Introduction
Mesenchymal stem cells (MSCs) are non-hematopoietic
multi-potent stem cells that possess therapeutic potential due to
their capacity for self-renewal, expansion and differentiation, in
addition to the ease of isolation from bone marrow, the umbilical
cord and other tissues (1–4). Human umbilical cord MSCs (hUCMSCs)
belong to a population of stem cells (5) that are used to treat certain
intractable diseases (6,7). Experimental and clinical studies have
indicated that transplantation with MSCs may be a promising
strategy for the regeneration and repair of damaged tissues
(4,8). However, the primary challenge in stem
cell therapy remains the need to reduce the apoptosis of these
cells, which occurs when exposed to harsh, proapoptotic
microenvironments, such as the presence of excessive inflammatory
stimuli, oxidative stress or cytotoxic free radicals (9,10).
Therefore, it is important to identify the factors that are
involved in the induction of apoptosis of MSCs, as well as those
which promote their survival.
Lipopolysaccharide (LPS), a component of the outer
membrane of gram-negative bacteria, is an important mediator of
endotoxemia, multiple organ dysfunction syndrome and endotoxic
shock. These may occur in patients as a result of sepsis, severe
burns or trauma (11,12). In addition to stimulating innate
immunity via regulation of the production of inflammatory
mediators, LPS may also result in apoptosis and cell death in
multiple cell types, including macrophages (13), endothelial cells (14) and microglial cells (15). However, other studies have
suggested that an appropriate concentration of LPS pretreatment may
protect neurons (16,17), myocardial cells (18) and bone marrow stem cells (19) from apoptosis induced by ischemic
injury or oxidative stress. Although LPS pretreatment has been
extensively investigated, little is known regarding the effect of
pretreatment with LPS on endotoxin-induced apoptosis in MSCs.
It is well-established that apoptosis is a tightly
regulated process (20). One of
the primary proteins responsible for regulating apoptosis is the
cellular FADD-like IL-1β-converting enzyme (FLICE)-inhibitory
protein (c-FLIP), which was originally identified as an inhibitor
of caspase 8. In addition to the full-length form of c-FLIP (55
kDa), also termed p55-FLIP or c-FLIPL, which contains
two death effector domains (DED) and a caspase-like domain, two
proteolytically processed forms of c-FLIPL have been
characterized, including p43-FLIP (43 kDa) and the shorter,
p22-FLIP (22 kDa), which contain the two DED domains without the
caspase-like domain (21). c-FLIP
binds to either Fas-associated protein with death domain (FADD) or
caspase 8, through DED-DED interactions, thus inhibiting the
activation of caspase 8 (22). A
number of studies have demonstrated that LPS and tumor necrosis
factor-α (TNF-α) upregulate c-FLIP and suppress Fas-FasL mediated
caspase 8 and 3 activation, as well as cell apoptosis (23,24).
The present study was designed to investigate
whether repetitive exposure to LPS protects hUCMSCs against the
apoptotic consequences of subsequent endoxin insults, and whether
LPS-induced adaptive cytoprotection is associated with
overexpression of the c-FLIP protein.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM)/F12,
trypsin, fetal bovine serum (FBS) and type-II collagenase were
purchased from Gibco Life Technologies (Carlsbad, CA, USA);
penicillin-streptomycin was from GE Healthcare Bio-Sciences
(Pittsburgh, PA, USA); lipopolysaccharides (Escherichia coli
serotype 055:B5) and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were from Sigma-Aldrich (St. Louis, MO, USA); Annexin V: FITC
Apoptosis Detection kit was from BD Biosciences (San Diego, CA,
USA); the Cell Death Detection ELISAPLUS Assay kit was
from Roche Diagnostics (Mannheim, Germany); anti-c-FLIP rabbit
monoclonal antibody (#8510) was from Cell Signaling Technology,
Inc. (Danvers, MA, USA); rabbit anti-GAPDH polyclonal antibody
(bs-2188R) and goat anti-rabbit IgG polyclonal antibody (bs-0295G)
were purchased from Bioss Company (Beijing, China); caspase 8 and 3
Activity Assay kits were purchased from Beyotime Institute of
Biotechnology (Haimen, China).
Preparation of hUCMSCs
hUCMSCs were isolated, as previously described
(25). Umbilical cord tissues
(15–20 cm) from three full-term healthy babies delivered by
caesarean section at the First Affiliated Hospital of PLA General
Hospital (Beijing, China), were thoroughly rinsed with
phosphate-buffered saline (PBS) and cut into 1-mm3
samples, following removal of the umbilical vessels and external
membrane. The tissues were placed in culture flasks (Corning,
Tewksbury, MA, USA) at a distance of 0.5 cm with DMEM/F12,
supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C in
a humidified atmosphere of 5% CO2. The medium was
replaced slowly and gradually every 3 days, to ensure that fixation
of the tissue. When cells in the tissue samples reached 80–85%
confluence, the tissues were removed and the cells were digested
with trypsin-EDTA (Gibco Life Technologies) and transferred to T-75
culture flasks for propagation and culture. Passage 3 cells were
stored for use in subsequent studies. The protocol of the current
study was approved by the ethics committee of the First Affiliated
Hospital of PLA General Hospital (Beijing, China).
Cell viability assay
hUCMSCs were inoculated in 96-well plates at a
density of 2×103 cells/well for 24 h, and the medium was
replaced with media containing different concentrations of LPS (0,
0.01, 0.1, 1, 10, 20, 30, 40 or 50 μg/ml) for continuous
culturing. One plate from each concentration group was sampled at
each time point (12, 24 and 48 h) and 20 μl/well of 5 mg/ml
MTT was added. Following a 4-h incubation, the supernatant was
removed from the plate and dimethyl sulfoxide (200 μl/well)
was added. The samples were shaken for 5 min, the MTT formazan
product was measured using a microplate reader (Synergy 2; BioTek
Instruments, Ltd., Winooski, VT, USA) and the absorbance was
measured at 490 nm.
Flow cytometry analysis
hUCMSCs were cultured in 6-well plates and either
treated with LPS (0, 1, 40 or 50 μg/ml) for 24 h, or
pretreated with LPS (0, 0.1, 1, 10 or 20 μg/ml) for 12 h
with subsequent exposure to 50 μg/ml LPS for 24 h. Following
treatment, cells were harvested using trypsin-EDTA and collected by
centrifugation at 900 × g for 5 min at room temperature. Cells were
washed, resuspended in PBS, and labeled with Annexin V and
propidium iodide (PI; from the BD Apoptosis Detection kit) for 20
min. Analyses were performed by flow cytometry (FC500; Beckman
Coulter, Brea, CA, USA) using FC500 MPL CXP2.1 software.
