Introduction
MicroRNAs (miRs) are a class of small (21–24 bp),
noncoding RNAs, which are involved in the negative regulation of
gene expression. miRs bind to complementary sequences in the
3′-untranslated region (3′-UTR) of their target mRNAs, causing
destabilization of the transcript or inhibition of translation
(1–3). miRs are involved in a number of
biological and pathological processes, including cell
proliferation, cell differentiation and carcinogenesis (4,5).
Previous studies have suggested that miR expression is
tissue-specific and that a number of miRs are expressed in the
human placenta (6,7). A previous study demonstrated that
miR-18a is downregulated in pre-eclamptic placentas (8). miR-18a is a component of the
miR-17-92 gene cluster, which is located on chromosome 13q31.3.
Evidence has suggested that miR-18a targets the estrogen receptor
α-3′ (ESRα-3′) UTR in human hepatocellular carcinoma cells
(9).
ESRs are ligand-activated transcription factors.
They are members of the nuclear hormone receptor superfamily, which
mediate the pleiotropic effects of the steroid hormone, estrogen
via a range of developmental and physiological processes (10). There are two forms of ESR: ESRα and
ESRβ, which are encoded by ESR1 and ESR2, respectively. ESRα is
predominantly expressed in the ovaries, uterus and placenta
(11), while ESRβ is expressed in
a number of types of tissues (12). A previous study suggested that ESRα
mRNA and protein levels were significantly increased in
pre-eclamptic pregnancies compared with levels in healthy
pregnancies (13).
Previous studies have demonstrated that miR-18a is
under-expressed (8), and ESRα is
overexpressed, in pre-eclamptic placentas (13). The results of previous studies have
suggested that miR-18a may be involved in the pathogenesis of
pre-eclamptic pregnancies via ESRα regulation. In order to gain
further understanding of the association between miR-18a and ESRα
in human trophoblast cells, the present study analyzed the effect
of miR-18a on trophoblast invasion, cell apoptosis and ESRα
expression in JEG-3 cells.
Materials and methods
Cell culture and transient
transfection
JEG-3 human trophoblast choriocarcinoma cells were
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA). JEG-3 cells were cultured in Dulbecco’s modified Eagle’s
medium (DMEM; Gibco Life Technologies, Carlsbad, CA, USA),
containing 10% fetal bovine serum (FBS; Gibco Life Technologies,
Carlsbad, CA, USA). Cells were cultured at 37°C, in a humidified
atmosphere at 5% CO2.
Pre-miR-18a mimics (pre-miR-18a), pre-miR negative
control (NC), an miR-18a inhibitor and miR-18a inhibitor
FAM-labeled controls were used to determine the transfection
efficiency and were purchased from Jima (Beijing, China). JEG-3
cells were transfected with pre-miR-18a or miR-18a inhibitors using
Lipofectamine 2000® (Gibco Life Technologies) according
to the manufacturer’s instructions. The medium was replaced with
fresh growth medium following 12 h of transfection. Following 48–72
h of transfection, a Transwell invasion assay was conducted.
Transwell invasion assay
Transfected cells were seeded at 4×105
cells/insert in Transwell inserts (8-μm pore size; Costar,
Corning, Cambridge, MA, USA) pre-coated with BD matrigel matrix (BD
Biosciences, USA), and they were incubated with DMEM medium,
without FBS. Lower chambers were loaded with DMEM medium,
containing 10% FBS.
Cells were incubated at 37°C for 24 h. Following
incubation, invading cells were fixed with 95% ethanol and stained
using crystal violet (Beyotime Institue of Biotechnology, Nanjing,
China). Non-invading cells were removed with a cotton swab. The
cell invasion index was determined by counting the number of
stained cells in ten randomly selected non-overlapping fields of
the membranes, using a light microscope (TE2000S; Nikon, Japan;
magnification, x200).
Cell apoptosis assay via flow
cytometry
Following transfection for 48 h, JEG-3 cell
apoptosis was analyzed using flow cytometry. 5×105
treated cells/well were seeded into 24-well plates and were
incubated with annexin V and propidium iodide (BD Biosciences, San
Diego, CA, USA) for 15 min at room temperature, in darkness. Cells
were then analyzed using a fluorescence activated cell sorting
(FACS) flow cytometer (BD Biosciences). Experiments were repeated
three times.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was isolated from JEG-3 cells using
TRIzol® (Invitrogen Life Technologies, Carlsbad, CA,
USA). RNA integrity was confirmed using electrophoresis in a 1.5%
agarose denaturing gel (Sangon Biotech, Shanghai, China). In order
to detect the presence of ESRα, 1 μg total RNA was reverse
transcribed into cDNA and primed with oligonucleotides, using a
RevertAid first-strand cDNA synthesis kit (Thermo Fisher Scientific
Baltics, Vilnius, Lithuania). β-Actin was used as a positive
control.
