Introduction
Ovarian cancer (OC) is the gynecological cancer with
the highest mortality rate. There were an estimated 22,280 new
cases and 15,500 fatalities in the United States in 2012 and a
survival rate of ~44% (1).
OC metastases include transcoelomic, lymphogenous
and hematogenous spread (2).
Lymphogenous metastasis is an indicator of disease aggressiveness,
recurrence and prognosis (3,4).
Kleppe et al (5) reported a
14.2% incidence of lymphatic metastases in stage I–II epithelial
OC, predominantly in the para-aortic and pelvic regions. In
advanced-stage disease, lymphatic metastases exceeds 50% (3,6–9).
Preclinical mouse models of lymphatic metastasis in OC remain
limited and the majority of models do not mimic the clinical
disease (10–14).
In the present study, a novel epithelial OC cell
line SKOV-3/LN403 with high metastatic potential for lymph node
metastasis was established by selection from the parental SKOV-3
cell line. The biological characteristics and potential genes and
pathways associated with the cell line were further investigated.
Microarray analysis was used to screen SKOV-3 and SKOV-3/LN403 for
genes and pathways involved in the molecular mechanism of lymphatic
metastasis.
Materials and methods
Cell culture and experimental
animals
The SKOV-3 human ovarian adenocarcinoma cell line
was obtained from the Shanghai Institute of Cell Biology of Chinese
Academy of Sciences (Shanghai, China). Cells were grown in RPMI
1640 medium supplemented with 10% (v/v) fetal bovine serum, 100
U/ml penicillin and 100 mg/ml streptomycin (all purchased from
Gibco Life Technologies, Grand Island, NY, USA). They were
maintained at 37°C in a humidified incubator containing 5%
CO2 and cultures were split twice per week.
Six-week-old female BALB/c nude mice were purchased
from the Animal Center of the Chinese Academy of Sciences
(Shanghai, China) and were maintained under specific pathogen-free
conditions. The current study was performed with the approval of
the Animal Ethics Committee of the Obstetrics and Gynecology
Hospital, Fudan University (Shanghai, China) and in accordance with
the Guide for the Ethical Treatment of Laboratory Animals from the
Ministry of Science and Technology of the People’s Republic of
China (publication no. 2006-398) (15).
Establishment of a novel cell
subline
SKOV-3/LN403 was selected following four rounds of
the following procedure: SKOV-3 cells were detached with 0.25%
trypsin (Gibco Life Technologies) and resuspended at a density of
1×107 cells/ml. The cells were implanted into the
abdominal cavities of five BALB/c nude mice by intraperitoneal
injection (2×106/mouse). Three weeks later, the mice
were sacrificed via cervical dislocation and all visible
retroperitoneal and para-aortic lymph nodes were removed. The nodes
were then grouped according to the mouse (designated 101–105).
Lymph nodes were trypsinized and cultivated under the conditions
described above, but with one passage every 3 days. Once stable
populations of SKOV-3/LN(101–105) were established, the suspended
cells were randomly selected and re-inoculated into five new
recipient BALB/c nude mice (mice 201–205). Following the same
procedures, SKOV-3/LN(201–205) populations were established and
re-inoculated. Following four rounds of selection from the parental
SKOV-3 cell line, SKOV-3/LN401-SKOV-3/LN405 were obtained.
SKOV-3/LN403 was selected at random and used in the following
experiments.
In vitro cell growth assay
SKOV-3 and SKOV-3/LN403 cells were seeded into eight
parallel wells of a quantitative cell analyzer (RTCA DP, Roche
Diagnostics GmbH, Mannheim, Germany) at a concentration of
1×105 cells/ml and were observed using phase-contrast
microscopy. Each well was seeded with 200 μl cells and the
cell index was measured over 96 h. Data were analyzed using RTCA
software, version 1.2 (ACEA Biosciences Inc., San Diego, CA,
USA).
Cell migration assay
Serum-starved SKOV-3 or SKOV-3/LN403 cells were
suspended in serum-free medium (SFM) and plated at a density of
1×105 cells/well onto gelatin-soaked membranes
(Osmonics, Inc., Livermore, CA, USA) in a membrane invasion culture
system (16,17). SFM was used as a chemoattractant in
the lower wells. Following incubation for 6 h in RPMI-1640 at 37°C,
the lower chambers were treated with 0.1% EDTA to retrieve the
cells. Randomly selected fields of fixed and stained migrating
cells were quantified with Image J software version 1.48 (National
Institutes of Health, Bethesda, MD, USA) (18). Experiments were performed in
triplicate.
Cell invasion assay
The membrane invasion culture system was also used
to perform invasion assays. In brief, 1×105 viable
serum-starved SKOV-3 or SKOV-3/LN403 cells were placed onto the
upper wells of a defined basement membrane matrix consisting of
human laminin (Sigma-Aldrich, St. Louis, MO, USA), type IV collagen
(Sigma-Aldrich) and gelatin (MP Biomedicals LLC, Santa Ana, CA,
USA) as the barrier to invasion. SFM was used as the
chemoattractant in the lower wells. The chambers were incubated for
24 h at 37°C and analyzed as described for the migration assay.
Invasion assays were performed in triplicate.
Lymph node metastases in vivo
SKOV-3 and SKOV-3/LN403 cells in the logarithmic
phase were harvested and injected into the abdominal cavities of 10
BALB/c nude mice (2×106 cells/mouse). Mouse health, the
degree of ascites and survival were observed every 2 days.
Following spontaneous death, the mice were dissected and the lymph
nodes and organ metastases were examined. The lymphatic nodes were
fixed in 10% neutral formalin, embedded in paraffin, cut into 5
μm tissue sections and stained with hematoxylin and eosin
(HE). The HE images were captured on an Olympus BX53 microscope
(Olympus, Tokyo, Japan) and cellSens Standard software
(Olympus).
