Introduction
Cyclosporin A (CsA), which was initially isolated
from the fungus Tolypocladiuminflatum
(Beauverianivea) by Hans Peter Frey in a soil sample
obtained in 1969 from Hardangervidda, Norway (1), is an effective immunosuppressant,
which is widely used against graft rejection and has clinical
applications in the treatment of various autoimmune disorders
(2–5). However, its application has been
limited due to the severe toxicity caused by CsA, particularly in
patients with renal injury (6,7). At
present, in order to reduce the CsA-induced toxicity, the plasma
concentration of CsA is adjusted and maintained at a non-toxic
range by monitoring the drug concentration in the patient’s blood,
however this approach required specialized equipment and
professional staff (7). Therefore,
investigations have attempted to identify effective components from
other drugs or natural sources, which inhibit the renal toxicity of
CsA. Protection against nephrotoxicity induced by CsA was observed
in several reagents, including shallot, black grape, garlic
extracts (8,9), L-arginine, N-acetylcysteine, vitamin
E and curcumin (10–13). Certain reagents are involved in the
inhibition of glycolysis, therefore, the present study investigated
whether 2-deoxy-D-glucose (2-DG), a typical inhibitor of
glycolysis, can protect against CsA-induced nephrotoxicity.
2-DG is a type of deoxyglucose and exists in all
types of microbes. Its predominant role is to inhibit glycolysis,
which induces cell death in tumor cells and has a protective effect
on normal cells. Therefore, 2-DG has been widely investigated as an
adjuvant of antitumor drugs (14,15).
The protective mechanism of 2-DG may be associated with it
increasing the expression levels of the heat shock protein (HSP)70
stress protein and phosphorylated-AKT, and inhibiting the
production of reactive oxygen species (ROS) (16–18).
The present study investigated the protective mechanism of 2-DG on
the cytotoxicity of CsA in vitro.
Materials and methods
Cell culture
The rat tubular cell line, NRK-52E, was cultured in
6-, 24- or 96-well plates with America Type Culture
Collection-modified Dulbecco’s modified Eagle’s medium (DMEM),
containing 4.5 g/l glucose and 1.5 g/l sodium bicarbonate, which
was supplemented with 10% fetal bovine serum (Takara Biotech Co.,
Ltd., Dalian, China), 100 U/ml penicillin and 100 U/ml streptomycin
(TaKaRa Biotech Co., Ltd.) at 37°C with 5% CO2.
Determination of lactate dehydrogenase
(LDH) activity
LDH activity was determined using an LDH assay kit
(Beyotime Institue of Biotechnology Co. Ltd., Shanghai, China),
according to the manufacturer’s instructions. Briefly, the NRK-52E
cells were seeded into 24-well plates at a density of
2×105 cells/well in DMEM, containing 10% fetal bovine
serum, and cultured at 37°C for 24 h. The medium was subsequently
replaced with DMEM without 10% fetal bovine serum, and the cells
were cultured for 12 h prior to replacing the medium again and
pretreating the cells with 2-DG (2, 10 and 25 mM), Nec-1 (50
μM) and z-VAD-fmk (20 μM; Beyotime Institute of
Biotechnology, Co. Ltd.) for 30 min. CsA (10 μM; Beyotime
Institute of Biotechnology, Co. Ltd.) was added to the cells and
cultured at 37°C for 24 h. The cell culture supernatants were
collected by centrifugation at 15,000 × g for 5 min and the LDH
levels were measured spectrophotometrically by nicotinamide adenine
dinucleotide oxidation at 440 nm on a PhotoLab 6100.