In order to detect the miR, 2 μg of total RNA
was reverse transcribed into cDNA with a gene-specific RT primer,
which is capable of folding into a stem-loop structure. The highly
conserved and universally expressed small nuclear RNA, U6, was used
as an endogenous control. Primer sequences and PCR conditions are
provided in Table I. A
25-μl PCR master mix was prepared as follows: RT products (1
μl), dNTPs (200 μmol/l), MgCl2 (2 mmol/l),
Taq DNA polymerase (1 IU)and primers (10 pmol).
 | Table IPrimer sequences and reaction
conditions for reverse transcription-polymerase chain reaction. |
Table I
Primer sequences and reaction
conditions for reverse transcription-polymerase chain reaction.
| Gene | Primer sequences | Annealing temperature
(°C) | Cycle (n) |
|---|
| ESRα | F:
CCTGGCTAGAGATCCTGAT | 56 | 31 |
| R:
CCCTGGTTCCTGTCCAAGA |
| β-actin | F:
TCATCACTATTGGCAACGAGC | 55 | 25 |
| R:
AACAGTCCGCCTAGAAGCAC |
| miR-18a | RT:
GTCGTATCCAGTGCAGGGTCCGAG | 50 | 30 |
|
GTATTCGCACTGGATACGACTATCTG |
| F:
GTGCTAAGGTGCATCTAGTGCAG |
| R:
GTGCAGGGTCCGAGGT |
| U6 | RT:
AACGCTTCACGAATTTGCGT | 55 | 29 |
| F:
CTCGCTTCGGCAGCACA |
| R:
AACGCTTCACGAATTTGCGT |
PCR amplification was verified within the
exponential phase of PCR using preliminary experiments. PCR
products were subjected to electrophoresis on agarose gels, and the
relative densities of target genes, standardized against that of
the control genes was analyzed, using Image-Pro Plus (version 6.0;
Media Cybernetics, Inc., Rockville, MD, USA).
Western blot analysis
Infected cells were washed three times with ice-cold
phosphate-buffered saline (PBS) and then lysed with 1X lysis buffer
(Cell Signaling Technology, Inc., Danvers, MA, USA). The
supernatant was collected following centrifugation at 12,000 × g
for 10 min at 4°C and protein estimation was conducted using a
Bradford assay (14). Protein (30
μg) per lane was separated using 10% SDS-polyacry1-amide gel
electrophoresis and transferred onto a polyvinylidene fluoride
membrane (Hybond; GE Healthcare, Chalfont, UK) via semidry
electroblotting. The membrane was blocked in 5% non-fat milk in PBS
with Tween-20® (PBST) for 1 h at room temperature, and
incubated with rabbit antibodies against human ESRα (1:2,500;
rabbit monolonal; cat. no. ab32063; Epitomics, Burlingame, CA,
USA), in PBS at 4°C overnight.
The membrane was washed PBST and incubated with a
horseradish peroxidase-conjugated secondary antibody (1:5,000; cat.
no. cw0111; Kangwei, Beijing, China) for 1 h at room temperature.
Expression signals were detected using the Enhanced
Chemiluminescent Substrate (Pierce Biotechnology, Inc., Rockford,
IL, USA). The membrane was stripped and reprobed with the
rabbit-anti-human β-actin antibody (1:3,000; Abcam, Cambridge, UK),
which was used as a positive control. ESRα expression was
standardized against that of β-actin, which was determined using
densi-tometric analysis (Image-Pro Plus 6.0; Media Cybernetics,
Inc., Rockville, MD, USA).
Luciferase reporter assay
In order to perform a luciferase reporter assay, the
3′-UTR segment of ESRα, which contains the miR-18a binding site
(NM_000125), was PCR amplified using the total RNA extracted from
JEG-3 cells and the following primers for ESRα: Forward:
5′-CCTAGCTAGCCAATGACCCAGGTGAGCTGCTCG-3′ and reverse:
5′-CCTAGCTAGCCATTCAATTGTCTGATAAACAAGC-3′ (9). The reporter plasmid was cloned by
inserting the 3′-UTR segment of ESRα into the XbaI site of a
pGL3-Promoter vector (Promega Corporation, Madison, WI, USA), in
order to generate pMIR-UTR. The mutant ESRα-3′-UTR fragment was
generated using a QuickChange II Site-Directed Mutagenesis kit
(Agilent Technologies, Inc., Santa Clara, CA, USA). Transfection
was performed using a Lipofectamine 2000 reagent.
