Introduction
MicroRNAs (miRNAs) are a family of endogenous and
noncoding RNAs that regulate gene expression by inhibiting mRNA
translation or inducing mRNA degradation, usually by binding to the
3′-untranslated region of target mRNAs (1–3). In
terms of their use as biomarkers or therapeutic targets, miRNAs are
more stable than mRNAs, which are sensitive to degradation
(4). The importance of miRNAs has
been focused upon during the past decade, and their dysregulation
has been identified as a feature of tumorigenesis.
Spinal cord injury (SCI), which commonly occurs as a
result of trauma, is a significant cause of disability (5). The cost is high in personal, social
and financial terms. In addition to motor and sensory dysfunction,
patients with SCI are also at risk of developing multiple
complications, such as bladder cancer, which may be induced by a
variety of mechanisms (6–9). The incidence of bladder cancer
appears to be increased following SCI; a number of retrospective
studies have reported that the risk of bladder cancer risk in
patients with SCI is 16–28 times higher than that of the general
population (10,11). Numerous risk factors have been
proposed to account for this increased incidence. However, there is
currently little consensus regarding the risk factors for bladder
cancer in patients with SCI. In a study involving 43,561 patients
with SCI, it was concluded that long-term indwelling
catheterization is a risk factor for bladder cancer in patients
with SCI (12). Other risk factors
identified for bladder cancer in patients with SCI, include urinary
tract infections (UTI) and the nature of the neurogenic bladder
itself (12,13). However, no studies have focused on
the role of miRNAs.
After consulting a high number of studies, it was
apparent that the majority of reports regarding bladder cancer
following SCI are derived from epidemiological studies. To the best
of our knowledge, the present study is the first to examine this
phenomenon through identification of changes in the miRNA
expression profile (the ‘microRNAome’) (14).
The Rb1 gene, located on chromosome 13q14, was the
first tumor suppressor gene to be isolated (15). The Rb1 protein is a nuclear
phosphoprotein, which is involved in a number of pathways of
bladder cancer, either at its initiation or during its progression,
including cell-cycle regulation and apoptosis (16,17).
Previous studies have confirmed that alteration or deletion of the
Rb1 protein is observed in human bladder cancer, suggesting that
the Rb1 gene is critical in bladder tumorigenesis (16). In addition, tumorigenicity may be
suppressed by transfecting the Rb1 gene into bladder cancer cells
(16). Therefore, the aims of
present study were to determine the miRNA expression of rat bladder
tissues following SCI, and the possible association between
miR-1949, Rb1 and the development of bladder cancer.
Materials and methods
Ethics statement
Seventy-two adult female Wistar rats (weight, 250±10
g) employed in the present study were obtained from the Radiation
Study Institute-Animal Center (Tianjin, China). All experimental
protocols involving animals were conducted in accordance with
guidelines published in the National Institutes of Health Guide for
the Care and Use of Laboratory Animals (NIH Publications no. 85–23,
revised 1996) and guidelines published by the United Kingdom
Coordinating Committee for Cancer Research (18), and were approved by the Ethics
Committee of Tianjin Medical University.
The experimental grouping
In the miRNA array experiment, 27 rats were ised.
There were 12 rats in the SCI group, 12 rats in the sham group and
three rats in control group. The SCI group and sham group included
four checkpoints, which in the SCI group were 3, 6, 9 and 12 months
following SCI or laminectomy (T1-T4). There were three rats in each
checkpoint. The control group was not given any checkpoint. To
validate if Rb1 is the target gene of miR1949, 15 rats were used
for immunohistochemisty, with three rats used at each checkpoint
(3, 6, 9 and 12 months following SCI) and three rats used as the
control group. To investigate the influence of age on the
expression levels of miR-1949 and Rb1, nine rats were used for
reverse transcription quantitative polymerase chain reaction
(RT-qPCR) and western blotting, and a further nine rats were used
for immunohistochemisty. In these experiments, there were three
checkpoints. Rats in the T1-T3 groups without SCI and laminectomy
were grouped together with SCI rats and were sacrificed at an
identical as the SCI rats from T2-T4 for sample extraction. To
determine the protein expression levels of miR-1949 and Rb1, 12
rats were added, which were used to supply three new checkpoints
C1-C3 (1, 14 days and 1 month following SCI) and a control group.
There were three rats in the C1-C3 and three rats in the control
group. Finally, the protein expression levels of miR-1949 and Rb1
in the C1-C3 and C4-C7 were used togther to determine the protein
expression levels of miR-1949 and Rb1 protein.
Surgical methods and sample
collection
Forty-five adult female Wistar rats were
anesthetized by intraperitoneal injection with 10% chloral hydrate
(0.3 ml/100 g; Tianjin Medical University General Hospital,
Tianjin, China). The area to be operated on was shaved and
sterilized with 70% ethanol and betadine (Lierkang, Dezhou, China).