Hoechst 33342/propidium iodide (PI)
double staining
The rates of cellular apoptosis or necrosis were
assessed with Hoechst 33342/PI staining. Briefly, 5×105
cells/well were seeded into 6-well plates with DMEM, containing 10%
fetal bovine serum, and cultured at 37°C for 24 h. The medium was
replaced with DMEM without 10% fetal bovine serum, and the cells
were cultured for 12 h prior to replacing the medium and
pretreating with 2-DG (25 mM), Nec-1 (50 μM) and z-VAD-fmk
(20 μM) for 30 min. CsA (10 μM) was added and the
cells were cultured at 37°C for 24 h. The cells were subsequently
stained with Hoechst 33342 and PI (10 μM) for 15 min. The
stained cells were observed under an IX71 inverted fluorescence
microscope (Olympus IX710; Olympus, Tokyo, Japan).
Analysis of apoptosis and necrosis by
flow cytometry
Apoptotic and necrotic cell death were assessed
using an annexin V-fluorescein isothiocyanate (FITC)-conjugated)/PI
apoptosis kit and a fluorescent activated cell sorter (FACS) flow
cytometer (BD, San Jose, CA, USA). Briefly, 5×105
cells/well were seeded into 6 -well plates with DMEM, containing
10% fetal bovine serum, and cultured at 37°C for 24 h. The medium
was replaced with DMEM without serum and the cells were cultured at
37°C for a further 12 h. The medium was replaced again, and the
cells were pretreated with 2-DG (25 mM), Nec-1 (50 μM) and
z-VAD-fmk (20 μM) for 30 min prior to the addition of 10
μM CsA and the cells were cultured for 24 h. The cells were
harvested and resuspended in 500 μl binding buffer. The
cells were incubated with 5 μl annexin V-FITC in the dark at
37°C for 15 min prior to the addition of 10 μl PI (50 mg/ml)
to the cell suspension and incubation for an additional 5 min. The
cells were immediately analyzed using a FACSCalibur flow cytometer.
For all samples, the fluorescence of 10,000 cells was gated and
quantified. The percentages of the cells in the lower right (early
apoptotic cells) and upper right (late apoptotic/necrotic cells)
region of the scatter plot of annexin V-FITC were calculated using
CellQuest software (BD Biosciences) for comparison.
Western blotting
The expression levels of caspase 3 and
receptor-interacting protein kinase 3 (RIP3) were assessed by
western blotting. Briefly, 5×105 cells/well were seeded
into 6-well plates with DMEM, containing 10% fetal bovine serum,
and cultured at 37°C for 24 h. The medium was replaced with DMEM
without serum and cultured at 37°C for 12 h. The medium was
replaced again, and the cells were pretreated with 2-DG (25 mM),
Nec-1 (50 μM) and z-VAD-fmk (20 μM) for 30 min prior
to the addition of 10 μM CsA and the cells were cultured at
37°C for 24 h. The cells were washed twice with cold
phosphate-buffered saline (PBS; Takara Biotech Co., Ltd.) and lysed
with radioimmunoprecipitation buffer, containing 150 mM NaCl, 1%
NP-40, 0.1% SDS, 2 μg/ml leupeptin, 1.5 mM EDTA, 1 mM
NaVanadate, for 25 min on ice. The lysates were centrifuged at
15,000 × g for 10 min at 4°C and the protein concentrations were
detected using a Bradford protein assay (Beyotime Institute of
Biotechnology Co. Ltd.). Briefly, a series of protein standards
were diluted with 0.15 M NaCl to final concentrations of 0 (blank =
NaCl only), 250, 500, 750 and 1,500 μg/ml. Serial dilutions
of the unknown sample to be measured were also prepared. The
standards and samples (100 μl) were added to a separate test
tube and 5 ml Coomassie Blue was added to each tube, and vortexed.