Hoechst staining analysis
hUCMSCs were cultured in 6-well plates. Following
treatment, cultured cells were washed twice with PBS and treated
with 4% paraformaldehyde (Guge Biological Technology Co., Ltd.,
Wuhan, China) for 10 min. After three washes with PBS, the cells
were stained with 5 μg/ml Hoechst 33258 (Sigma-Aldrich) for
10 min. The morphology of the nuclei of the hUCMSCs was then
analyzed under an inverted phase contrast microscope (DMI6000B;
Leica Microsystems GmbH, Wetzlar, Germany).
Western blot analysis
hUCMSCs, with or without LPS stimulation, were
rinsed twice with ice-cold PBS and lysed with ice-cold lysis buffer
[1% Triton X-100, 20 mmol/l HEPES (pH 7.5), 1 mmol/l each of EDTA,
DTT and PMSF, and 1 mg/ml each of leupeptin, aprotinin and
pepstatin; Macgene (Beijing) Biotechnology Ltd., Beijing, China]
for 30 min. Cell and nuclear lysates were centrifuged at 12,000 × g
for 10 min at 4°C, and the supernatants were mixed with 5X SDS
sample buffer (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China), boiled for 5 min and separated on 15% SDS-PAGE
gels (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Following
electrophoresis, proteins were transferred to nitrocellulose
blotting membranes by electrophoretic transfer. Non-specific
binding was blocked with 5% non-fat milk for 1 h. The membrane
(Merck Millipore, Darmstadt, Germany) was then rinsed with TBST and
incubated overnight at 4°C with the primary antibody (c-FLIP,
1:1,000 or GAPDH, 1:1,500). It was then washed in TBST and
incubated for 1 h with horseradish peroxidase-conjugated secondary
antibody. After washing in TBST, bands were visualized using
enhanced chemoluminescence and exposed to radiography film (Kodak,
Tokyo, Japan).
Silencing of c-FLIP expression with small
interfering RNA (siRNA)
The targeting sequence of human c-FLIP is
GGAGCAGGGACAAGTTACA (26).
Double-stranded, siRNA for c-FLIP was chemically synthesized by
GeneChem, Inc. (Shanghai, China). The transfection of siRNA was
performed using Lipofectamine 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA), according to the manufacturer’s instructions.
In brief, cells were harvested using 0.25% trypsin, 1 mM EDTA in
PBS without Ca2+ and Mg2+, and plated in
6-well plates at 105 cells/cm2. Next, 5
μl siRNA (100 lM) solution was mixed with 125 μl
serum-free culture medium for 5 min at room temperature. During
this incubation period, 5 μl Lipofectamine 2000 was diluted
in 125 μl serum-free culture medium. These two mixtures were
combined, mixed gently, and incubated for 20 min at room
temperature to allow complex formation. The resulting 250 μl
siRNA-Lipofectamine mixture was then added to the cells to produce
a final volume of 2.5 ml/well. Forty-eight hours after infection,
cells were washed and then incubated with LPS prior to use.
Knockdown efficiencies were routinely monitored by western
blotting.
Analysis of caspase activity and
internucleosomal degradation of genomic DNA
Caspase 3 and caspase 8 activity levels were
measured with the Caspase-Glo Assay System (Promega Corporation,
Madison, WI, USA), according to the manufacturer’s instructions.
Internucleosomal degradation of genomic DNA was detected using the
Cell Death Detection ELISAPLUS assay. hUCMSCs that were
either untreated or treated with LPS, with or without transfection
of siRNA, were washed twice with cold PBS and lysed on ice in a
cold lysis buffer. Cell lysates were centrifuged at 12,000 × g for
10 min in order to precipitate cellular debris. The assay was
performed in a 96-well plate, according to the manufacturer’s
instructions, using the microplate reader, and the absorbance was
measured at 405 nm.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Significance was analyzed using SPSS software, version
16.0 (SPSS Inc., Chicago, IL, USA). Statistical analysis was
performed by one-way analysis of variance, followed by Bonferroni
multiple-comparison test from >3 independent experiments.
P<0.05 was considered to indicate a statistically significant
difference.
Results
LPS induces apoptosis in hUCMSCs in a
dose-dependent manner
In order to investigate the cytotoxicity of LPS,
hUCMSCs were incubated with various concentrations of LPS for 12,
24 and 48 h, and cell viability was examined using an MTT assay. As
shown in Fig. 1A, exposure of
hUCMSCs to 40 or 50 μg/ml LPS resulted in a significant
reduction in cell viability compared with a dose of 0 μg/ml,
for all three time periods, while the cell viability was increased
following treatment with low concentrations of LPS. Flow cytometric
analysis using Annexin V/PI staining, further confirmed that cells
exposed to 40 and 50 μg/ml LPS for 24 h exhibited a
significant increase in apoptosis (Fig. 1B and C; P<0.01). By contrast,
LPS (0.01–30 μg/ml) produced little or no cytotoxicity in
hUCMSCs, indicating that LPS may exert antithetical effects,
depending upon its concentration; low doses of LPS appeared to
enhance cell viability while higher doses exhibited cytotoxicity in
hUCMSCs. The current data indicates that LPS induces apoptosis in
hUCMSCs in a dose-dependent manner.