Transfections of JEG-3 cells were performed using
pMIR-UTR and pre-miR-18a for the experimental group. For the
negative control group, transfections were performed using pMIR-UTR
and pre-miR-control. Transfections were performed with
pMIR-UTR-mutant and pre-miR-18a, for the mutant control group.
Following 48 h of transfection, cells were lysed and measured for
luciferase activity using a luciferase assay system (Promega
Corporation, Madison, WI, USA), according to the manufacturer’s
instructions. Cells were also transfected with 50 ng pRL-TK vector
as an internal standard.
Statistical analysis
Values are presented as the mean ± standard
deviation of three individual experiments. Comparisons between
groups were performed using one-way analysis of variance. In all
cases P<0.05 was considered to indicate a statistically
significant difference. Statistical analyses were performed using
SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA).
Results
Suppression of miR-18a expression
inhibits JEG-3 cell invasion
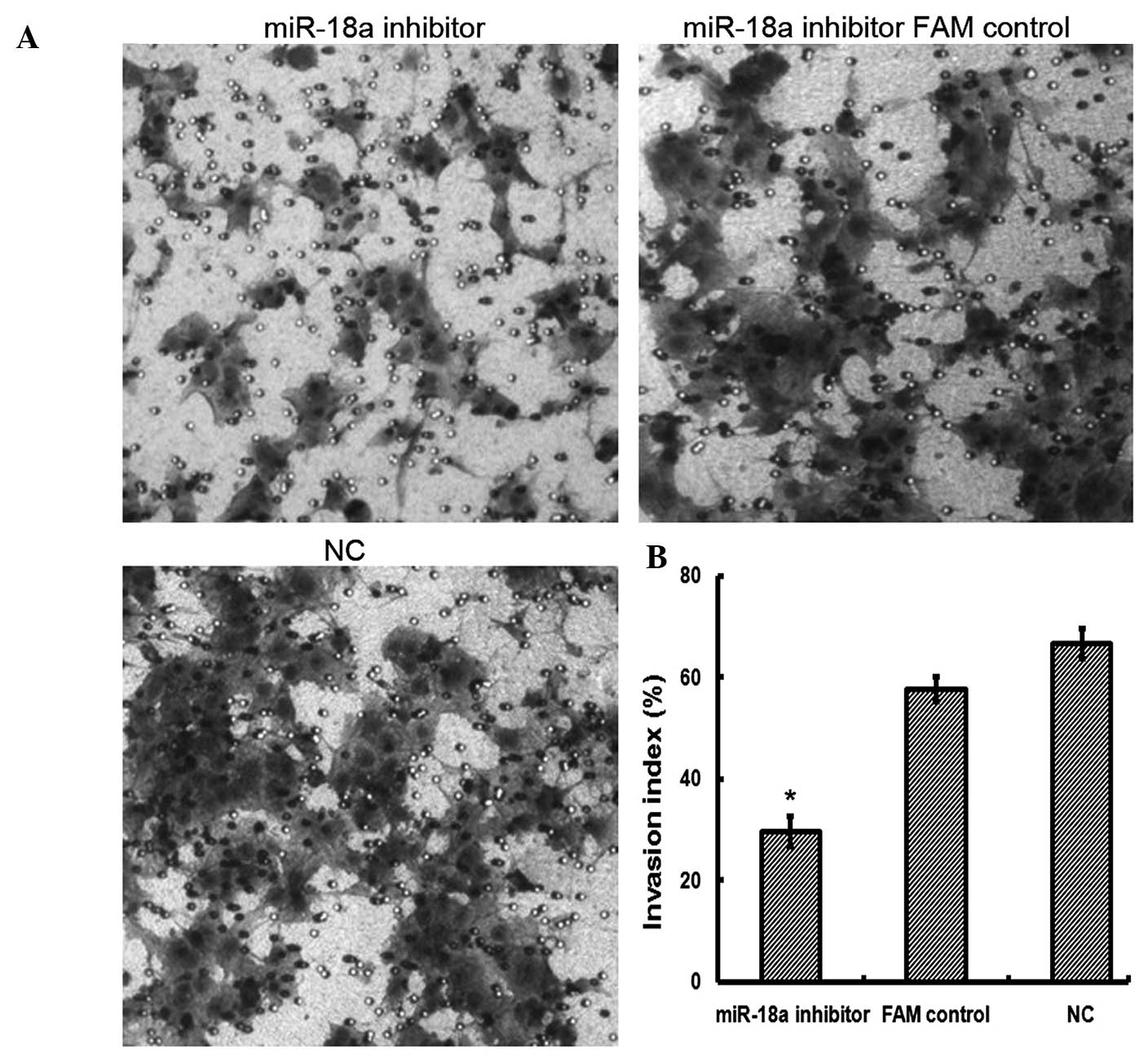
A cell invasion assay was performed in order to
investigate the effect of miR-18a on JEG-3 cell invasion. As shown
in Fig. 1, the suppression of
miR-18a significantly inhibited cell invasion in JEG-3 cells
(29.67±3.06; P<0.05) compared with that in the NC and FAM
control cells (66.67±3.06 and 57.67±2.52, respectively). The data
suggested that suppression of miR-18a may inhibit the invasive
capacity of JEG-3 cells.