SPECT-CT imaging in vivo
Small animal SPECT-CT (Nano SPECT/CT PLUS; Bioscan,
Inc., Washington, DC, USA) was used to analyze the lymph nodes of
an independent group of BALB/c nude mice anesthetized in an
anesthetic chamber with 4% isoflurane (Abbott Laboratories,
Chicago, IL, USA) to induce anesthesia, which was maintained with
2% isoflurane. Each mouse was confined in a gantry and injected
with 0.1 mCi99m Tc-sulfur colloid (Beijing Syncor Star
Medicinal Technology Co., Ltd., Beijing, China) in one footpad;
although the recommended concentration is 0.3 mCi, a smaller
concentration was used due to experimental limitations (19). Subsequent to injection, the mice
were forced to exercise in the animal cage for 1 h, then were
examined by NanoSPECT/CT small animal imaging tomographic γ-camera
for 10 min with static views. Images were analyzed with in
vivo Scope software version 1.44 (Bioscan, Inc.). SPECT-CT
imaging was performed at the Department of Nuclear Medicine, Fudan
University Shanghai Cancer Center (Shanghai, China).
Drug cytotoxicity assay
The IC50 was determined as described
previously (20). Briefly, 3,000
cells were seeded in each well of a 96-well plate and allowed to
adhere overnight. Following a resting period of 24 h, the medium
was exchanged for one containing increasing concentrations of
paclitaxel (Sigma-Aldrich). Following 72 h, a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was conducted. Absorbance was measured at 570 nm using a
multiwell scanning spectrophotometer (Multiskan; Spectrum, Thermo
Electron Co., Vantaa, Finland).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) analysis
RT-qPCR was performed to assess FAK mRNA expression
in SKOV-3 and SKOV-3/LN403 cells using the RNAqueous kit (Ambion,
Austin, TX, USA), following the manufacturer’s instructions. The
mRNA was then reverse-transcribed into cDNA using the Verso cDNA
synthesis kit (Thermo Scientific, Pittsburgh, PA, USA). The cDNA
was subjected to PCR amplification using the following primers
(Sangon Biotech, Shanghai, China): FAK, sense 5′-CCAGGGATTATGAGA
TTC-3′ and antisense 5′-GACACCAGAACATTCCGAGCA-3′ in the Applied
Biosystems 7500 system using SYBR Green master mix (Applied
Biosystems, Foster City, CA, USA). The amplification reactions were
performed for 35 cycles at 94°C for 50 sec, 55°C for 30 sec and
72°C for 2 min. The products were electrophoresed on a 1% agarose
gel (Sigma-Alrdich) containing ethidium bromide (Sigma-Aldrich) and
images of the bands were captured under UV light. β-actin was used
as an endogenous control.
Western blot analysis
Cells at 80% confluence were harvested and lysed in
modified radioimmunoprecipitation assay buffer (50 mmol/l Tris, 150
mmol/l NaCl, 1% Triton X-100, 0.5% deoxycholate, 25 μg/ml
leupeptin, 10 μg/ml aprotinin, 2 mmol/l EDTA and 1 mmol/l
sodium orthovanadate). Typically, 30 μg protein was
fractionated by 10% SDS-PAGE, transferred onto a nitrocellulose
membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA), blocked
with 5% nonfat milk in Tris-buffered saline with Tween-20 (10
mmol/l Tris (pH 8.0), 150 mmol/l NaCl and 0.05% Tween-20) for 1 h
at ambient temperature, and incubated with primary antibodies
against purified mouse anti-FAK (cat. no. 610088; 1:500) and
pFAK397 (cat. no. 611807; 1:1,000), each purchased from BD
Biosciences (San Jose, CA, USA) at 4°C overnight. Antibodies were
detected using 0.167 μg/ml horseradish peroxidase-conjugated
secondary antibody (Jackson Laboratory, Bar Harbor, ME, USA) and
developed using an enhanced chemiluminescence detection kit (Pierce
Chemical, Rockford, IL, USA). A densitometric analysis was
performed using Image J software (version 1.48) to interpret the
differences in western blot results.
cDNA microarray expression analysis and
target gene prediction
Total RNA was isolated from SKOV-3 and SKOV-3/LN403
cells with TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA)
and the RNeasy kit (Qiagen, Hilden, Germany) according to the
manufacturer’s instructions, including a DNase digestion step. RNA
quantity and purity were determined using an ND-1000
Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA)
and with denaturing gel electrophoresis. The samples were amplified
and labeled with the Agilent Quick Amp Labeling kit and hybridized
to an Agilent Whole Human Genome Oligo Microarray (Agilent
Technologies, Inc., Santa Clara, CA, USA) in Agilent’s SureHyb
Hybridization Chambers. The array contained 45,000 probes for human
genes. Microarray experiments were performed in triplicate
according to the manufacturer’s instructions. RNA from SKOV-3 and
SKOV-3/LN403 cells was reverse-transcribed to cDNA using the Verso
cDNA synthesis kit (Thermo Scientific). and labeled with Cy3 and
Cy5 dye (GE Healthcare, Chalfont St. Giles, UK), respectively.
Hybridization was performed using Agilent’s Surehyb chambers at
65°C for 16 h. The hybridized slides were washed using Agilent Gene
Expression wash buffers (part no. 5188-5327) then scanned with the
Agilent DNA microarray scanner using settings recommended by the
manufacturer. The microarray datasets were normalized in GeneSpring
GX (Agilent Technologies, Inc.) using the Agilent FE one-color
scenario (mainly median normalization; Agilent Technologies, Inc.).
Fold change (FC) analysis was performed by calculating the ratio of
SKOV-3 to SKOV-3/LN403 to identify differentially expressed genes.
Genes with an FC >2 were identified as upregulated and
alterations <0.5 fold represented downregulated expression.
Functional analysis was performed with the Kyoto Encyclopedia of
Genes and Genomes (KEGG) Pathway database (http://www.genome.jp/kegg/pathway.html) using the
WEB-based GEne SeT AnaLysis Toolkit (WebGestalt; http://bioinfo.vanderbilt.edu/webgestalt/). The
microarray experiment was performed by Shanghai KangChen Biotech,
Co., Ltd. (Shanghai, China).
Statistical analysis
Statistical analysis was performed in SPSS, version
10.0 (SPSS, Inc., Chicago, IL, USA) and P<0.05 was considered to
indicate a statistically significant difference. All results are
expressed as the mean ± standard error of the mean. Continuous
variables were compared using the Student’s t-test or an analysis
of variance.