The T10 spinous process and vertebral plate were removed, and the
dura mater was opened. The exposed spinal cord was struck by a 10
g, 25 mm weight-drop, using a New York University impactor device,
as described previously (19). A
valid contusive SCI model should comply with the criteria of an SCI
model, such that the hindlimbs of the rat twitch involuntarily and
the tail moves (20). Sham rats
received a laminectomy only. Following surgery, rats were placed in
rooms with controlled temperature and humidity, and bladder
evacuation was applied daily, prior to the return of reflexive
bladder control (21). The rats
were sacrificed by intracardial perfusion with 200 ml normal saline
(Baiteyiliaoyongping, Shanghai, China).
Following sacrifice of the rats, bladders were
excised, and the tissue was either fixed with formalin and embedded
in paraffin for immunohistochemistry, or rapidly cut into small
sections and snap-frozen in liquid nitrogen, then stored at −80°C,
for subsequent RNA isolation and western blotting.
RNA isolation
The mirVana™ RNA Isolation kit (Applied Biosystems,
Carlsbad, CA, USA) was used for total RNA isolation. For each
tissue mass, 10 volumes of lysis/binding buffer were placed into a
plastic tube on ice. Powdered tissue was transferred to the
Lysis/Binding buffer, using a pre-chilled metal spatula. The
mixture was mixed rapidly and transferred to a vessel. The mixture
was then processed to homogeneity using a pulsing vortex mixer
(SI-P246; Scientific Industries, New York, NY, USA). miRNA
homogenate additive (1/10 volume) was added and chilled for 10 min
on ice. A volume of acid-phenol: chloroform, equal to the lysate
volume, was added, prior to adding the miRNA homogenate additive
and mixing via vortex for 30–60 sec. In order to separate the
organic and aqueous phases, centrifugation was performed for 5 min
at room temperature, at the maximum speed (10,000 x g) and the
aqueous phase was transferred to a fresh tube without disturbing
the organic phase. The volume removed was recorded, and 1.25 volume
100% ethanol was added and mixed thoroughly. A filter cartridge was
put into one of the collection tubes supplied for each sample. The
lysate/ethanol mixture was applied onto the filter cartridge using
a pipette. The mixture was centrifuged for 15 sec at 10,000 x g, in
order to pass it through the filter. Alternatively, vacuum pressure
may be used to pass samples through the filter. This process was
repeated until the entire lysate/ethanol mixture had passed through
the filter and the flow-through was discarded. The collection tube
was reused for the washing steps. The filter was washed with 700
μl miRNA wash solution 1 and then washed twice with 500
μl wash solution 2/3. Elution solution (100 μl) that
had been preheated to 95°C, was applied to the center of the
filter, which was covered with the cap. Samples were spun for 20–30
sec at 10,000 x g in order to recover the eluate, which contained
the RNA, and were stored at −70°C.
miRNA array and data analysis
In order to assess the differential expression of
miRNAs, 27 samples were used from the T1-T4 time points (3, 6, 9
and 12 months following SCI or laminectomy). Four SCI groups and
four sham groups from each of the four time points (T1-T4), and one
control group (rats without SCI and laminectomy), were included.
Each group contained three samples. The NanoDrop ND-2100 (Thermo
Fisher Scientific, Waltham, MA, USA) was used to quantify total
RNA, and the Agilent 2100 (Agilent Technologies, Inc., Santa Clara,
CA, USA) was used to assess RNA integrity. Labeling, hybridization
and washing were performed according to the manufacturer’s
instructions. Briefly, Poly A was tailed to total RNA, which was
then labeled by Biotin. After they were labeled, RNAs were
hybridized onto the microarray (Affymetrix 902017, Affymetrix Santa
Clara, USA). Following washing and staining, scanning was performed
with the Affymetrix Scanner 3000 (Affymetrix, Inc., Santa Clara,
CA, USA).
Expression Console software (version1.3.1,
Affymetrix, Inc.) was used to analyze array images in order to
extract raw data and provide robust multi-array average (RMA)
normalization. The resulting data was processed by Genespring
software (version 12.5; Agilent Technologies, Inc.). Differential
expression of miRNAs was then identified by evaluation of fold
changes. The Targetscan database (http://www.targetscan.org) was used to predict the
target genes of differentially expressed miRNAs. The
distinguishable expression pattern of miRNAs among samples was
demonstrated by performing Hierarchical Clustering using Multi
Experiment Viewer 4.9 software.