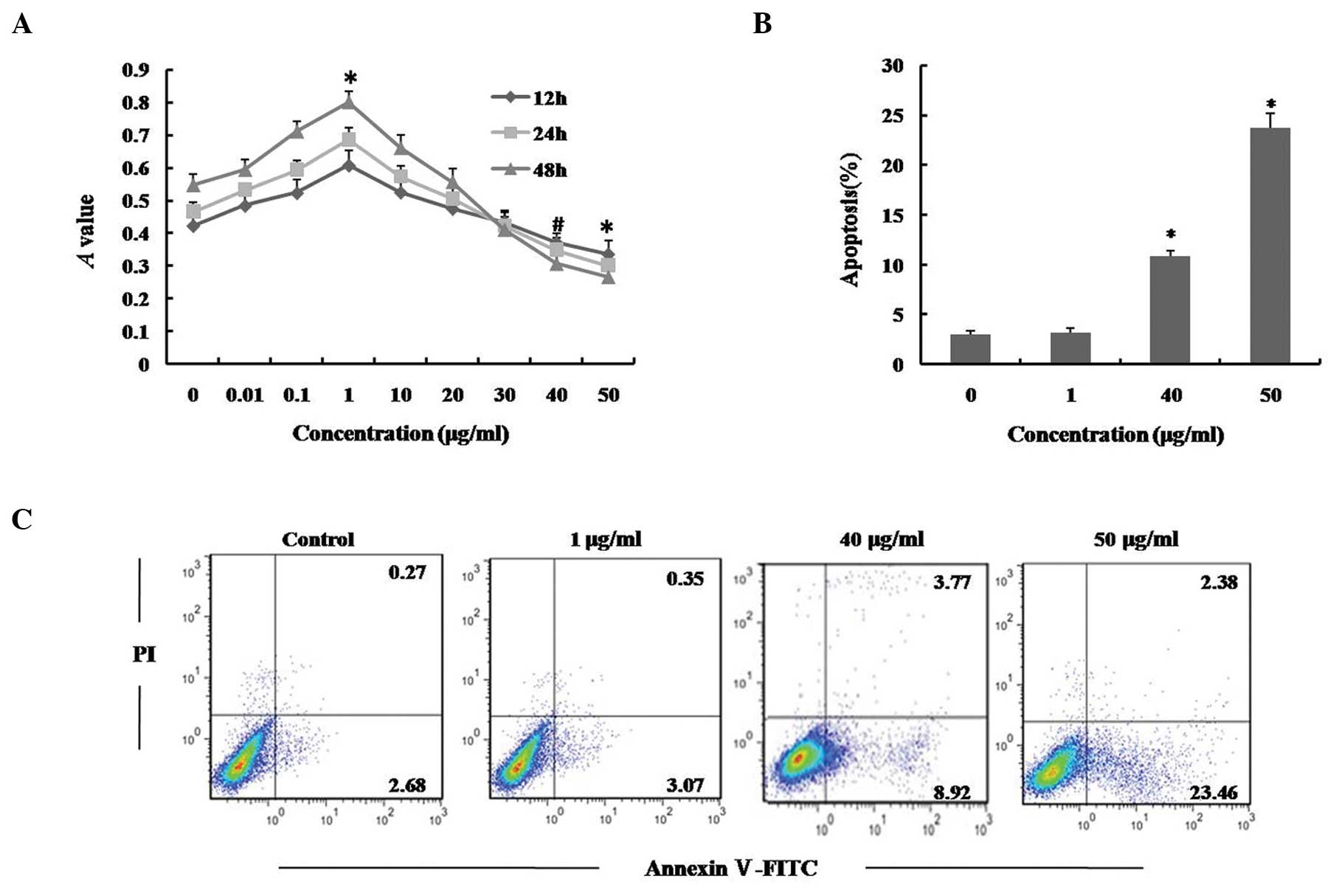 | Figure 1Effect of LPS on viability and
apoptosis of hUCMSCs. (A) hUCMSCs were treated with 0, 0.01, 0.1,
1, 10, 20, 30, 40 or 50 μg/ml LPS for 12, 24 and 48 h, and
cell viability was determined using an MTT assay. (B) and (C)
hUCMSCs were treated with 0, 1, 40 or 50 μg/ml LPS for 24 h.
The cells were stained with Annexin V/PI and apoptosis levels were
determined using flow cytometry. Data are presented as the mean ±
standard deviation. *P<0.01, #P<0.05
vs. control; n=5. LPS, lipopolysaccharide; hUCMSCs, human umbilical
cord mesenchymal stem cells; PI, propidium iodide. |
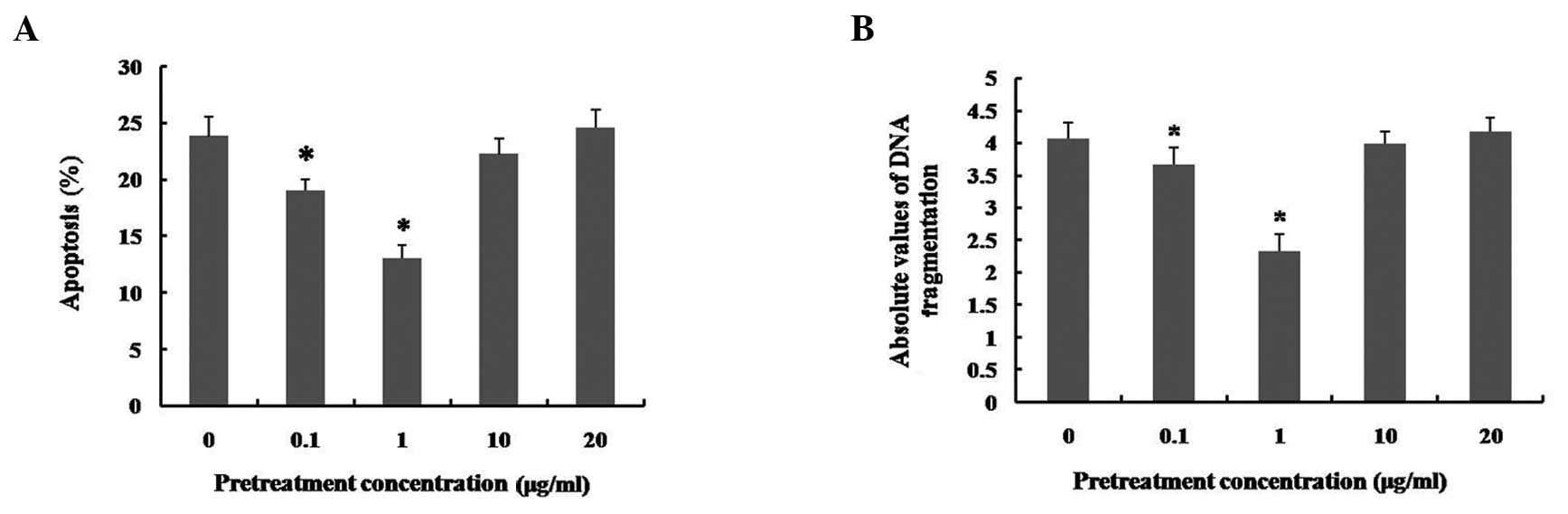
Effects of pretreatment with different
concentrations of LPS on LPS-induced apoptosis in hUCMSCs
An increasing number of studies have reported that
low-dose LPS pretreatment results in cytoprotection in several cell
types (16–19). Fig.