Results
Selection of lymph node-metastatic
cells
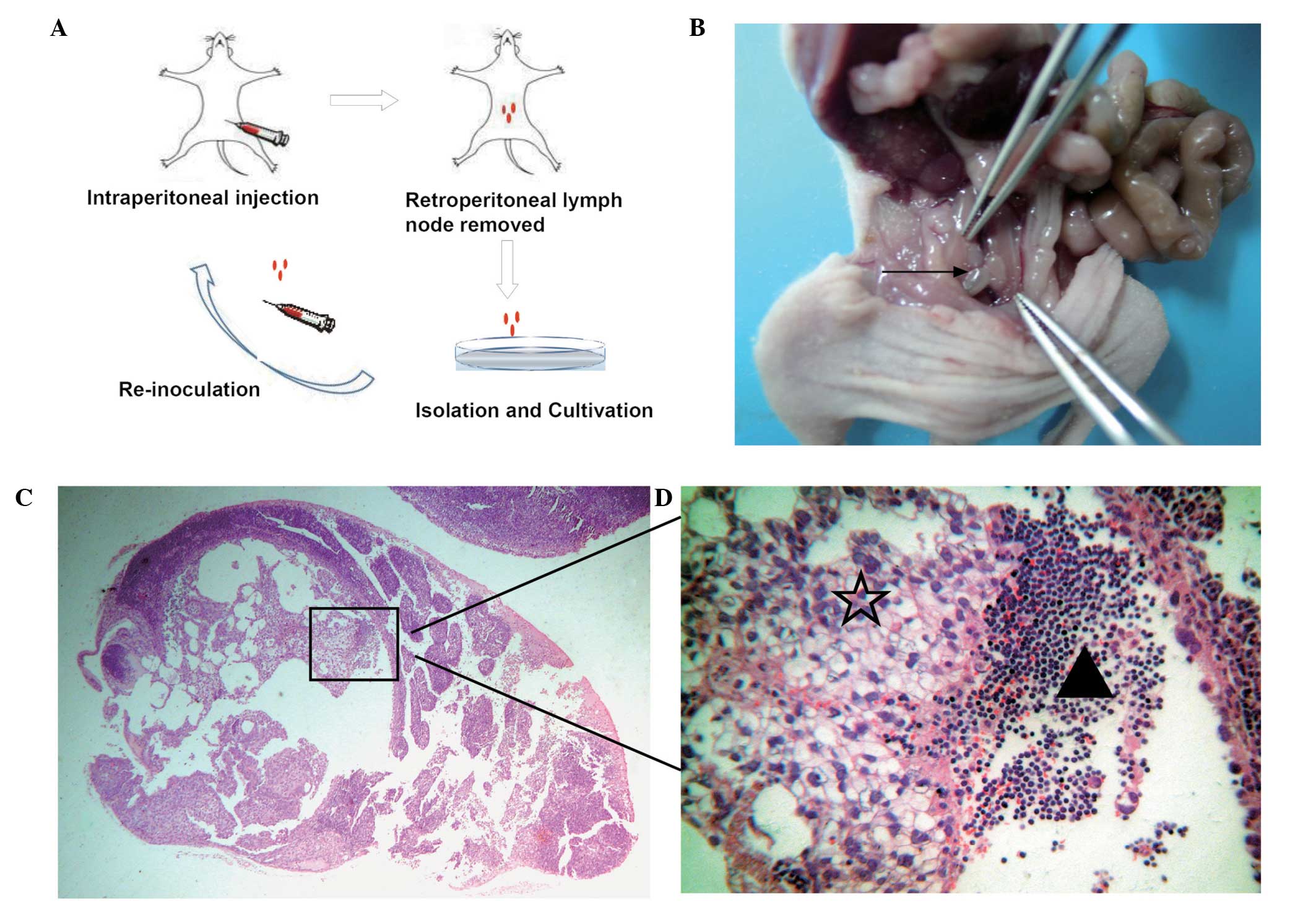
A flow diagram illustrating the establishment of a
highly lymphatic metastatic subline via in vivo selection is
presented in Fig. 1A. SKOV-3/LN403
cells were isolated by a series of steps including multiple
intraperitoneal injection and continuous screening of the
retroperitoneal lymph nodes (Fig.
1B), amplification and in vitro subculture.
Hematoxylin-eosin staining illustrated lymphatic node metastasis
(Fig. 1C and D).
In vitro growth curves of SKOV-3 and
SKOV-3/LN403 generated by quantitative cell counting demonstrated
SKOV-3/LN403 cells to have increased proliferation rates compared
with SKOV-3 cells. Data was representative of eight independent
experiments (Fig. 2A).
The morphological characteristics of SKOV-3
(Fig. 2B) and SKOV-3/LN403
(Fig. 2C) were observed and
compared by phase-contrast microscopy. SKOV-3 cells presented an
irregular appearance, whilst SKOV-3/LN403 cells grew in
clusters.
