furthermore, HIT exerted more
favorable effects with regard to alleviating oxidative stress,
ameliorating glucolipid metabolism and enhancing ATP production,
than those of CMT. However, HIT and CMT were almost equal in their
ability to attenuate apoptosis. The current study provided
underlying evidence that HIT is more preferable and effective for
cardiac rehabilitation as a novel form of aerobic exercise
training.
Acknowledgments
The present study was funded by grants from the 12th
Five-year Science and Technology Support Program of the Ministry of
Science and Technology of China (grant no. 2013BAI06B02). The
authors would like to express their gratitude to Professors Tiemin
Ma and Xin Ma from Beijing University of Chinese Medicine (Beijing,
China) for their assistance in establishing the rat model.
References
|
1
|
Nagayama M, Itoh H and Maeda T: Cardiac
rehabilitation for patients with acute myocardial infarction. Nihon
Rinsho. 69(Suppl 9): S203–S209. 2011.In Japanese.
|
|
2
|
Achttien RJ, Staal JB, van der Voort S, et
al Practice Recommendations Development Group: Exercise-based
cardiac rehabilitation in patients with coronary heart disease: a
practice guideline. Neth Heart J. 21:429–438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oldridge N: Exercise-based cardiac
rehabilitation in patients with coronary heart disease:
meta-analysis outcomes revisited. Future Cardiol. 8:729–751. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moholdt T, Aamot IL, Granøien I, et al:
Aerobic interval training increases peak oxygen uptake more than
usual care exercise training in myocardial infarction patients: a
randomized controlled study. Clin Rehabil. 26:33–44. 2012.
View Article : Google Scholar
|
|
5
|
Moholdt T, Aamot IL, Granøien I, et al:
Long-term follow-up after cardiac rehabilitation: a randomized
study of usual care exercise training versus aerobic interval
training after myocardial infarction. Int J Cardiol. 152:388–390.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maiorana A: Interval training confers
greater gains than continuous training in people with heart
failure. J Physiother. 58:1992012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kemi OJ and Wisløff U: Mechanisms of
exercise-induced improvements in the contractile apparatus of the
mammalian myocardium. Acta Physiol (Oxf). 199:425–439. 2010.
View Article : Google Scholar
|
|
8
|
Garza MA, Wason EA and Zhang JQ: Cardiac
remodeling and physical training post myocardial infarction. World
J Cardiol. 7:52–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kida K, Osada N, Akashi YJ, Sekizuka H,
Omiya K and Miyake F: The exercise training effects of skeletal
muscle strength and muscle volume to improve functional capacity in
patients with myocardial infarction. Int J Cardiol. 129:180–186.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moreira JB, Bechara LR, Bozi LH, et al:
High-versus moderate-intensity aerobic exercise training effects on
skeletal muscle of infarcted rats. J Appl Physiol (1985).
114:1029–1041. 2013. View Article : Google Scholar
|
|
11
|
Lee Y and Gustafsson AB: Role of apoptosis
in cardiovascular disease. Apoptosis. 14:536–548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tullio F, Angotti C, Perrelli MG, Penna C
and Pagliaro P: Redox balance and cardioprotection. Basic Res
Cardiol. 108:3922013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Quindry JC, Hamilton KL, French JP, et al:
Exercise-induced HSP-72 elevation and cardioprotection against
infarct and apoptosis. J Appl Physiol 1985. 103:1056–1062. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen TI, Shen YJ, Wang IC and Yang KT:
Short-term exercise provides left ventricular myocardial protection
against intermittent hypoxia-induced apoptosis in rats. Eur J Appl
Physiol. 111:1939–1950. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wambolt RB, Lopaschuk GD, Brownsey RW and
Allard MF: Dichloroacetate improves postischemic function of
hypertrophied rat hearts. J Am Coll Cardiol. 36:1378–1385. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lopaschuk GD, Spafford MA, Davies NJ and
Wall SR: Glucose and palmitate oxidation in isolated working rat
hearts reperfused after a period of transient global ischemia. Circ
Res. 66:546–553. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Egstrup M, Kistorp CN, Schou M, et al:
Abnormal glucose metabolism is associated with reduced left
ventricular contractile reserve and exercise intolerance in
patients with chronic heart failure. Eur Heart J Cardiovasc
Imaging. 14:349–357. 2013. View Article : Google Scholar
|
|
18
|
Reichkendler MH, Auerbach P, Rosenkilde M,
et al: Exercise training favors increased insulin-stimulated
glucose uptake in skeletal muscle in contrast to adipose tissue: a
randomized study using FDG PET imaging. Am J Physiol Endocrinol
Metab. 305:E496–E506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bourlier V, Saint-Laurent C, Louche K, et
al: Enhanced glucose metabolism is preserved in cultured primary
myotubes from obese donors in response to exercise training. J Clin
Endocrinol Metab. 98:3739–3747. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Castorena CM, Arias EB, Sharma N and
Cartee GD: Postexercise improvement in insulin-stimulated glucose
uptake occurs concomitant with greater AS160 phosphorylation in
muscle from normal and insulin-resistant rats. Diabetes.