RT-qPCR
Quantification was performed with a two-step
reaction proces, RT and PCR. Each RT reaction consisted of 0.5
μg RNA, 2 μl PrimerScript Buffer, 0.5 μl oligo
dT, 0.5 μl random 6mers and 0.5 μl PrimerScript RT
Enzyme Mix I (Takara, Otsu, Japan), in a total volume of 10
μl. The reactions were performed in a GeneAmp®
PCR System 9700 (Applied Biosystems) for 15 min at 37°C, followed
by heat inactivation of RT for 5 sec at 85°C. For miRNA, each RT
reaction consisted of 1 μg RNA, 4 μl miScript HiSpec
Buffer, 2 μl Nucleics mix and 2 μl miScript Reverse
Transcriptase mix (Qiagen, Hilden, Germany) in a total volume of 20
μl. Reactions were performed in a GeneAmp® PCR
System 9700 (Applied Biosystems, USA) for 60 min at 37°C, followed
by heat inactivation of RT for 5 min at 95°C. For mRNA, the
expression of miRNAs and mRNA was normalized to that of U6 or
GADPH, respectively. Each sample was performed in triplicate. The
primer sequences (Oebiotech, Shanghai, China) were as follows: U6,
5′-CAAGGATGACACGCAAATTCG-3′; rno-miR-1949,
5′-TATACCAGGATGTCAGCATAGTT-3′; rno-miR-205,
5′-TCCTTCATTCCACCGGAGTCTGT-3′; rno-miR-150-3p, forward
5′-ATTTACTGGTACAGGCCTGGG-3′ and reverse 5′-CAGTGCAGGGTCCGAGGTAT-3′;
rno-miR- 652-5p, forward 5′-AAGGACAACCCTAGGAGGGG-3′ and reverse
5′-CGCAGGGTCCGAGGTATTC-3′; Rb1, forward 5′-TGTGATGTTTGCTCTTGGT-3′
and reverse 5′-GAATGTGGACAATCAATCAACT-3′; GAPDH, forward
5′-GCGAGATCCCGCTAACATCA-3′ and reverse
5′-CTCGTGGTTCACACCCATCA-3′.
Western blotting
In order to investigate the expression of the Rb1
protein, western blotting was performed. Briefly, bladder samples
of rats were lysed in RIPA buffer (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). Following supplementation with protease inhibitor
cocktail (Sigma-Aldrich, St. Louis, MO, USA), the lysates were
centrifuged in order to isolate protein. Protein samples were
separated using SDS-polyacrylamide gel electrophoresis (Tuheshiye,
Shanghai, China) and then transferred to nitrocellulose membranes
(Tuheshiye). Membranes were blocked with 5% non-fat milk in PBS,
containing 0.05% Tween-20, in order to reduce non-specific antibody
binding. Blots were then incubated with rabbit anti-human Rb
antibody primary antibodies (1:500; cat. no. GTX50459; GeneTex).
β-actin was used as internal control. A got anti-rabbit
immunoglobulain G horseradish perxoidase linked secondary antibody
(1:2,000; cat. no. HA1001; Huaan, Hangzhou, China) was used to
detect bound primary antibody. All experiments were conducted in
triplicate.
Immunohistochemistry
In order to demonstrate the presence of the Rb1
protein, immunohistochemistry was used. In brief, formalin-fixed
and paraffin-embedded samples were cut into sections. The
histological sections were deparaffinised with xylene, rehydrated
through graded ethanol solutions and washed in distilled water.
After endogenous peroxidase activity had been blocked using 0.3%
hydrogen peroxide for 15 min, the sections were submitted to
antigen retrieval by microwaving 3 times for 5 min each and then
cooling to room temperature. Following washing with distilled water
and TBS, the primary antibody to Rb was added at room temperature
in a wet chamber. Following thorough washing with TBS, the sections
were incubated with the rabbit secondary antibody (ImmunoCruz™ ABC
staining system; 1:500; cat. no. sc-2018; Santa Cruz Biotechnology,
Dallas, USA) in a wet chamber at room temperature and stained with
DAB (Saichi, Beijing, China). The sections were then
counter-stained with hematoxylin.
Statistical analysis
Data are reported as the mean ± standard deviation.
Comparisons between two groups were analyzed using Student’s
t-test using SPSS 18.0 software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression profiles of miRNAs in the rat
bladder following SCI
Following array processing, Expression Console
software (version1.3.1, Affymetrix, Inc.) was used to analyze array
images in order to obtain raw data and then to provide RMA
normalization. Based on the literature pertaining to the duration
of SCI at the time of diagnosis of bladder cancer, and using a
formula to adjust these times to the lifespan of a rat, miRNA
expression data were collected from rat bladders at 3, 6, 9 and 12
months following SCI (time points, T1-T4) (8,22,23).
Microarray analysis demonstrated that 381 miRNAs
were differentially expressed in the adult rat bladder following
SCI. Hierarchical Clustering was performed in order to illustrate
the expression pattern of the distinguishable miRNAs among the
samples (Fig. 1A).
