Introduction
Mesenchymal stem cells (MSCs) are multipotent cells
which abundantly exist in adult bone marrow and adipose tissue
(1). MSCs are able to
differentiate into numerous lineages of cell types (2); therefore, they are widely used in
various fields, including rheumatology, orthopedic surgery,
gastroenterology, and transplant surgery (3). Beneficial effects of MSCs have also
been demonstrated in a variety of inflammatory disorders, including
systemic lupus erythematosis (4),
rheumatoid arthritis (5),
inflammatory bowel disease (6) and
graft-versus-host disease (7).
Recently, the role of MSCs in ameliorating the characteristics of
asthma has also been reported (8–10);
however, the exact mechanism of the inhibitory role of MSCs in
asthma remains to be elucidated.
Allergic asthma is a T helper type 2 (Th2)
lymphocyte-associated inflammatory airway disease characterized by
airway eosinophilia, goblet-cell hyperplasia, variable airway
obstruction and bronchial hyper-responsiveness (BHR) to
non-specific stimuli. A variety of cells are recruited into the
allergically inflamed lungs, including eosinophils, mast cells, T
lymphocytes and antigen-presenting dendritic cells (DCs) (11). DCs are the most important
antigen-presenting cells (APC) in the immune system, mainly
characterized by their capacity to induce primary immune responses
(12). In rats, allergen-primed
lung DCs induced allergen-specific Th2-mediated immunoglobulin
(Ig)G1 with little Th1-directed allergen-specific IgG2b generation
(13). Lung DCs are potent
regulators of Th2-biased responses to inhalant allergens (14). Pulmonary DCs reside near the
epithelium in an immature state, where they sample the airway lumen
and are specialized in antigen (Ag) uptake. Upon the triggering of
danger signals, DCs upregulate co-stimulatory molecules, such as
CD86, and migrate to the mediastinal lymph nodes (MLNs) (15). At arrival in the MLNs, DCs present
Ags to naive T cells and induce a polarized T-cell response
(15). It is known that the number
and maturation state of lung DCs is elevated during secondary
immune challenge with allergens and during chronic airway
inflammation (16–18). These studies show that maturation,
migration to draining lymph nodes and expression of co-stimulatory
molecules on DCs are all essential for the priming of T cells and
for the development of allergic airway inflammation. A number of
studies have focused on the influence of MSCs on DC function
(19,20), and demonstrated that MSCs disrupt
the three major functions that characterize the transition of DCs
from an immature state to a mature state, namely the upregulation
of antigen presentation and co-stimulatory molecule expression, the
ability to present defined antigens and the capacity of migration
(21).
Therefore, the present study aimed to investigate
whether transplantation of MSCs was able to suppress experimental
asthma, particularly focusing on how MSCs exert effects on DC
function.
Materials and methods
Experimental animals
Female BALB/c mice and ovalbumin (OVA)-T-cell
receptor (TCR) transgenic mice (DO11.10 BALB/c) (n= 6/group),
weighing 16–24 g, aged 6–8 weeks, were obtained from the Laboratory
Animal Center of Wuhan University (Wuhan, China). The mice were
housed in specific pathogen-free conditions, under a 12:12 h
light/dark cycle, with ad libitum access to water and a
standard laboratory diet. The mice were maintained at 18–29°C, with
40–70% relative humidity. Animal care and handling protocols were
in accordance with the Guide for the Care and Use of Laboratory
Animals (22). All experimental
procedures were approved by the Animal Care Committee of Wuhan
University (Wuhan, China).
Isolation and culture of bone
marrow-derived MSCs
The mice were sacrificed by cervical dislocation and
the femur and tibia were harvested and cleaned of all connective
tissue. The bones were then placed in ice-cold isolation medium,
which consisted of RPMI-1640 (Invitrogen Life Technologies,
Carlsbad, CA, USA), supplemented with 10% heat-inactivated fetal
bovine serum (Gibco Life Technologies, Carlsbad, CA, USA), 10%
(v/v) equine serum (HyClone, GE Healthcare, Logan, UT, USA), 1%
(v/v) penicillin/streptomycin (Gibco Life Technologies), and 1%
(v/v) L-glutamine (Gibco Life Technologies). The ends of the bones
were then cut to expose the marrow. The cells were flushed out with
isolation medium, using a 5 ml syringe with a 27-gauge needle
(Dakewei Biotechnology Co., Ltd., Shanghai, China). Cell clumps
were disaggregated using a 21-gauge needle and syringe, followed by
filtration through a 70 μm nylon mesh filter (Dakewei
Biotechnology Co., Ltd). The cells were then centrifuged at 600 × g
for 5 min, resuspended in 15 ml isolation medium, and cultured at
37°C and 5% CO2. After 24 h, non-adherent cells were
removed by washing with sterile PBS, and the isolation medium was
replaced. This process was repeated every 3–4 days for 28 days.
After 28 days, the cells were removed from the flask by mild
trypsinization. Passage 2 cells were seeded at low density (50
cells/cm2) and cultured in expansion medium [isolation
medium with RPMI-1640 replaced with α-minimum essential medium
(Gibco Life Technologies)]. Cells retained their differentiation
capacity (23).
Determination of MSC surface antigen
expression
A total of 1×105 cells were incubated
with 1:200 dilutions of fluorochrome-conjugated specific or isotype
control antibodies for 30 min at 4°C. Specific or isotype control
antibodies included: PE/Cy5-rat IgG2b, κ isotype control antibody
(Abcam, Cambridge, UK; cat.no. ab18538); PE-rat IgG2a, κ isotype
control antibody (BD Pharmingen; cat. no. 554689); PE-armenian
hamster IgG2, κ isotype control antibody (BD Pharmingen; cat. no.
550085); APC-armenian hamster IgG1, λ2 isotype control antibody
(BioLegend, San Diego, CA, USA; cat. no. 400912); FITC-Rat IgG2a, κ
isotype control antibody (Abcam; cat. no. ab18446); biotin-rat
IgG2a, κ isotype control antibody (Abcam; cat. no. ab18445). The
following antibodies were used: Biotin-conjugated anti-major
histocompatibility complex (MHC)I (cat. no. ab25240), fluorescein
isothiocyanate (FITC)-conjugated anti-Sca-1 (cat. no. ab25031),
phycoerythrin (PE)/Cy5-conjugated anti-CD44 (cat. no. ab25579),
FITC-conjugated anti-CD106 (cat. no. ab24853), FITC-conjugated
anti-CD45 (cat. no. ab24917), PE-conjugated anti-CD34 (cat. no.
ab23830), FITC-conjugated anti-CD117 (cat. no. ab24870), and
PE/Cy5-conjugated anti-CD11b (cat. no. ab25533) (Abcam); and
FITC-conjugated anti-MHCII (cat. no. 562009) and allophycocyanin
(APC)-conjugated anti-CD11c (cat. no. 561119) (BD Pharmingen, San
Diego, CA, USA). Fluorescence histograms were obtained by recording
2×104 cells/sample, at a flow rate of ~200
cells/event(s). Experiments were conducted using a FACSCalibur flow
cytometer, with CellQuest version 3.3 (BD Biosciences, San Jose,
CA, USA), and FlowJo version 6.4.7 (FlowJo, LLC, Ashton, OR,
USA).
All MSCs were MHCI+, Sca-1+,
CD44low, CD106low, MHCII−,
CD11b−, CD11c−, CD34−,
CD45− and CD117−. MSCs were used between
passages 3 and 10 and rigorous purification and quality control
were performed to ensure MSC purity as previously described
(24).
OVA sensitization and airway
challenge
BALB/c mice (n=6–8 per group) were immunized on days
0 and 7 by intraperitoneal (i.p.) injection of OVA/alum (10
μg OVA emulsified in 1 mg aluminium hydroxide; grade V;
Sigma-Aldrich, St Louis, MO, USA) and were exposed to OVA aerosol
challenges (grade III) on days 17–19; aerosol challenges were
generated from a jet nebulizer delivering 1% OVA in
phosphate-buffered saline (PBS) for 30 min. To examine the effect
of MSC transplantation, 24 h prior to the first OVA aerosol
challenge, a number of the mice received an intravenous injection
of 106 MSCs. The positive control mice received PBS
instead of MSCs. The negative control mice were neither sensitized
with OVA nor given MSC treatment prior to OVA aerosol challenges.
Twenty-four hours after the last OVA exposure, bronchoalveolar
lavage fluid (BALF) was collected with 1-ml washes of
Ca2+- and Mg2+-free Hank’s balanced salt
solution (HBSS; Invitrogen Life Technologies) supplemented with 0.1
mM EDTA, and slides were prepared with Cytospin III (Shandon
Scientific, Pittsburgh, PA, USA) and stained with May-Giemsa
(Sigma-Aldrich) for determination of cell counts. Immediately after
the collection of BALF, lungs were resected and stored in optimum
cutting temperature freezing medium. In certain experiments, MLNs
and lungs were digested with collage-nase/DNAse (Sigma-Aldrich) as
previously described (25).