Suppression of miR-18a leads to increased
cell apoptosis in JEG-3 cells
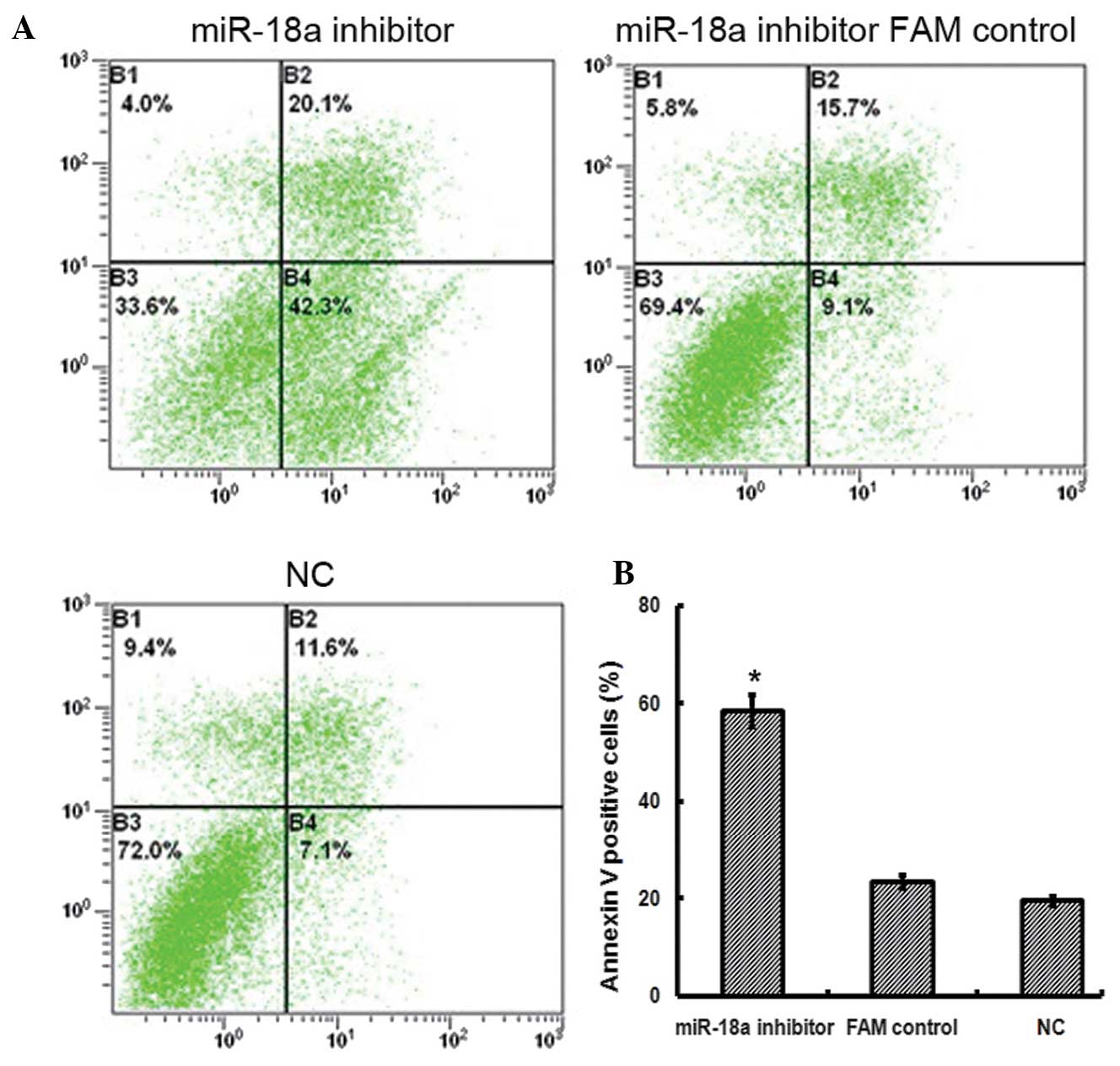
A cell apoptosis assay was performed using flow
cytometry, in order to investigate whether decreased miR-18a
expression in JEG-3 cells was associated with a change in cell
apoptosis. Following transfection with the miR-18a inhibitor, JEG-3
cell apoptosis was higher (58.63±3.27; P<0.05) than that in the
control cells (NC, 19.50±0.92; FAM control, 23.30±1.37; Fig. 2). The results of the present study,
therefore, suggested that the suppression of miR-18a expression may
promote JEG-3 cell apoptosis.
