Introduction
Retinal ischemia reperfusion (RIR) injury exists in
various eye diseases, including glaucoma, diabetic retinopathy and
other ocular vascular disorders (1–3).
Production of reactive oxygen species (ROS) in RIR induces
oxidative stress, which leads to lipid oxidation, protein synthetic
disorder and DNA oxidation (4,5). It
has been verified that nuclear and mitochondrial DNA oxidation
leads to neuron death (6–8). Therefore, the prevention of DNA
injury may be an effective strategy to promote retinal cellular
survival.
ROS, particularly peroxynitrite, can induce nuclear
DNA oxidative breaks, followed by activation of poly (ADP-ribose)
polymerase-1 (PARP-1), a nuclear enzyme involved in the regulation
of multiple pathophysiological cellular procedures, including DNA
repair, gene transcription and cell death (9–11).
PARP-1 catalyzes the formation of poly (ADP-ribose) polymers, which
triggers the translocation of apoptosis-inducing factor (AIF) from
the mitochondria into the nucleus, causing DNA condensation and
caspase-independent cell death. In addition, ROS induced
mitochondrial DNA stress and lipid oxidation and can also induce
mitochondrial structural breakdown and the release of cytochrome
c, which promotes caspase family activation and apoptotic
cell death (8,12).
Hydrogen, the most common gas in the atmosphere, was
initially recognized as a therapeutic reductive substance in
medicine in 1975 (13). Several
studies (14–17) regarding its usage and underlying
molecular mechanism have been conducted since Ohsawa et al
identified that the inhalation of hydrogen gas markedly suppressed
ischemia-reperfusion injury in the brain by alleviating oxidative
stress caused by ROS in 2007 (18). Hydrogen can be dissolved in water
up to 0.8 mM under atmospheric pressure at room temperature and its
solubilized form, hydrogen-rich saline (HRS), is beneficial since
it is a portable, easily administered and a safe means of
delivering hydrogen (14). In
addition, administration of HRS has been demonstrated to enhance
cell survival in different disorders in animal models, particularly
due to its anti-oxidative and anti-inflammatory properties
(15–17).
The present study aimed to investigate whether HRS
had a protective effect in rodent RIR injury. In addition, the
mechanism underlying the protective effects of HRS was investigated
by measuring DNA oxidative stress, PARP-1 expression and
apoptosis.
Materials and methods
Animals
A total of 48 3 month-old adult male Sprague-Dawley
rats weighing 300–350 g (Tianjin Medical University Animal Center,
Tianjin, China) were used in the present study. Animals were fed a
standard rodent diet with a normal light-dark cycle. All animals
were cared for with the approval of the Laboratory Animal Research
Committee at Tianjin Medical University Eye Center. All
experimental procedures involving animals were performed in
accordance with the ARVO Statement for the Use of Animals in
Ophthalmic and Vision Research.
HRS production
Hydrogen was dissolved and saturated in 0.9% normal
saline (NS) for 6 h under a high pressure of 0.4 MPa using
hydrogen-producing apparatus (GCH-3000; Tianjin Tongpu Analytical
Instrument Tech Co., Ltd., Tianjin, China) according to the method
reported by Cai et al (17). The saturated HRS was stored at 4°C
under normal pressure in an aluminum bag. HRS was freshly prepared
and sterilized by γ radiation 24 h prior to the experiment,
ensuring an effective concentration of 0.6 mM. The content of
hydrogen in NS was confirmed through gas chromatography as
described previously by Ohsawa et al (14).
Rat RIR model and peritoneal
administration of HRS
All surgery was performed under general anesthesia
with intraperitoneal administration of chloral hydrate (300 mg/kg).
The anterior chamber of the left eye was cannulated with a 30-gauge
needle connected to a reservoir containing NS. Intraocular pressure
was increased to 130 mmHg for 60 min and ocular ischemia was
confirmed by interruption of the ocular circulation, as described
by Sun et al (2).
Thereafter, the cannula was immediately retracted and the adequacy
of retinal reperfusion was confirmed visually by ophthalmoscopy.
Rats that did not recover from retinal perfusion 3 min after the
end of the ischemic period and those with lens injury, which
prevents retinal ganglion cell (RGC) death and promotes axonal
regeneration (19), were excluded
from the investigation. Following surgery, levofloxacin ophthalmic
solution 0.3% (Cravit®; Santen Pharmaceutical Co., Ltd.,
Osaka, Japan) was dropped into the conjunctival sac of the left eye
to prevent infection. The model of IR injury was fully induced 24 h
after reperfusion.
All the rats were classified into the following four
groups: IR injury with HRS group, IR injury with NS group,
HRS-treated control group and NS-treated control group. The animals
in the IR injury with HRS and NS groups were peritoneally injected
with HRS or NS (5 ml/g) at the beginning of reperfusion.
Consecutive HRS or NS treatment was administered daily until the
rats were sacrificed with overdose anaesthesia. The rats in the
HRS-treated and NS-treated control groups were peritoneally
injected with HRS or NS (5 ml/kg) at the same time points without
initial reperfusion. As molecular hydrogen can easily diffuse into
tissues and penetrate the cellular membrane, HRS (5 ml/kg) was
injected peritoneally only at the beginning of reperfusion.
Furthermore, consecutive HRS, compared with the equivalent quantity
of NS, was administered daily until the rats were sacrificed.
Histological staining
Freshly enucleated eyeballs (n=6) were fixed in 4%
paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) for 30
min. Subsequently, corneal paracentesis was performed using a 25 G
needle. Following that, the entire eye cups were further fixed
overnight and then processed for paraffin embedding. Sections (4
µm) were cut along the vertical meridian of the eye, 2 mm
away from the optic nerve head and mounted on pre-coated glass
slides. Following deparaffinization and rehydration, sections were
stained with hematoxylin and eosin. Retinal damage was assessed by
measuring the thickness of the retina and cell loss in the ganglion
cell layer (GCL). The retinal thickness is defined as the total
width between the inner limiting membrane to the interface of the
outer plexiform layer and the outer nuclear layer (20). The number of cells in the GCL was
calculated under a light microscope (magnification, x40) (BX51;
Olympus Corporation, Tokyo, Japan). Three different sections were
randomly selected and each section was measured at four different
points between 1 and 2 mm from the optic disc. The average value
was appointed as the thickness of retina and the number of cells in
the GCL of the eye.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL)
Cryosections (n=6) were fixed with 4%
paraformaldehyde in 0.1 M PBS (pH 7.4) for 20 min. Cell death was
detected by a TUNEL assay using the In Situ Cell Death
Detection kit, POD (Roche Diagnostics Deutschland GmbH, Mannheim,
Germany) and color-developed with 3,3′-diaminobenzidine (DAB)
substrate (Roche Diagnostics Deutschland GmbH) according to the
manufacturer’s instructions. The number of TUNEL-positive cells in
the GCL was counted at x400 magnification for each section using a
light microscope (BX51; Olympus Corporation).
