Introduction
Neurogenic lower urinary tract dysfunction (NLUTD)
is a major problem in patients with various neurological disorders,
and may result in debilitating symptoms and serious complications,
including chronic renal failure and recurrent urinary tract
infections. Important causes of NLUTD include cerebrovascular
accident, Parkinson’s disease, spinal cord injury (SCI), multiple
sclerosis, diabetes mellitus and peripheral nerve injury due to
radical abdominal or pelvic surgery. NLUTD is also common in stroke
patients, and the evaluation of bladder function and management of
this disorder should be recognized as part of routine
rehabilitation (1–3). Occasionally, successful treatment is
difficult to quantify due to the irreversibility or progressive
nature of strokes. Therefore, neurological investigation of NLUTD
in stroke patients is urgently required.
The majority of animal experimental studies of NLUTD
have been directed towards the development of models of overactive
bladder (OAB) (4). Various animal
models of OAB have been analyzed, including a spontaneous
hypertensive rat model and a rat model of dopaminergic brain
lesions, and various methods have been used to induce OAB,
including autoimmune encephalomyelitis, experimental fluid
percussion injury to the brain and cerebral infarction using
ligation of the common carotid arteries (4). SCI animal models generated by spinal
cord compression using electromagnetically controlled watch-maker
forceps or spinal cord transection using iridectomy microscissors
have also been used to investigate OAB (5,6).
Furthermore, bladder outlet obstruction (BOO) models to examine OAB
have been generated using a small polyethylene catheter to produce
partial urethral obstruction or by intraperitoneal (i.p.)
cyclophosphamide (CYP) injection, due to the ease and simplicity of
these techniques (7,8). However, these models may not
adequately address the characteristics of hemorrhage in stroke
patients, although cerebral infarction models are appropriate for
ischemic stroke studies. In addition, the relevance of observed
changes following SCI, BOO and CYP injection may not reflect
conditions in human stroke patients in terms of the possible
pathophysiology of NLUTD.
An intracerebral hemorrhage (ICH) model obtained by
direct collagenase injection into the brain has been used in
numerous neurological studies to evaluate the pathophysiological
changes in the brain following ICH and to determine the therapeutic
options for treating ICH (9,10).
To the best of our knowledge, in the urological literature, no
studies that use an ICH model to analyze NLUTD, including OAB and
lower urinary tract function, have been reported. Thus, in the
present study, lower urinary tract function in ICH-induced rats was
investigated and compared with that in normal rats. The effects of
ICH on NLUTD with regard to peripheral bladder function and central
micturition centers [medial preoptic area (MPA), ventrolateral
periaqueductal gray (vlPAG), pontaine micturition center (PMC) and
spinal cord (lumbar 4 (L4)-L5)] were also examined.
Materials and methods
Experimental animals and treatments
Adult female Sprague-Dawley rats, weighing 260±10 g
and aged 10 weeks, were obtained from a commercial breeder (Orient
Co., Seoul, Korea). The present study was performed in accordance
with the animal care guidelines of the National Institutes of
Health and the Korean Academy of Medical Sciences (Seoul, Korea),
and was approved by the Kyung Hee University Institutional Animal
Care and Use Committee (Seoul, Korea). Each animal was housed under
controlled temperature (23±2°C) and lighting (8:00 a.m.–8:00 p.m.)
conditions with food and water available ad libitum prior
and subsequent to experiments. The animals were randomly divided
into the following two groups (n=10 in each group): Control and
ICH-induced.
ICH induction
To induce ICH, the rats were anesthetized with
Zoletil® 50 (10 mg/kg, i.p.; Vibac Laboratories, Carros,
France) and placed in a stereotaxic frame. The needle of a 10
µl Hamilton syringe (Micro 701; Hamilton company, Reno, NV,
USA) was inserted through a burr hole into the right hippocampus to
the following coordinates: 2.2 mm anterior, 2.2 mm lateral and 4.2
mm depth to bregma. A solution (1 µl) containing 0.2 U
collagenase (Type IV; Sigma-Aldrich, St. Louis, MO, USA) was
infused over 1 min. The needle was maintained in place for an
additional 3 min following the infusion and was subsequently
withdrawn slowly. The animals in the sham-operation group received
1 µl physiological saline by the same method.