The samples were measured at 595 nm wavelength and the absorbance
of the standards, vs. their concentration was plotted. The
extinction coefficient was calculated and the concentrations of the
unknown samples were determined. The total proteins were separated
on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis
gels (Takara Biotech Co., Ltd.) and were transferred onto
polyvinylidene difluoride membranes (Millipore Co., Billerica, MA,
USA). The membranes were blocked with 5% non-fat milk in 10 mM
Tris-HCl (pH 8.0), 150 mM NaCl, and 0.1% Tween-20 (TBST; Takara
Biotech Co., Ltd.) for 2 h and were then incubated with the
following primary antibodies: Rabbit anti-RIP3 (1:1,000;
Antibodies-online Inc., Atlanta, GA, USA, cat. no. ABIN360159),
anti-cleaved caspase 3 rabbit polyclonal (1:1,000; Trevigen, Inc,
Gaithersburg, MD, USA, cat. no. 2305-PC-020) or mouse anti-tubulin
(1:10,000; Antibodies-online Inc; cat. no. ABIN125953) at 4°C
overnight. The membranes were incubated with secondary antibodies
conjugated to horseradish peroxidase at room temperature for 1 h.
Antibody binding was detected using an enhanced chemiluminescence
detection kit (Beyotime Institute of Biotechnology Co. Ltd.). The
images were analyzed using Quantity One software (Bio-Rad).
Determination of ROS
The effect of 2-DG on the production of ROS induced
by CsA was evaluated using flow cytometry. Briefly,
5×105 cells/well were seeded into 6-well plates with
DMEM, containing 10% fetal bovine serum, and cultured at 37°C for
24 h. The medium was replaced with DMEM without serum and the cells
were cultured at 37°C for a further 12 h. The medium was changed
and the cells were treated with 2-DG (25 mM) for 30 min prior to
the addition of 10 μM CsA. Rosup was added 30 min prior to
cell digestion. The cells were incubated at 37°C for 12 h, and
changes in the levels of ROS were detected using flow
cytometry.
Determination of glutathione (GSH)
GSH was detected using a GSH assay kit (Beyotime
Institute of Biotechnology Co. Ltd.), according to the
manufacturer’s instructions. Briefly, 5×105 cells/well
were seeded into 6-well plates with DMEM, containing 10% fetal
bovine serum, and cultured at 37°C for 24 h. The medium was
replaced with DMEM without serum and the cells were cultured at
37°C for a further 12 h. The medium was replaced again, and the
cells were treated with 2-DG (25 mM) for 30 min prior to the
addition of 10 μM CsA and culture for 24 h. The cells were
then washed with cold PBS and lysed. The supernatant was assessed
using an ELISA reader to determine the absorbance at the wavelength
of 405 nm.
Determination of malondialdehyde
(MDA)
MDA is a natural product of lipid oxidation,
therefore, the levels of MDA represent the lipid peroxidation
level. MDA was detected using a lipid peroxidation MDA assay kit
(Beyotime Institute of Biotechnology Co. Ltd.). Briefly,
5×105 cells/well were seeded into 6-well plates with
DMEM, containing 10% fetal bovine serum and cultured at 37°C for 24
h. The medium was replaced with DMEM without serum and the cells
were cultured at 37°C for 12 h. The medium was replaced again and
the cells were treated with 2-DG (25 mM) for 30 min, prior to the
addition of 10 μM CsA and cultured at 37°C for 24 h. The
cells were subsequently washed with cold PBS and lysed with
radioimmunoprecipitation buffer. The supernatant was assessed using
an ELISA reader (Peiou 318C) to determine the absorbance at a
wavelength of 532nm and 600nm.
Data analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). The differences between
experimental groups were analyzed by one-way analysis of variance
and Kruskal-Wallis tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of 2-DG on LDH
As shown in Fig. 1,
treatment with 2, 10 or 25 mM 2-DG significantly inhibited the
release of LDH induced by CsA. The protective effects of 10 and 25
mM 2-DG were higher compared with that of 50 μM Nec-1 and 20
μM z-VAD-fmk. However, no difference was observed between 10
and 25 mM 2-DG.