Immunofluorescence staining of
8-hydroxy-2-deoxyguano-sine (8-OHdG)
Eyes (n=6) in optimal cutting temperature compound
were cut into 6 µm sections (RM2165; Leica, Wetzlar,
Germany) following being flash frozen in liquid nitrogen. The
cryosections were fixed in ice-cold acetone for 10 min, washed in
PBS three times and blocked with 1% bovine serum albumin (BSA) in
PBST for 30 min at room temperature. Subsequently, primary goat
anti-8-hydroxyguanine (8-OHdG, a classical DNA oxidative product)
polyclonal antibody (1:100; cat. no. ab10802; Abcam, San Francisco,
CA, USA) in 1% BSA in PBST were added to the samples and incubated
in a humidified chamber overnight at 4°C. Following three washes in
PBS, the sections were incubated with tetramethyl rhodamine
isothiocyanate-conjugated rabbit anti-goat IgG secondary antibody
(1:200; cat. no. BA1091; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) for 1 h in the dark at
37°C, followed by 4′,6-diamidino-2-phenylindole staining (0.1
µg/ml; Sigma-Aldrich, St. Louis, MO, USA) and another three
washes in PBS. Coverslides were mounted and the immunoreactivity of
8-OHdG was detected under a fluorescence microscope (DFC500; Leica
Microsystems, Renens, Switzerland).
Western blot analysis
Total retinal tissue lysates (n=6) were prepared by
the addition of 500 µl sodium dodecyl sulfate (SDS) buffer
[250 mM Tris (pH 6.8), 10% SDS, 500 mM dithiothreitol, 50%
glycerol, 0.5% bromophenol blue and a 1:100 protease inhibitor
cocktail (cat. no. P8340; Sigma-Aldrich)]. Total tissue extracts
(20 µg) were separated on a 12% SDS polyacrylamide gel.
Proteins were transferred onto an Immobilon-P membrane (Millipore,
Billerica, MA, USA) and blocked with 5% skim milk in TBST (TBS
containing 0.05% Tween-20) at room temperature for 2 h.
Subsequently, the membranes were hybridized at 4°C overnight in
TBST with the primary antibodies: Anti-rPARP-1 (1:5,000; cat. no.
1051-1; Epitomics, Burlingame, CA, USA), and anti-rCaspase-3
(1:1,000; cat. no. 9661; Cell Signaling Technology, Boston, MA,
USA) overnight at 4°C. Following incubation with a horseradish
peroxidase-conjugated anti-rabbit immunoglobulin antibody, proteins
were visualized with an enhanced chemiluminescence kit (Kirkegaard
& Perry Laboratories, Inc., Gaithersburg, MD, USA). For
normalization, blots were probed with β-actin, a housekeeping
antibody (anti-β-actin; 1:1,000; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.). The signal was detected by exposing X-ray
films to the processed blots and analyzed by laser scanning
densitometry (Personal Densitometer; GE Healthcare, Piscataway, NJ,
USA).
Immunohistochemical staining of
PARP-1
Paraffin-embedded sections (n=6, 4 µm thick)
were deparaffinized and rehydrated. Antigen retrieval was achieved
by boiling in 10 mmol/l sodium citrate buffer for 10 min and then
steadily cooling to room temperature. Subsequently, the sections
were blocked using 3.0% H2O2 in methanol for
15 min in order to inhibit endogenous peroxidase activity.
Following washing in PBS, the sections were incubated overnight at
4°C with monoclonal rabbit anti-PARP-1 primary antibody (cat. no.
1051-1) at a dilution of 1:40 (Epitomics). The sections were