Cystometry
Rat bladder function was evaluated by cystometry 14
days after the ICH surgery. The rats were anesthetized with 10
mg/kg Zoletil® 50 (i.p.). A sterile polyethylene catheter (PE50)
was inserted into the urethra through the bladder dome. The
catheter was connected to a pressure transducer (Harvard Apparatus,
Holliston, MA, USA) and syringe pump (Harvard Apparatus) via a
three-way stopcock to record the intravesical pressure. When the
bladder had emptied, cystometry was conducted by infusing 0.5 ml
saline into the bladder. Bladder contraction pressure and time were
monitored using Labscribe (iWork System Inc., Dover, NH, USA).
Preparation of tissues
The rats were sacrificed immediately following
cystometric determination of the contraction pressure and time in
the bladder. Briefly, the animals were anesthetized with Zoletil
50® (10 mg/kg, i.p.; Vibac Laboratories), and were
transcardially perfused with 50 µm phosphate-buffered saline
(PBS), followed by 4% paraformaldehyde in 100 mm sodium phosphate
buffer at pH 7.4. The brain was removed, postfixed in the same
fixative overnight and transferred to a 30% sucrose solution for
cryoprotection. Subsequently, 40 µm serial coronal sections
were produced with a freezing microtome (Leica Biosystems Nussloch
GmbH, Nussloch, Germany). The PMC was defined as the region
spanning Bregma −9.68 to −9.80 mm, the vlPAG as the region spanning
Bregma −7.64 to −8.00 mm, the MPA as the region spanning Bregma
−0.26 to 0.80 mm and the spinal cord as the L4–L5 region. An
average of 10 sections were collected from each rat for each region
and were used for immunohistochemical analysis.
Immunohistochemistry for c-Fos and nerve
growth factor (NGF)
Rabbit polyclonal anti-c-Fos (cat. no. sc-52) or
mouse monoclonal anti-NGF (cat. no. sc-365944) antibodies (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 1:1,000 dilutions
were incubated overnight with free-floating tissue sections at 4°C.
The sections were washed three times with PBS for 3 min, and were
subsequently incubated for 1 h at room temperature with
biotinylated anti-rabbit (cat. no. BA1000) or anti-mouse (cat. no.
BA2000) secondary antibodies (1:200 dilution; Vector Laboratories,
Burlingame, CA, USA) as appropriate. After washing three times with
PBS for 3 min, the sections were incubated for 1 h at room
temperature with avidin-biotin-peroxidase complex (Vector
Laboratories). The sections were washed a further three times with