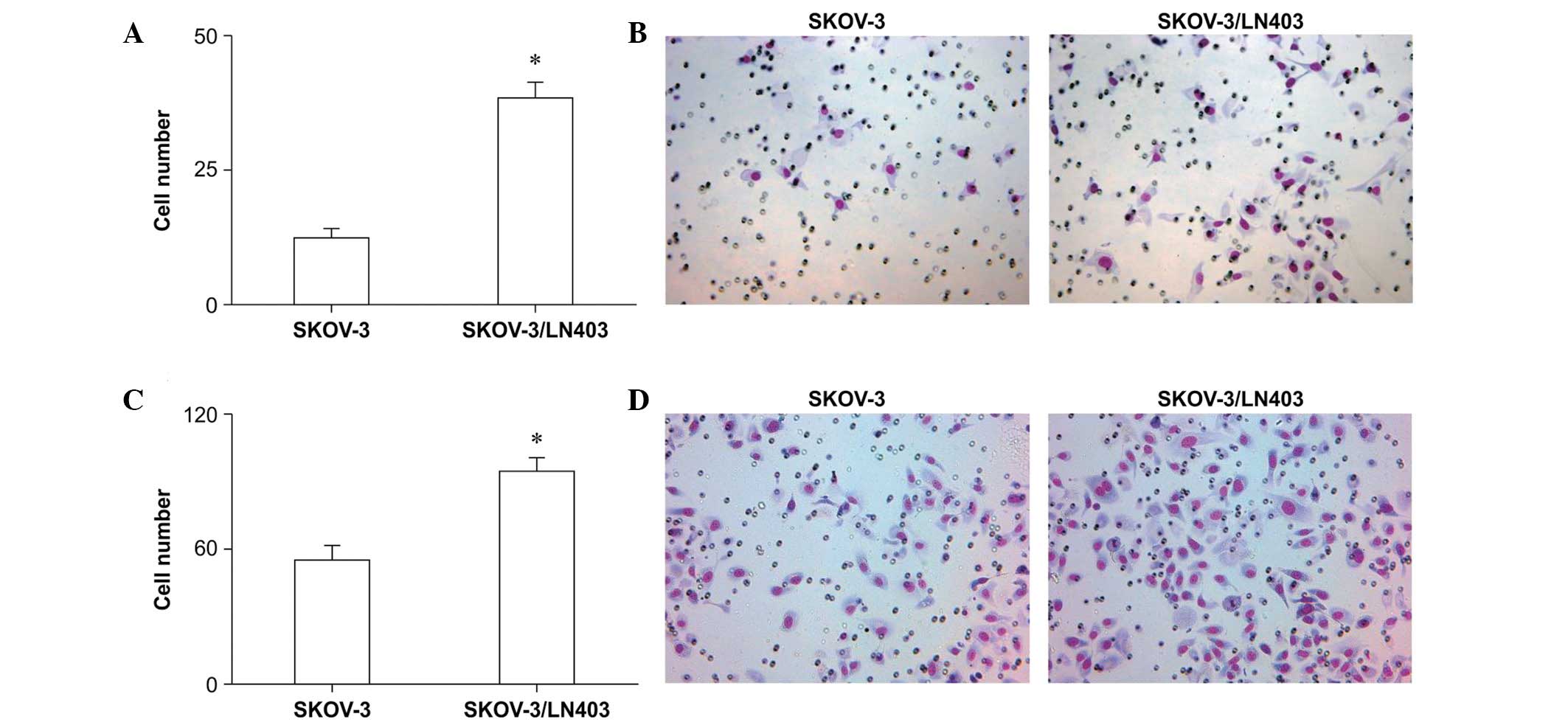
Cell migration and invasion assay
SKOV-3 and SKOV-3/LN403 cells were seeded and
allowed to migrate for 6 h. During this period, 12.40±1.514 SKOV-3
cells and 38.50±2.841 SKOV-3/LN403 cells migrated to the lower
chamber; this difference was statistically significant (Fig. 3A and B, P<0.0001). For the 24-h
invasion assay, the SKOV-3 and SKOV-3/LN403 cell numbers were
55.00±5.578 and 94.00±5.455 (Fig. 3C
and D, P<0.0001), respectively, suggesting that SKOV-3/LN403
cells are more invasive.
Drug cytotoxicity assay
The effect of paclitaxel on SKOV-3 and SKOV-3/LN403
cell growth is presented in Fig.
4. The IC50 values of SKOV-3/LN403 and SKOV-3 cells
were 104.72±23.889 and 5.33±1.215 nmol, respectively (Fig. 4).
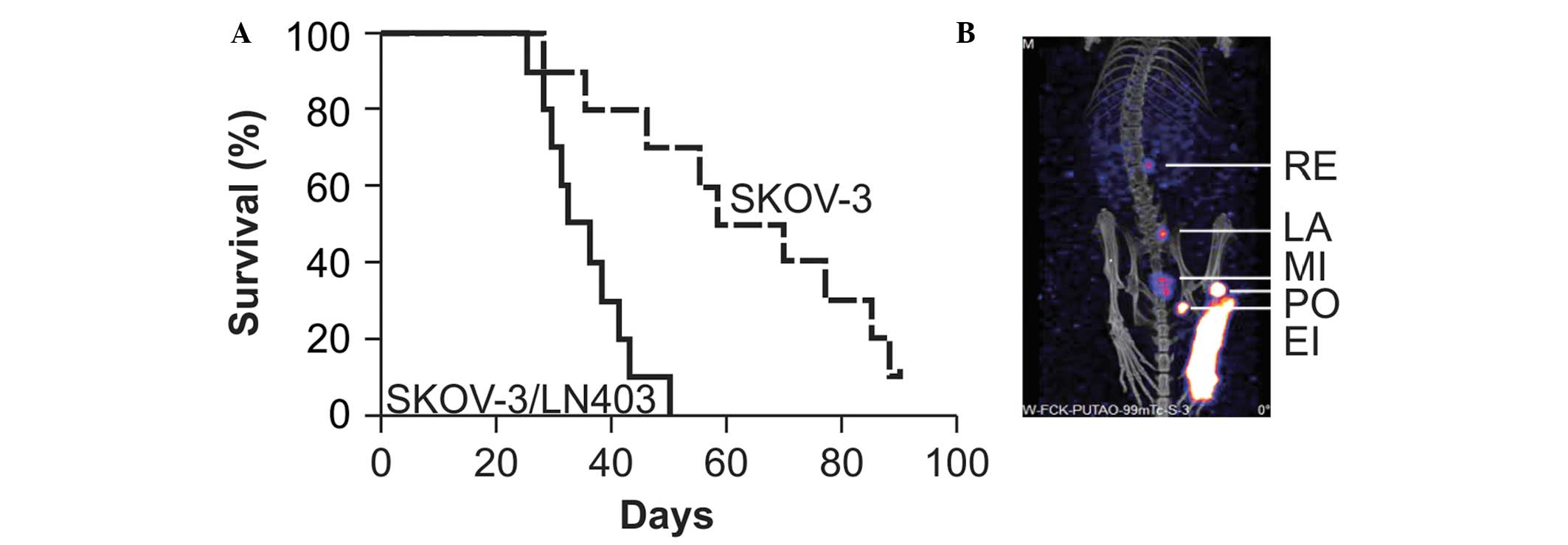
In vivo spontaneous lymphatic metastasis
via intraperitoneal injection
The average survival of nude mice with SKOV-3/LN403
cell implants was observed to be 35.1±8.3 days. The rate of lymph
node metastasis was 90% (9/10), abdominal tumors were widely
distributed and grew into an omental cake and bloody ascites were
detected in six mice. The average survival time of nude mice with
SKOV-3 cell implants was 64.5±21.2 days. The rate of lymph node
metastasis was 10% (1/10) and bloody ascites were detected in only
one mouse (Fig. 5A). Pathological
examination identified no metastasis of SKOV-3/LN403 cells to other
immune organs (data not shown). In addition, SPECT-CT enabled clear
visualization of the drainage lymph nodes (Fig. 5B).