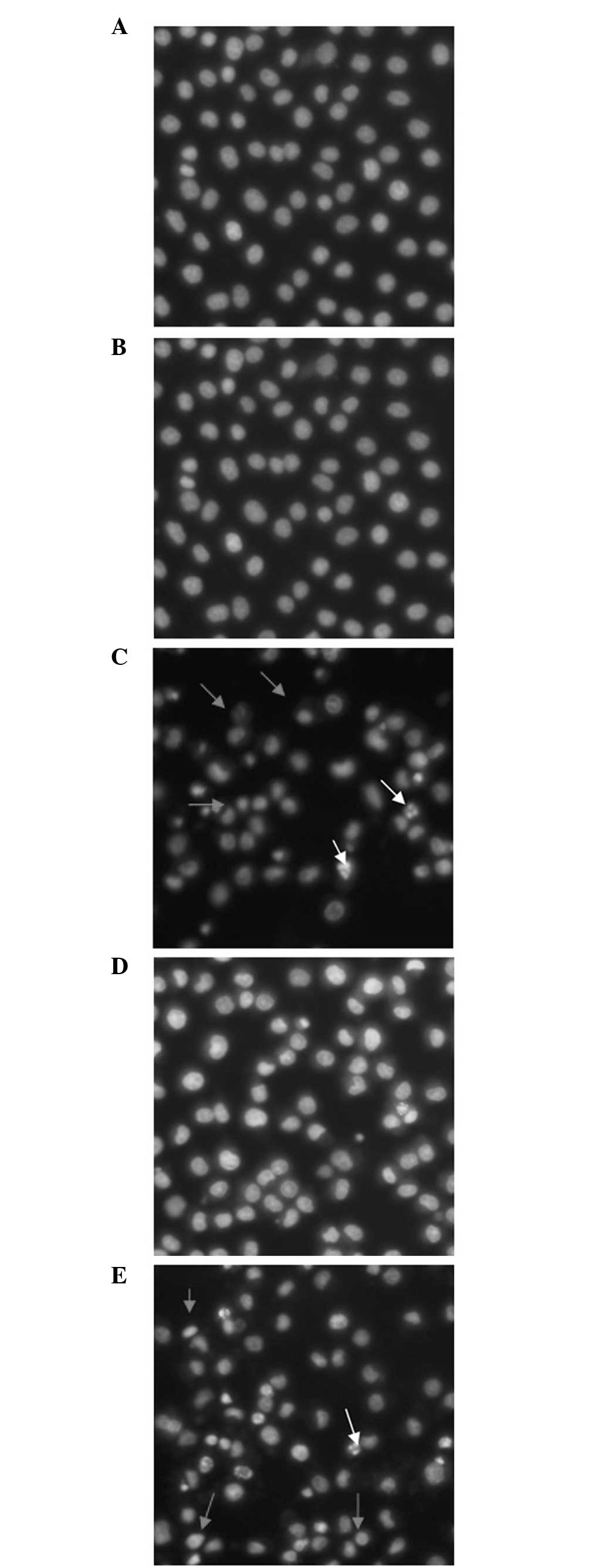
Hoechst 33342/PI double staining
The morphological changes of apoptotic and necrotic
cells were observed by Hoechst 33342/PI double staining. As shown
in Fig. 2, treatment with 25 mM
2-DG was not toxic to the NRK-52E cells. This treatment reduced the
rate of CsA-induced cellular apoptosis and necrosis, and the
inhibition of apoptosis was similar to that of z-VAD-fmk.
Flow cytometric analysis of the effects
of 2-DG on necrosis and apotosis
The results of the flow cytometry were the same as
those obtained by Hoechst33342/PI double staining. Treatment with
25 mM 2-DG reduced the rate of CsA-induced cellular apoptosis and
necrosis. The inhibitory effect of 25 mM 2-DG on cellular apoptosis
and necrosis was higher compared with treatment with 50 μM
Nec-1 and was similar to that of z-VAD-fmk (Fig. 3).