Mouse model of asthma induced by
endogenous myeloid airway DCs
Mice received 3 i.p. injections of the plasmacytoid
(p)DC-depleting antibody (Ab) Gr-1 (RB6-8C5) to deplete tolerogenic
plasmacytoid DCs (pDCs) on days 1, 0, and 1 as described previously
(26). On day 0, 800 μg OVA
(low in lipopolysaccharide; Worthington Biochemicals, Lakewood, NJ,
USA) was injected intrathecally (i.t.) with or without venous
injection of 106 MSCs. 10 days later, mice were exposed
to OVA aerosols for 30 min on 3 consecutive days and sacrificed 24
h after the last dose. Briefly, the mice were anesthetized with
Avertin (2% v/v in PBS; Dakewei Biotechnology Co., Ltd.) and were
sacrificed by cervical dislocation. BALF, MLNs and lung tissues
were obtained from the mice for further experiments.
Induction of a mouse model of asthma by
adoptive transfer of BMDCs
A model in which sensitization to inhaled OVA was
induced by i.t. injection of OVA-pulsed bone marrow-derived mDCs
has been previously developed (27). Briefly, bone marrow was flushed
with RPMI 1640 from femurs and tibiae of BALB/c mice. Cells were
washed, counted and plated in bacteriological 100-mm-diameter Petri
dishes. The cell-culture medium used was RPMI 1640 supplemented
with gentamicin (60 μg/ml; Invitrogen Life Technologies),
2-mercaptoethanol (5×10−5 mol/l; Invitrogen Life
Technologies) and 5% fetal calf serum (HyClone). At day 0 of
culture, the cells were seeded at a concentration of
2×106/dish in medium containing recombinant murine
granulocyte macrophage colony-stimulating factor (GM-CSF; 200
IU/ml; Biolegend, San Diego, CA, USA). On days 3, 6 and 8, the
medium was refreshed and GM-CSF was added. More than 90% of the
cells were myeloid (m) DCs. On day 9 of culture, cells were pulsed
overnight with 100 μg/ml OVA (Worthington Biochemicals)
(indicated as OVA-DCs). As a control, DCs were incubated with PBS
(indicated as PBS-DCs). Prior to their transfer, a number of DCs
were co-cultured with MSCs, while they were pulsed with OVA
(indicated as MSC-OVA-DCs). After antigen pulsing, non-adherent DCs
were collected, washed 3 times with PBS to remove free OVA and
re-suspended in PBS at a concentration of 12.5×106
cells/ml. For in vivo experiments, on day 0 under
anesthesia, BALB/c mice were instilled through the trachea with
1×106 PBS-treated DCs (PBS-DCs), OVA-pulsed DCs
(OVA-DCs) or MSCs-treated OVA-DCs (MSC-OVA-DCs) as described
previously (27). Ten days after
DC transfer, mice were exposed to a 30-min OVA aerosol once per day
for 3 consecutive days and sacrificed 24 h after the last
challenge.
Flow cytometry and cell sorting
For determination of the DC number in the MLNs, MLN
cells were stained for DCs [FITC-labeled anti-MHCII (cat. no.
ab93561; Abcam), APC-labeled anti-CD11c). The absolute cell number
was calculated by multiplying the total leukocyte number by the
percentage of each population of interest. For analysis of DC
maturation, bone marrow, lung or MLN cell suspensions were stained
with FITC-labeled anti-I-Ad/I-Ed; phycoerythrin PE-labeled
anti-CD40 (cat. no. 553791), anti-CD80 (cat. no. 553769) and
anti-CD86 (cat. no. 553692); and APC-labeled anti-CD11c (cat. no.
561119) Abs (BD Pharmingen). To address migration of lung DCs
(25), 80 μl FITC-OVA (10
mg/ml) was administered i.t., with or without venous injection of
106 MSCs. Control mice received 80 μl PBS. After
24 h, migrating DCs were counted in the MLNs as
CD11c+MHCII+ cells carrying FITC+
material. In all experiments, dead cells and debris were excluded
using propidium iodide (Abcam). Analysis was performed on a
FacsCalibur flow cytometer using CellQuest version 3.3 and FlowJo
version 6.4.7 software.
Evaluation of BHR
Assessment of BHR was undertaken 24 h after the last
OVA inhalation using a single-chamber, barometric whole-body
plethysmograph (Buxco Electronics, Troy, NY, USA) as previously
described (28). Briefly,
conscious mice were placed in the main chamber and baseline
readings were taken and averaged for 3 min. Aerosolized normal
saline or acetyl-β-methycholine chloride (MCh; Sigma-Aldrich) at
increasing concentrations (1.5625, 3.125, 6.25, 12.5, and 25 mg/ml)
were nebulized through an inlet of the main chamber for 3 min.
Recordings were taken and averaged for 3 min after each
nebulization. The average Heuristic parameter (PenH) values were
expressed for each methacholine concentration as the percentage
increase over baseline PenH values.
Activation of OVA-specific naive and
effector T cells by mDCs
PBS-treated DCs (PBS-DCs), OVA-pulsed DCs (OVA-DCs)
or MSC-treated OVA-DCs (MSC-OVA-DCs) were collected and co-cultured
with OVA-specific naive CD4+ T cells, which were
purified from DO11.10 TCR transgenic mice. In the co-culture
system, the ratio of DCs to CD4+ T cells from DO11.10
TCR transgenic mice r was 1×104:1×105. In
separate experiments, naive DO11.10 T cells were first
differentiated for seven days into effector Th2 cells in the
presence of interleukin (IL)-4, anti-interferon (IFN)-γ and
anti-IL-12 as previously described (29). After washing, these effector Th2
cells were stimulated with DCs as for naive T cells. After 4 days,
supernatants were harvested and the concentration of IFN-γ, IL-4,
IL-5 and IL-13 cytokines were assayed by specific mouse ELISA kits
(Abcam). In separate wells, proliferation was measured by pulsing
with [3H]thymidine (1 μCi/well; GE Healthcare
Life Sciences, Little Chalfont, UK) for the last 16 h of culture.
Cells were then harvested onto glass filters by an automated
multi-sample harvester and counted with a dry scintillation counter
(Perkin-Elmer, Waltham, MA, USA) and results are presented in cpm
(mean ± standard error of the mean) of triplicate cultures.