PBS, and were then incubated in a solution consisting of 0.05%
3,3′-diaminobenzidine (Sigma-Aldrich) and 0.01%
H2O2 in 50 mm Tris-buffered saline (pH 7.6)
for ~3 min to visualize immunoreactivity. The sections were mounted
onto gelatine-coated slides subsequent to washing three times with
PBS. The slides were air-dried at room temperature overnight and
coverslips were mounted using Permount™ Mounting Medium (Fisher
Scientific, Waltham, MA, USA).
Data analysis and statistics
The numbers of c-Fos-positive and NGF-positive cells
in the neuronal voiding centers [MPA, vlPAG, PMC and spinal cord
(L4–L5)] were counted hemilaterally through a light microscope
(BX-51; Olympus Corporation, Tokyo, Japan). The area of the
neuronal voiding center in each slice was measured using the
Image-Pro® Plus computer-assisted image analysis system
(Media Cyberbetics Inc., Silver Spring, MD, USA) attached to the
light microscope (BX-51; Olympus Corporation). The data were
analyzed using SPSS software (ver. 20.2; IBM, Armonk, NY, USA) and
are expressed as the mean ± standard error of the mean. The results
of the sham surgery and ICH-induced groups were compared using an
independent paired Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
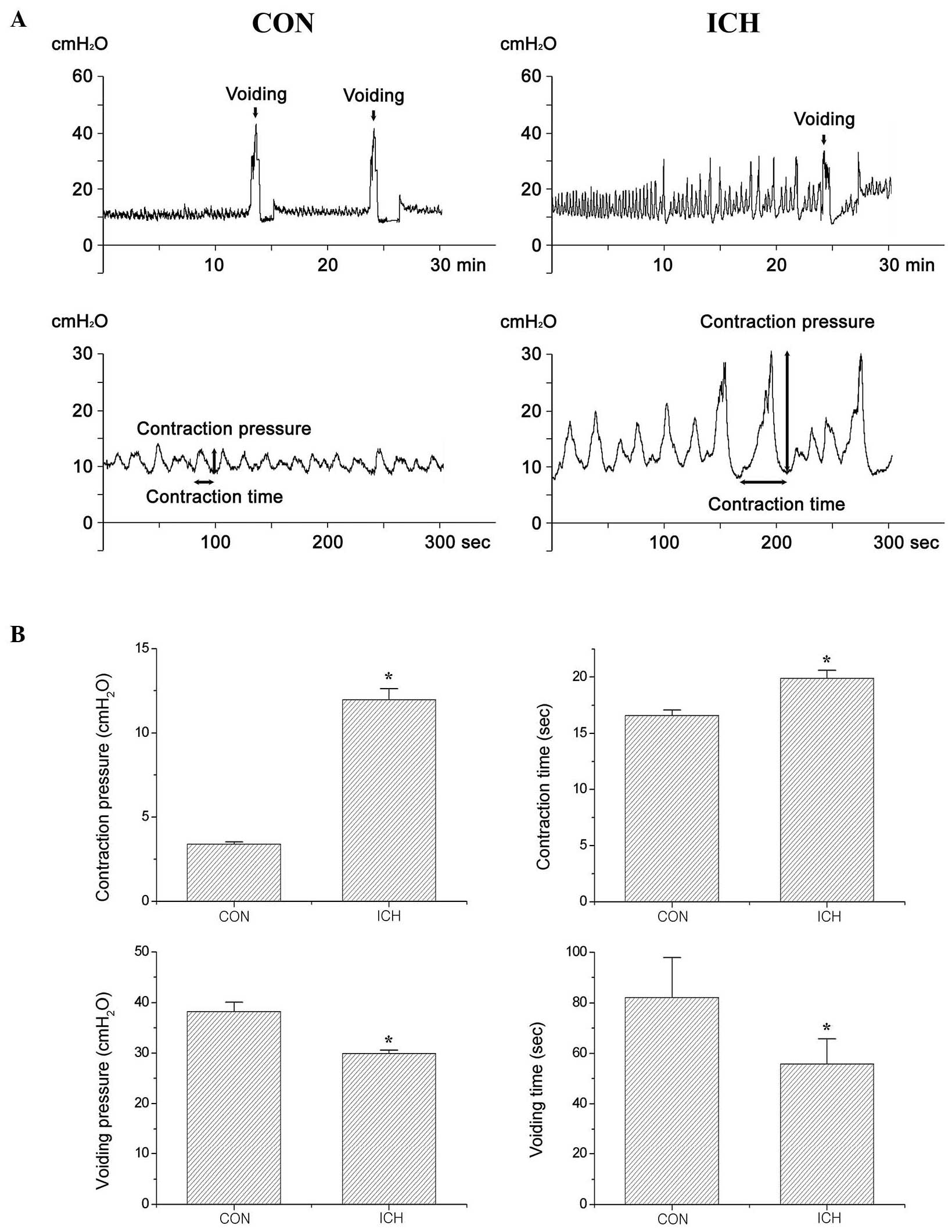
Effect of ICH on urodynamic
parameters
The bladder contraction and voiding parameters
(pressure and time) obtained by cystometry are presented in
Fig. 1 and Table I. These results indicate that
bladder contraction pressure (P<0.05) and time (P<0.05) were
significantly increased by the induction of ICH as compared with
the control treatment. By contrast, the voiding pressure
(P<0.05) and time (P<0.05) were significantly reduced in the
rats with ICH induction compared with the control rats.
 | Table IChanges in urodynamic parameters,
c-Fos and NGF expression following the induction of ICH. |
Table I
Changes in urodynamic parameters,
c-Fos and NGF expression following the induction of ICH.
| Variable | Control | ICH |
|---|
| Bladder contraction
parameter | | |
| Pressure (cm
H2O) | 3.30±0.13 | 11.97±0.64a |
| Time (sec) | 16.58±0.50 | 19.89±0.70a |
| Voiding
parameter | | |
| Pressure (cm
H2O) | 38.23±1.86 | 29.93±1.50a |
| Time (sec) | 82.00±15.90 | 55.78±10.00a |
| c-Fos expression (no.
cells/section) | | |
| MPA | 33.22±3.11 | 87.20±5.19a |
| vlPAG | 39.50±2.88 | 81.20±5.69a |
| PMC | 22.44±3.09 | 70.10±4.01a |
| L4–L5 | 13.55±1.80 | 41.09±4.11a |
| NGF expression (no.