1A also indicates that low doses of LPS may promote hUCMSC
viability. In order to explore the possible cytoprotective effects
of low doses of LPS, prior to administration of a high-dose of LPS
(50 μg/ml), LPS pretreatment was performed in hUCMSC
cultures by incubation with a range of doses (0, 0.1, 1, 10 and 20
μg/ml) of LPS for 12 h. The apoptosis induced by LPS at 50
μg/ml was inhibited by pretreatment with 0.1 and 1
μg/ml LPS (particularly with 1 μg/ml; P<0.01).
However, pretreatment with 10 and 20 μg/ml LPS did not
significantly alter the apoptosis induced by treatment with
high-dose LPS (Fig. 2A). As
indicated by the cell death detection ELISAPLUS assay,
the severity of DNA fragmentation in hUCMSCs was consistent with
the flow cytometry results (Fig.
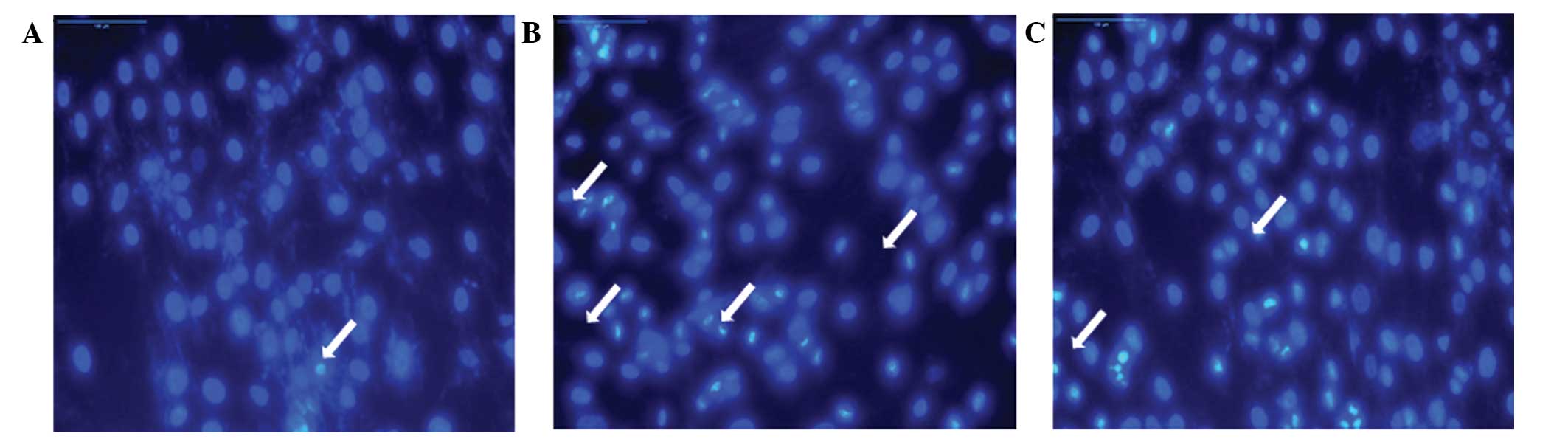
2B). In addition, the apoptotic nuclear condensation in hUCMSCs
was detected by Hoechst 33258 staining. In addition, as shown by
Hoechst 33258 staining, cells treated with 50 μg/ml LPS
presented apoptotic phenotypes, with clear chromatin conden sation,
and cellular and nuclear shrinkage (Fig. 3A and B). LPS (1 μg/ml)
pretreatment markedly reduced 50 μg/ml LPS-induced hUCMSC
cell apoptosis (Fig. 3C). These
results indicated that early exposure to low-dose LPS may protect
hUCMSCs from the apoptotic consequences of subsequent lethal LPS
insults, and that this effect is dependent upon the concentration
of LPS used. The most effective cytoprotective effect was observed
using 1 μg/ml LPS, which had not effect on the induction of
apoptosis in hUCMSCs. Therefore, this dose of LPS was selected for
LPS pretreatment in the subsequent experiments.
LPS pretreatment induces overexpression
of c-FLIP
c-FLIP has been demonstrated to be involved in the
prevention of apoptosis in a number of systems (22,27).
In order to explore whether pretreatment with low concentrations
(0, 0.1, 1, 10 or 20 μg/ml) of LPS modulates expression of
c-FLIP in hUCMSCs, and to assess the response to high-dose LPS, the
expression levels of c-FLIP were measured by western blot analysis.
Following pretreatment with LPS for 12 h, the expression of c-FLIP
was markedly increased in the hUCMSCs that had been pretreated with
0.1 and 1 μg/ml LPS. Statistical analysis indicated that
pretreatment with 0.1 and 1 μg/ml LPS increased the
expression level of c-FLIP to 1.63±0.11 and 2.36±0.15 times that of
the control (P<0.01), respectively. However, pretreatment with
10 and 20 μg/ml LPS did not significantly enhance c-FLIP
expression levels (Fig. 4A).