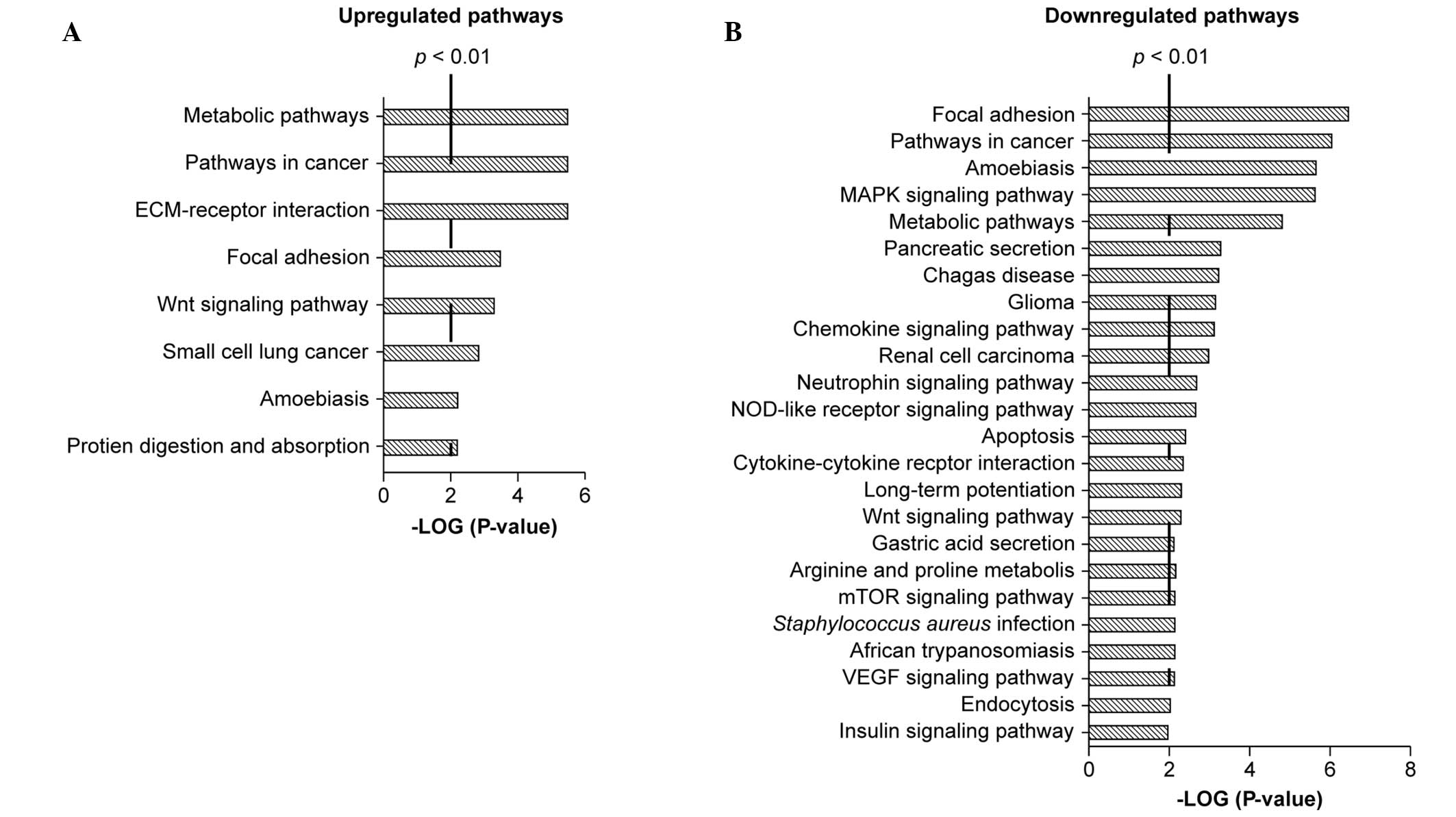
Candidate gene targets in lymphatic
metastasis
Microarray analyses of SKOV-3 and SKOV-3/LN403 cells
was conducted in order to screen for the genes and pathways
involved in the lymphatic metastaticity of the SKOV-3/LN403 cell
line. A total of 8 upregulated pathways (Fig. 6A) and 24 downregulated pathways
were identified in the KEGG database (Fig. 6B); these included pathways involved
in cancer, focal adhesion, metabolism, amebiasis and the Wnt
signaling pathway. The results identified differential expression
of the focal adhesion pathway components ECM, ITGA, ITGB, RTK and
CycD in SKOV-3 and SKOV-3/LN403 cells. Since the focal adhesion
kinase (FAK) is a prominent member of the focal adhesion pathway,
analysis of FAK expression was undertaken in the two cell lines.