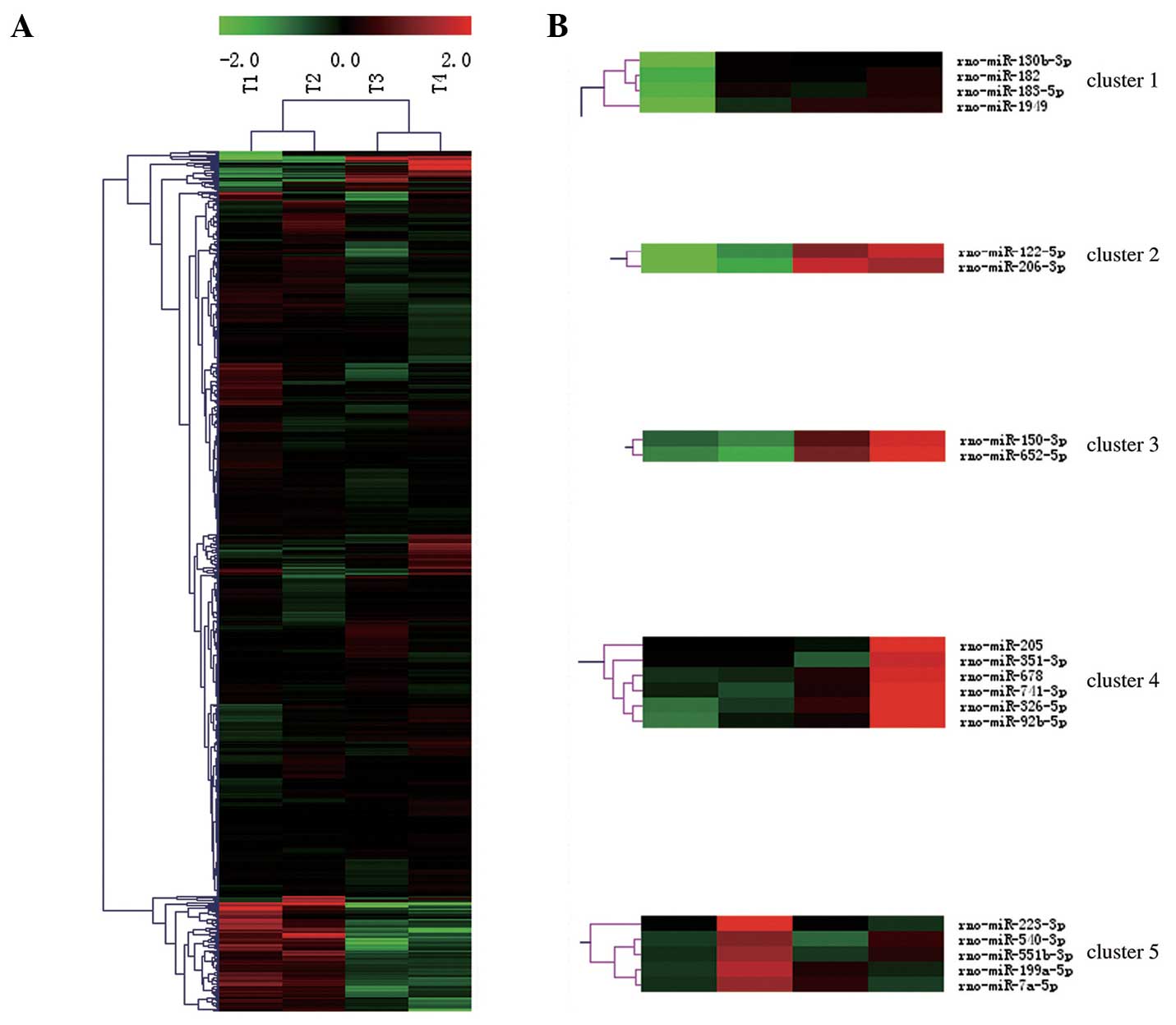 | Figure 1Expression profiles of miRNAs in rat
bladder following SCI. (A) Heat map of microarray expression data
from four time points (T1-T4). The expression of miRNAs, analyzed
by Hierarchical Clustering, is shown on the y-axis, and the four
time points, analyzed by Hierarchical Clustering, are shown on the
x-axis. The relative miRNA expression is depicted according to the
color scale above. Red indicates upregulation; green,
downregulation. (B) miRNAs is which the RMA normalization signal
value of the experimental group was ≥5, the RMA normalization
signal value ratio of experimental group to that of the sham group
was ≥2, and the fold change was ≥4), belong to these five clusters.
T1-T4 = 3, 6, 9 and 12 months after SCI. miRNA, microRNA; SCI,
spinal cord injury; RMA, robust multi-array average. |
Since only a small proportion of miRNAs are
abundantly expressed in the microRNAome, and as they seem to be
more important in bladder biology and carcinogenic effects, only
those miRNAs which were fulfilled following criteria that the RMA
normalization signal value was ≥5 at one time point, the ratio of
the RMA normalization signal value of the experimental group to
that of the sham group was ≥2, and the fold change was ≥4, were
considered likely to be involved in bladder cancer pathogenesis
(3,4,14).
Based on these criteria, seven miRNAs were identified, which are
shown in Table I.
 | Table IDeregulated abundant microRNAs in the
rat bladder following SCI. |
Table I
Deregulated abundant microRNAs in the
rat bladder following SCI.