incubated with peroxidase-conjugated goat anti-rabbit IgG (Beijing
Zhongshan Goldenbridge Biotechnology Co., Ltd.) at the same
dilution of 1:500 for 2 h at 37°C. The sections were washed in PBS,
developed in prepared DAB chromogen solution, lightly
counterstained with hematoxylin, dehydrated and mounted.
Statistical analysis
All data are presented as the mean ± standard
deviation. Data were analyzed using one-way analysis of variance
conducted between groups with post hoc analysis through Bonferroni
multiple comparisons test. Student’s t-test was used to compare
data between two groups. Statistical analyses were conducted using
SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
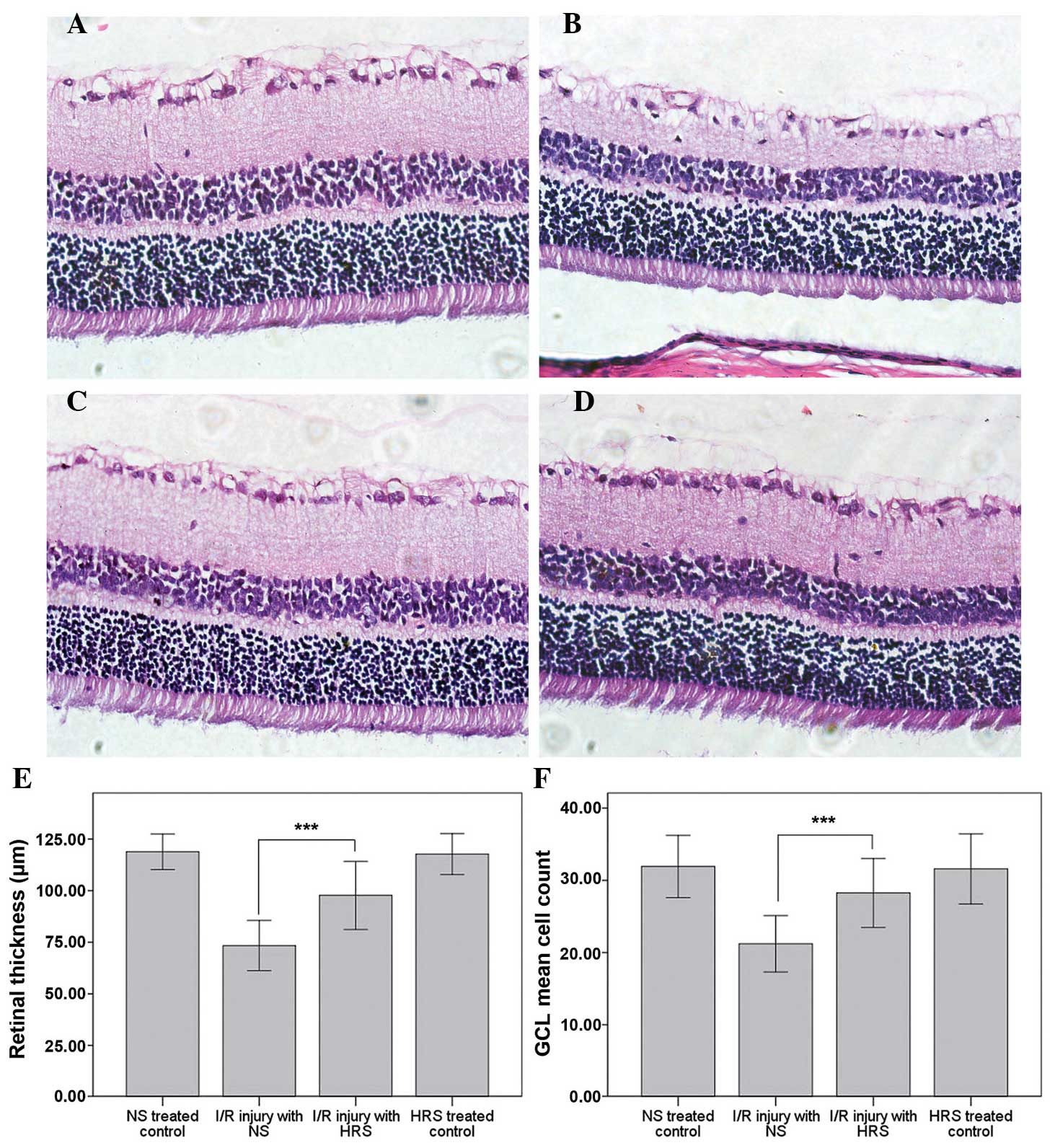
HRS administration reduces histological
disorder following RIR injury
The histological alterations of RIR injury occurred
after 1 week of reperfusion (21).
The thinning of retinas and cell loss in the GCL demonstrated the
successful induction of this model. Abdominal administration of HRS
effectively reversed the morphological alterations, including
thinning of retinas and cell loss in the GCL (Fig. 1). By contrast, the retinal
thickness of the RIR injury with NS group was 73.39±6.07 µm,
which was markedly thinner than the retinal thickness in the
NS-treated control group (118.85±4.31 µm; P<0.001). The
retinal thickness in the HRS-treated control group was 117.71±4.95
µm, which demonstrated that hydrogen did not alter the
thickness of the retina in normal rats. However, following HRS
administration the thinning of retina following RIR injury
partially recovered to 97.72±8.25 µm, which was
significantly different to the NS-treated control (P<0.001).
Furthermore, the number of cells in the GCL in the
IR injury with NS group decreased to 21.2±1.9, compared with
31.9±2.2 in the NS-treated control (P<0.001). No significant
difference was identified between the HRS-treated (31.6±2.4) or
NS-treated controls. By contrast, the number of cells in the IR
injury with HRS group was 28.3±2.4 (P<0.001).
HRS administration alleviates death of
RGCs in RIR injury
The NS-treated control and HRS-treated control had
few TUNEL positive cells, which illustrated that HRS did not induce
cell death. By contrast, retinas with RIR injury experienced severe
cell death in the GCL 7 days after reperfusion, which was in
accordance with previous studies (22,23).
Treatment with HRS significantly decreased the number of
TUNEL-positive cells in the GCL of RIR rats on the seventh day
after reperfusion (P<0.001), suggesting that HRS may have a
protective effect on cell apoptosis (Fig. 2).
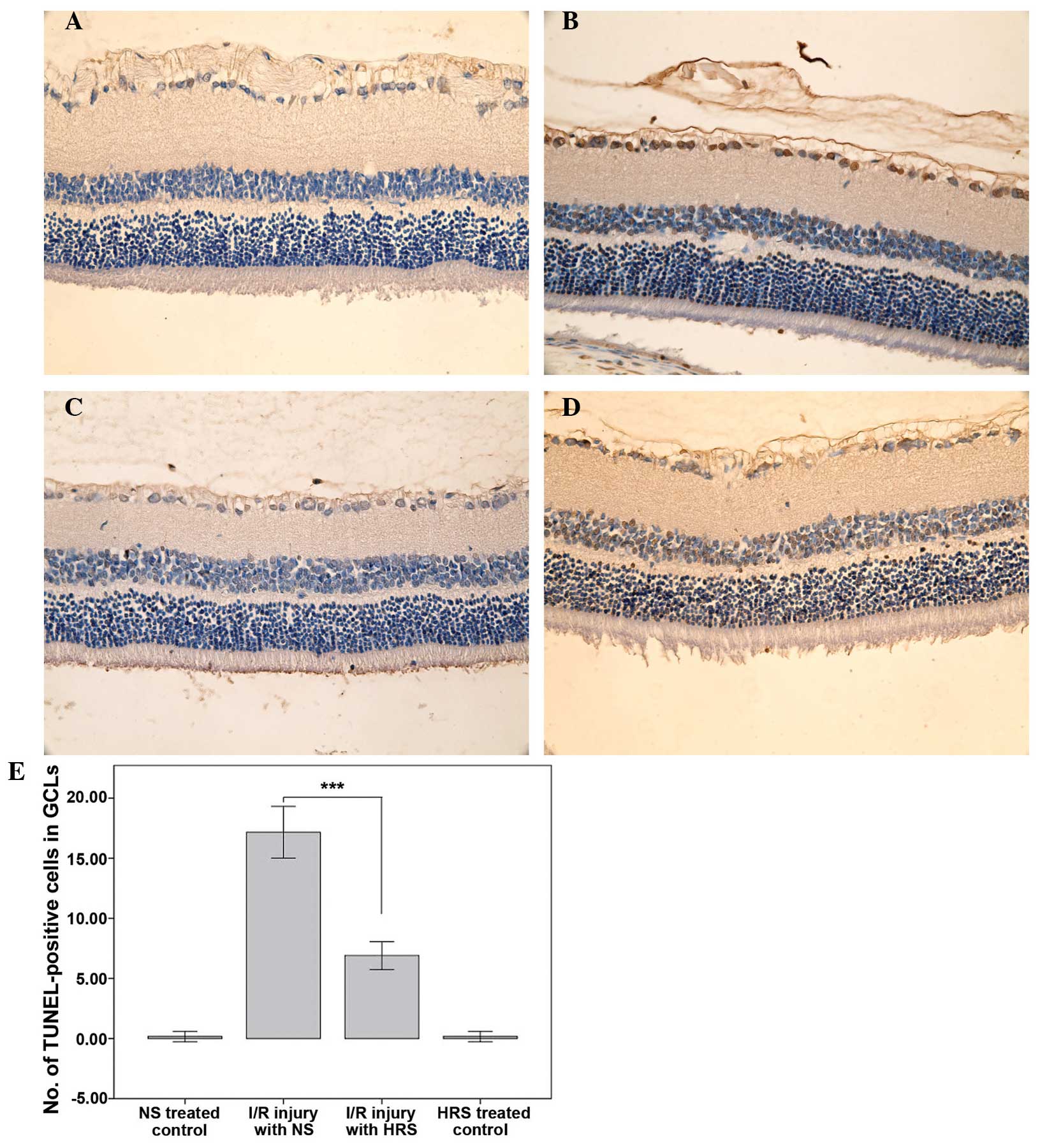 | Figure 2HRS alleviates cell death in the GCL
in a rat model of RIR (magnification, x400). Retinal cryosections
(n=6) were stained with TUNEL using the in situ cell death
detection kit 1 week after RIR injury and cell death was detected
as brown nuclei by 3,3′-diaminobenzidine staining. (A) Images of
representative sections of the NS-treated control group, (B) RIR
injury with NS group, (C) the HRS-treated control group and (D) the
RIR injury with HRS group are shown. (E) The administration of HRS
significantly alleviated cell death in the GCL.