Validation of ESRα as a target of miR-18a
in JEG-3 cells
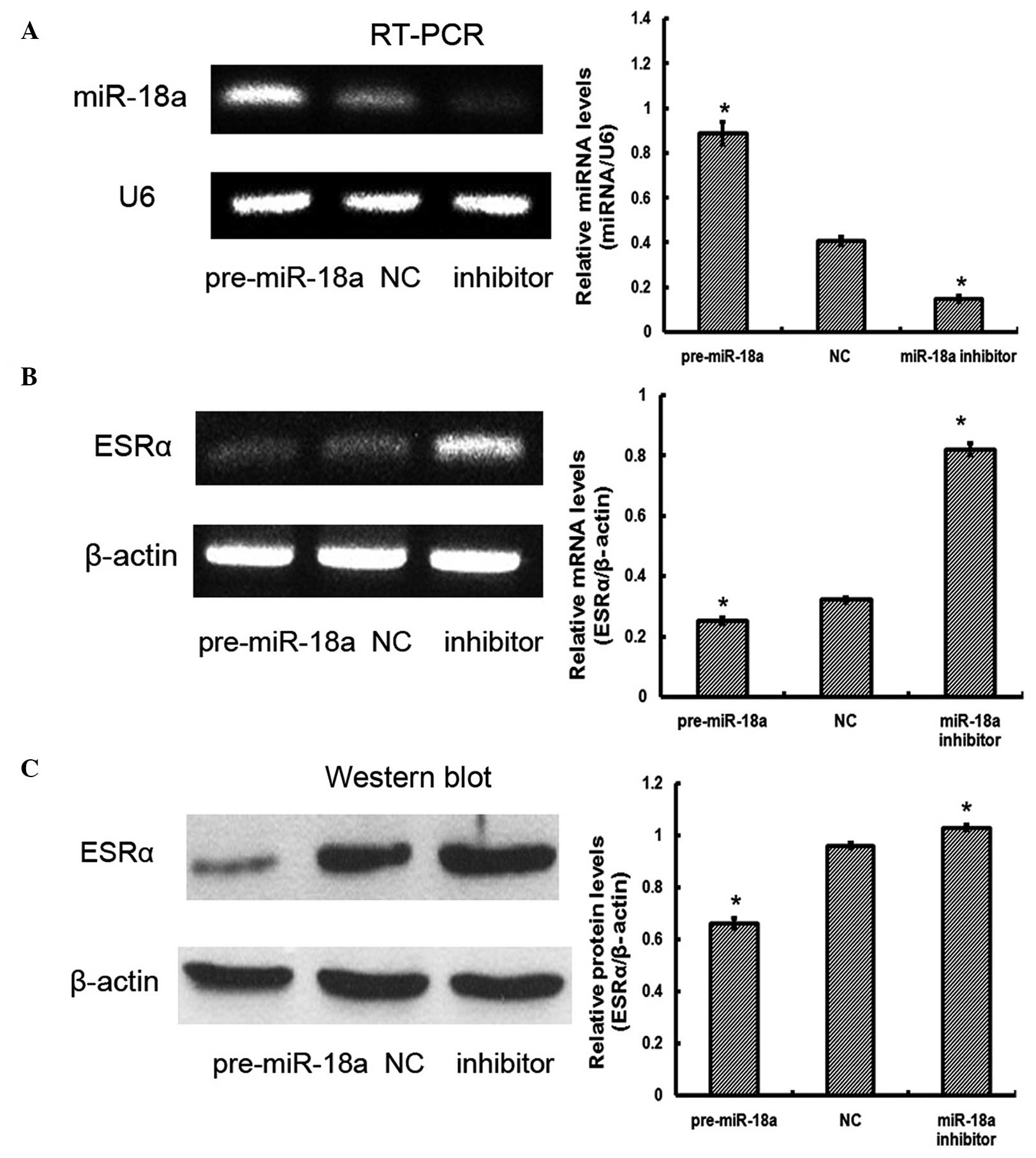
In order to assess whether miR-18a affects ESRα
expression, the levels of ESRα mRNA and protein were measured in
JEG-3 cells that had been transfected with pre-miR-18a or miR-18a
inhibitor. RT-PCR analysis suggested that transfection was
effective (Fig. 3A). Significant
differences were observed in ESRα mRNA expression levels:
Pre-miR-18a, 0.25±0.01; NC, 0.32±0.01; miR-18a inhibitor,
0.82±0.02; P<0.05 (Fig. 3B).