| miRBase name | T1a
| T2b
| T3c
| T4d
|
|---|
| Fold change | SV1e | SV2f | Fold change | SV1e | SV2f | Fold change | SV1e | SV2f | Fold change | SV1e | SV2f |
|---|
| miR-223-3pg | 1.38 | 2.45 | 1.36 | 18.34h | 6.19h | 2.38h | 1.45 | 2.52 | 1.33 | 1.08 | 2.10 | 2.45 |
| miR-122-5pg | 1.04 | 3.01 | 5.40 | 7.10 | 5.79 | 3.79 | 35.07 | 8.09 | 5.42 | 52.60h | 8.68h | 3.01h |
| miR-1949g | −2.98 | 2.19 | 1.01 | 2.89 | 5.29 | 2.20 | 4.59h | 5.96h | 1.92h | 4.56h | 5.95h | 2.19h |
| miR-92b-5pg | 1.48 | 3.56 | 3.65 | 2.63 | 4.39 | 2.77 | 3.22 | 4.68 | 3.56 | 20.04h | 7.32h | 3.56h |
| miR-205g | −1.29 | 1.51 | 1.36 | −1.29 | 1.51 | 2.34 | −1.39 | 1.41 | 1.29 | 11.36h | 5.38h | 1.51h |
| miR-652-5pg | −1.28 | 2.41 | 2.92 | −1.67 | 2.03 | 2.44 | 3.39 | 4.53 | 1.96 | 7.29h | 5.63h | 2.41h |
| miR-150-3pg | 1.01 | 2.58 | 2.59 | −1.22 | 2.27 | 1.99 | 2.94 | 4.12 | 1.79 | 6.27h | 5.21h | 2.58h |
Deregulated abundant miRNAs in the rat
bladder following SCI
A more thorough investigation of the miRNAs in
Table I was conducted in order to
identify the potential mechanisms underlying the development of
bladder cancer, and the miRNAs responsible, by examining numerous
published studies. To the best of our knowledge, there is no
evidence suggesting that six of the seven miRNAs (miR-223-3p,
miR-122-5p, miR-1949, miR-92b-5p, miR-652-5p and miR-150-3p) are
associated with the development of bladder cancer. A number of
studies have reported that miR-205 is a biomarker for the
diagnosis, staging and prognosis of bladder cancer. However, it has
not been reported that miR-205 or its target genes induce bladder
cancer (24–31).
Subsequently, clusters of the miRNAs in Table I were investigated, according to
the hypothesis that miRNAs in the same cluster, as analyzed by
Hierarchical Clustering, may perform similar biological functions
and exhibit similar expression patterns. The seven miRNAs in
Table I belonged to five clusters
(Fig. 1B).
To the best of our knowledge, there are no previous
reports that miRNAs in three of these clusters (2, 3 and 5) are
associated with bladder cancer. Among the miRNAs in cluster 4, none
are associated with bladder cancer, with the exception of miR-205.
miR-205, a biomarker for the diagnosis, grading and prognosis of
bladder cancer, is not associated with the biological mechanisms
underlying tumorigenesis in the bladder (24–31).
Previous reports have shown that all members of
cluster 1, with the exception of miR-1949, that is, miR-130b-3p,
miR-182 and miR-183-5p, are biomarkers of bladder cancer, and their
upregulation was also observed in the present study (32–37).
As shown in Fig. 2A, the change in
expression of miR-1949 over time, was similar to that of miR-182
and miR-183-5p. miR-182 and miR-183-5p are the most frequently
reported deregulated miRNAs in studies of bladder cancer studies.
However, the increase in their expression was not as marked as that
of miR-1949. Therefore, miR-1949 was further investigated.
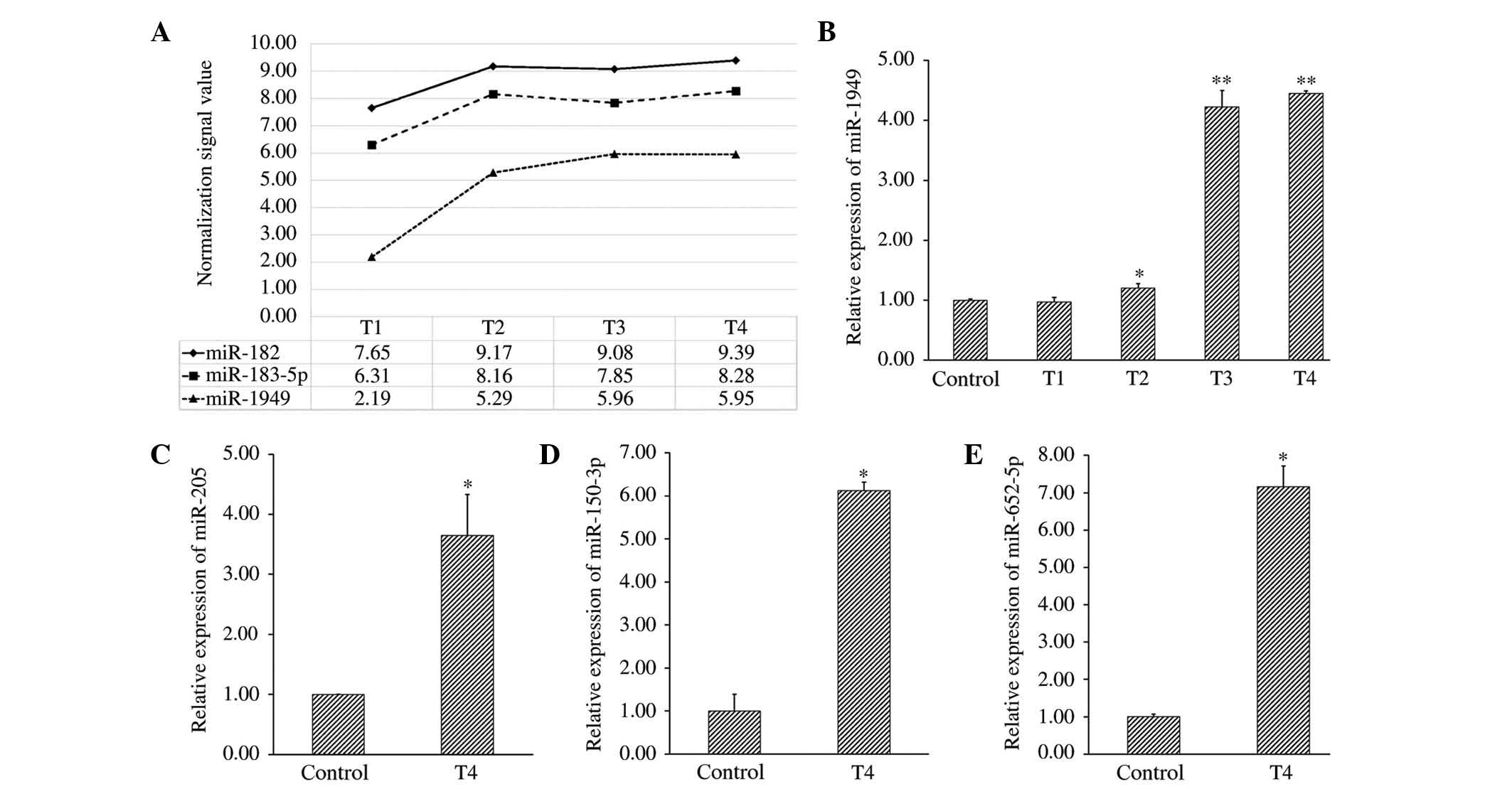 | Figure 2Expression trends of miR-182,
miR-183-5p and miR-1949, and validation of microarray data by
qRT-PCR. (A) Expression trends of miR-182, miR-183-5p and miR-1949
over four time points (T1-T4) were analyzed by microarray. (B) The
relative expression of miR-1949 at four time points (T1-T4) was
measured by qRT-PCR and normalized to that of the internal control
(U6). (C), (D) and (E) The relative expression of miR-205,