cells/section) | | |
| MPA | 46.38±9.01 | 99.44±10.19a |
| vlPAG | 39.14±6.90 | 104.45±11.39a |
| PMC | 30.61±5.10 | 79.50±7.20a |
| L4–L5 | 21.45±3.10 | 56.11±5.10a |
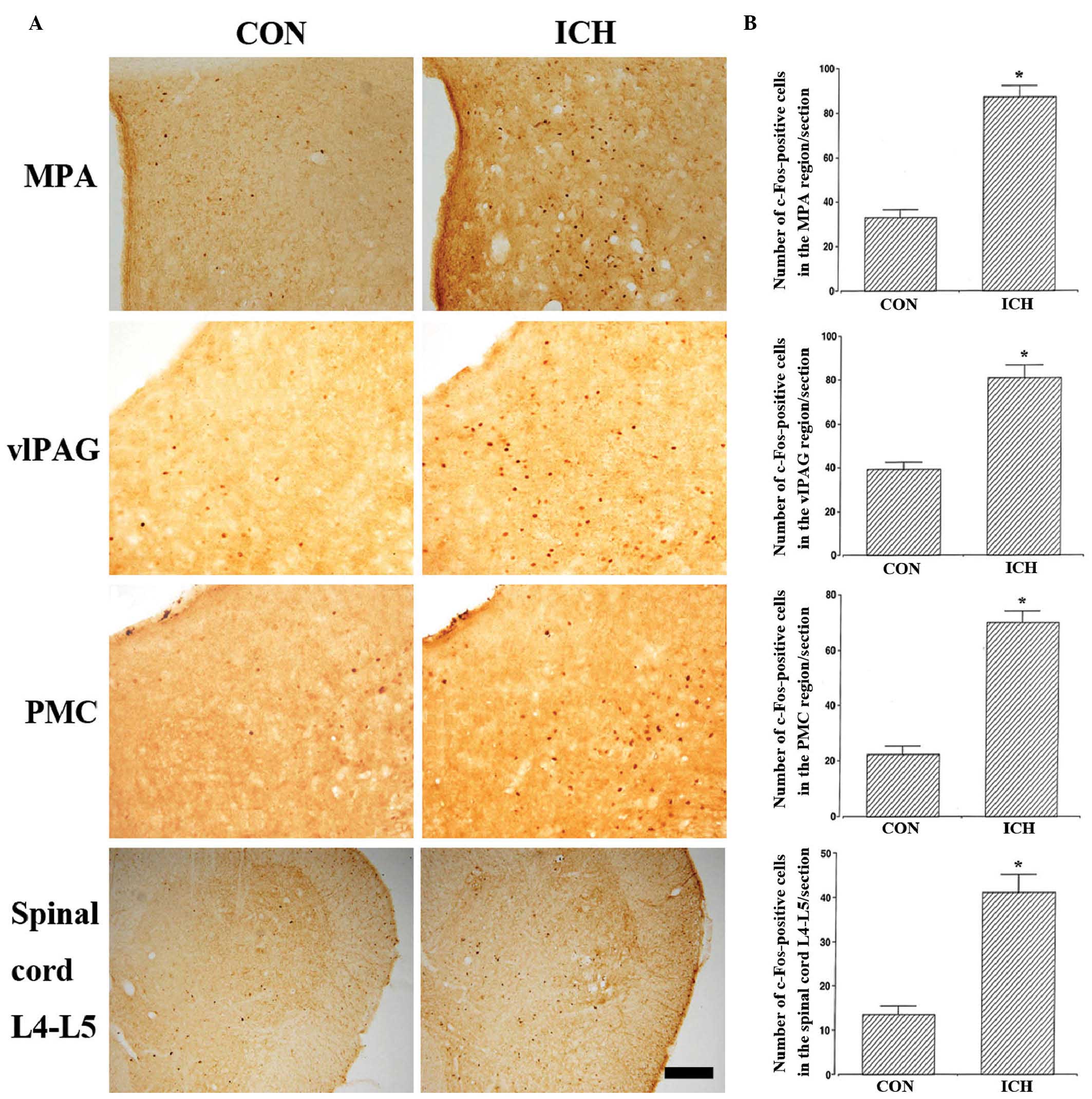
Effect of ICH induction on c-Fos
expression in the neuronal voiding centers
The results of the analysis of c-Fos-positive cells
in the neuronal voiding centers are presented in Fig. 2 and Table I. These results indicate that the
c-Fos expression levels in the neuronal voiding centers were
significantly increased in the rats with induction of ICH compared
with the control rats (P<0.05).