Significant differences were also observed in ESRα protein
expression levels: pre-miR-18a, 0.66±0.02; NC, 0.96±0.01; miR-18a
inhibitor, 1.03±0.01; P<0.05 (Fig.
3C). The results of the present study indicated that miR-18a
downregulated ESRα mRNA and protein expression in JEG-3 cells
compared with that in control cells.
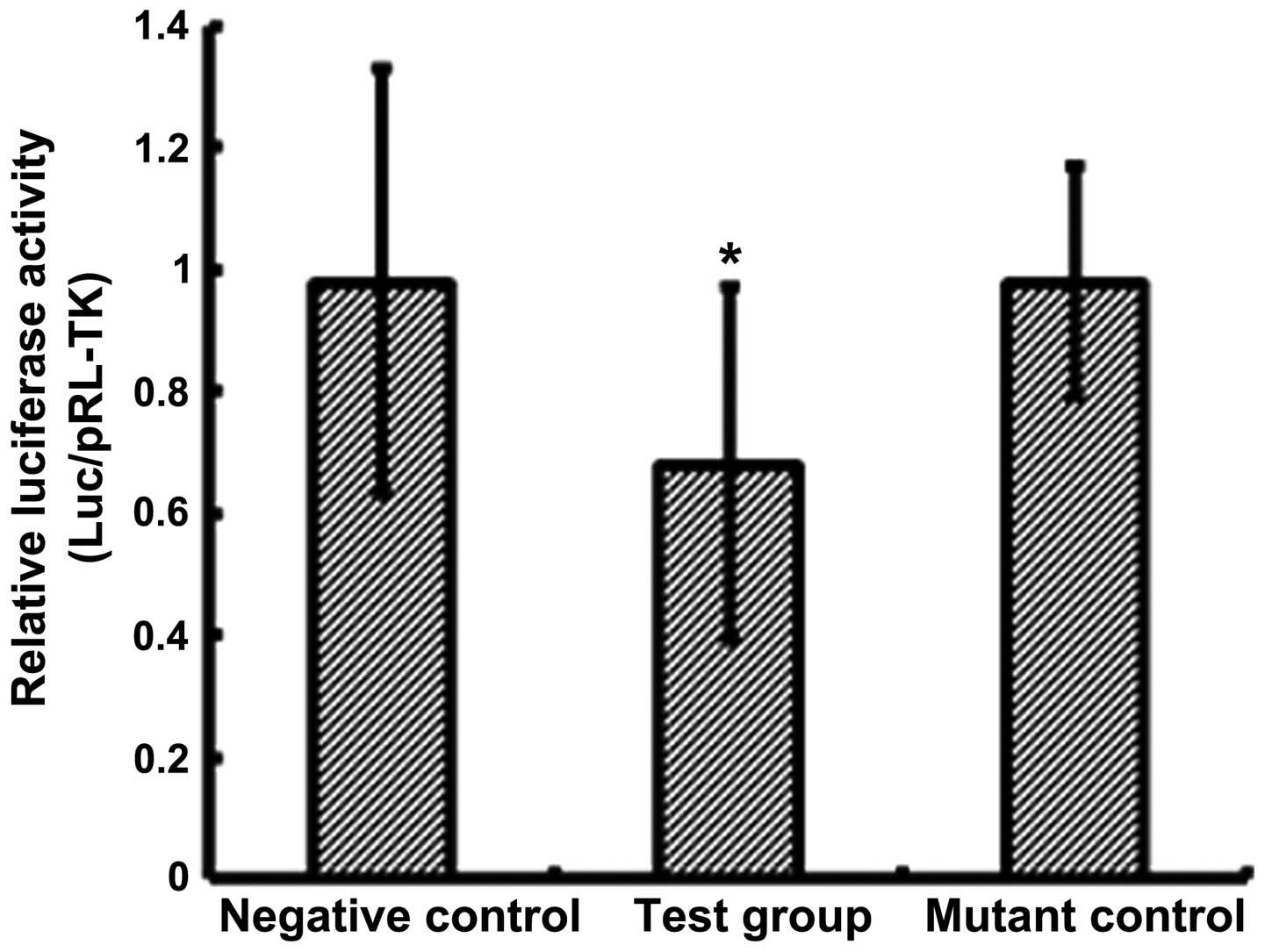
It has been shown that miRs modulate gene expression
through association with the 3′-UTR region of target genes
(1–3). A luciferase reporter assay was
conducted, and a significant reduction (0.68±0.29; P<0.05) in
luciferase activity was observed in pre-miR-18a-transfected cells,
compared with cells in the control groups (NC, 0.98±0.35; mutant
control, 0.98±0.19; Fig. 4). The
results of the present study suggested that ESRα is involved in the
regulation of miR-18a expression in JEG-3 cells.