Analysis of thymus and activation
regulated chemokine (TARC)/chemokine (C-C motif) ligand 17 (CCL17)
and macrophage-derived chemokine (MDC)/CCL22 produced by DCs
At day 9 of culture, DCs were collected and seeded
at 6×105 cells/ml in 24-well cultures (Nunc, Rorsklide,
Denmark) and either not stimulated or pulsed overnight with 100
μg/ml OVA in the presence or absence of MSCs
(2×105/ml). Following 24 h, culture supernatants were
centrifuged and collected for analysis of TARC/CCL17 and MDC/CCL22
by specific mouse ELISA kits (Abcam).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. All experimental data were analyzed by SPSS 18.0
(International Business Machines, Armonk, NY, USA). Statistical
analysis was performed using one-way analysis of variance followed
by Dunnett’s post hoc test. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
MSC transplantation reduces the cardinal
features of asthma
Asthma is a chronic pulmonary inflammatory disease
characterized by airway and tissue infiltration of eosinophils and
other cell types (11). To
evaluate the immunoregulatory role of MSCs on pulmonary
inflammation, a murine model was generated in which mice were
sensitized using i.p. injection of OVA in an alum solution as a Th2
adjuvant. 10 days later, these mice were challenged by exposure to
OVA aerosols three times on three consecutive days. After the last
challenge, BALF was collected and the various cell types were
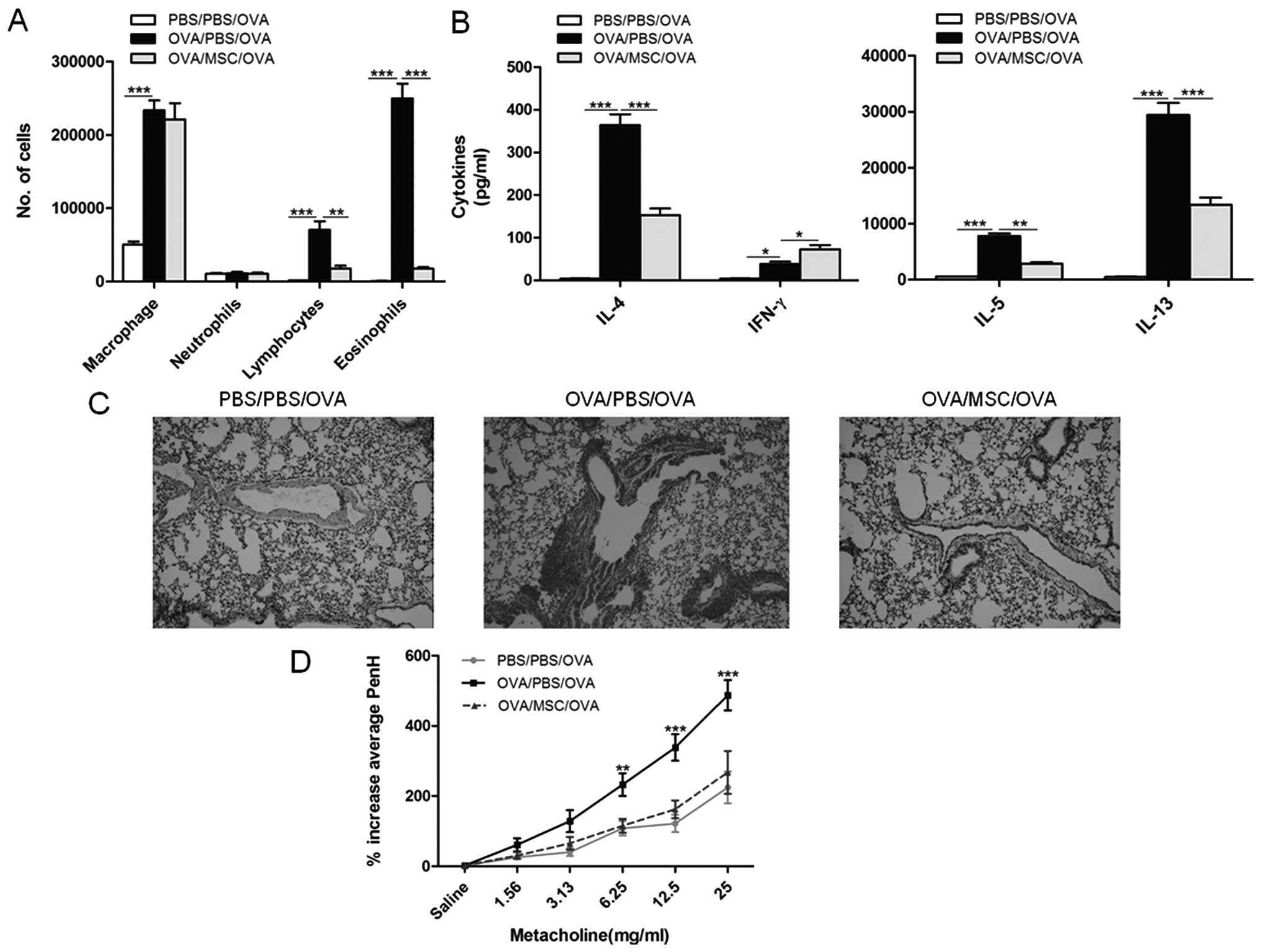
counted. As expected, upon OVA aerosol challenge, OVA-sensitized
mice showed a marked elevation of macrophages, lymphocytes and
eosinophils in BALF compared with those in PBS-sensitized mice;
however, in the treatment group, MSC transplantation significantly
suppressed eosinophil infiltration (Fig. 1A). Cytokine production was also
compared between groups in MLN cell cultures (Fig. 1B). Significantly reduced production
of IL-4, IL-5 and IL-13 and a minor increase of IFN-γ were found in
the MLN cell cultures in the MSC-treated group compared with that
in the OVA-challenged group. Cellular tissue infiltration in lung
sections in different groups of mice was also assessed. As shown in
Fig. 1B, OVA challenge induced
marked perivascular and peribronchial inflammatory cell
infiltration, and MSC transplantation significantly reduced
OVA-induced eosinophilrich leucocyte infiltration and mucus
hypersecretion into the airways. Increased airway responsiveness
and sensitivity to non-specific stimulation is a major pathological
characteristic of asthma. As shown in Fig. 1C, exposure of OVA-sensitized mice
to aerosolized allergen resulted in BHR to inhaled MCh compared
with that of sham-sensitized mice, but administration of MSCs
strikingly attenuated the MCh-induced airflow obstruction. These
results demonstrated that MSC transplantation reduced the cadinal
features of asthma, including pulmonary inflammation, airway
remodelling and bronchial hyperresponsiveness.
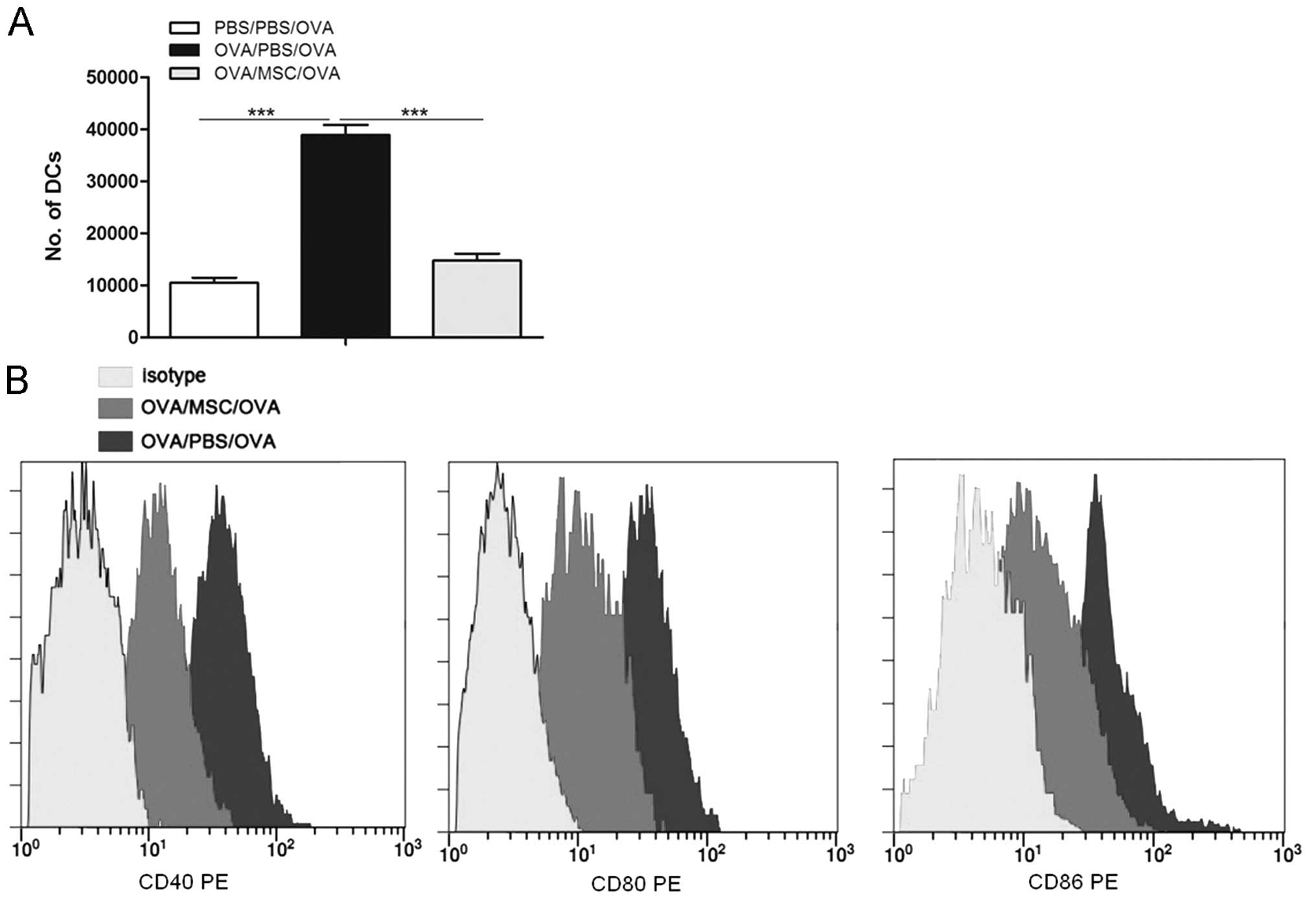
MSC transplantation reduces the presence
of DCs in MLN and suppresses DC maturation
As administration of MSCs prior to allergen
challenge abolished the characteristics of asthma, it was
hypothesized that this response may result from direct alteration
of DC function. The total number of DCs
(MHCIIhighCD11chigh) in MLNs was determined
24 h after the last OVA challenge. As shown in Fig. 2A, in OVA-sensitized mice, OVA
challenge led to an increase of DCs in the MLNs compared with those
in sham-sensitized mice. Of note, intravenous injection of MSCs
prior to OVA challenge markedly reduced this increase (Fig. 2A).
The maturation status of DCs is associated with
their capacity to initiate immune responses. Next, it was
investigated whether MSC transplantation affected lung DC
maturation. As shown in Fig. 2B,
transplantation of MSC markedly reduced the expression of the
co-stimulatory molecules CD40, CD80 and CD86 on
CD11c+MHCII+ lung DCs from
allergen-challenged mice.
MSC transplantation reduces lung DC
migration to the MLNs
The present study examined whether MSC
transplantation was able to reduce the migratory ability of DCs.