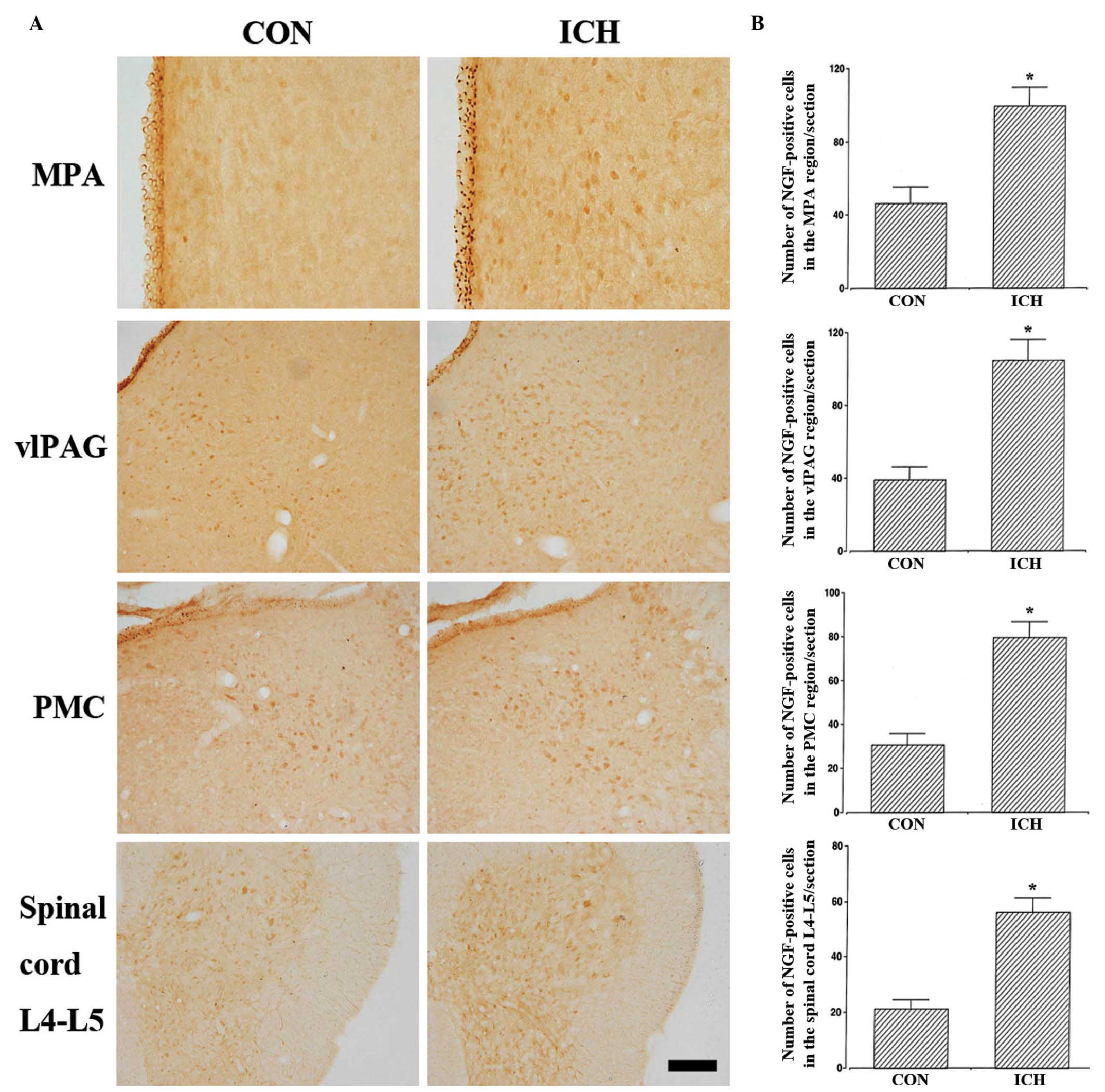
Effects of ICH induction on NGF
expression in the neuronal voiding centers
The results from the analysis of NGF-positive cells
in the neuronal voiding centers are presented in Fig. 3 and Table I. These results indicate that NGF
expression levels in the neuronal voiding centers were
significantly increased in the rats with induction of ICH compared
with the control rats (P<0.05).
Discussion
The terms ICH and hemorrhagic stroke are used
interchangeably and epidemiological studies indicate that 8–18% of
strokes result from hemorrhagic insult (11). In ICH, bleeding occurs directly
into the brain parenchyma. The predominant mechanism is considered
to be leakage from small intracerebral arteries damaged by chronic
hypertension (11). Furthermore,
in 20–40% of patients with ischemic infarction, hemorrhagic
transformation may occur within one week of ictus (12). Therefore, hemorrhage is an
important pathophysiological factor in hemorrhagic and ischemic
stroke.