***P<0.0001, compared with I/R-injured retina treated
with NS. Histograms represent the mean ± standard deviation. HRS,
hydrogen-rich saline; RIR, retinal ischemia reperfusion; TUNEL,
terminal deoxynucleotidyl transferase dUTP nick end labeling; GCL,
ganglion cell layer; NS, normal saline; I/R,
ischemia/reperfusion. |
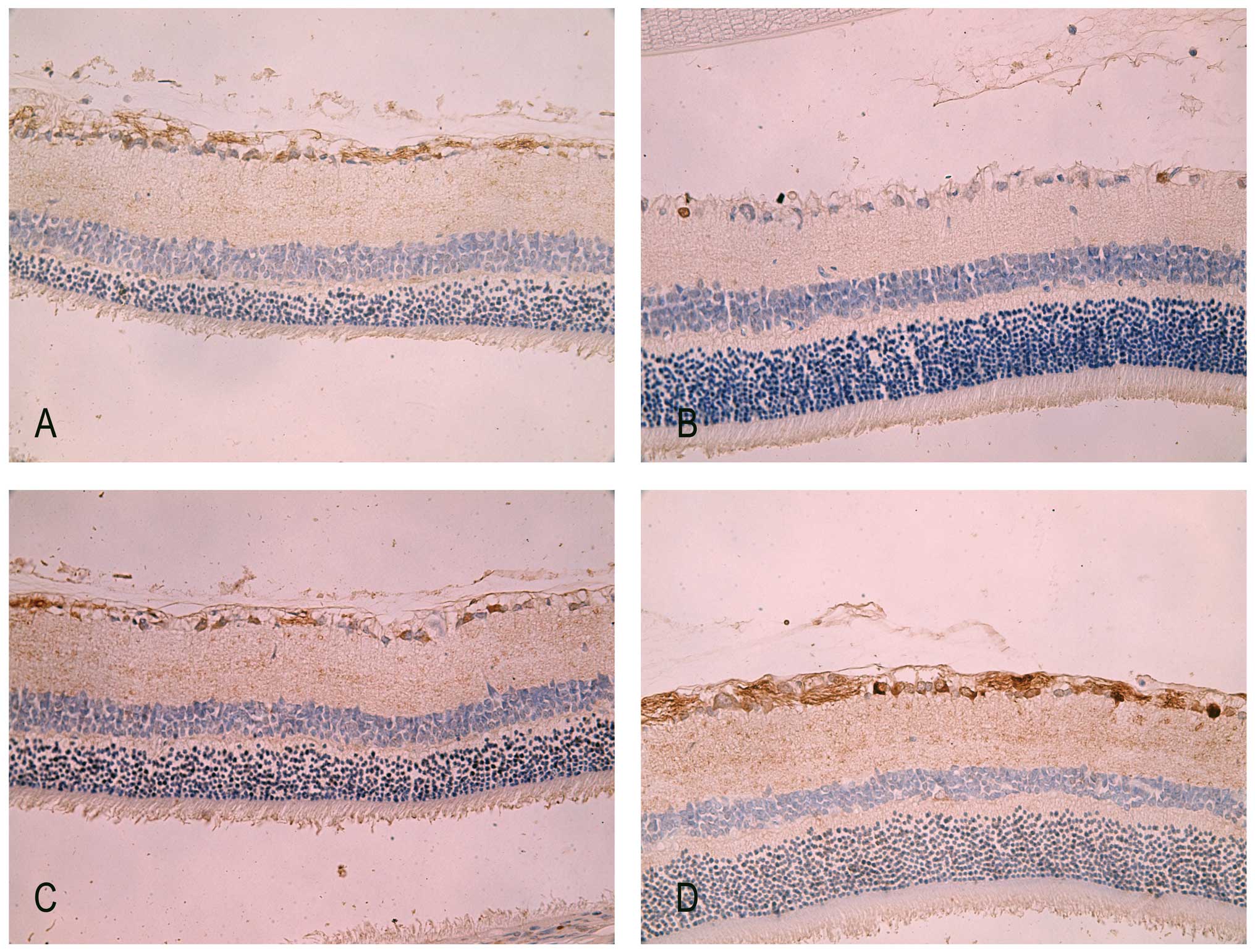
HRS administration alleviates DNA
oxidative breaks in RIR injury
The DNA oxidative breaks in RIR injury were detected
through immunofluorescent staining of 8-OHdG, a DNA oxidative
product (Fig. 3). There was
moderate 8-OHdG immunoreactivity in the photoreceptor cell layer
compared with faint immunostaining in the GCL in the untreated
control. Following administration of HRS, 8-OHdG immunoreactivity
partially decreased. In addition, IR injury increased the strength
of 8-OHdG immunoreactivity in the GCL as well as in photoreceptor
cell layers, suggesting severe DNA oxidative stress due to ROS. By
contrast, the immunoreactivity of 8-OHdG decreased significantly in
the GCL immediately following HRS intervention in IR injury, which
may confirm the reductive potential of hydrogen to DNA oxidative
stress.