Discussion
Suppression of miR-18a expression leads to decreased
cell invasion and increased cell apoptosis in JEG-3 human
trophoblast cells, by inducing ESRα expression. The results of the
present study supported the findings of Liu et al (9), which demonstrated that miR-18a may
suppress ESRα expression in human hepatocellular carcinoma
cells.
The JEG-3 cell line is a well-established human
trophoblast cell model that has been used in studies investigating
failed pregnancies (15–17). In the present study, in
vitro experiments were performed in order to determine the
effect of miR-18a on JEG-3 cell invasion. The results suggested
that the suppression of miR-18a expression may inhibit the invasive
capacity of JEG-3 cells. Xu et al (18) demonstrated that overexpression of
miR-18a may promote cell invasion in HTR8/SVneo human trophoblast
cells by targeting Smad2 expression. Smad2 mediates TGF-β
signaling, which is involved in regulating trophoblast cell
invasion (19–21). Therefore, miR-18a may regulate
trophoblast cell invasion via the TGF-β signaling pathways.
Furthermore, different types of miRs may be involved in the
regulation of a single gene (22).
Therefore, it will useful to examine other targets of miR-18a in
future studies.
The suppression of miR-18a expression led to an
increase in JEG-3 cell apoptosis. The results of the present study
suggested that miR-18a may function as an antiapoptotic factor in
human trophoblast cells. Previous studies have demonstrated that
miR-18a is downregulated (8),
whilst ESRα expression is upregulated (13), in pre-eclamptic placentas, compared
with levels in healthy placentas. The expression of miR-18a was
negatively correlated with ESRα expression in pre-eclampsia, which
suggests that miR-18a is involved in the pathogenesis of
pre-eclampsia, via negative regulation of ESRα expression (8–13).
Recent studies have shown that ESRα expression is involved in cell
apoptosis (23), and that a change
in cell apoptosis is associated with the pathogenesis of
pre-eclampsia (24). Therefore,
the low expression of miR-18a may affect trophoblast cell apoptosis
by regulating ESRα expression, which may contribute to
pre-eclampsia. Further investigation is therefore required, in
order to clarify the involvement of miR-18a in pre-eclamptic
pregnancies.
The results of the present study demonstrated that
miR-18a suppressed ESRα expression by targeting its 3′-UTR binding
sites. However, ESRα expression was not completely inhibited in
response to miR-18a overexpression (Fig. 4). miR-18a gene overexpression led
to a decrease in luciferase reporter gene expression by ~30%
(Fig. 4), which suggests that
additional miRs may participate in the regulation of ESRα
expression. Studies have demonstrated that a single gene may be
regulated by a number of different miRs (1–3,22).
Therefore, further studies are required, in order to investigate
the involvement of other miRs in the regulation of ESRα
expression.
In conclusion, the present study demonstrated that
miR-18a expression suppresses ESRα expression by targeting binding
sites in the 3′-UTR. The suppression of miR-18a led to a decrease
in invasion and an increase in the apoptosis of JEG-3 cells. The
present study, therefore, provides evidence for the involvement of
miR-18a in the pathogenesis of pre-eclampsia.
Acknowledgments
This study was supported by the Chinese Natural
Science Foundation (grant nos. 81471474/31000660 and 81170582), the
Postdoctoral Science Foundation of China (grant nos. 2014T70964 and
2013M532203), and Tangdu Hospital Reserve Personnel Fund.
References
|
1
|
Zeng Y: Principles of micro-RNA production
and maturation. Oncogene. 25:6156–62. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lai EC: microRNAs: runts of the genome
assert themselves. Curr Biol. 13:R925–R936. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ke XS, Liu CM, Liu DP and Liang CC:
MicroRNAs: key participants in gene regulatory networks. Curr Opin
Chem Biol. 7:516–23. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bushati N and Cohen SM: microRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun BK and Tsao H: Small RNAs in
development and disease. J Am Acad Dermatol. 59:725–737. 2008.