Urinary symptoms are associated with disability and
are reported to exert a considerable impact on the quality of life
of stroke survivors (13). Urinary
incontinence (UI) has been reported in 47% of stroke patients in
the acute phase and in 19% of patients at the six-month follow-up
(2). Urinary retention has been
reported in 47% of stroke patients within 72 h of the
cerebrovascular accident (1) and
in 29% within four weeks of the stroke (3). Urinary symptoms, including
alterations in urinary frequency, nocturia and a significantly
higher rate of urinary tract infections, are observed in patients
with urinary retention or incomplete bladder emptying (3). In a previous study, the frequencies
of urine storage and emptying disorders in hemorrhagic and ischemic
stroke patients with persistent bladder dysfunction were similar:
73.3% in the hemorrhagic stroke group versus 63.6% in the ischemic
stroke group (14).
However, the majority of experimental studies of the
pathophysiology and treatment of NLUTD in stroke have used a
cerebral infarction animal model (4). This experimental model, however, does
not accurately reflect hemorrhagic stroke. Therefore, this is not
an optimal animal model in which to analyze NLUTD associated with
hemorrhagic stroke. The induction of ICH by direct collagenase
injection into the brain has been used in previous neurological
studies (9,10). In the present study, previously
reported procedures for ICH induction in the hippocampal CA1 region
using a stereotaxic frame and type IV collagenase were adopted, and
ICH-induced changes in bladder function and neuronal voiding
centers were investigated.
Bladder contraction pressure and time were found to
be significantly increased in ICH-induced rats compared with normal
rats, whereas the voiding pressure and time were significantly
reduced by the induction of ICH, which indicated that ICH resulted
in a deterioration in bladder function and as a result, the
induction of OAB. Furthermore, the reductions observed in voiding
pressure and time in the ICH-induced rat model are consistent with
the findings in various previous OAB models (6–8),
demonstrating that OAB was induced in the ICH-induced rat model in
the present study. The findings from the present study provide
further support for those from a previous study that reported a
significant reduction in bladder capacity 2 h after middle cerebral
artery (MCA) occlusion in rats (15). Furthermore, the mean micturition
threshold pressure increased significantly in the cerebral
infarction group in that previous study, but the bladder
contraction pressure was not significantly affected by left MCA
occlusion compared with a sham group (15). In another study, cerebral
infarction induced by MCA occlusion reduced bladder capacity in
rats, but did not produce a change in bladder contraction pressure
(16). The discrepancy in bladder
contraction results between the present study and previous studies
may be due to differences in the stroke mechanism, namely
hemorrhage versus ischemia. This highlights the importance of the
method used to induce stroke in experimental animal NLUTD
models.
The PMC is important in the control of urinary
bladder function. The hypothalamic PAG and MPA regions have been
associated with the PMC (17). The
PAG-PMC projection is hypothesized to be involved in the
micturition reflex. The vlPAG functions as a central sensorimotor
integrative relay of the micturition reflex via the reception of
sensory information concerning bladder fullness and this area
directly projects to the PMC (18). Neurons in the PAG regulate the
micturition reflex in animals and humans, since lesions in the PAG
cause severe urinary dysfunction (18,19).
In addition, the PMC is densely innervated by the MPA (18). The MPA sends projections to the PMC
that synapse on neurons directly through projections to the spinal
cord (17).
c-Fos expression occasionally serves as a marker for
stimuli-induced changes in the metabolic activity of neurons under
various conditions (20). In a
previous study, stimulation of lower urinary tract symptoms (LUTS)
was shown to cause changes in neuronal activity in neuronal
micturition centers (21).
Increased NGF levels have been observed in the urine of patients
with interstitial cystitis and painful bladder contractions
(22). NGF overexpression in the
bladder and urethra is associated with modulation disability of
micturition in patients with UI (23,24).
One biochemical study demonstrated that NGF regulates afferent
bladder neuronal plasticity subsequent to partial urethral
obstruction in female rats (24).
Increased levels of NGF in the bladder, spinal cord and dorsal root
ganglia have been associated with bladder hyperreflexia following
SCI in rats (25).