Mice received i.t. injection of OVA-FITC 24 h after instillation,
and FITC+ DCs (CD11c+ and MHC-II+
cells) in the MLNs were counted. As shown in Fig. 3A, MSC transplantation significantly
inhibited the migration of lung DCs to the MLNs, and significantly
more FITC+ DCs were retained in the lungs after MSC
treatment (Fig. 3B).
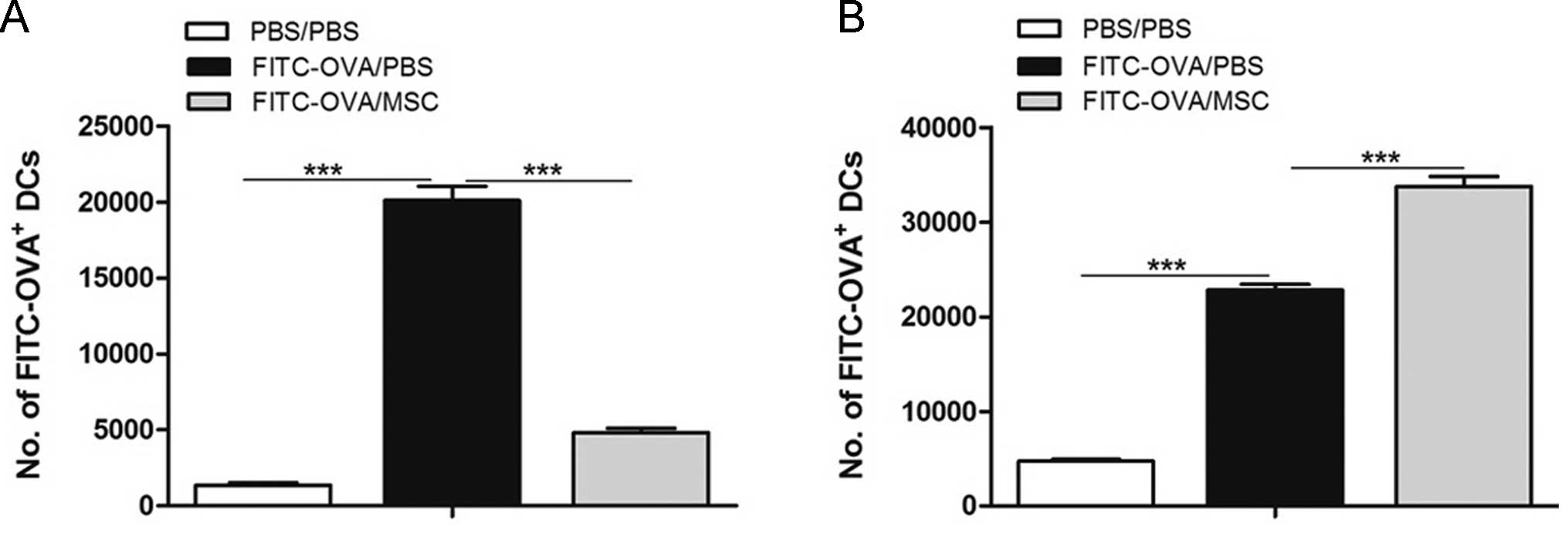 | Figure 3MSCs suppress lung DC migration to
the MLNs. (A) On day 0, naive mice were instilled intrathecally
with FITC-OVA with or without tail intravenous injection of MSCs.
On day 1, the presence of FITC+ migrating DCs in MLNs
was analyzed by flow cytometry. (B) In the same mice, lung DCs were
also counted. Lungs were enzymatically digested and stained for the
presence of FITC+MHCII+CD11c+ DCs.
Values are expressed as thee mean ± standard error of the mean
(n=8). ***P<0.001. FITC, fluorescein isothiocyanate;
DC, dendritic cell; OVA, ovalbumin; PBS, phosphate-buffered saline;
MSC, mesenchymal stem cell; MHC, major histocompatibility complex;
MLN, mediastinal lymph node. |
Effect of MSCs on Th2 priming induced by
lung mDCs
To elucidate whether MSC administration is able to
suppress the function of lung DCs in vivo, a model was used
in which sensitization to OVA occurs via the airways by endogenous
mDCs (30). In the preceding
experiments, mice were depleted of pDCs with anti-Gr-1 Abs and then
primed with a single injection of 800 mg of LPS-low OVA. In the
anti-Gr-1-treated group, but not in the isotype-treated control
group, OVA inhalation led to the development of cardinal features
of asthma, as shown by eosinophilia in the BALF (Fig. 4A), goblet cell hyperplasia in the
lung (Fig. 4B) and Th2 cytokine
production in the MLNs (Fig. 4C).
However, mice depleted of pDCs and receiving MSC transplantation at
the time of OVA priming did not develop any signs of airway
inflammation and lymph node cytokine production was reduced in
these animals.
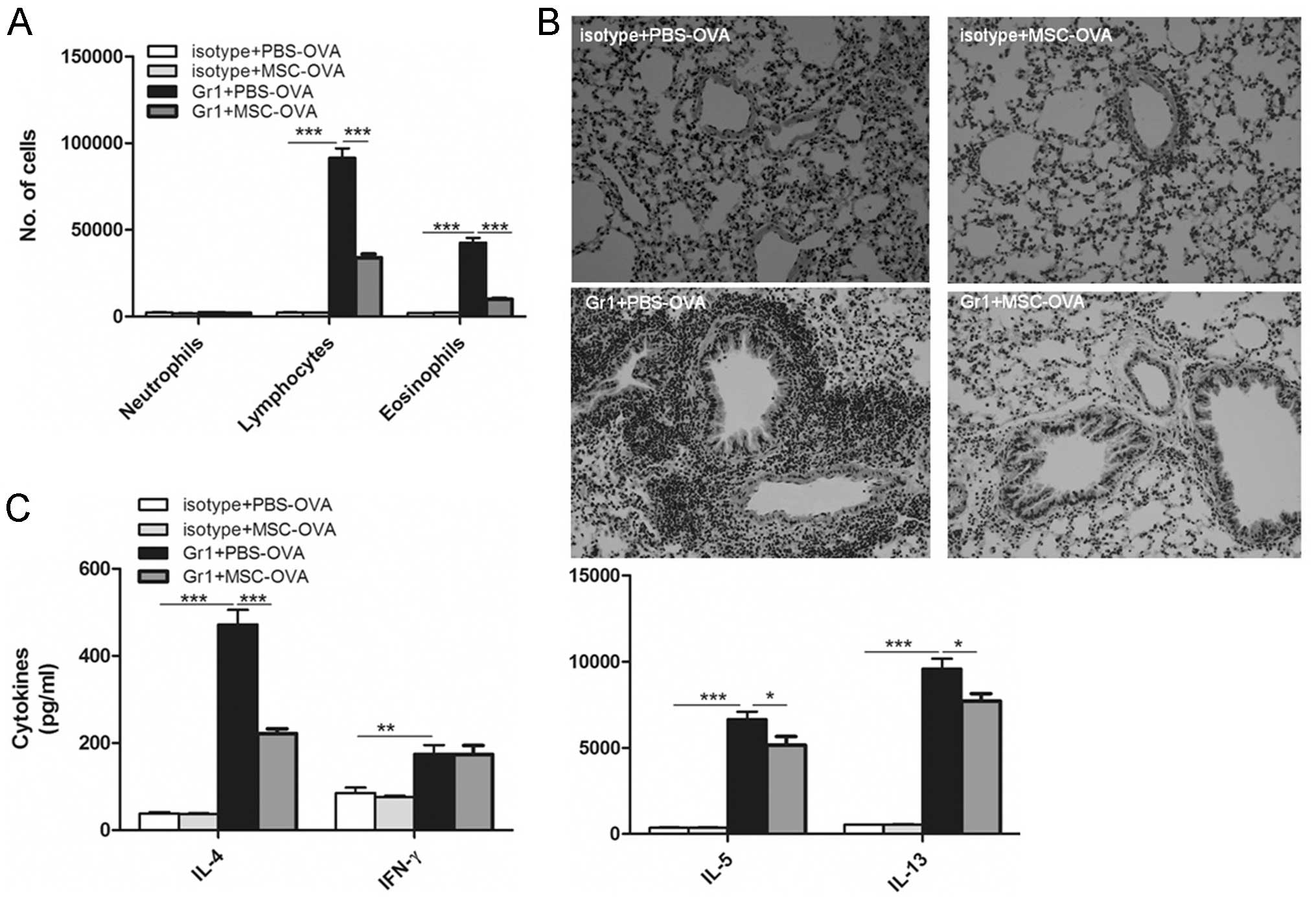 | Figure 4Transplantation of MSCs prevents
sensitization induced by DCs. On day 0, mice received an
intrathecal injection of OVA with or without intravenous injection
of MSCs. From days 1 to 2, mice were injected intraperitoneally
with anti-Gr-1 antibodies to deplete plasmacytoid DCs or isotype
control antibodies. Ten days later, mice were exposed to OVA
aerosols three times on three consecutive days. (A) Bronchoalveolar
lavage fluid was analyzed by flow cytometry. (B) Hematoxylin and
eosin staining of lung sections (magnification, x100). (C)
Mediastinal lymph node cells were re-stimulated in vitro for
4 days with OVA, and cytokines were measured in the supernatant.