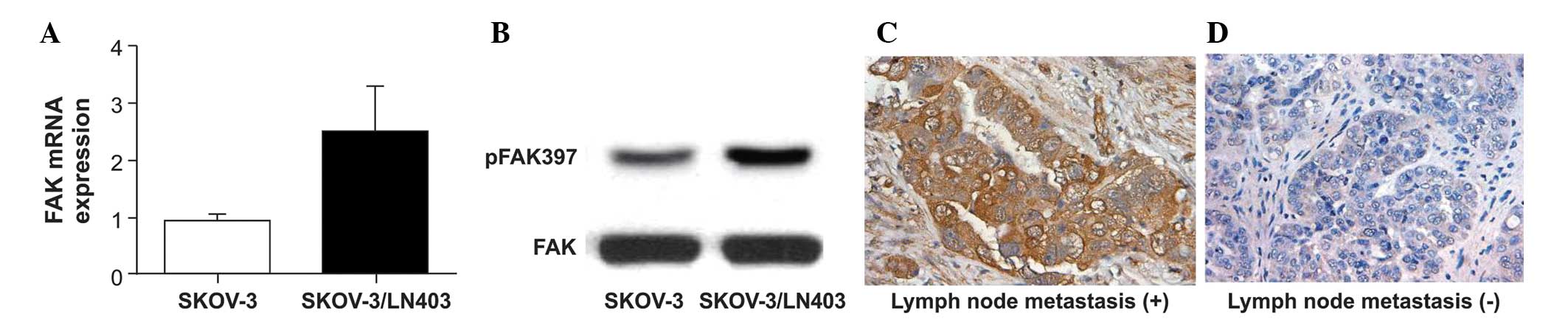
RT-qPCR, western blotting and immunohistochemistry identified
significant FAK induction in the SKOV-3/LN403 cell line (Fig. 7A and B) and in clinical
pathological specimens from three patients with OC with lymph node
metastasis and three patients with OC without metastases (Fig. 7C and D).
Discussion
An OC animal model was prepared by injecting
SKOV-3/LN403 cells into mice and was clinically similar to patients
with OC, with respect to clinical features including: Widespread
intra-abdominal metastasis, bloody ascites, up to 90% lymph node
metastasis involving retroperitoneal lymph nodes, significantly
shorter average survival times and faster-spreading tumor cells
in vivo. The shorter average survival times significantly
shortens the experimental cycle, making it convenient to use
SKOV-3/LN403 in OC metastasis studies. With reference to data on
the anatomy and nomenclature of murine lymph nodes provided by Van
den Broeck et al (21), it
was observed that SPECT-CT analysis of the BALB/c nude mice used in
the current study clearly illustrated the retroperitoneal lymph
nodes.
In 1996, Onda et al (22) suggested that the para-aortic lymph
nodes and the internal and external iliac and obturator lymph nodes
are the most frequent sites of metastasis of ovarian carcinomas.
Lymphatic metastasis is a critical factor in cancer staging and
prognosis (23,24), thus preclinical evaluation of lymph
nodes in small animal models is important. Since the first use of
xenografts of human cancer cells into immunodeficient mice in 1969
(25), they have been widely used
to explore tumorigenesis and therapeutic efficacy. The existing
human OC cell lines HEY, OVCA429, OCC1, OVCAR3, SKOV-3, A2780-s,
A2780-cp, OV2008 and ES-2 have been used for tumor transplant
models in mice, however, lymph node metastasis seldom occurs in
subcutaneous xenograft or abdomen metastasis tumor models with
these cell lines in nude mice (11,12,14,26).
A2780-cp cells have been reported to metastasize to one or both
ovaries; however, regardless of the thickened bursal membrane, the
tumor cells were localized inside the ovary and no metastasis
occurred (26). The OC
high-metastasis strain HD-8910PM was established from lung
metastases by Xu et al (12). Using orthotopic transplantation,
Tamada et al (14)
established the first model of lymph node metastasis of ovarian
carcinomas with the serous-type cell lines MH and KF. The
lymph-fixing metastasis cell line SKOV-3-pm3, whose
lymph metastasis rate can reach up to 100%, was isolated from the
SKOV-3 OC cell line with lesser metastatic potential (11). However, in this OC model,
SKOV-3-pm3 cells were injected into the footpads of nude
mice. Unlike human patients with OC, this model does not exhibit
abdominal cavity metastasis or ascites.
Using existing SKOV-3 cells, the novel SKOV-3/LN403
cell line was established, which has high lymph node
metastasticity, a well-characterized genetic background and
stability through repeated subculture. In comparison to the
parental SKOV-3 cell line, SKOV-3/LN403 exhibits a higher rate of
proliferation and invasion and a greater resistance to
paclitaxel.
A total of 8 upregulated pathways and 24
downregulated pathways were identified using the Agilent Whole
Human Genome Oligo Microarray. FAK was selected as a target for
experimental confirmation. FAK is a non-receptor protein-tyrosine
kinase that mediates integrin interaction with the extracellular
matrix (27–29). In the context of cancer, FAK is
involved in tumor cell growth, migration and invasion, in addition
to epithelial-to-mesenchymal transition and angiogenesis (28,30,31).
In 1999, overexpression of FAK was first reported in ovarian
carcinoma (32). Sood et al
(16) evaluated FAK-expressing
ovarian cell lines, benign ovarian samples and epithelial OCs, and
observed a significant association between FAK overexpression and
cancer migration and invasion. Several studies also identified
cofactors contributing to FAK overexpression (33–36).
FAK overexpression has been associated with breast cancer lung
metastasis (37,38), and FAK inhibitors have been
introduced in novel therapeutic strategies (20,39–41).
An association between FAK overexpression and lymphatic metastasis
has been identified in non-small-cell lung, oral squamous cell,
esophageal, gastric, tongue, laryngeal and endometrial cancer by
retrospective analysis of clinical samples (42–49).
Upregulation of FAK in the SKOV-3/LN403 cell line and clinical
pathological specimens suggests that it may contribute to the high
lymphatic metastaticity of the SKOV-3/LN403 cell line. To the best
of our knowledge, the current study is the first to report
alterations in FAK expression in OC lymphatic metastatic cell
lines.
In conclusion, the SKOV-3/LN403 cell line and a
novel mouse model of OC lymphatic metastasis were established in
the current study. Microarray analysis of SKOV-3 and SKOV-3/LN403
identified several candidate genes and pathways involved in
lymphatic metastasis of OC. Induction of FAK expression provides a
potential therapeutic target for OC lymphatic metastasis.
Acknowledgments
The current study was supported by The National
Natural Science Foundation of China (grant no. 81472423); The
Shanghai Natural Science Foundation of China (grant no.
13ZR1404300) and The Experimental Animal Special Fund of Science
and Technology Commission of Shanghai (grant no. 13140901500).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan DS, Agarwal R and Kaye SB: Mechanisms
of transcoelomic metastasis in ovarian cancer. Lancet Oncol.