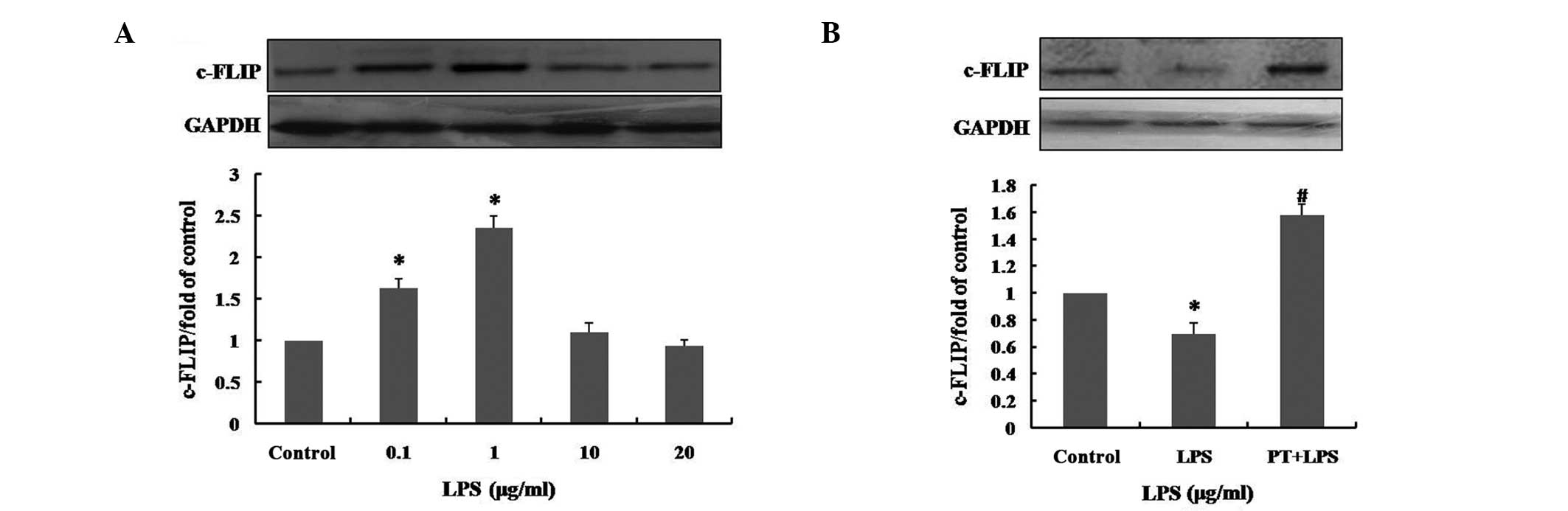 | Figure 4Effect of LPS pretreatment on c-FLIP
expression levels in hUCMSCs. (A) Following pretreatment with 0,
0.1, 1, 10 or 20 μg/ml LPS for 12 h, c-FLIP expression
levels in hUCMSCs were determined by western blotting. (B) The
c-FLIP expression levels, following exposure to 50 μg/ml for
24 h with or without pretreatment with 1 μg/ml LPS for 12 h
were determined by western blotting. Control, medium only; LPS, 50
μg/ml LPS treatment for 24 h; PT, 1 μg/ml LPS
pretreatment for 12 h. Data are presented as the mean ± standard
deviation and are expressed as a percentage of the control value.
*P<0.01. vs. control and #P<0.01, vs.
LPS; n=5. LPS, lipopolysaccharide; c-FLIP, cellular
FLICE-inhibitory protein; hUCMSCs, human umbilical cord mesenchymal
stem cells. |
Following exposure of hUCMSCs to 50 μg/ml LPS
for 24 h, the level of c-FLIP expression was markedly reduced, to
0.69±0.09-fold that of the control (P<0.01; Fig. 4B). However, the inhibition of the
expression of c-FLIP induced by 50 μg/ml LPS, was
significantly reduced by pretreatment with 1 μg/ml LPS for
12 h (P<0.01). This suggested that LPS pretreatment promotes the
expression of c-FLIP and also prevents the reduction of expression
induced by high-dose LPS treatment. It was thus inferred that the
cytoprotective effects of pretreatment with low doses of LPS in
hUCMSCs may be associated with overexpression of c-FLIP.
LPS pretreatment prevents LPS-induced
apoptosis by upregulating c-FLIP expression levels
LPS has been used to induce a model of
caspase-dependent apoptosis (26,28).
As shown in Fig. 5, 50
μg/ml LPS induced apoptosis, and enhanced the activity of
caspase 8 and 3 in hUCMSCs, and these effects were inhibited by the
caspase inhibitor Z-VAD-fmk. This indicates that LPS-mediated
hUCMSC apoptosis is also caspase-dependent. c-FLIP has been shown
to block the apoptotic pathway by interacting with caspase 8 at the
death-inducing signaling complex, through DED-DED interactions
(22,29). The present study aimed to determine
whether the upregulation of c-FLIP induced by 1 μg/ml LPS
inhibits LPS-induced apoptosis in hUCMSCs that have been pretreated
with low-dose LPS. Following treatment with 50 μg/ml LPS for
24 h, the activity of caspase 8 and 3 in LPS-pretreated hUCMSCs was
significantly reduced compared with that in the group without LPS
pretreatment (Fig. 5C and D;
P<0.01). In addition, following siRNA c-FLIP inhibition, caspase
8 and 3 activity, and the level of apoptosis in hUCMSCs subjected
to high-dose LPS exposure, were significantly elevated (P<0.01),
and this effect was reduced by pretreatment with 1 μg/ml LPS
for 12 h prior to exposure to the high-dose LPS. This result
confirmed that LPS pretreatment prevents caspase-dependent
apoptosis induced by high-dose LPS in hUCMSCs, via the upregulation
of c-FLIP.
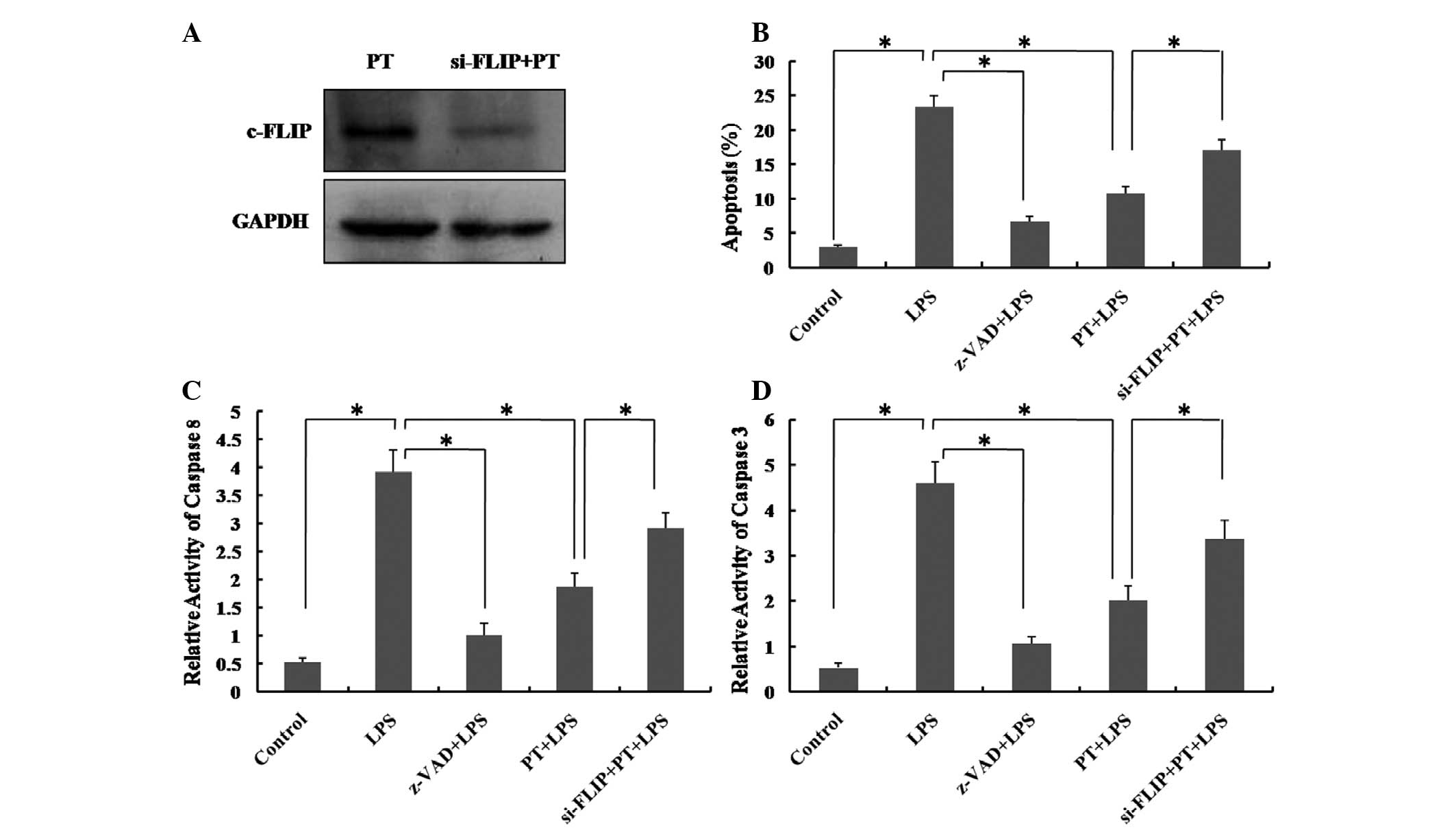 | Figure 5Effect of c-FLIP siRNA on LPS-induced
apoptotic activity in hUCMSCs preconditioned with LPS was measured
using flow cytometry, and caspase 8 and 3 ELISA assays. (A)
Knockdown efficiency of c-FLIP was monitored by western blotting.
(B) The percentage of apoptotic hUCMSCs was analyzed by flow
cytometry. (C) and (D) The activity of caspase 8 and 3 was detected
by ELISA. Control, medium only; LPS, hUCMSCs treated with LPS 50
μg/ml for 24 h; Z-VAD, caspase inhibitor (20 μM); PT,
hUCMSCs preconditioned with LPS 1 μg/ml for 12 h; si-FLIP,
hUCMSCs transfected with siRNA of c-FLIP. Data are expressed as the
mean ± standard deviation. *P<0.01; n=5. c-FLIP,
cellular FLICE-inhibitory protein; LPS, lipopolysaccharide;
hUCMSCs, human umbilical cord mesenchymal stem cells; siRNA, small
interfering RNA. |
Discussion
As a population of stem cells, hUCMSCs are able to
self-renew, rapidly proliferate, differentiate and are involved in
tissue repair and microenvironment regulation. These functions
initially depend on the ability of cells to survive in a complex
microenvironment. The findings of the present study indicate that