63:2297–2308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Franke TF, Hornik CP, Segev L, Shostak GA
and Sugimoto C: PI3K/Akt and apoptosis: size matters. Oncogene.
22:8983–8998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blanc A, Pandey NR and Srivastava AK:
Synchronous activation of ERK 1/2, p38mapk and PKB/Akt signaling by
H2O2 vascular smooth muscle cells: Potential
involvement in vascular in disease (Review). Int J Mol Med.
11:229–234. 2003.PubMed/NCBI
|
|
23
|
Bijland S, Mancini SJ and Salt IP: Role of
AMP-activated protein kinase in adipose tissue metabolism and
inflammation. Clin Sci (Lond). 124:491–507. 2013. View Article : Google Scholar
|
|
24
|
Pfeffer MA, Pfeffer JM, Fishbein MC, et
al: Myocardial infarct size and ventricular function in rats. Circ
Res. 44:503–512. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Høydal MA, Wisløff U, Kemi OJ and
Ellingsen O: Running speed and maximal oxygen uptake in rats and
mice: practical implications for exercise training. Eur J
Cardiovasc Prev Rehabil. 14:753–760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kraljevic J, Marinovic J, Pravdic D, et
al: Aerobic interval training attenuates remodelling and
mitochondrial dysfunction in the post-infarction failing rat heart.
Cardiovasc Res. 99:55–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pauly DF and McMillin JB: Importance of
acyl-CoA availability in interpretation of carnitine
palmitoyltransferase I kinetics. J Biol Chem. 263:18160–18167.
1988.PubMed/NCBI
|
|
28
|
Dumas JF, Goupille C, Julienne CM, et al:
Efficiency of oxidative phosphorylation in liver mitochondria is
decreased in a rat model of peritoneal carcinosis. J Hepatol.
54:320–327. 2011. View Article : Google Scholar
|
|
29
|
Zhang KR, Liu HT, Zhang HF, et al:
Long-term aerobic exercise protects the heart against
ischemia/reperfusion injury via PI3 kinase-dependent and
Akt-mediated mechanism. Apoptosis. 12:1579–1588. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Powers SK, Criswell D, Lawler J, et al:
Rigorous exercise training increases superoxide dismutase activity
in ventricular myocardium. Am J Physiol. 265(6 Pt 2): H2094–H2098.
1993.PubMed/NCBI
|
|
31
|
Jiang HK, Miao Y, Wang YH, et al: Aerobic
interval training protects against myocardial infarction-induced
oxidative injury by enhancing antioxidase system and mitochondrial
biosyn-thesis. Clin Exp Pharmacol Physiol. 41:192–201. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Barbosa VA, Luciano TF, Vitto MF, et al:
Exercise training plays cardioprotection through the oxidative
stress reduction in obese rats submitted to myocardial infarction.
Int J Cardiol. 157:422–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pinho CA, Tromm CB, Tavares AM, et al:
Effects of different physical training protocols on ventricular
oxidative stress parameters in infarction-induced rats. Life Sci.
90:553–559. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Frederico MJ, Justo SL, Da Luz G, et al:
Exercise training provides cardioprotection via a reduction in
reactive oxygen species in rats submitted to myocardial infarction
induced by isoproterenol. Free Radic Res. 43:957–964. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Broderick TL, Poirier P and Gillis M:
Exercise training restores abnormal myocardial glucose utilization
and cardiac function in diabetes. Diabetes Metab Res Rev. 21:44–50.
2005. View
Article : Google Scholar
|
|
36
|
Burelle Y, Wambolt RB, Grist M, et al:
Regular exercise is associated with a protective metabolic
phenotype in the rat heart. Am J Physiol Heart Circ Physiol.
287:H1055–H1063. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Garvey WT, Hardin D, Juhaszova M and
Dominguez JH: Effects of diabetes on myocardial glucose transport
system in rats: implications for diabetic cardiomyopathy. Am J
Physiol. 264(3 Pt 2): H837–H844. 1993.PubMed/NCBI
|
|
38
|
Richter EA and Hargreaves M: Exercise,
GLUT4 and skeletal muscle glucose uptake. Physiol Rev. 93:993–1017.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hardie DG and Pan DA: Regulation of fatty
acid synthesis and oxidation by the AMP-activated protein kinase.
Biochem Soc Trans. 30:1064–1070. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zonderland ML, Bär PR, Reijneveld JC,
Spruijt BM, Keizer HA and Glatz JF: Different metabolic adaptation
of heart and skeletal muscles to moderate-intensity treadmill
training in the rat. Eur J Appl Physiol Occup Physiol. 79:391–396.
1999. View Article : Google Scholar : PubMed/NCBI
|