7:925–934. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
di Re F, Baiocchi G, Fontanelli R, Grosso
G, Cobellis L, Raspagliesi F and di Re E: Systematic pelvic and
paraaortic lymphadenectomy for advanced ovarian cancer: Prognostic
significance of node metastases. Gynecol Oncol. 62:360–365. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pereira A, Magrina JF, Rey V, Cortes M and
Magtibay PM: Pelvic and aortic lymph node metastasis in epithelial
ovarian cancer. Gynecol Oncol. 105:604–608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kleppe M, Wang T, Van Gorp T, Slangen BF,
Kruse AJ and Kruitwagen RF: Lymph node metastasis in stages I and
II ovarian cancer: a review. Gynecol Oncol. 123:610–614. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ayhan A, Gultekin M, Dursun P, Dogan NU,
Aksan G, Guven S, Velipasaoglu M and Yuce K: Metastatic lymph node
number in epithelial ovarian carcinoma: Does it have any clinical
significance? Gynecol Oncol. 108:428–432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
du Bois A, Reuss A, Harter P,
Pujade-Lauraine E, Ray-Coquard I and Pfisterer J;
Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe
Ovarialkarzinom; Groupe d’Investigateurs Nationaux pour l’Etude des
Cancers Ovariens: Potential role of lymphadenectomy in advanced
ovarian cancer: A combined exploratory analysis of three
prospectively randomized phase III multicenter trials. J Clin
Oncol. 28:1733–1739. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hacker NF, Valmadre S and Robertson G:
Management of retroperitoneal lymph nodes in advanced ovarian
cancer. Int J Gynecol Cancer. 18(Suppl 1): 7–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harter P, Gnauert K, Hils R, Lehmann TG,
Fisseler-Eckhoff A, Traut A and du Bois A: Pattern and clinical
predictors of lymph node metastases in epithelial ovarian cancer.
Int J Gynecol Cancer. 17:1238–1244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
House CD, Hernandez L and Annunziata CM:
Recent technological advances in using mouse models to study
ovarian cancer. Front Oncol. 4:262014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ruan HY, Li DR, Li L, Guan X and Zhang W:
Establishment of human ovarian carcinoma cell lines with
directional highly lymphatic metastasis and study of their
biological characteristics. Zhonghua Fu Chan Ke Za Zhi. 42:482–486.
2007.In Chinese. PubMed/NCBI
|
|
12
|
Shenhua X, Lijuan Q, Hanzhou N, Xinghao N,
Chihong Z, Gu Z, Weifang D and Yongliang G: Establishment of a
highly metastatic human ovarian cancer cell line (HO-8910PM) and
its characterization. J Exp Clin Cancer Res. 18:233–239.
1999.PubMed/NCBI
|
|
13
|
Servais EL, Colovos C, Bograd AJ, White J,
Sadelain M and Adusumilli PS: Animal models and molecular imaging
tools to investigate lymph node metastases. J Mol Med (Berl).
89:753–769. 2011. View Article : Google Scholar
|
|
14
|
Tamada Y, Aoki D, Nozawa S and Irimura T:
Model for paraaortic lymph node metastasis produced by orthotopic
implantation of ovarian carcinoma cells in athymic nude mice. Eur J
Cancer. 40:158–163. 2004. View Article : Google Scholar
|
|
15
|
Kang Y, Chen CM and Xu CJ: Secretion of
oestrogen from murine-induced pluripotent stem cells co-cultured
with ovarian granulosa cells in vitro. Cell Bio Int. 35:871–874.
2011. View Article : Google Scholar
|
|
16
|
Sood AK, Coffin JE, Schneider GB, Fletcher
MS, DeYoung BR, Gruman LM, Gershenson DM, Schaller MD and Hendrix
MJ: Biological significance of focal adhesion kinase in ovarian
cancer: role in migration and invasion. Am J Pathol. 165:1087–1095.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sood AK, Bhatty R, Kamat AA, Landen CN,
Han L, Thaker PH, Li Y, Gershenson DM, Lutgendorf S and Cole SW:
Stress hormone-mediated invasion of ovarian cancer cells. Clin
Cancer Res. 12:369–375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spannuth WA, Mangala LS, Stone RL, et al:
Converging evidence for efficacy from parallel EphB4-targeted
approaches in ovarian carcinoma. Mol Cancer Ther. 9:2377–2388.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khan IU, Shahid A, Ahmad F, Dar UK, Ahmad
M and Javed M: Studying the biological feasibility of
[99mTc(CO)3]-dextran-cystein
e-cysteine-mannose as a potential molecular radiopharmaceutical for
sentinel node detection. Ann Nucl Med. 28:248–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang Y, Hu W, Ivan C, et al: Role of focal
adhesion kinase in regulating YB-1-mediated paclitaxel resistance
in ovarian cancer. J Natl Cancer Inst. 105:1485–1495. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Van den Broeck W, Derore A and Simoens P:
Anatomy and nomenclature of murine lymph nodes: Descriptive study
and nomenclatory standardization in BALB/cAnNCrl mice. J Immunol
Methods. 312:12–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Onda T, Yoshikawa H, Yokota H, Yasugi T
and Taketani Y: Assessment of metastases to aortic and pelvic lymph
nodes in epithelial ovarian carcinoma. A proposal for essential
sites for lymph node biopsy. Cancer. 78:803–808. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Benedet JL, Bender H, Jones H III, Ngan HY
and Pecorelli S: FIGO staging classifications and clinical practice
guidelines in the management of gynecologic cancers. FIGO Committee
on Gynecologic Oncology. Int J Gynaecol Obstet. 70:209–262. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim HS, Ju W, Jee BC, Kim YB, Park NH,
Song YS, Kim SC, Kang SB and Kim JW: Systematic lymphadenectomy for
survival in epithelial ovarian cancer: a meta-analysis. Int J
Gynecol Cancer. 20:520–528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rygaard J and Povlsen CO:
Heterotransplantation of a human malignant tumour to ‘Nude’ mice.