 | Figure 3DNA oxidative injury detected by
immunoreactivity of 8-OHdG is ameliorated following HRS
intervention between 24 h and 7 days after reperfusion in a rat RIR
model (magnification, x200). Retinal cryosections (n=6) were
stained with primary goat anti 8-OHdG polyclonal antibody (1:100)
overnight at 4°C and were incubated with tetramethyl rhodamine
isothiocyanate-conjugated rabbit IgG secondary antibody (1:200 for
1 h in the dark at 37°C, followed by 4′,6-diamidino-2-phenylindole
staining; 0.1 µg/ml). The immunoreactivity of 8-OHdG was
detected under a fluorescence microscope. (A) NS-treated control
group, (B) RIR injury with NS group 24 h after reperfusion, (C) RIR
injury with NS group 7 days after reperfusion, (D) HRS-treated
control group, (E) RIR injury with HRS group 24 h after reperfusion
and (F) the RIR injury with HRS group 7 days after reperfusion.
HRS, hydrogen-rich saline; 8OHdg, 8-hydroxy-2-deoxyguanosine; RIR,
retinal ischemia reperfusion; NS, normal saline. |
PARP-1 and cleaved PARP-1 are
downregulated following hydrogen intervention, concordant with the
expression of caspase-3
Excessive DNA breaks may induce overactivation of
PARP-1 and cell death. In addition, as the substrate of caspase-3,
activated PARP-1 can be cleaved into 29 and 84 kDa fragments. The
primary anti-capase-3 antibody could react with the whole molecule
and the 19 kDa fragment of caspase-3, while anti-PARP-1 antibody
recognized full length PARP-1 and its 29 kDa fragment.
Cleavage of PARP-1 was significantly elevated in RIR
retina 24 h and 7 days after reperfusion (Fig. 4). Compared with the relatively low
immunofluorescent staining of PARP-1 at 116 kDa in the untreated
control and hydrogen control, the RIR model demonstrated strong
immunoreactivity of the 29 kDa C-terminal catalytic domain. At the
same time, the pro-form of PARP-1 decreased 24 h after RIR injury,
but increased and surpassed the control 7 days after RIR injury,
while the 29 kDa cleaved fragment remained visible. Following
peritoneal administration of HRS, cleaved PARP-1 was reduced 24 h
and 7 days after reperfusion, demonstrated as decreased
immunoreactivity of 29 kDa PARP-1 fragments.
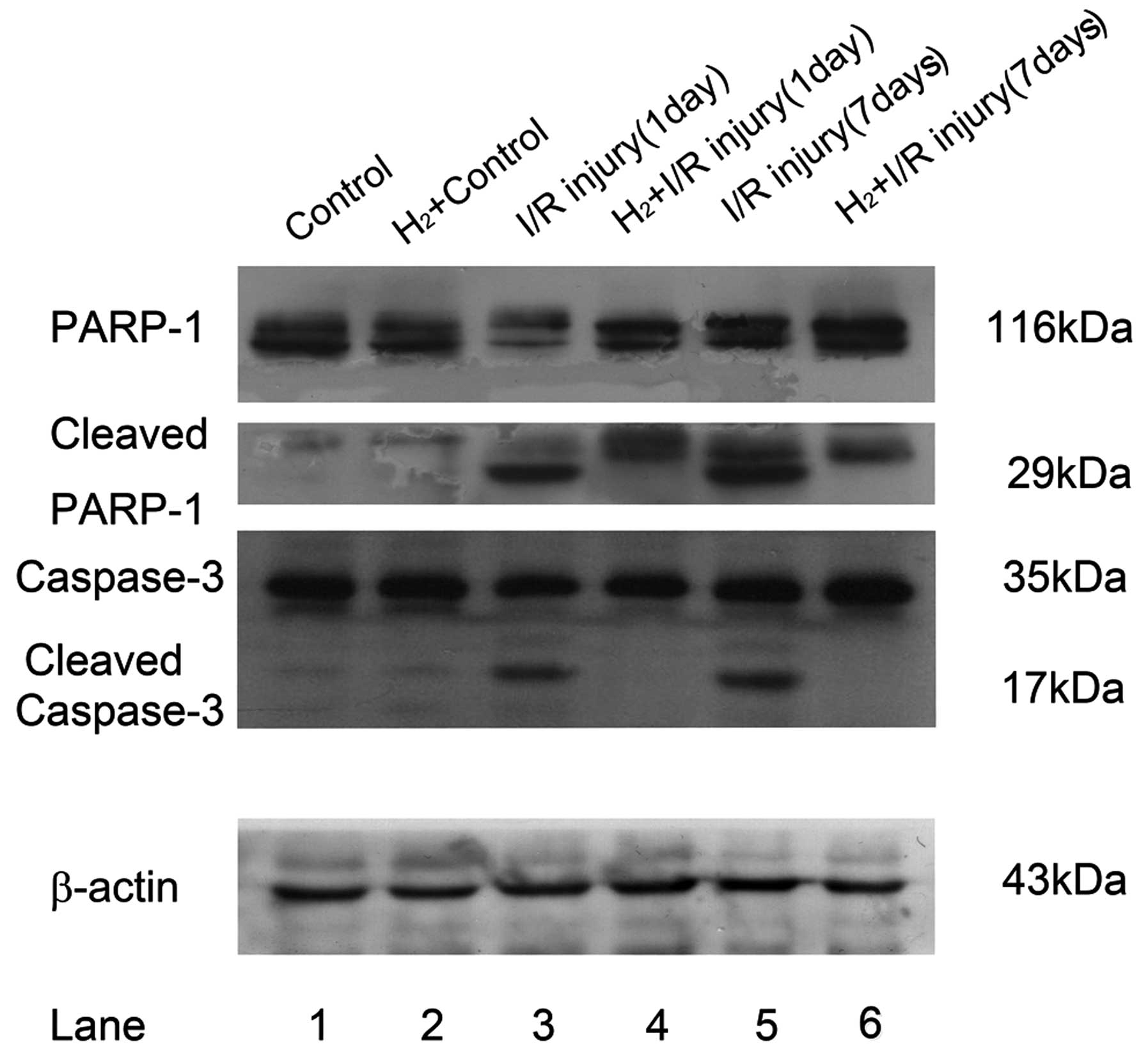 | Figure 4Alterations in PARP-1 and caspase-3
cleavage following administration of HRS in a retinal I/R model in
rats. Retinas (n=6) were lysed with RIPA mixed with a protease
inhibitor cocktail. Lysates (20 µg) from each group were
added into their corresponding layers of 12% SDS-PAGE. Following
being transferred onto a polyvinylidene fluoride membrane, primary
antibodies (anti-rPARP-1; 1:5,000; anti-rCaspase-3, 1:1,000) were
cultured at 4°C overnight followed by horseradish
peroxidase-conjugated secondary antibody cultured for 2 h at 37°C.