 | Figure 3Effect of 2-DG on CsA-induced necrosis
and apoptosis. The rates of apoptosis and necrosis were analyzed by
flow cytometric analysis. The experimental groups were divided into
(A) untreated cells (control), (B) cells treated with 25 mM 2-DG
for 24 h, (C) cells were treated with 10 μM CsA for 24 h,
(D) pretreatment with 25 mM 2-DG 30 min prior to treatment with
CsA, (E) pretreatment with 50 μM Nec-1 30 min prior to
treatment with CsA and (F) pretreatment with 20 μM z-VAD-fmk
30 min prior to treatment with CsA. In the graphs of the results,
data are expressed as the mean ± standard deviation (*P<0.05,
vs. control group; #P<0.05, vs. CsA group). CsA,
cyclosporin A; 2-DG, 2-deoxy-D-glucose; FITC, fluorescein
isothiocyanate; PI, propidium iodide. |
Effects of 2-DG on the activation of
caspase 3
Caspase 3 was activated by CsA and this activation
was inhibited by treatment with 2-DG. Treatment with z-VAD-fmk also
inhibited the activation of caspase 3 (Fig. 4).
Effects of 2-DG on the expression of
RIP3
Western blot analysis was performed to investigate
the effect of 2-DG on the expression of RIP3. The results
demonstrated that 2-DG had no effect on the expression of RIP3
(Fig. 5).
Effect of 2-DG on the production of
CsA-induced ROS
The effect of 2-DG on the production of ROS, induced
by CsA, was assessed using flow cytometry. Treatment with CsA
revealed a significant increase in the generation of ROS, whereas
treatment with 2-DG suppressed the generation of ROS. As a ROS
scavenger, vitamin E decreased the cellular level of ROS following
CsA treatment. The effects of 2-DG were more marked compared with
that of VE (Fig. 6).
Effect of 2-DG on cellular levels of
CsA-induced GSH
The intracellular levels of GSH decreased following
treatment with CsA for 12 h. By contrast, the intracellular level
of GSH increased in the cells pre-treated with 2-DG prior to CsA
treatment, which occurred in a dose-dependent manner (Fig. 7).
Effect of 2-DG on the CsA-induced
cellular levels of MDA
The intracellular levels of MDA increased following
treatment with CsA for 12 h. By contrast, the levels of MDA in the
cells decreased following pre-treatment with 2-DG prior to
treatment with CsA, which occurred in a dose-dependent manner
(Fig. 8).
Discussion
2-DG is an inhibitor of glucose transport and
glycolysis, and is present naturally in bacteria. At present, 2-DG
is predominantly used in the treatment of tumor cells, as it has a
selective effect on tumor cells and is non-toxic to normal cells at
the conventional dose (15,19).
The present study hypothesized that this may be associated with the
inhibition of glycolysis. LDH is an enzyme widely present in the
cytosol. When the plasma membrane integrity is disrupted, LDH leaks
into the culture media and its extracellular level is increased
(20). In the present study, the
protective effect of 2-DG on CsA-induced cellular toxicity was
investigated. The present study revealed that 2-DG inhibited the
release of LDH in the CsA-induced NRK-52E cells, which suggested
that 2-DG inhibited CsA-induced cellular necrosis and apoptosis.
The results of the flow cytometric and morphological analyses
confirmed that 2-DG almost completely inhibited the toxicity of CsA
on the NRK-52E cells, which may be indirect evidence that
acceleration in the rate of cellular glycolysis by CsA is the
predominant reason for its cytotoxicity.
ROS is a by-product of the oxidative respiratory
chain and is produced predominantly in the mitochondria, which are
rich in renal tubular epithelial cells (21). A low level of ROS can eliminate the
invading bacteria, however, excess ROS can lead to cell dysfunction
and cause cellular apoptosis or necrosis (21–23).
The present study found that 2-DG reduced the increased production
of ROS, which was induced by CsA. Reducing the production of ROS
may be the predominant mechanism underlying the effect of 2-DG
against CsA cytotoxicity. In addition, the levels of the
intracellular antioxidant, GSH, and the oxidation product, MDA,
were determined. This revealed that 2-DG inhibited the CsA-induced
reduction in GSH and increase in MDA content, which demonstrated
that CsA altered the cellular redox status and 2-DG enabled the
cell recovery to the normal level.