Values are expressed as the mean ± standard error of the mean
(n=8). *P<0.05; **P<0.01;
***P<0.001. OVA, ovalbumin; PBS, phosphate-buffered
saline; MSC, mesenchymal stem cell; DC, dendritic cell; IL,
interleukin; IFN, interferon. |
MSC reduces the potential of mDCs to
induce Th2 development in vivo
MSC transplantation resulted in reduced allergic
sensitization and may have resulted from direct or indirect
influence on DCs to prime Th2 differentiation in vivo. To
rule out any indirect effects, mDCs were pre-treated with MSCs
prior to their i.t. transfer into naive mice. As previously
reported, adoptive i.t. transfer of OVA-pulsed mDCs led to Th2
priming and subsequent development of features of asthma upon OVA
aerosol challenge 10 days later, a function associated with the
number of DCs injected (27). As
expected, mice receiving unpulsed DCs showed a lower amount of
inflammatory cells accumulated in the BALF (Fig. 5A) and lung tissue (Fig. 5B) compared with mice that had
received pulsed DCs. By contrast, mice that had received OVA-pulsed
DCs showed asthmatic inflammation characterized by marked cellular
infiltration of lymphocytes and eosinophils in the BALF (Fig. 5A) as well as in the peribronchial
and perivascular area (Fig. 5B).
Pre-treatment of OVA-pulsed mDCs with MSCs significantly reduced
the capacity of these cells to induce eosinophilic airway
inflammation and goblet cell hyperplasia in mice (Fig. 5B). This was accompanied by a
significant decrease in IL-4, IL-5 and IL-13 levels in the MLNs,
while the concentration of IFN-γ was not significantly changed
(Fig. 5C).
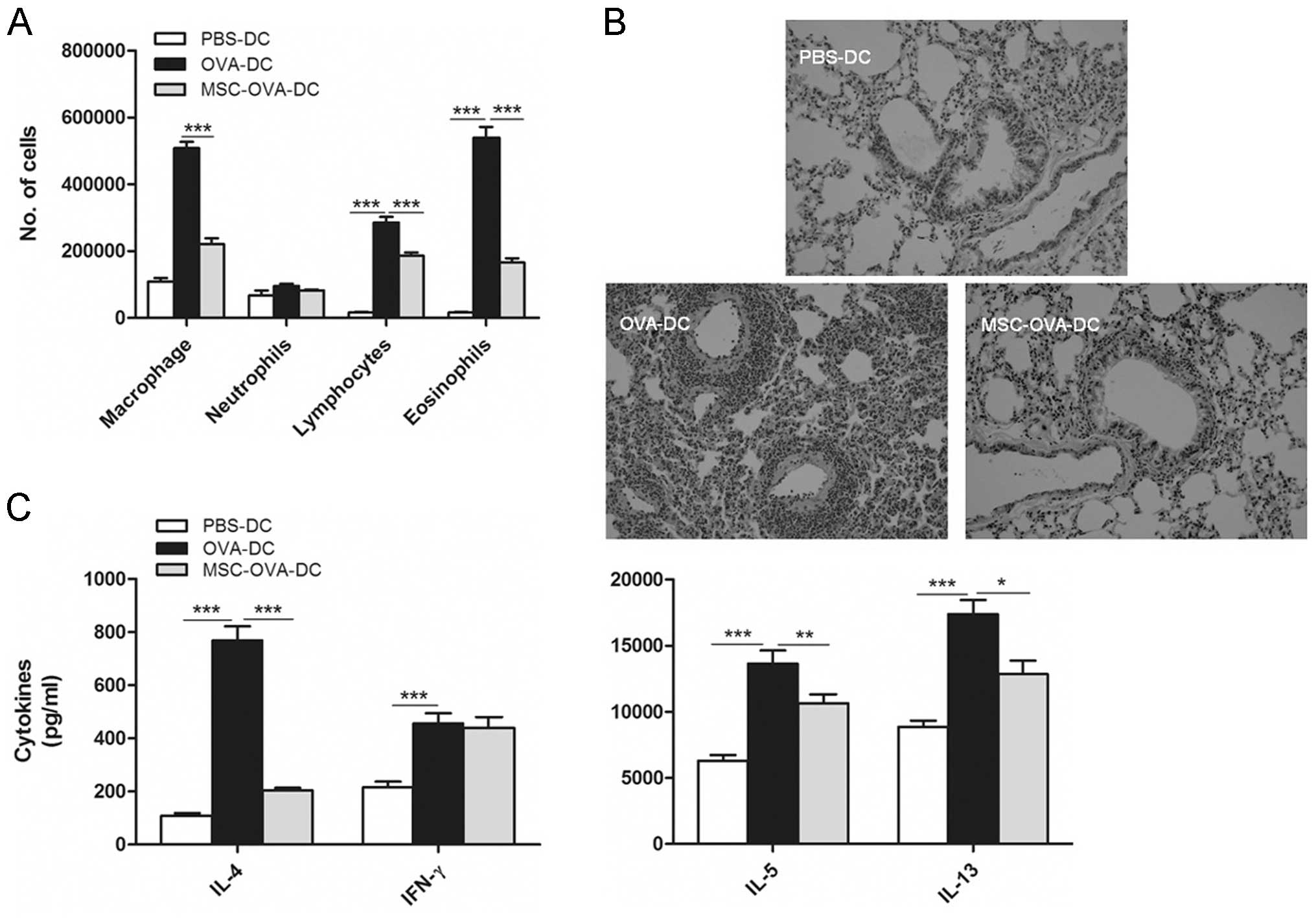 | Figure 5MSC treatment inhibits the potential
of DCs to induce asthmatic inflammation. (A and B) On day 0, mice
received an intrathecal injection of un-pulsed DCs (PBS-DC),
OVA-pulsed DCs (OVA-DC) or MSC-treated OVA-DCs (MSC-OVA-DC). From
days 10–13, all mice were exposed to OVA aerosols. (A)
Bronchoalveolar lavage fluid was analyzed by flow cytometry. (B)
Hematoxylin and eosin staining of lung sections (magnification,
×100). (C) MLN cells were re-stimulated in vitro for 4 days
with OVA, and cytokines were measured using ELISA. Values are
expressed as the mean ± standard error of the mean (n=8).