miR-150-3p and miR-652-5p at the check point for which the RMA
normalization signal value of the experimental group was ≥5, the
RMA normalization signal value ratio of the experimental group to
that of the sham group was ≥2, and the fold change ≥4) was analyzed
by qRT-PCR and normalized to the internal control (U6).
*P<0.05 and **P<0.005, compared with the control
group). T1-T4=3, 6, 9 and 12 months following spinal cord injury.
miRNA, microRNA; qRT-PCT, quantitative reverse
transcription-polymerase chain reaction; RMA, robust multi-array
average. |
qRT-PCR validates the profiling data
In order to confirm the accuracy of the microarray
data, qRT-PCR was used to measure the expression of miR-1949 at
four time points (T1-T4), and that of miR-205, miR-652-5p and
miR-150-3p at the time point for which the change in their
expression, as assessed by the microarray, was consistent with the
criteria already described. The correlation between microarray and
qRT-PCR data was strong for these miRNAs, with the exception of
miR-205. The fold change of miR-205 according to qRT-PCR, was 3.65,
which was not consistent with the microarray data, although a
statistical difference still existed, compared withe the control
group. The relative expression of each miRNA compared with that in
control subjects is shown in Fig.
2B–E.
miR-1949 targets Rb1
As miRNA functions primarily by inhibiting the mRNA
of target genes, the target of miR-1949 that is known to be
involved in the pathogenesis of bladder cancer was further
analyzed. The target genes of miR-1949 were computationally
predicted by TargetScan. Computational prediction indicated that
miR-1949 may target Rb1. It has been shown that the absence of the
Rb1 protein is involved in several pathways in bladder cancer,
either at its initiation or during its progression, including
cell-cycle regulation and apoptosis (16,17).
It is hypothesized that the Rb1 gene is involved in bladder tumori
In order to assess the differential expression of miRNAs, 27
samples were used from the T1-T4 time points (3, 6, 9 and 12 months
following SCI or laminectomy). Four SCI groups and four sham groups
from each of the four time points (T1-T4), and one control group
(rats without SCI and laminectomy), were included genesis, as
tumorigenicity may be suppressed by transfecting the Rb1 gene into
bladder cancer cells (16).
In order to investigate the regulation of Rb1 by
miR-1949, the expression of Rb1 mRNA was measured using qRT-PCR.
The relative expression of Rb1 at four time points (T1-T4) compared
with that in normal control subjects is shown in Fig. 3A. The level of Rb1 mRNA was not
significantly influenced by the expression of miR-1949. Further,
western blotting of four time points (T1-T4) samples demonstrated
that the level of the Rb1 protein was decreased at T3 and T4
(Fig. 3B), which was further
confirmed by immunohistochemistry (Fig. 3C–G). This suggested that Rb1
expression may be inhibited by miR-1949, primarily through
translational inhibition. miRNA has two modalities to inhibit it
target proteins: One is to inhibit its target mRNA and another is
to degrade its target mRNA, if the miRNA is high compared with its
target mRNA (1–3). In the first modality, the level of
target mRNA remains unchanged. These data indicate that endogenous
Rb1 is targeted and regulated by miR-1949, and also suggest that
the potential carcinogenic effect of miR-1949 is due to the
inhibition of Rb1.