The present study also revealed that CsA activated
and cleaved caspase-3, whereas 2-DG inhibited this activation. This
suggested that the inhibitory action of 2-DG on the activity of
caspase was also involved in the protective effects on CsA-induced
toxicity.
The importance of RIP3 in the signal transduction
cascade of necrosis has been reported (24,25).
In our previous study, RIP3 and ROS were found to be involved in
CsA-induced necroptosis (21).
However, 2-DG did not alter the expression of RIP3 in the present
study, therefore, whether 2-DG inhibits the activity of the RIP3
phosphokinase and the necroptosis induced by CsA remains to be
elucidated.
In conclusions, 2-DG inhibited CsA-induced cellular
apoptosis and necroptosis, which may be through reducing the
CsA-induced production of ROS. The inhibition of caspase 3
activation was demonstrated as one of the protective mechanism of
2-DG, however, the expression of RIP3 was not affected. Whether
2-DG affects the phosphorylation of RIP3 and inhibits CsA-induced
necroptosis through inhibition of the RIP3 signaling pathway
remains to be elucidated.
References
|
1
|
Svarstad H, Bugge HC and Dhillion SS: From
Norway to Novartis: Cyclosporin from Tolypocladium inflatum in an
open access bioprospecting regime. Biodivers Conserv. 9:1521–1541.
2000. View Article : Google Scholar
|
|
2
|
Kahan BD: Immunosuppressive therapy. Curr
Opin Immunol. 4:553–560. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hariharan S, Johnson CP, Bresnahan BA,
Taranto SE, McIntosh MJ and Stablein D: Improved graft survival
after renal transplantation in the United States, 1988 to 1996. N
Engl J Med. 342:605–612. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Laupacis A, Keown PA, Ulan RA, McKenzie N
and Stiller CR: Cyclosporin A: a powerful immunosuppressant. Can
Med Assoc J. 126:1041–1046. 1982.PubMed/NCBI
|
|
5
|
Borel JF, Baumann G, Chapman I, Donatsch
P, Fahr A, Mueller EA and Vigouret JM: In vivo pharmacological
effects of ciclosporin and some analogues. Adv Pharmacol.
35:115–246. 1996.PubMed/NCBI
|
|
6
|
Myers BD, Sibley R, Newton L, Tomlanovich
SJ, Boshkos C, Stinson E, Luetscher JA, Whitney DJ, Krasny D,
Coplon NS, et al: The long-term course of cyclosporine-associated
chronic nephropathy. Kidney Int. 33:590–600. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakamura T, Nozu K, Iijima K, Yoshikawa N,
Moriya Y, Yamamori M, Kako A, Matsuo M, Sakurai A, Okamura N,
Ishikawa T, Okumura K and Sakaeda T: Association of cumulative
cyclosporine dose with its irreversible nephrotoxicity in Japanese
patients with pediatric-onset autoimmune diseases. Biol Pharm Bull.
30:2371–2375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wongmekiat O, Leelarugrayub N and
Thamprasert K: Beneficial effect of shallot (Allium ascalonicum L.)
extract on cyclosporine nephrotoxicity in rats. Food Chem Toxicol.
46:1844–1850. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Durak I, Cetin R, Candir O, Devrim E,
Kiliçoğlu B and Avci A: Black grape and garlic extracts protect
against cyclosporine a nephrotoxicity. Immunol Invest. 36:105–114.