*P<0.05; **P<0.01;
***P<0.001. OVA, ovalbumin; PBS, phosphate-buffered
saline; MSC, mesenchymal stem cell; DC, dendritic cell; IL,
interleukin; IFN, interferon. |
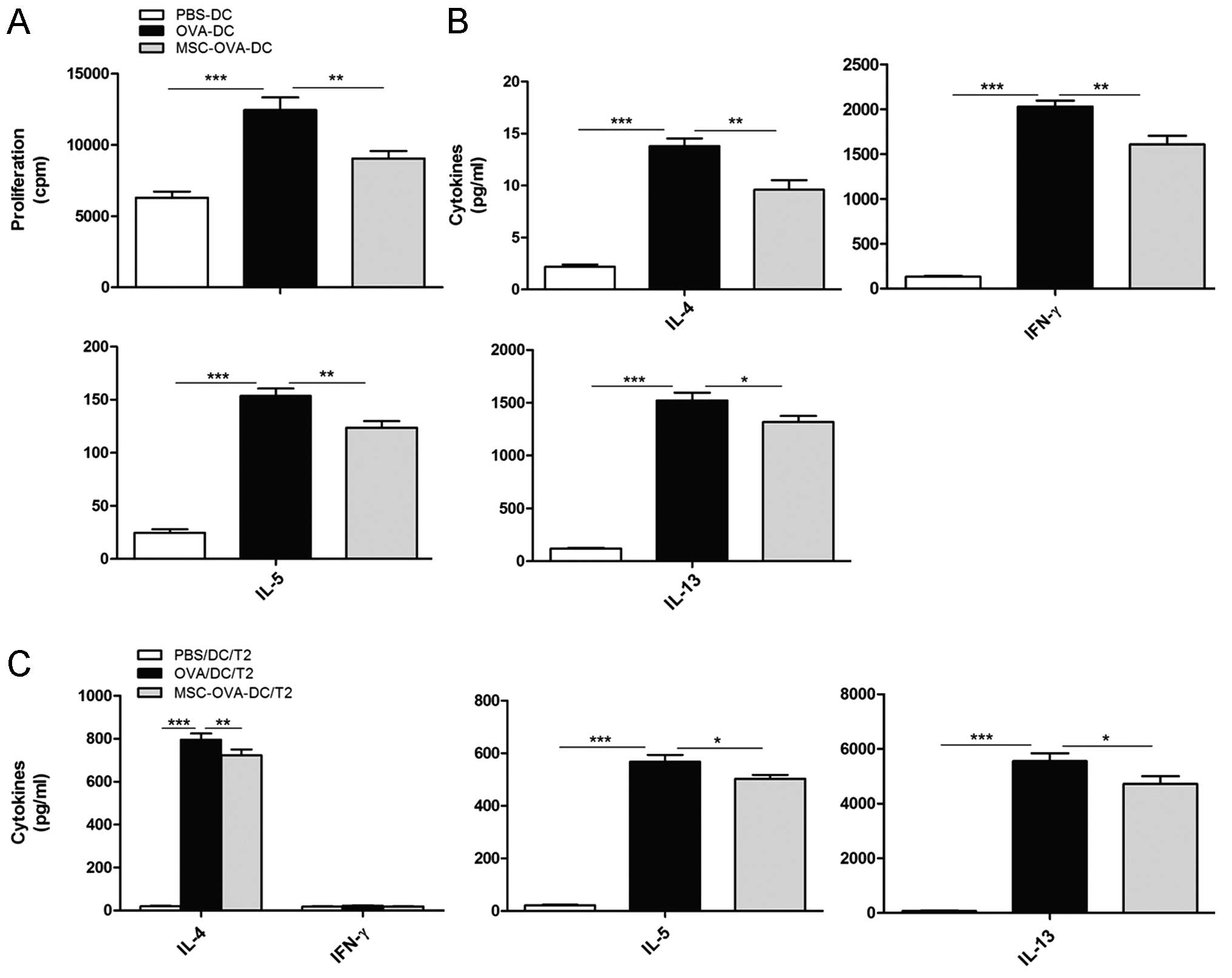
Effect of in vitro MSC treatment on the
capacity of DCs to activate and polarize Ag-specific T cells in
vitro
As MSC treatment profoundly impaired the migration
of DCs to MLNs, it was next investigated whether this could impact
the potential of DCs to activate naive T cells. To this point, the
effect of MSCs on DC-driven OVA-specific T-cell (DO11.10)
proliferation and cytokine production had been tested in
vitro. As shown in Fig. 6A,
MSC-treated OVA-DCs (MSC-OVA-DCs) induced the proliferation of
OVA-specific naive T cells to a lesser extent than OVA-pulsed DCs
(OVA-DCs). Moreover, T cells stimulated by MSC-OVA-DCs produced
lower levels of Th2 cytokines than T cells stimulated with OVA-DCs
(Fig. 6B).
In a separate experiment, MSC-OVA-DCs were
co-cultured with in vitro-differentiated DO11.10 Th2 cells,
which were obtained by stimulating MLN cells with OVA in the
presence of IL-4, anti-IFN-γ and anti-IL-12 (29). As shown in Fig. 6C, pre-treatment of bone
marrow-derived MSCs with MSCs markedly reduced the production of
Th2 cytokines by stimulating effector Th2 cells.
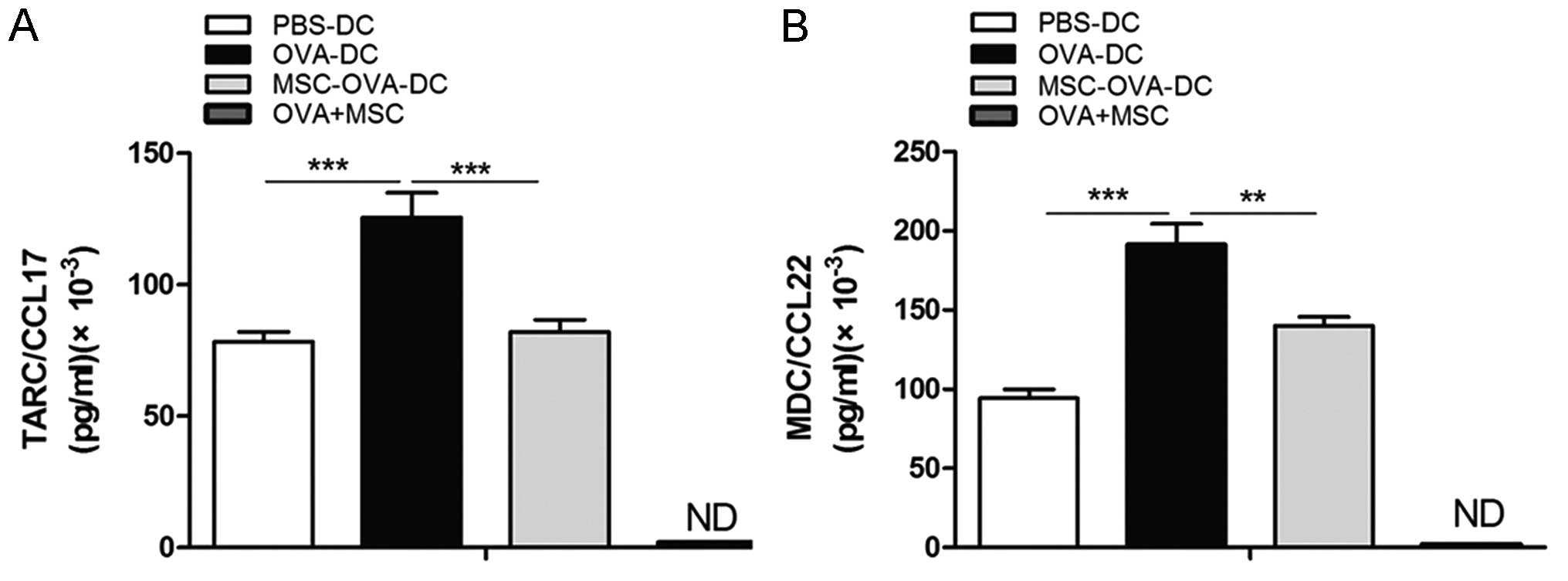
MSCs suppress the production of
TARC/CCL17 and MDC/CCL22 by DC
To investigate the effects of MSCs on TARC/CCL17 and
MDC/CCL22 production by DCs, supernatants of non-pulsed-DCs
(PBS-DCs), DCs pulsed with OVA in the presence or absence of MSCs
(OVA-DCs and MSC-OVA-DCs, respectively) and OVA-pulsed MSCs
(OVA+MSCs) were collected, and the concentrations of TARC/CCL17 and
MDC/CCL22 were determined by ELISA. As shown in Fig. 7, DCs, upon stimulation with OVA,
produced large quantities of TARC/CCL17 and MDC/CCL22 compared with
un-pulsed DCs; however, this upregulation was signifi-cantly
inhibited by MSCs. In addition, it was found that when MSCs were
cultured independently and stimulated with OVA, TARC/CCL17 and
MDC/CCL22 levels were undetectably low. This indicated that MSCs
were able to reduce the TARC/CCL17 and MDC/CCL22 production by DCs,
but MSCs could not produce any TARC/CCL17 and MDC/CCL22.
Discussion
The present study, clearly demonstrated the immune
regulatory role of MSCs. To date, the role of MSCs in
immune-mediated disorders such as asthma has not been elucidated.
The present study clarified that MSCs potently suppressed
Ag-induced immune responses through inhibition of DCs.
Given their vast proliferative potential,
immunosuppressive properties and the ease of access to available
tissue sources, therapies with autologous or allogeneic MSCs have
been tested in a variety of immune-mediated disease models,
including experimental allergic encephalomyelitis (31), diabetic NOD/scid mice (32), collagen-induced arthritis (33) and several murine models of lupus
(34). The role of MSCs in
treating asthma has gained significant interest. In a study by
Kapoor et al (35) MSCs
suppressed the proliferation of dust mite (DM)-challenged
peripheral blood mononuclear cells (PBMCs) from allergic asthmatic
subjects but not from allergic subjects without asthma. Repeated
exposure to low-dose DMs and MSCs, as well as pre-conditioning of
PBMCs with MSCs, caused refractoriness to DMs. Firinci et al
(36) reported that MSCs migrated
to lung tissue and ameliorated bronchial asthma in a murine model.
In an experimental toluene diisocyanate (TDI)-induced animal model
of severe asthma, MSC transfer significantly reduced the
TDI-induced increase in the inflammatory index as well as numbers
of eosinophils and neutrophils in BALF; the MSC transfer also
significantly reduced the number of goblet cells, collagen
deposition and immune staining for smooth muscle actin and
proliferating cell nuclear antigen with concomitant normalization
of the airway response to MCh. In the present study, it was
demonstrated that transplantation of MSCs during the challenge
phase strongly reduced the cardinal features of asthma, including
eosinophilic inflammaion, goblet cell hyperplasia, Th2 cytokine
production and, most importantly, BHR to inhaled MCh. Studies have
reported that intravenously introduced human MSCs localize in the
lung prior to dispersing into the peripheral tissues and seemingly
homing to injured tissue (37,38).
However, how MSCs perform out their ’regulatory’ role in asthma
remains to be elucidated.