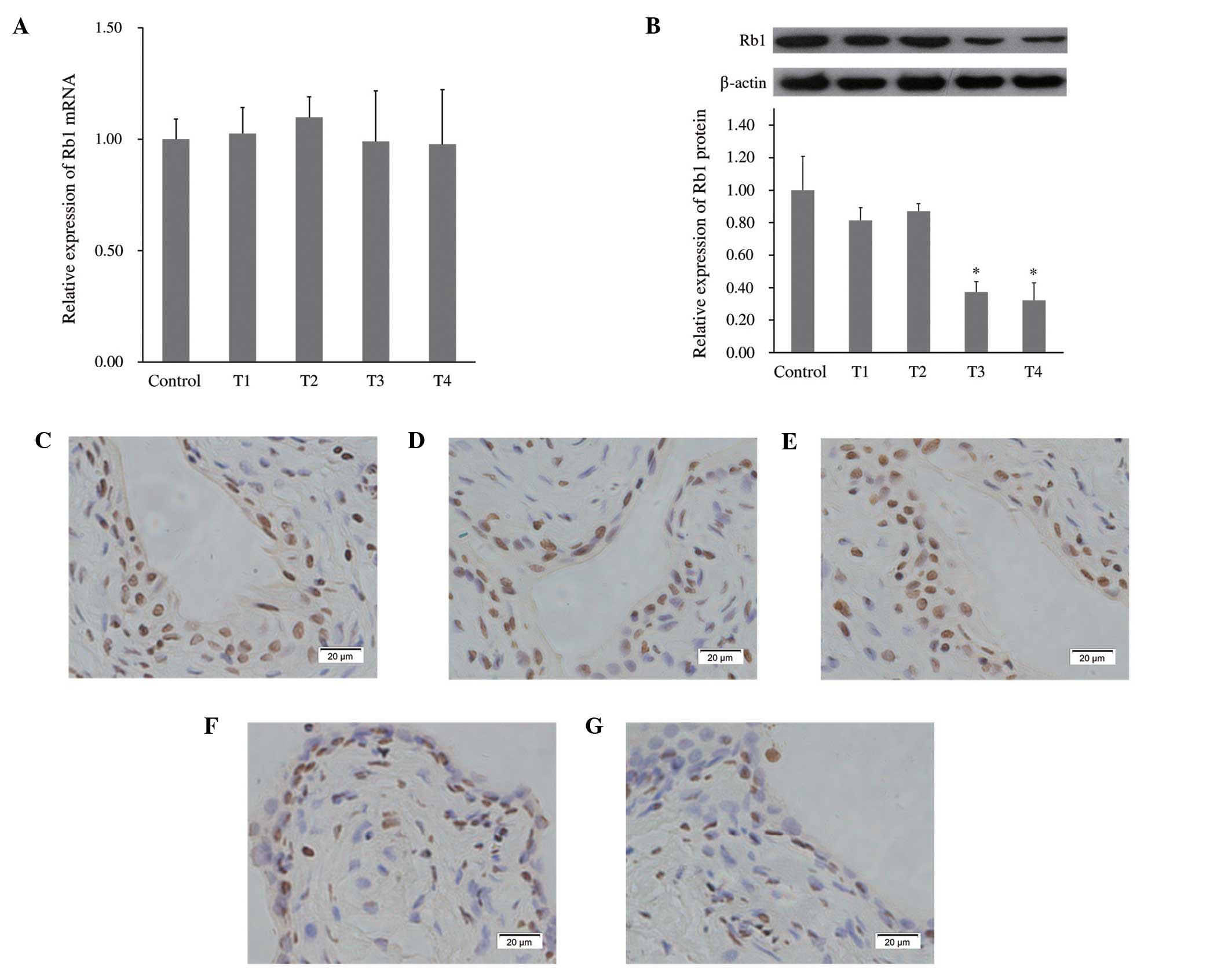 | Figure 3miR-1949 targets Rb1. (A) Relative
expression of Rb1 mRNA at four time points (T1-T4) was measured
using qRT-PCR and normalized to the expression of the internal
control (GADPH). (B) The protein level of Rb1 at four time points
(T1-T4) was analyzed by western blotting and normalized to the
level of β-actin. Rb1 protein levels significantly decreased at T3
and T4. *P<0.05, compared with the control group.