Subsequently, electrochemiluminescnce-assisted exposure was
performed to gain 116 kDa full PARP-1 and its cleaved 29 kDa
fragments, the intact caspase-3 (35 kDa) and its 17 kDa cleaved
fragment. For normalization, blots were probed with β-actin
(anti-β-actin; 1:1,000). Compared with the control, RIR injury led
to significant cleavage of full length PARP-1 between 24 h and 7
days after reperfusion (lane 1 and 2 versus lane 3 and 5). The
cleavage of caspase-3 had a similar change to PARP-1. HRS
intervention of RIR injury significantly reduced the cleavage of
these two proteins (lane 3 and 5 versus lane 4 and 6). PARP-1, poly
(ADP-ribose) polymerase 1; HRS, hydrogen-rich saline; I/R,
ischemia/reperfusion. |
The expression of capase-3 was detected in RIR
injury by immunostaining. In the untreated control retina and
hydrogen control retina, there was almost no caspase-3
immunoreactivity. However, cleaved caspase-3 was detected between
24 h and 7 days after reperfusion. However, hydrogen intervention
inhibited caspase-3 cleavage, showing the loss of cleaved fragment
immunoreactivity 24 h and 7 days after reperfusion.
Immunohistochemistry staining of PARP-1
in retina
In normal adult retina, PARP-1 was mildly detected
in the cytoplasm of the GCL and nerve fiber layer (Fig. 5A). There were markedly fewer PARP-1
positive cells in the IR injury groups at 7 days. However, the
immunoreactivity of PARP-1 was concentrated predominantly in the
nuclei of the GCL in IR retina (Fig.
5C). This altered location of PARP-1 from the cytoplasm to the
nuclei was previously observed by Huang et al (24) in the same rodent experiment model.
Since the cleaved 29 kDa fragment of PARP-1 can translocate to the
nuclei and bind to the oxidized DNA breaks, the translocation of
PARP-1 immunoreactivity illustrated the sustained existence of DNA
breaks in RIR injury. By contrast, after 7 days of intraperitoneal
administration of HRS in IR rats, there was apparent
immunoreactivity of PARP-1 not only in the nuclei but also in the
cytoplasm of the GCL as well as in the nerve fiber layer (Fig. 5D).
Discussion
DNA damage due to oxidative stress is a crucial
factor in the pathogenesis of neurodegenerative diseases (25). It has been demonstrated that DNA
oxidation is involved in the pathophysiology of retinal disorder
(4,26), including RIR injury, which
typically manifests as ganglion cell degeneration. Peroxynitrite,
generated from NO and O2−, destroys lipid, protein and
nucleic acid and is considered to be the most effective promoter of
cell death (27,28). Since peroxynitrite induces the
selective oxidation of guanine in DNA, its oxidative product,
8-OHdG, is the most widely used ‘marker’ for oxidative DNA damage
(29–32).
Activation of PARP-1 is recognized to perform
central roles in DNA repair, as its DNA binding domain may seal the
breaks on DNA strands and prevent incorrect DNA recombination
(33). However, severely sustained
DNA injury induces overactivation of PARP-1, which in turn leads to
increased PAR production that augments the translocation of AIF
into the nuclei to induce DNA cleavage into large fragments. This
process is known as PARP-1-mediated cell death (PARthanatos)
(34–37). Furthermore, the overproduction of
PAR can cause lethal consumption of cellular NAD+,
consequently leading to energy exhausted cell death (37).
In addition, oxidative stress can also cause
mitochondria and endoplasmic reticulum to trigger cell apoptosis.
For instance, oxidative stress leads to the opening of
mitochondrial membrane permeability transition pores and the
release of cytochrome c, followed by the activation of
caspase-3 in the form of cleaved fragments. Since activated
caspase-3 has the potential to cleave PARP-1, cell death may be
partially inhibited due to its inhibition of PARP-1 activity.
Simultaneously, cleaved caspase-3 induces apoptosis, which may be
cellular compensation for the severe destruction caused by
PARthanatos (38,39). Therefore, PARP-1 and caspase-3 may
be simultaneously involved in the procedure and affect each other
mutually.
The powerful reductive potential of hydrogen in
medicine has been recently recognized (14,16–18).
It selectively neutralizes peroxynitrite and hydroxyl radicals
generated from oxidative stress and promotes cell survival in
vitro and in vivo (14,40,41).
Furthermore, HRS is more conveniently used in vivo. The
present study demonstrated that peritoneal administration of HRS
successfully promoted cell survival in the GCL in a rat RIR model.
This result was in accordance with the results of the study by
Oharazawa et al in which hydrogen loaded eyedrops reduced
TUNEL positive cells in nuclear layers of an RIR model (42).
DNA oxidation was significantly reduced following
administration of HRS, demonstrating that molecular hydrogen
successfully rescued cells by preventing DNA oxidative breaks.
Further investigation of the expression of PARP-1 fragments
demonstrated its elevated cleavage from the caspase family
following IR injury, which was further confirmed by the condensed
nuclear aggregation of intact PARP-1 and its 29 kDa cleaved
fragment in the GCL in rodent RIR. This result illustrates the
overactivation of PARP-1 can cause cell death in RIR. Notably,
following treatment with hydrogen, the integrity of PARP-1 was
significantly well preserved, suggesting the recovery of its
repairing function to maintain stability of neurons and endothelia
in retinas.
In addition, caspase-3 cleavage was significantly
elevated following IR injury whereas hydrogen intervention reduced
caspase-3 cleavage, suggesting that caspase-mediated apoptosis is
inhibited by molecular hydrogen.