Acta Pathol Microbiol Scand. 77:758–760. 1969. View Article : Google Scholar
|
|
26
|
Shaw TJ, Senterman MK, Dawson K, Crane CA
and Vanderhyden BC: Characterization of intraperitoneal, orthotopic
and metastatic xenograft models of human ovarian cancer. Mol Ther.
10:1032–1042. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hanks SK and Polte TR: Signaling through
focal adhesion kinase. BioEssays. 19:137–145. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao J and Guan JL: Signal transduction by
focal adhesion kinase in cancer. Cancer Metastasis Rev. 28:35–49.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hanks SK, Ryzhova L, Shin NY and Brábek J:
Focal adhesion kinase signaling activities and their implications
in the control of cell survival and motility. Front Biosci.
8:d982–996. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schlaepfer DD, Hauck CR and Sieg DJ:
Signaling through focal adhesion kinase. Prog Biophys Mol Biol.
71:435–478. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McLean GW, Carragher NO, Avizienyte E,
Evans J, Brunton VG and Frame MC: The role of focal-adhesion kinase
in cancer-a new therapeutic opportunity. Nat Rev Cancer. 5:505–515.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Judson PL, He X, Cance WG and Van Le L:
Overexpression of focal adhesion kinase, a protein tyrosine kinase,
in ovarian carcinoma. Cancer. 86:1551–1556. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gabriel B, Mildenberger S, Weisser CW,
Metzger E, Gitsch G, Schüle R and Müller JM: Focal adhesion kinase
interacts with the transcriptional coactivator FHL2 and both are
overexpressed in epithelial ovarian cancer. Anticancer Res.
24:921–927. 2004.PubMed/NCBI
|
|
34
|
Grisaru-Granovsky S, Salah Z, Maoz M,
Pruss D, Beller U and Bar-Shavit R: Differential expression of
protease activated receptor 1 (Par1) and pY397FAK in benign and
malignant human ovarian tissue samples. Int J Cancer. 113:372–378.
2005. View Article : Google Scholar
|
|
35
|
Shibata K, Kikkawa F, Nawa A, Thant AA,
Naruse K, Mizutani S and Hamaguchi M: Both focal adhesion kinase
and c-Ras are required for the enhanced matrix metalloproteinase 9
secretion by fibronectin in ovarian cancer cells. Cancer Res.
58:900–903. 1998.PubMed/NCBI
|
|
36
|
Wang X, Urvalek AM, Liu J and Zhao J:
Activation of KLF8 transcription by focal adhesion kinase in human
ovarian epithelial and cancer cells. J Biol Chem. 283:13934–13942.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
van Nimwegen MJ, Verkoeijen S, van Buren
L, Burg D and van de Water B: Requirement for focal adhesion kinase
in the early phase of mammary adenocarcinoma lung metastasis
formation. Cancer Res. 65:4698–4706. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mitra SK, Lim ST, Chi A and Schlaepfer DD:
Intrinsic focal adhesion kinase activity controls orthotopic breast
carcinoma metastasis via the regulation of urokinase plasminogen
activator expression in a syngeneic tumor model. Oncogene.
25:4429–4440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Halder J, Lin YG, Merritt WM, et al:
Therapeutic efficacy of a novel focal adhesion kinase inhibitor
TAE226 in ovarian carcinoma. Cancer Res. 67:10976–10983. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Infante JR, Camidge DR, Mileshkin LR, et
al: Safety, pharma-cokinetic and pharmacodynamic phaseI
dose-escalation trial of PF-, an inhibitor of focal adhesion
kinase, in advanced solid tumors. J Clin Oncol. 30:1527–1533. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun H, Pisle S, Gardner ER and Figg WD:
Bioluminescent imaging study: FAK inhibitor, PF-562,271,
preclinical study in PC3M-luc-C6 local implant and metastasis
xenograft models. Cancer Biol Ther. 10:38–43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ji HF, Pang D, Fu SB, Jin Y, Yao L, Qi JP
and Bai J: Overexpression of focal adhesion kinase correlates with
increased lymph node metastasis and poor prognosis in
non-small-cell lung cancer. J Cancer Res Clin Oncol. 139:429–435.
2013. View Article : Google Scholar
|
|
43
|
de Vicente JC, Rosado P,
Lequerica-Fernández P, Allonca E, Villallaín L and
Hernández-Vallejo G: Focal adhesion kinase overexpression:
Correlation with lymph node metastasis and shorter survival in oral
squamous cell carcinoma. Head Neck. 35:826–830. 2013. View Article : Google Scholar
|
|
44
|
Cai HX, Yang LC, Song XH, Liu ZR, Chen YB
and Dong GK: Expression of paxillin and FAK mRNA and the related
clinical significance in esophageal carcinoma. Mol Med Rep.
5:469–472. 2012.
|
|
45
|
Park JH, Lee BL, Yoon J, Kim J, Kim MA,
Yang HK and Kim WH: Focal adhesion kinase (FAK) gene amplification
and its clinical implications in gastric cancer. Hum Pathol.
41:1664–1673. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jiang H, Liu L, Ye J, Liu H, Xing S and Wu
Y: Focal adhesion kinase serves as a marker of cervical lymph node
metastasis and is a potential therapeutic target in tongue cancer.
J Cancer Res Clin Oncol. 136:1295–1302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gabriel B, Hasenburg A, Waizenegger M,
Orlowska-Volk M, Stickeler E and zur Hausen A: Expression of focal
adhesion kinase in patients with endometrial cancer: a
clinicopathologic study. Int J Gynecol Cancer. 19:1221–1225. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rodrigo JP, Dominguez F, Suárez V, Canel
M, Secades P and Chiara MD: Focal adhesion kinase and E-cadherin as
markers for nodal metastasis in laryngeal cancer. Arch Otolaryngol
Head Neck Surg. 133:145–150. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Miyazaki T, Kato H, Nakajima M, Sohda M,
Fukai Y, Masuda N, Manda R, Fukuchi M, Tsukada K and Kuwano H: FAK
overexpression is correlated with tumour invasiveness and lymph
node metastasis in oesophageal squamous cell carcinoma. Br J
Cancer. 89:140–145. 2003. View Article : Google Scholar : PubMed/NCBI
|