In the present study, the expression levels of c-Fos
and NGF in the neuronal voiding centers was significantly increased
following the induction of ICH, indicating that ICH facilities
bladder instability through enhanced neuronal activation in central
micturition areas. These findings are consistent with those of a
previous study that reported increased NGF levels in the bladder
tissues and urine of patients with OAB and detrusor overactivity
(26), and increased c-Fos
expression levels in the neuronal voiding centers in OAB model rats
(27). Understanding the
underlying mechanisms by which ICH affects urinary function is
important. The limbic system is a complex set of neurological
structures that lies on the two sides of the thalamus immediately
under the cerebrum, and includes the hypothalamus, hippocampus,
amygdala and a number of proximal areas (28). These limbic systems are known
modulators of urinary function. In particular, the hypothalamus has
monosynaptic connections with the PAG and PMC (28), and modulates the bladder reflex and
therefore control of urinary function. Animal studies have shown
connections between the thalamus and the prefrontal regions and
also the PAG, which indicates that this structure is important in
the relay of information, presumably including bladder sensation
(21). Matsuura et al
(29) noted that the thalamus was
activated during the ‘full bladder’ state. Therefore, hemorrhagic
attacks of the limbic system result in changes in the synapses of
limbic areas, including the hypothalamus and hippocampus, and
neuronal activity alteration in the neuronal voiding areas. NLUTD,
including OAB and UI, may result from these alterations.
The ICH-induced NLUTD rat model in the present study
may therefore be a more appropriate model to analyze NLUTD in
stroke patients than the cerebral infarction model, as the model in
the present study more accurately reflects the nature of the
hemorrhage in hemorrhagic and ischemic stroke. Little consensus has
been reached with regard to how stroke patients with NLUTD should
be treated or monitored, and current treatment options for NLUTD
are also limited. Animal studies are required for the examination
of the efficacy or toxicity of novel medications. In the present
study, ICH-induced expression of c-Fos and NGF in the neuronal
voiding centers was observed in an animal model of NLUTD. Enhanced
c-Fos and NGF expression levels in the voiding centers indicates
activated neuronal systems that presumably induce LUTS associated
with NLUTD. Thus, the animal model developed in the present study
may be useful for future investigations of NLUTD associated with
stroke, particularly hemorrhagic stroke.
Acknowledgments
The abstract and figures of the present study were
presented as a poster during podium session 28 of the International
Continence Society 2014 annual meeting, held in Rio de Janeiro,
Brazil between the 20th and 24th October 2014. The present study
was supported by a grant from the National Research Foundation of
Korea (grant no. NRF-2012R1A1A1013173).
References
|
1
|
Burney TL, Senapati M, Desai S, Choudhary
ST and Badlani GH: Acute cerebrovascular accident and lower urinary
tract dysfunction: a prospective correlation of the site of brain
injury with urodynamic findings. J Urol. 156:1748–1750. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakayama H, Jørgensen HS, Pedersen PM,
Raaschou HO and Olsen TS: Prevalence and risk factors of
incontinence after stroke. The Copenhagen Stroke Study. Stroke.
28:58–62. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kong KH and Young S: Incidence and outcome
of poststroke urinary retention: A prospective study. Arch Phys Med
Rehabil. 81:1464–1467. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fry CH, Daneshgari F, Thor K, et al:
Animal models and their use in understanding lower urinary tract
dysfunction. Neurourol Urodyn. 29:603–608. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kajbafzadeh AM, Mohammadinejad P, Hojjat
A, et al: The timing of established detrusor hyperreflexia in a rat
model of neuropathic bladder. J Surg Res. 178:346–351. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ozsoy O, Ozsoy U, Stein G, et al:
Functional deficits and morphological changes in the neurogenic
bladder match the severity of spinal cord compression. Restor
Neurol Neurosci. 30:363–381. 2012.PubMed/NCBI
|
|
7
|
Kitta T, Kakizaki H, Tanaka H, et al: An
alpha-amino-3-hydrox y-5-methyl-4-isoxazolepropionate
glutamate-receptor antagonist can inhibit premicturition
contractions in rats with bladder outlet obstruction. BJU Int.
100:181–186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Juszczak K, Gil K, Wyczolkowski M and Thor
PJ: Functional, histological structure and mastocytes alterations
in rat urinary bladders following acute and [corrected] chronic
cyclophosphamide treatment. J Physiol Pharmacol. 61:477–482.