In terms of oxidative injury in retina, there are
several studies which support that PARP-1-mediated cell death may
be an important mechanism of RIR injury. Li et al (43) found that hydrogen peroxide, a
strong oxidizer, induced RGC-5 cell death, which induced abundant
production of ROS. The authors found that there was no activation
of caspase-3 following oxidative injury, however, PARP and its
downstream effector, AIF, were involved (43). Furthermore, Li et al
(44) investigated
D-galactose-induced oxidative stress in neuroblastoma cells and
observed significant necrotic cell death, which could not be
alleviated by the caspase inhibitor z-VAD-fmk, suggesting that
caspase-dependent cell death did not occur. It is possible that
PARP-1-mediated cell death co-exists with caspase-dependent
apoptosis in RIR injury and molecular hydrogen can modulate these
two procedure simultaneously in neuroretina.
Considering the data presented, hydrogen-mediated
neuroprotection in the rat retina may result from inhibition of
PARP-1. The histological findings strengthen these results,
providing morphological evidence of RGC survival. However, further
studies on apoptosis and PARthanatos are required in order to
confirm the mechanism of hydrogen intervention.
Acknowledgments
The authors would like to thank Professor Yongming
Wang and Professor Jinyan He (Tianjin Medical University) for their
assistance with the animal procedures and for consultation on the
experimental technique.
This study was supported by research grants from the
National Natural Science Foundation of China (nos. 81071533 and
81101409), Tianjin Municipal Science and Technology Commission
Foundation (nos. 11JCYBJC12900 and 13JCQNJC11400), the Foundation
of Tianjin Bureau of Public Health (no. 2011KZ108) and the
Foundation of Tianjin Municipal Education Commission (no.
20120129).
References
|
1
|
Bringmann A, Uckermann O, Pannicke T,
Iandiev I, Reichenbach A and Wiedemann P: Neuronal versus glial
cell swelling in the ischaemic retina. Acta Ophthalmol Scand.
83:528–538. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun MH, Pang JH, Chen SL, Han WH, Ho TC,
Chen KJ, Kao LY, Lin KK and Tsao YP: Retinal protection from acute
glaucoma-induced ischemia-reperfusion injury through pharmacologic
induction of heme oxygenase-1. Invest Ophthalmol Vis Sci.
51:4798–4808. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Makita J, Hosoya K, Zhang P and Kador PF:
Response of rat retinal capillary pericytes and endothelial cells
to glucose. J Ocul Pharmacol Ther. 27:7–15. 2011. View Article : Google Scholar :
|
|
4
|
Liu Y, Tang L and Chen B: Effects of
antioxidant gene therapy on retinal neurons and oxidative stress in
a model of retinal ischemia/reperfusion. Free Radic Biol Med.
52:909–915. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barakat DJ, Dvoriantchikova G, Ivanov D
and Shestopalov VI: Astroglial NF-κB mediates oxidative stress by
regulation of NADPH oxidase in a model of retinal ischemia
reperfusion injury. J Neurochem. 120:586–597. 2012. View Article : Google Scholar :
|
|
6
|
Pazdro R and Burgess JR: Differential
effects of α-tocopherol and N-acetyl-cysteine on advanced glycation
end product-induced oxidative damage and neurite degeneration in
SH-SY5Y cells. Biochim Biophys Acta. 1822:550–556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Poulsen HE, Specht E, Broedbaek K,
Henriksen T, Ellervik C, Mandrup-Poulsen T, Tonnesen M, Nielsen PE,
Andersen HU and Weimann A: RNA modifications by oxidation: A novel
disease mechanism? Free Radic Biol Med. 52:1353–1361. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jaeschke H, McGill MR and Ramachandran A:
Oxidant stress, mitochondria and cell death mechanisms in
drug-induced liver injury: lessons learned from acetaminophen
hepatotoxicity. Drug Metab Rev. 44:88–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Virág L and Szabó C: The therapeutic
potential of poly (ADP-ribose) polymerase inhibitors. Pharmacol
Rev. 54:375–429. 2002. View Article : Google Scholar
|
|
10
|
Jagtap P and Szabó C: Poly (ADP-ribose)
polymerase and the therapeutic effects of its inhibitors. Nat Rev
Drug Discov. 4:421–440. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hassa PO and Hottiger MO: The diverse
biological roles of mammalian PARPS, a small but powerful family of
poly-ADP-ribose polymerases. Front Biosci. 13:3046–3082. 2008.
View Article : Google Scholar
|
|
12
|
Rodrigues FP, Pestana CR, Polizello AC,
Pardo-Andreu GL, Uyemura SA, Santos AC, Alberici LC, da Silva RS
and Curti C: Release of NO from a nitrosyl ruthenium complex
through oxidation of mitochondrial NADH and effects on
mitochondria. Nitric Oxide. 26:174–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dole M, Wilson FR and Fife WP: Hyperbaric
hydrogen therapy: a possible treatment for cancer. Science.
190:152–154. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagata K, Nakashima-Kamimura N, Mikami T,
Ohsawa I and Ohta S: Consumption of molecular hydrogen prevents the
stress-induced impairments in hippocampus-dependent learning tasks
during chronic physical restraint in mice. Neuropsychopharmacology.
34:501–508. 2009. View Article : Google Scholar
|
|
15
|
Terasaki Y, Ohsawa I, Terasaki M,
Takahashi M, Kunugi S, Dedong K, Urushiyama H, Amenomori S,
Kaneko-Togashi M, Kuwahara N, Ishikawa A, et al: Hydrogen therapy
attenuates irradiation-induced lung damage by reducing oxidative
stress. Am J Physiol Lung Cell Mol Physiol. 301:L415–L426. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai J, Kang Z, Liu K, Liu W, Li R, Zhang
JH, Luo X and Sun X: Neuroprotective effects of hydrogen saline in
neonatal hypoxia-ischemia rat model. Brain Res. 1256:129–137. 2009.
View Article : Google Scholar
|
|
17
|
Spulber S, Edoff K, Hong L, Morisawa S,
Shirahata S and Ceccatelli S: Molecular hydrogen reduces
LPS-induced neuroinflammation and promotes recovery from sickness
behaviour in mice. PLoS One. 7:e420782012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohsawa I, Ishikawa M, Takahashi K,
Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S
and Ohta S: Hydrogen acts as a therapeutic antioxidant by
selectively reducing cytotoxic oxygen radicals. Nat Med.
13:688–694. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fischer D, Pavlidis M and Thanos S:
Cataractogenic lens injury prevents traumatic ganglion cell death
and promotes axonal regeneration both in vivo and in culture.