LPS induces apoptosis in hUCMSCs by activating caspases in a
dose-dependent manner, and that pretreatment with low
concentrations of LPS markedly increases the survival of hUCMSCs
under high-dose endotoxin conditions. In addition, the
cytoprotective effect of LPS pretreatment against apoptosis induced
by LPS in hUCMSCs is, in part, associated with the overexpression
of c-FLIP.
As a result of bacterial infections and enterogenous
endotoxin translocation, a large quantity of endotoxins is often
present in patients who have received burns or other significant
trauma (30). As a constitutive
component of the bacterial cell wall in Gram-negative bacteria, LPS
is an important mediator of endotoxemia and its invariant molecular
pattern, lipid A, is specifically recognized by Toll-like receptor
4 (TLR4). TLR4s are expressed by MSCs and may be deleterious in
MSC-mediated protection (31,32).
TLR4 has been shown to induce apoptosis and inhibit proliferation
in neuronal progenitor cells and various other cell types (13,15).
A TLR4-mediated apoptotic pathway may be triggered by LPS and
blocked by caspase inhibitors in endothelial cells (28). Other studies have demonstrated that
LPS induces apoptosis in multiple cellular systems (15,28,33).
The present study demonstrated that LPS induced apoptosis in
hUCMSCs in a dose-dependent manner, indicating that a high level of
endotoxins is a factor that influences hUCMSC survival. A hallmark
of apoptosis is the activation of highly specific effector
proteases of the caspase family. LPS has been reported to activate
caspase 8 and 3 (34,35). Haase et al (13) also suggested that LPS/TLR4 induces
apoptosis in macrophages via activation of caspase 8 and 3 in a
FADD protein-dependent pathway (13). In the present study, it was shown
that 50 μg/ml LPS enhanced the activation of caspase 8 and
3, which is consistent with previous studies conducted in other
cell systems (13,35).
Pretreatment creates a potent protective phenomenon
in which tissues or cells develop resistance to the proapoptotic
microenvironment, following exposure to a variety of injurious
stimuli, including brief ischemia, hypoxia and low-dose LPS
treatment (36–38). It has been shown that ischemic or
oxidative pretreatment produces protective effects in the heart
(39), brain (40,41)
and kidneys (42). A number of
studies have indicated that low-dose LPS pretreatment also elicits
neuroprotection and protects MSCs from oxidative stress-induced
apoptosis (16,43,44).
The core principle of cytoprotection using pretreatment, is that
the dose of the pretreatment stimulus should be high enough to
produce an effect, while not causing cytotoxicity. The results of
the current study indicated that repeated exposed to 0.1 and 1
μg/ml LPS protected hUCMSCs against the apoptotic
consequences of subsequent high-dose LPS insults, whereas 10 and 20
μg/ml LPS did not. The reason for this may be that higher
doses of LPS elicit deleterious effects. Although an increasing
number of studies report a cytoprotective effect of LPS
pretreatment, the molecular mechanism underlying the regulation of
cytoprotection by LPS pretreatment remains unclear.
c-FLIP is a cytoplasmic protein, with sequence
homology to FLICE. It binds to either FADD or caspase 8 via DED-DED
interactions, resulting in inhibition of caspase 8 activation and
apoptosis (45). The functional
role of c-FLIP depends upon its level of expression; low levels of
c-FLIP typically induce apoptosis while higher levels are
cytoprotective (22,46). It has been demonstrated that LPS
exposure rapidly upregulates the expression of c-FLIP in human
dendritic cells (47). Other
studies have reported that TNF-α and LPS induced the nuclear
factor-κB-mediated expression of c-FLIP in a variety of cell types,
promoting inducible resistance to death-receptor signaling
(23,24). Using in vitro culture of
hUCMSCs, the present study demonstrated that low concentrations of
LPS enhance expression of c-FLIP, while high concentrations do not.