View Article : Google Scholar
|
|
6
|
Barad O, Meiri E, Avniel A, Aharonov R,
Barzilai A, Bentwich I, Einav U, Gilad S, Hurban P, Karov Y, et al:
MicroRNA expression detected by oligonucleotide microarrays: system
establishment and expression profiling in human tissues. Genome
Res. 14:2486–2494. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lim LP, Lau NC, Garrett-Engele P, Grimson
A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu XM, Han T, Sargent IL, Yin GW and Yao
YQ: Differential expression profile of microRNAs in human placentas
from preeclamptic pregnancies vs normal pregnancies. Am J Obstet
Gynecol. 200:661.e1–e7. 2009. View Article : Google Scholar
|
|
9
|
Liu WH, Yeh SH, Lu CC, Yu SL, Chen HY, Lin
CY, Chen DS and Chen J: MicroRNA-18a prevents estrogen
receptor-alpha expression, promoting proliferation of
hepatocellular carcinoma cells. Gastroenterology. 136(2): 683–693.
2009. View Article : Google Scholar
|
|
10
|
Shao W and Brown M: Advances in estrogen
receptor biology: prospects for improvements in targeted breast
cancer therapy. Breast Cancer Res. 6:39–52. 2004. View Article : Google Scholar :
|
|
11
|
Kuiper GG, Carlsson B, Grandien K, Enmark
E, Häggblad J, Nilsson S and Gustafsson JA: Comparison of the
ligand binding specificity and transcript tissue distribution of
estrogen receptors alpha and beta. Endocrinology. 138:863–870.
1997.PubMed/NCBI
|
|
12
|
Kuiper GG, Enmark E, Pelto-Huikko M,
Nilsson S and Gustafsson JA: Cloning of a novel receptor expressed
in rat prostate and ovary. Proc Natl Acad Sci USA. 93:5925–5930.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yin G, Zhu X, Guo C, Yang Y, Han T, Chen
L, Yin W, Gao P, Zhang H, Geng J, et al: Differential expression of
estradiol and estrogen receptor α in severe preeclamptic
pregnancies compared with normal pregnancies. Mol Med Rep.
7:981–985. 2013.PubMed/NCBI
|
|
14
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle ofproteindye binding. Anal Biochem. 72:248–254. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kilburn BA, Wang J, Duniec-Dmuchowski ZM,
Leach RE, Romero R and Armant DR: Extracellular matrix composition
and hypoxia regulate the expression of HLA-G and integrins in a
human trophoblast cell line. Biol Reprod. 62:739–747. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mouillot G, Marcou C, Zidi I, Guillard C,
Sangrouber D, Carosella ED and Moreau P: Hypoxia modulates HLA-G
gene expression in tumor cells. Hum Immunol. 68:277–285. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yie SM, Li LH, Li GM, Xiao R and Librach
CL: Progesterone enhances HLA-G gene expression in JEG-3
choriocarcinoma cells and human cytotrophoblasts in vitro. Hum
Reprod. 21:46–51. 2006. View Article : Google Scholar
|
|
18
|
Xu P, Zhao Y, Liu M, Wang Y, Wang H, Li
YX, Zhu X, Yao Y, Wang H, Qiao J, et al: Variations of microRNAs in
human placentas and plasma from preeclamptic pregnancy.
Hypertension. 63:1276–1284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Irving JA and Lala PK: Functional role of
cell surface integrins on human trophoblast cell migration:
regulation by TGF-beta, IGF-II, and IGFBP-1. Exp Cell Res.
217:419–427. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Morrish DW, Dakour J and Li H: Functional
regulation of human trophoblast differentiation. J Reprod Immunol.
39:179–195. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Caniggia I, Grisaru-Gravnosky S,
Kuliszewsky M, Post M and Lye SJ: Inhibition of TGF-beta 3 restores
the invasive capability of extravillous trophoblasts in
preeclamptic pregnancies. J Clin Invest. 103:1641–1650. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jackson RJ and Standart N: How do
microRNAs regulate gene expression? Sci STKE. 2007:re12007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen P, Wang H, Duan Z, Zou JX, Chen H, He
W and Wang J: Estrogen-related receptor alpha confers methotrexate
resistance via attenuation of reactive oxygen species production
and P53 mediated apoptosis in osteosarcoma cells. Biomed Res Int.
2014:6160252014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Levy R: The role of apoptosis in
preeclampsia. Isr Med Assoc J. 7:178–181. 2005.PubMed/NCBI
|