2007. View Article : Google Scholar
|
|
10
|
Mansour M, Daba MH, Gado A, Al-Rikabi A
and Al-Majed A: Protective effect of L-arginine against
nephrotoxicity induced by cyclosporine in normal rats. Pharmacol
Res. 45:441–446. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Magendiramani V, Umesalma S, Kalayarasan
S, Nagendraprabhu P, Arunkumar J and Sudhandiran G: S-allylcysteine
attenuates renal injury by altering the expressions of iNOS and
matrix metallo proteinase-2 during cyclosporine-induced
nephrotoxicity in Wistar rats. J Appl Toxicol. 29:522–530. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Duru M, Nacar A, Yönden Z, Kuvandik G,
Helvaci MR, Koç A, Akaydin Y, Oksüz H and Söğüt S: Protective
effects of N-acetylcysteine on cyclosporine-A-induced
nephrotoxicity. Ren Fail. 30:453–459. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abdel Fattah EA, Hashem HE, Ahmed FA,
Ghallab MA, Va rga I and Polak S: Prophylactic role of curcumin
against cyclosporine-induced nephrotoxicity: histological and
immuno-histological study. Gen Physiol Biophys. 29:85–94. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khaitan D, Chandna S, Arya MB and
Dwarakanath BS: Differential mechanisms of radiosensitization by
2-deoxy-D-glucose in the monolayers and multicellular spheroids of
a human glioma cell line. Cancer Biol Ther. 5:1142–1151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Venkataramanaa NK, Venkatesh PK,
Dwarakanath BS and Vani S: Protective effect on normal brain tissue
during a combinational therapy of 2-deoxy-d-glucose and
hypofractionated irradiation in malignant gliomas. Asian J
Neurosurg. 8:9–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee J, Bruce-Keller AJ, Kruman Y, Chan SL
and Mattson MP: 2-Deoxy-D-glucose protects hippocampal neurons
against excitotoxic and oxidative injury: evidence for the
involvement of stress proteins. J Neurosci Res. 57:48–61. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong D, Liu X, Schafer-Hales K, Marcus
AI, Khuri FR, Sun SY and Zhou W: 2-Deoxyglucose induces Akt
phosphorylation via a mechanism independent of LKB1/AMP-activated
protein kinase signaling activation or glycolysis inhibition. Mol
Cancer Ther. 7:809–817. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Han H, Wang J, Wang H, Yang B and
Wang Z: Impairment of human ether-á-go-go-related gene (HERG) K+
channel function by hypoglycemia and hyperglycemia. Similar
phenotypes but different mechanisms. J Biol Chem. 278:10417–10426.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vilalta A and Brown GC: Deoxyglucose
prevents neurodegeneration in culture by eliminating microglia. J
Neuroinflammation. 11:582014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wroblewski F and Ladue JS: Lactic
dehydrogenase activity in blood. Proc Soc Exp Biol Med. 90:210–213.
1955. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ouyang Z, Zhu S, Jin J, Li J, Qiu Y, Huang
M and Huang Z: Necroptosis contributes to the cyclosporin A-induced
cytotoxicity in NRK-52E cells. Pharmazie. 67:725–732.
2012.PubMed/NCBI
|
|
22
|
Thapa RJ, Basagoudanavar S, Nogusa S,
Irrinki K, Mallilankaraman K, Slifker MJ, Beg AA, Madesh M and
Balachandran S: NF-kappaB protects cells from
interferon-gamma-induced RIP1-dependent necroptosis. Mol Cell Biol.
31:2934–2946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song KJ, Jang YS, Lee YA, Kim KA, Lee SK
and Shin MH: Reactive oxygen species dependent necroptosis in
Jurkat T cells induced by pathogenic free-living Naegleria fowleri.
Parasite Immunol. 33:390–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vandenabeele P, Galluzzi L, Vanden Berghe
T and Kroemer G: Molecular mechanisms of necroptosis: an ordered
cellular explosion. Nat Rev Mol Cell Biol. 11:700–714. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cho YS, Challa S, Moquin D, Genga R, Ray
TD, Guildford M and Chan FK: Phosphorylation-driven assembly of the
RIP1-RIP3 complex regulates programmed necrosis and virus-induced
inflammation. Cell. 137:1112–1123. 2009. View Article : Google Scholar : PubMed/NCBI
|