Additionally, the results indicated that pretreatment with 1
μg/ml LPS induced overexpression of c-FLIP and blocked
high-dose LPS-induced inhibition of c-FLIP. It was hypothesized
that overexpression of c-FLIP may be important for LPS
pretreatment-mediated cytoprotection against high-dose LPS-induced
apoptosis. In order to examine the association between the
cytoprotection of LPS pretreatment and c-FLIP expression levels in
hUCMSCs, c-FLIP siRNA was employed. The antiapoptotic effect of LPS
pretreatment was weakened following the use of c-FLIP siRNA. This
data confirmed that LPS pretreatment prevents the LPS-inducing
caspase-dependent apoptosis in hUCMSCs through the induction of
c-FLIP expression.
In conclusion, LPS induced apoptosis in hUCMSCs via
activation of caspase in a dose-dependent manner. Pretreatment with
low concentrations of LPS protected hUCMSCs against apoptosis
induced by subsequent high-dose LPS insults. The cytoprotection
effected by the LPS pretreatment occurred, in part, as a result of
the overexpression of c-FLIP. However, the proapoptotic and
antiapoptotic mechanisms are complex, and the antiapoptotic effect
of LPS pretreatment may be associated with other unknown
mechanisms. Additionally, the effect of LPS stimulation on the MSC
phenotype and differentiation is unclear. Further investigations
are required to address these remaining issues.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81372052 and 81471873), the
First Affiliated Hospital of PLA General Hospital Science Research
Foundation of China (grant no. QN201207) and General Financial
Grant from the China Postdoctoral Science Foundation (grant no.
2013M532200).
References
|
1
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, et al: Multilineage potential of adult human
mesenchymal stem cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ivanova-Todorova E, Bochev I, Mourdjeva M,
Dimitrov R, et al: Adipose tissue-derived mesenchymal stem cells
are more potent suppressors of dendritic cells differentiation
compared to bone marrow-derived mesenchymal stem cells. Immunol
Lett. 126:37–42. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baksh D, Yao R and Tuan RS: Comparison of
proliferative and multilineage differentiation potential of human
mesenchymal stem cells derived from umbilical cord and bone marrow.
Stem Cells. 25:1384–1392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Orlic D, Kajstura J, Chimenti S, Jakoniuk
I, Anderson SM, Li B, et al: Bone marrow cells regenerate infarcted
myocardium. Nature. 410:701–705. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Troyer DL and Weiss ML: Wharton’s
jelly-derived cells are a primitive stromal cell population. Stem
Cells. 26:591–599. 2008. View Article : Google Scholar
|
|
6
|
Lee PH, Lee JE, Kim HS, Song SK, Lee HS,
Nam HS, et al: A randomized trial of mesenchymal stem cells in
multiple system atrophy. Ann Neurol. 72:32–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Z, Lin H, Shi M, Xu R, Fu J, Lv J,
Chen L, Lv S, et al: Human umbilical cord mesenchymal stem cells
improve liver function and ascites in decompensated liver cirrhosis
patients. J Gastroenterol Hepatol. 27(Suppl 2): 112–120. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dimarino AM, Caplan AI and Bonfield TL:
Mesenchymal stem cells in tissue repair. Front Immunol. 4:2012013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Müller-Ehmsen J, Krausgrill B, Burst V,
Schenk K, et al: Effective engraftment but poor mid-term
persistence of mononuclear and mesenchymal bone marrow cells in
acute and chronic rat myocardial infarction. J Mol Cell Cardiol.
41:876–884. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burlacu A: Tracking the mesenchymal stem
cell fate after transplantation into the infarcted myocardium. Curr
Stem Cell Res Ther. 8:284–291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yin HN, Chai JK, Yao YM, Shen CA, et al:
Effect of ubiquitin-proteasome pathway on inflammatory reaction in
intestine and its barrier function in rats with postburn sepsis.
Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 18:649–652. 2006.In Chinese.
PubMed/NCBI
|
|
12
|
Caroff M and Karibian D: Structure of
bacterial lipopolysaccharides. Carbohydr Res. 338:2431–2447. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haase R, Kirschning CJ, Sing A, Schröttner
P, et al: A dominant role of Toll-like receptor 4 in the signaling
of apoptosis in bacteria-faced macrophages. J Immunol.
171:4294–4303. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bannerman DD, Erwert RD, Winn RK and
Harlan JM: TIRAP mediates endotoxin-induced NF-kappaB activation
and apoptosis in endothelial cells. Biochem Biophys Res Commun.
295:157–162. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung DY, Lee H, Jung BY, Ock J, et al:
TLR4, but not TLR2, signals autoregulatory apoptosis of cultured
microglia: A critical role of IFN-beta as a decision maker. J
Immunol. 174:6467–6476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vartanian KB, Stevens SL, Marsh BJ, et al:
LPS preconditioning redirects TLR signaling following stroke:
TRIF-IRF3 plays a seminal role in mediating tolerance to ischemic
injury. J Neuroinflammation. 8:1402011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li WC, Jiang DM, Hu N, Qi XT, Qiao B and
Luo XJ: Lipopolysaccharide preconditioning attenuates
neuroapoptosis and improves functional recovery through activation
of Nrf2 in traumatic spinal cord injury rats. Int J Neurosci.
123:240–247. 2013. View Article : Google Scholar
|
|
18
|
Ha T, Hua F, Liu X, Ma J, McMullen JR,
Shioi T, Izumo S, et al: Lipopolysaccharide-induced myocardial
protection against ischaemia/reperfusion injury is mediated through
a PI3K/Akt-dependent mechanism. Cardiovasc Res. 78:546–553. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao Y, Zhang F, Wang L, Zhang G, Wang Z,
Chen J and Gao X: Lipopolysaccharide preconditioning enhances the
efficacy of mesenchymal stem cells transplantation in a rat model
of acute myocardial infarction. J Biomed Sci. 16:742009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chetoui N, Boisvert M, Gendron S and
Aoudjit F: Interleukin-7 promotes the survival of human CD4+
effector/memory T cells by up-regulating Bcl-2 proteins and
activating the JAK/STAT signalling pathway. Immunology.