It has known that pulmonary DCs are crucial
mediators in regulating immune responses in the lung and that these
DCs bridge the innate and adaptive immune response. In asthma, for
example, DCs are important in the induction and maintenance of the
disease (15). Depletion of airway
DCs during secondary challenge in sensitized mice abolished all
cardinal features of asthma (including airway eosinophilia, goblet
cell hyperplasia and BHR to MCh), an effect that was completely
restored by adoptive transfer of wild-type DCs (29). DCs are crucial as they can locally
activate Th2 effector cells in the airway wall by providing
chemotactic cues for Th2 cells (CCL17 and CCL22) and by delivering
MHC and co-stimulatory signals (39–42).
In addition, at times of allergen challenge, DCs migrate from the
site of allergic inflammation to MLNs to stimulate new rounds of
division in re-circulating central memory cells or from naive T
cells, thus feeding the inflammatory response with new waves of
effector cells (18,43–45).
The present study found that transplantation of MSCs decreased the
number of mDCs in MLNs of allergen-challenged mice. Although a
reduction in lung inflammation may have been responsible for
reduced input of immigrating lung-derived DCs in MLNs, it was also
observed that MSC transplantation suppressed the migration of DCs
fluorescently labeled with OVA to MLNs in naive mice, concomitant
with an accumulation of DCs in the lung compartment. A previous
in vitro study demonstrated that DCs matured in the presence
of MSCs and showed significantly reduced migration to MLNs;
furthermore, MSCs prevented loss of expression of the
tissue-anchoring protein E-cadherin (21), which was downregulated when DCs
migrated to the local lymph node. As the level of maturation is the
hallmark of DC activity and is critical for priming naive T-cell
responses, the present study assessed the maturation status of
pulmonary DCs upon encountering a soluble allergen. The results
showed that pulmonary DCs from OVA-sensitized and -challenged mice
treated with MSCs showed reduced expression of CD40, CD80 and CD86
compared with those in the PBS-treated asthma group. It was
recently shown that CD80/CD86 co-stimulation on DCs is necessary
for differentiation of Th2 cells from naive T cells and for
re-stimulation of effector Th2 cells in the lung (40,46,47).
Thus, in the present study, MSC transplantation hampered DC
maturation, leading to the reduced capacity of priming the naive
T-cell response with concomitant reduced airway inflammation.
In pulmonary tissue, various DC subsets can be
found, based on anatomical location and function (48,49):
Classic/myeloid (c)DCs, subdivided into CD11b+ and
CD11b-cDCs and pDCs. CD11b-cDCs are located adjacent to the
epithelium and extend their dendrites between epithelial cells to
sample the airway lumen (50),
whereas CD11b+cDCs are located underneath the epithelium
and pick up Ags that have passed the basal membrane.
CD11b−cDCs were shown to be prolific producers of
inflammatory chemokines, and in allergen-challenged mice,
CD11b−cDCs were shown to produce the highest amounts of
Th2 cell-attracting chemokines (51). cDCs, however, are important in
inducing sensitization, while pDCs are involved in the induction of
immune tolerance; depletion of pDCs during exposure to harmless Ags
was previously shown to induce sensitization (26). Inhalation of LPS-low OVA is
normally a tolerogenic event, in which pDCs inhibit the potential
of mDCs to prime effector Th2 cells (39). In the present study, pDCs were
depleted, which abolished this tolerogenic response and led to a
robust Th2 priming by mDCs. Transplantation of MSCs reduced the
development of the Th2 immune response, and consequently, asthma
did not develop upon repeated OVA challenge.
The effects of MSCs on DCs may be direct or
indirect. In support of the direct effects of MSCs on DCs, the
present study found that in vitro pre-treatment of
OVA-pulsed mDCs with MSCs prior to transfer to the airways
significantly reduced their potential to induce Th2 priming, and
consequently, asthmatic inflammation did not develop. The in
vitro study also indicated that when DCs were pre-treated with
MSCs, they were defective in priming naive and memory T cells due
to an intrinsic defect in T-cell stimulatory capacity. When
OVA-pulsed bone marrow-derived MSCs were co-cultured with
OVA-specific T cells from naive mice, reduced proliferation and
production of Th1 and Th2 effector cytokines were observed.
Furthermore, pre-treatment of DCs with MSCs inhibited their
potential to stimulate primed effector Th2 cells generated under
polarized Th2 conditions in vitro. Similar effects were also
reported by English et al (21), who used an antigen-specific T-cell
proliferation assay and specific peptide:MHCII antigen display to
demonstrate that MSCs interfered with the antigen presentation
ability of DCs.
Previous studies showed that TARC/CCL17 and
MDC/CCL22 induce the selective migration of Th2 cells but not Th1
cells through triggering of CCR4 (52), and the BALF levels of TARC/CCL17
and MDC/CCL22 but not Th1-selective chemokines are increased upon
allergen challenge to the lung (53,54).
Furthermore, in vitro house DM exposure of DCs from house
DM-allergic patients but not that of healthy controls caused CCL17
and CCL22 release resulting in chemoattraction of polarized human
Th2 cells in a CCR4-dependent manner (55). The present in vitro study
also demonstrated that when DCs were co-cultured with MSCs, their
capacity of producing CCL17 and CCL22 was significantly suppressed.
This may explain why in the murine model, transplantation of MSCs
suppressed CCL17 and CCL22 production by DCs, leading to reduced
recruitment of Th2 cells and the concomitantly reduced level of
airway inflammation.
In conclusion, the present study demonstrated that
MSCs inhibit Th2-mediated cardinal features of asthma by altering
the function of lung DCs, including the state of maturation,
capacity of migration, ability of antigen presentation and the
capacity of chemokine production. MSCs may be applicable as novel
therapeutics for the treatment of airway remodeling and
inflammation associated with chronic asthma.
Acknowledgments
The authors would like to thank Mr. Bo-quan Pan
(Biomedical Research Center, Wuhan University) and Mrs. Shu-fang
Guo (Institute of Virology, Wuhan University Medical College) for
their skillful assistance. This study was supported by the National
Natural Science Foundation of China (no. 30871122, to Jiong
Yang).
References
|
1
|
Rasmusson I: Immune modulation by
mesenchymal stem cells. Exp Cell Res. 312:2169–2179. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uccelli A, Moretta L and Pistoia V:
Mesenchymal stem cells in health and disease. Nat Rev Immunol.
8:726–736. 2008. View
Article : Google Scholar
|
|
3
|
Prockop DJ, Kota DJ, Bazhanov N and Reger
RL: Evolving paradigms for repair of tissues by adult
stem/progenitor cells (MSCs). J Cell Mol Med. 14:2190–2199. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liang J, Gu F, Wang H, et al: Mesenchymal
stem cell transplantation for diffuse alveolar hemorrhage in SLE.
Nat Rev Rheumatol. 6:486–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gonzalez-Rey E, Gonzalez MA, Varela N, et
al: Human adipose-derived mesenchymal stem cells reduce
inflammatory and T cell responses and induce regulatory T cells in
vitro in rheumatoid arthritis. Ann Rheum Dis. 69:241–248. 2010.
View Article : Google Scholar
|
|
6
|
Parekkadan B, Tilles AW and Yarmush ML:
Bone marrow-derived mesenchymal stem cells ameliorate autoimmune
enteropathy independently of regulatory T cells. Stem Cells.
26:1913–1919. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ringdén O, Uzunel M, Rasmusson I, et al:
Mesenchymal stem cells for treatment of therapy-resistant
graft-versus-host disease. Transplantation. 81:1390–1397. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Firinci F, Karaman M, Baran Y, et al:
Mesenchymal stem cells ameliorate the histopathological changes in
a murine model of chronic asthma. Int Immunopharmacol.
11:1120–1126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SH, Jang AS, Kwon JH, Park SK, Won JH
and Park CS: Mesenchymal stem cell transfer suppresses airway
remodeling in a toluene diisocyanate-induced murine asthma model.
Allergy Asthma Immunol Res. 3:205–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ge X, Bai C, Yang J, Lou G, Li Q and Chen
R: Effect of mesenchymal stem cells on inhibiting airway remodeling
and airway inflammation in chronic asthma. J Cell Biochem.
114:1595–1605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wills-Karp M: Immunologic basis of
antigen-induced airway hyperresponsiveness. Annu Rev Immunol.
17:255–281. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stumbles PA, Thomas JA, Pimm CL, et al:
Resting respiratory tract dendritic cells preferentially stimulate
T helper cell type 2 (Th2) responses and require obligatory
cytokine signals for induction of Th1 immunity. J Exp Med.