2010.PubMed/NCBI
|
|
9
|
Ohnishi M, Katsuki H, Fujimoto S, et al:
Involvement of thrombin and mitogen-activated protein kinase
pathways in hemorrhagic brain injury. Exp Neurol. 206:43–52. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohnishi M, Katsuki H, Fukutomi C, et al:
HMGB1 inhibitor glycyrrhizin attenuates intracerebral
hemorrhage-induced injury in rats. Neuropharmacology. 61:975–980.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feigin VL, Lawes CM, Bennett DA and
Anderson CS: Stroke epidemiology: a review of population-based
studies of incidence, prevalence, and case-fatality in the late
20th century. Lancet Neurol. 2:43–53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mullins ME, Lev MH, Schellingerhout D,
Gonzalez RG and Schaefer PW: Intracranial Hemorrhage Complicating
Acute Stroke: How Common is Hemorrhagic Stroke on Initial Head CT
Scan and How Often is Initial Clinical Diagnosis of Acute Stroke
Eventually Confirmed? AJNR Am J Neuroradiol. 26:2207–2212.
2005.PubMed/NCBI
|
|
13
|
Brittain KR, Perry SI, Peet SM, et al:
Prevalence and impact of urinary symptoms among community-dwelling
stroke survivors. Stroke. 31:886–891. 2000. View Article : Google Scholar
|
|
14
|
Ersoz M, Tunc H, Akyuz M and Ozel S:
Bladder storage and emptying disorder frequencies in hemorrhagic
and ischemic stroke patients with bladder dysfunction. Cerebrovasc
Dis. 20:395–399. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagasaka Y, Yokoyama O, Komatsu K, et al:
Effects of opioid subtypes on detrusor overactivity in rats with
cerebral infarction. Int J Urol. 14:226–232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yusup A, Akino H, Miwa Y, et al: Effects
of antimuscarinics on voiding function after cerebral infarction in
a rat model of overactive bladder. Eur J Pharmacol. 577:143–149.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rickey LM, Sarkey S and DonCarlos LL:
Estrogen-sensitive projections from the medial preoptic area to the
dorsal pontine tegmentum, including Barrington’s nucleus, in the
rat. Neurourol Urodyn. 27:440–445. 2008. View Article : Google Scholar
|
|
18
|
Blok BF and Holstege G: Direct projections
from the periaqueductal gray to the pontine micturition center
(M-region). An anterograde and retrograde tracing study in the cat.
Neurosci Lett. 166:93–96. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakakibara R, Hattori T, Yasuda K, et al:
Micturitional disturbance in Wernicke’s encephalopathy. Neurourol
Urodyn. 16:111–115. 1997. View Article : Google Scholar
|
|
20
|
Dragunow M and Faull R: The use of c-fos
as a metabolic marker in neuronal pathway tracing. J Neurosci
Methods. 29:261–265. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kavia RB, Dasgupta R and Fowler CJ:
Functional imaging and the central control of the bladder. J Comp
Neurol. 493:27–32. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okragly AJ, Niles AL, Saban R, et al:
Elevated tryptase, nerve growth factor, neurotrophin-3 and glial
cell line-derived neurotrophic factor levels in the urine of
interstitial cystitis and bladder cancer patients. J Urol.
161:438–442. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Furuta A, Kita M, Suzuki Y, et al:
Association of overactive bladder and stress urinary incontinence
in rats with pudendal nerve ligation injury. Am J Physiol Regul
Integr Comp Physiol. 294:R1510–R1516. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schnegelsberg B, Sun TT, Cain G, et al:
Overexpression of NGF in mouse urothelium leads to neuronal
hyperinnervation, pelvic sensitivity, and changes in urinary
bladder function. Am J Physiol Regul Integr Comp Physiol.
298:R534–R547. 2010. View Article : Google Scholar :
|
|
25
|
Seki S, Sasaki K, Fraser MO, et al:
Immunoneutralization of Nerve Growth Factor in Lumbosacral Spinal
Cord Reduces Bladder Hyperreflexia in Spinal Cord Injured Rats. J
Urol. 168:2269–2274. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu HT, Chancellor MB and Kuo HC: Urinary
Nerve Growth Factor Levels are Elevated in Patients with Detrusor
Overactivity and Decreased in Responders to Detrusor Botulinum
Toxin-A Injection. Eur Urol. 56:700–706. 2009. View Article : Google Scholar
|
|
27
|
Kim SE, Shin MS, Kim CJ, et al: Effects of
Tamsulosin on Urinary Bladder Function and Neuronal Activity in the
Voiding Centers of Rats with Cyclophosphamide-induced Overactive
Bladder. Int Neurourol J. 16:13–22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Holstege G: Micturition and the soul. J
Comp Neurol. 493:15–20. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matsuura S, Kakizaki H, Mitsui T, et al:
Human brain region response to distention or cold stimulation of
the bladder: a positron emission tomography study. J Urol.
168:2035–2039. 2002. View Article : Google Scholar : PubMed/NCBI
|