130:418–426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peter ME: The flip side of FLIP. Biochem
J. 382:e1–e3. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Irmler M, Thome M, Hahne M, Schneider P,
Hofmann K, et al: Inhibition of death receptor signals by cellular
FLIP. Nature. 388:190–195. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Perlman H, Pagliari LJ, Nguyen N, Bradley
K, Liu H and Pope RM: The Fas-FasL death receptor and PI3K pathways
independently regulate monocyte homeostasis. Eur J Immunol.
31:2421–2430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bai S, Liu H, Chen KH, Eksarko P, et al:
NF-kappaB-regulated expression of cellular FLIP protects rheumatoid
arthritis synovial fibroblasts from tumor necrosis factor
alpha-mediated apoptosis. Arthritis Rheum. 50:3844–3855. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han Y, Chai J, Sun T, Li D and Tao R:
Differentiation of human umbilical cord mesenchymal stem cells into
dermal fibroblasts in vitro. Biochem Biophys Res Commun.
413:561–565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schmidt M, Hupe M, Endres N, et al: The
contact allergen nickel sensitizes primary human endothelial cells
and keratinocytes to TRAIL-mediated apoptosis. J Cell Mol Med.
14:1760–1776. 2010. View Article : Google Scholar
|
|
27
|
Chiou SH, Liu JH, Hsu WM, Chen SS, Chang
SY, Juan LJ, et al: Upregulation of Fas ligand expression by human
cytomegalovirus immediate-early gene product 2: A novel mechanism
in cytomegalovirus-induced apoptosis in human retina. J Immunol.
167:4098–4103. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu LC, Flynn AN, Turner JR and Buret AG:
SGLT-1-mediated glucose uptake protects intestinal epithelial cells
against LPS-induced apoptosis and barrier defects: A novel cellular
rescue mechanism? FASEB J. 19:1822–1835. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Piao X, Komazawa-Sakon S, Nishina T, Koike
M, et al: c-FLIP maintains tissue homeostasis by preventing
apoptosis and programmed necrosis. Sci Signal.
5:ra932012.PubMed/NCBI
|
|
30
|
Yao YM, Chai JK and Sheng ZY: Diagnostic
criterion and management of burn sepsis. Zhonghua Shao Shang Za
Zhi. 19:65–66. 2003.In Chinese. PubMed/NCBI
|
|
31
|
Pevsner-Fischer M, Morad V, Cohen-Sfady M,
Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR and Zipori D:
Toll-like receptors and their ligands control mesenchymal stem cell
functions. Blood. 109:1422–1432. 2007. View Article : Google Scholar
|
|
32
|
Wang Y, Abarbanell AM, Herrmann JL, Weil
BR, Manukyan MC, Poynter JA and Meldrum DR: TLR4 inhibits
mesenchymal stem cell (MSC) STAT3 activation and thereby exerts
deleterious effects on MSC-mediated cardioprotection. PLoS One.
5:e142062010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choi KB, Wong F, Harlan JM, Chaudhary PM,
Hood L and Karsan A: Lipopolysaccharide mediates endothelial
apoptosis by a FADD-dependent pathway. J Biol Chem.
273:20185–20188. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Karahashi H and Amano F: Changes of
caspase activities involved in apoptosis of a macrophage-like cell
line J774.1/JA-4 treated with lipopolysaccharide (LPS) and
cycloheximide. Biol Pharm Bull. 23:140–144. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hull C, McLean G, Wong F, Duriez PJ and
Karsan A: Lipopolysaccharide signals an endothelial apoptosis
pathway through TNF receptor-associated factor 6-mediated
activation of c-Jun NH2-terminal kinase. J Immunol. 169:2611–2618.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gidday JM: Cerebral preconditioning and
ischaemic tolerance. Nat Rev Neurosci. 7:437–448. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rosenzweig HL, Lessov NS, Henshall DC,
Minami M, Simon RP and Stenzel-Poore MP: Endotoxin preconditioning
prevents cellular inflammatory response during ischemic
neuroprotection in mice. Stroke. 35:2576–2581. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dirnagl U, Simon RP and Hallenbeck JM:
Ischemic tolerance and endogenous neuroprotection. Trends Neurosci.
26:248–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: A delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thompson JW, Dave KR, Young JI and
Perez-Pinzon MA: Ischemic preconditioning alters the epigenetic
profile of the brain from ischemic intolerance to ischemic
tolerance. Neurotherapeutics. 10:789–797. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen J and Simon R: Ischemic tolerance in
the brain. Neurology. 48:306–311. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Islam CF, Mathie RT, Dinneen MD, Kiely EA,
Peters AM and Grace PA: Ischaemia-reperfusion injury in the rat
kidney: The effect of preconditioning. Br J Urol. 79:842–847. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hickey E, Shi H, Van Arsdell G and Askalan
R: Lipopolysaccharide-induced preconditioning against ischemic
injury is associated with changes in toll-like receptor 4
expression in the rat developing brain. Pediatr Res. 70:10–14.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang ZJ, Zhang FM, Wang LS, Yao YW, Zhao Q
and Gao X: Lipopolysaccharides can protect mesenchymal stem cells
(MSCs) from oxidative stress-induced apoptosis and enhance
proliferation of MSCs via Toll-like receptor (TLR)-4 and PI3K/Akt.
Cell Biol Int. 33:665–674. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Krueger A, Baumann S, Krammer PH and
Kirchhoff S: FLICE-inhibitory proteins: regulators of death
receptor-mediated apoptosis. Mol Cell Biol. 21:8247–8254. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chang DW, Xing Z, Pan Y,
Algeciras-Schimnich A, Barnhart BC, Yaish-Ohad S, Peter ME and Yang
X: c-FLIP(L) is a dual function regulator for caspase-8 activation
and CD95-mediated apoptosis. EMBO J. 21:3704–3714. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Willems F, Amraoui Z, Vanderheyde N,
Verhasselt V, et al: Expression of c-FLIP(L) and resistance to
CD95-mediated apoptosis of monocyte-derived dendritic cells:
inhibition by bisindolylmaleimide. Blood. 95:3478–3482.
2000.PubMed/NCBI
|