188:2019–2031. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McWilliam AS, Nelson DJ and Holt PG: The
biology of airway dendritic cells. Immunol Cell Biol. 73:405–413.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hammad H and Lambrecht BN: Dendritic cells
and epithelial cells: linking innate and adaptive immunity in
asthma. Nat Rev Immunol. 8:193–204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jahnsen FL, Moloney ED, Hogan T, Upham JW,
Burke CM and Holt PG: Rapid dendritic cell recruitment to the
bronchial mucosa of patients with atopic asthma in response to
local allergen challenge. Thorax. 56:823–826. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moller GM, Overbeek SE, Van
Helden-Meeuwsen CG, et al: Increased numbers of dendritic cells in
the bronchial mucosa of atopic asthmatic patients: downregulation
by inhaled corticosteroids. Clin Exp Allergy. 26:517–524. 1996.
View Article : Google Scholar
|
|
18
|
van Rijt LS, Prins JB, Leenen PJ, et al:
Allergen-induced accumulation of airway dendritic cells is
supported by an increase in CD31(hi)Ly-6C(neg) bone marrow
precursors in a mouse model of asthma. Blood. 100:3663–3671. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang W, Ge W, Li C, et al: Effects of
mesenchymal stem cells on differentiation, maturation and function
of human monocyte-derived dendritic cells. Stem Cells Dev.
13:263–271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beyth S, Borovsky Z, Mevorach D, et al:
Human mesenchymal stem cells alter antigen-presenting cell
maturation and induce T-cell unresponsiveness. Blood.
105:2214–2219. 2005. View Article : Google Scholar
|
|
21
|
English K, Barry FP and Mahon BP: Murine
mesenchymal stem cells suppress dendritic cell migration,
maturation and antigen presentation. Immunol Lett. 115:50–58. 2008.
View Article : Google Scholar
|
|
22
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. Washington (DC): National Academies Press (US); 2011
|
|
23
|
Peister A, Mellad JA, Larson BL, Hall BM,
Gibson LF and Prockop DJ: Adult stem cells from bone marrow (MSCs)
isolated from different strains of inbred mice vary in surface
epitopes, rates of proliferation and differentiation potential.
Blood. 103:1662–1668. 2004. View Article : Google Scholar
|
|
24
|
English K, Barry FP, Field-Corbett CP and
Mahon BP: IFN-gamma and TNF-alpha differentially regulate
immunomodulation by murine mesenchymal stem cells. Immunol Lett.
110:91–100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vermaelen KY, Carro-Muino I, Lambrecht BN
and Pauwels RA: Specific migratory dendritic cells rapidly
transport antigen from the airways to the thoracic lymph nodes. J
Exp Med. 193:51–60. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de Heer HJ, Hammad H, Soullié T, et al:
Essential role of lung plasmacytoid dendritic cells in preventing
asthmatic reactions to harmless inhaled antigen. J Exp Med.
200:89–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lambrecht BN, De Veerman M, Coyle AJ,
Gutierrez-Ramos JC, Thielemans K and Pauwels RA: Myeloid dendritic
cells induce Th2 responses to inhaled antigen, leading to
eosinophilic airway inflammation. J Clin Invest. 106:551–559. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hamelmann E, Schwarze J, Takeda K, et al:
Noninvasive measurement of airway responsiveness in allergic mice
using barometric plethysmography. Am J Respir Crit Care Med.
156:766–775. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van Rijt LS, Jung S, Kleinjan A, et al: In
vivo depletion of lung CD11c+ dendritic cells during allergen
challenge abrogates the characteristic features of asthma. J Exp
Med. 201:981–991. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zumbuehl A, Stano P, Heer D, Walde P and
Carreira EM: Amphotericin B as a potential probe of the physical
state of vesicle membranes. Org Lett. 6:3683–3686. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rafei M, Campeau PM, Aguilar-Mahecha A, et
al: Mesenchymal stromal cells ameliorate experimental autoimmune
encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine
ligand 2-dependent manner. J Immunol. 182:5994–6002. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee RH, Seo MJ, Reger RL, et al:
Multipotent stromal cells from human marrow home to and promote
repair of pancreatic islets and renal glomeruli in diabetic
NOD/scid mice. Proc Natl Acad Sci USA. 103:17438–17443. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Augello A, Tasso R, Negrini SM, Cancedda R
and Pennesi G: Cell therapy using allogeneic bone marrow
mesenchymal stem cells prevents tissue damage in collagen-induced
arthritis. Arthritis Rheum. 56:1175–1186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun L, Akiyama K, Zhang H, et al:
Mesenchymal stem cell transplantation reverses multiorgan
dysfunction in systemic lupus erythematosus mice and humans. Stem
Cells. 27:1421–1432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kapoor S, Patel SA, Kartan S, Axelrod D,
Capitle E and Rameshwar P: Tolerance-like mediated suppression by
mesenchymal stem cells in patients with dust mite allergy-induced
asthma. J Allergy Clin Immunol. 129:1094–1101. 2012. View Article : Google Scholar
|
|
36
|
Firinci F, Karaman M, Baran Y, et al:
Mesenchymal stem cells ameliorate the histopathological changes in
a murine model of chronic asthma. Int Immunopharmacol.
11:1120–1126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Atsma DE, Fibbe WE and Rabelink TJ:
Opportunities and challenges for mesenchymal stem cell-mediated
heart repair. Curr Opin Lipidol. 18:645–649. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Le Blanc K and Ringdén O: Immunomodulation
by mesenchymal stem cells and clinical experience. J Intern Med.
262:509–525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kohl J, Baelder R, Lewkowich IP, et al: A
regulatory role for the C5a anaphylatoxin in type 2 immunity in
asthma. J Clin Invest. 116:783–796. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huh JC, Strickland DH, Jahnsen FL, et al:
Bidirectional interactions between antigen-bearing respiratory
tract dendritic cells (DCs) and T cells precede the late phase
reaction in experimental asthma: DC activation occurs in the airway
mucosa but not in the lung parenchyma. J Exp Med. 198:19–30. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vermaelen KY, Cataldo D, Tournoy K, et al:
Matrix metallopro-teinase-9-mediated dendritic cell recruitment
into the airways is a critical step in a mouse model of asthma. J
Immunol. 171:1016–1022. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou B, Comeau MR, De Smedt T, et al:
Thymic stromal lymphopoietin as a key initiator of allergic airway
inflammation in mice. Nat Immunol. 6:1047–1053. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lambrecht BN and Hammad H: Taking our
breath away: dendritic cells in the pathogenesis of asthma. Nat Rev
Immunol. 3:994–1003. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vermaelen K and Pauwels R: Accelerated
airway dendritic cell maturation, trafficking and elimination in a
mouse model of asthma. Am J Respir Cell Mol Biol. 29:405–409. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Harris NL, Watt V, Ronchese F and Le Gros
G: Differential T cell function and fate in lymph node and
nonlymphoid tissues. J Exp Med. 195:317–326. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lewkowich IP, Rempel JD and HayGlass KT:
Prevention of allergen-specific, Th2-biased immune responses in
vivo: role of increased IL-12 and IL-18 responsiveness. J Immunol.
175:4956–4962. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
van Rijt LS, Vos N, Willart M, et al:
Essential role of dendritic cell CD80/CD86 costimulation in the
induction, but not reactivation, of TH2 effector responses in a
mouse model of asthma. J Allergy Clin Immunol. 114:166–173. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wikstrom ME and Stumbles PA: Mouse
respiratory tract dendritic cell subsets and the immunological fate
of inhaled antigens. Immunol Cell Biol. 85:182–188. 2007.PubMed/NCBI
|
|
49
|
GeurtsvanKessel CH and Lambrecht BN:
Division of labor between dendritic cell subsets of the lung.
Mucosal Immunol. 1:442–450. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sung SS, Fu SM, Rose CE Jr, Gaskin F, Ju
ST and Beaty SR: A major lung CD103 (alphaE)-beta7
integrin-positive epithelial dendritic cell population expressing
Langerin and tight junction proteins. J Immunol. 176:2161–2172.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Beaty SR, Rose CE Jr and Sung SS: Diverse
and potent chemokine production by lung CD11bhigh dendritic cells
in homeostasis and in allergic lung inflammation. J Immunol.
178:1882–1895. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sallusto F, Lenig D, Mackay CR and
Lanzavecchia A: Flexible programs of chemokine receptor expression
on human polarized T helper 1 and 2 lymphocytes. J Exp Med.
187:875–883. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Panina-Bordignon P, Papi A, Mariani M, et
al: The C-C chemokine receptors CCR4 and CCR8 identify airway T
cells of allergen-challenged atopic asthmatics. J Clin Invest.
107:1357–1364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pilette C, Francis JN, Till SJ and Durham
SR: CCR4 ligands are up-regulated in the airways of atopic
asthmatics after segmental allergen challenge. Eur Respir J.
23:876–884. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Perros F, Hoogsteden HC, Coyle AJ,
Lambrecht BN and Hammad H: Blockade of CCR4 in a humanized model of
asthma reveals a critical role for DC-derived CCL17 and CCL22 in
attracting Th2 cells and inducing airway inflammation. Allergy.
64:995–1002. 2009. View Article : Google Scholar : PubMed/NCBI
|