Invest Ophthalmol Vis Sci. 41. pp. 3943–3954. 2000
|
|
20
|
Hughes WF: Quantitation of ischemic damage
in the rat retina. Exp Eye Res. 53:573–582. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sellés-Navarro I, Villegas-Pérez MP,
Salvador-Silva M, Ruiz-Gómez JM and Vidal-Sanz M: Retinal ganglion
cell death after different transient periods of pressure-induced
ischemia and survival intervals. A quantitative in vivo study.
Invest Ophthalmol Vis Sci. 37:2002–2014. 1996.PubMed/NCBI
|
|
22
|
Biermann J, Lagrèze WA, Dimitriu C,
Stoykow C and Goebel U: Preconditioning with inhalative carbon
monoxide protects rat retinal ganglion cells from
ischemia/reperfusion injury. Invest Ophthalmol Vis Sci.
51:3784–3791. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hirooka K, Miyamoto O, Jinming P, Du Y,
Itano T, Baba T, Tokuda M and Shiraga F: Neuroprotective effects of
D-allose against retinal ischemia-reperfusion injury. Invest
Ophthalmol Vis Sci. 47:1653–1657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang W, Dobberfuhl A, Filippopoulos T,
Ingelsson M, Fileta JB, Poulin NR and Grosskreutz CL:
Transcriptional up-regulation and activation of initiating caspases
in experimental glaucoma. Am J Pathol. 167:673–681. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hegde ML, Mantha AK, Hazra TK, Bhakat KK,
Mitra S and Szczesny B: Oxidative genome damage and its repair:
implications in aging and neurodegenerative diseases. Mech Ageing
Dev. 133:157–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ozawa Y, Sasaki M, Takahashi N, Kamoshita
M, Miyake S and Tsubota K: Neuroprotective effects of lutein in the
retina. Curr Pharm Des. 18:51–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guidarelli A, Cerioni L, Tommasini I,
Brüne B and Cantoni O: A downstream role for protein kinase Calpha
in the cytosolic phospholipase A2-dependent protective signalling
mediated by peroxynitrite in U937 cells. Biochem Pharmacol.
69:1275–1286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ali TK, Matragoon S, Pillai BA, Liou GI
and El-Remessy AB: Peroxynitrite mediates retinal neurodegeneration
by inhibiting nerve growth factor survival signaling in
experimental and human diabetes. Diabetes. 57:889–898. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tuo J, Liu L, Poulsen HE, Weimann A,
Svendsen O and Loft S: Importance of guanine nitration and
hydroxylation in DNA in vitro and in vivo. Free Radic Biol Med.
29:147–155. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Whiteman M, Hong HS, Jenner A and
Halliwell B: Loss of oxidized and chlorinated bases in DNA treated
with reactive oxygen species: implications for assessment of
oxidative damage in vivo. Biochem Biophys Res Commun. 296:883–889.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haider L, Fischer MT, Frischer JM, Bauer
J, Höftberger R, Botond G, Esterbauer H, Binder CJ, Witztum JL and
Lassmann H: Oxidative damage in multiple sclerosis lesions. Brain.
134:1914–1924. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Werner SR, Prahalad AK, Yang J and Hock
JM: RECQL4-deficient cells are hypersensitive to oxidative
stress/damage: Insights for osteosarcoma prevalence and
heterogeneity in Rothmund-Thomson syndrome. Biochem Biophys Res
Commun. 345:403–409. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chaitanya GV, Steven AJ and Babu PP:
PARP-1 cleavage fragments: signatures of cell-death proteases in
neurodegeneration. Cell Commun Signal. 8:312010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dantzer F, de La Rubia G, Ménissier-de
Murcia J, Hostomsky Z, de Murcia G and Schreiber V: Base excision
repair is impaired in mammalian cells lacking Poly (ADP-ribose)
polymerase-1. Biochemistry. 39:7559–7569. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Harraz MM, Dawson TM and Dawson VL:
Advances in neuronal cell death 2007. Stroke. 39:286–288. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Y, Dawson VL and Dawson TM: Poly
(ADP-ribose) signals to mitochondrial AIF a key event in
parthanatos. Exp Neurol. 218:193–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Kim NS, Haince JF, Kang HC, David
KK, Andrabi SA, Poirier GG, Dawson VL and Dawson TM: Poly
(ADP-ribose) (PAR) binding to apoptosis-inducing factor is critical
for PAR polymerase-1-dependent cell death (parthanatos). Sci
Signal. 4:ra202011.
|
|
38
|
Kliem H, Berisha B, Meyer HH and Schams D:
Regulatory changes of apoptotic factors in the bovine corpus luteum
after induced luteolysis. Mol Reprod Dev. 76:220–230. 2009.
View Article : Google Scholar
|
|
39
|
Mei YP, Zhou JM, Wang Y, Huang H, Deng R,
Feng GK, Zeng YX and Zhu XF: Silencing of LMP1 induces cell cycle
arrest and enhances chemosensitivity through inhibition of AKT
signaling pathway in EBV-positive nasopharyngeal carcinoma cells.
Cell Cycle. 6:1379–1385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cardinal JS, Zhan J, Wang Y, Sugimoto R,
Tsung A, McCurry KR, Billiar TR and Nakao A: Oral hydrogen water
prevents chronic allograft nephropathy in rats. Kidney Int.
77:101–109. 2010. View Article : Google Scholar
|
|
41
|
Hanaoka T, Kamimura N, Yokota T, Takai S
and Ohta S: Molecular hydrogen protects chondrocytes from oxidative
stress and indirectly alters gene expressions through reducing
peroxynitrite derived from nitric oxide. Med Gas Res. 1:182011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oharazawa H, Igarashi T, Yokota T, Fujii
H, Suzuki H, Machide M, Takahashi H, Ohta S and Ohsawa I:
Protection of the retina by rapid diffusion of hydrogen:
administration of hydrogen-loaded eye drops in retinal
ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 51:487–492.
2010. View Article : Google Scholar
|
|
43
|
Li GY and Osborne NN: Oxidative-induced
apoptosis to an immortalized ganglion cell line is caspase
independent but involves the activation of poly (ADP-ribose)
polymerase and apoptosis-inducing factor. Brain Res. 1188:35–43.
2008. View Article : Google Scholar
|
|
44
|
Li N, He Y, Wang L, Mo C, Zhang J, Zhang
W, Li J, Liao Z, Tang X and Xiao H: D-galactose induces necroptotic
cell death in neuroblastoma cell lines. J Cell Biochem.
112:3834–3844. 2011. View Article : Google Scholar : PubMed/NCBI
|