(C), (D), (E), (F) and (G) Immunohistochemical staining showing
bladder tissue Rb1 protein from the control group and T1-T4 time
points, respectively. Magnification, ×400. T1-T4=3, 6, 9 and 12
months following spinal cord injury. Rb1, retinoblastoma 1;
qRT-PCR, quantitative reverse transcription-polymerase chain
reaction. |
Influence of age on miR-1949 and Rb1
protein
As tumorigenesis and the expression of certain
miRNAs has been shown to be associated with aging (38–40),
the levels of miR-1949 and Rb1 protein were measured in rats of
different ages. The healthy rats from the control group and the
t1-t3 groups without SCI and laminectomy, which were employed
together with SCI rats, were sacrificed at the same time as the SCI
rats from the T1-T4 groups, in order to extract samples for
qRT-PCR. Each group included three rats. No significant differences
were detected, which suggested that miR-1949 levels are not
influenced by age (Fig. 4A). The
Rb1 protein levels were analyzed by western blotting at different
time points (t1-t3), and no significant difference was detected
among these groups, indicating that the level of the Rb1 protein is
also not influenced by age (Fig.
4B). Furthermore, this result was validated by
immunohistochemistry (Fig.
4C–E).
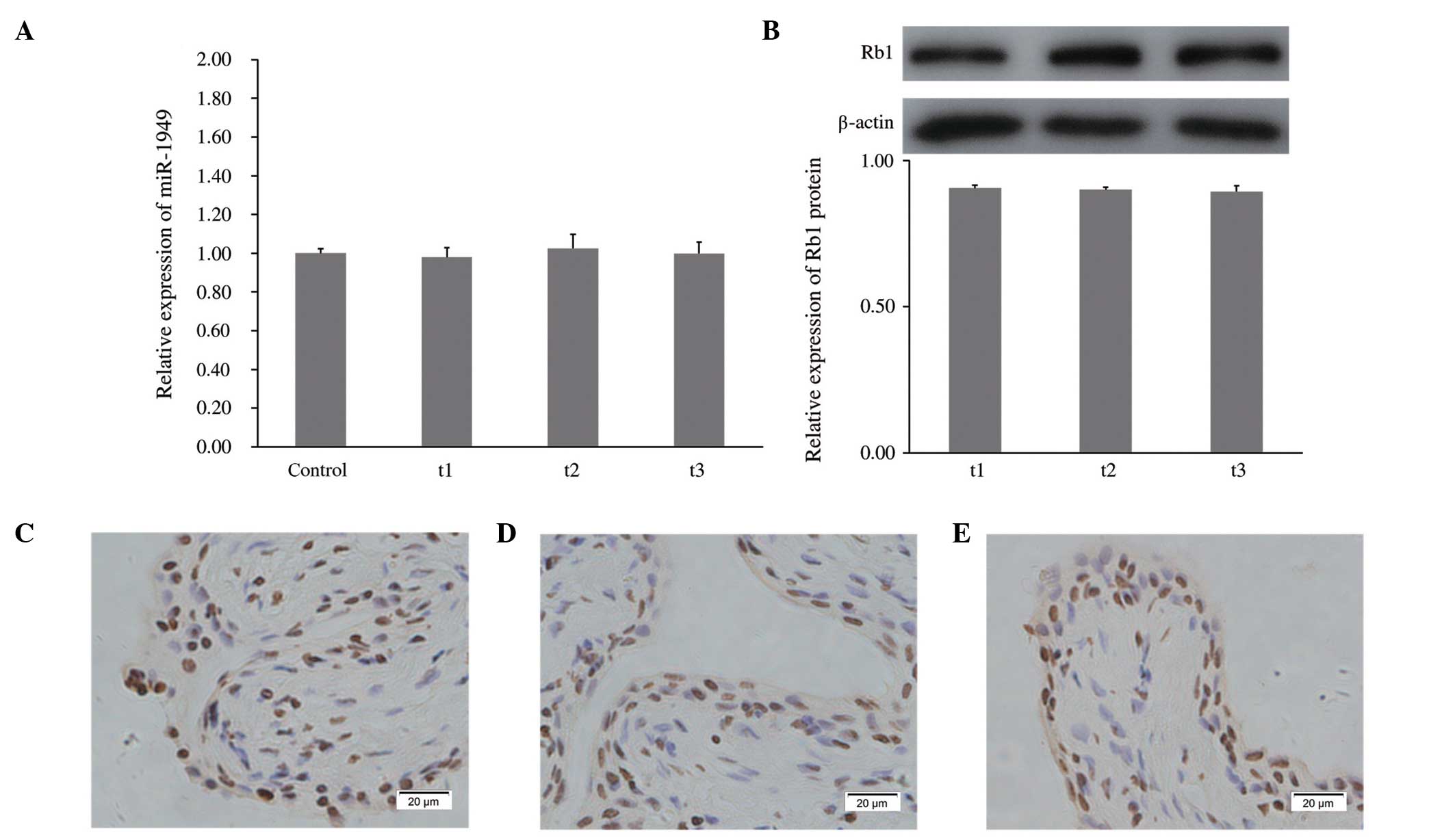 | Figure 4Influence of age on miR-1949 and Rb1
protein. (A) Relative expression of miR-1949 was analyzed by
qRT-PCR and normalized to the internal control (U6). (B) The
protein level of Rb1 was analyzed by western blotting and
normalized to β-actin. (C), (D) and (E) Immunohistochemical
staining showing bladder tissue Rb1 protein at t1, t2 and t3,
respectively. Magnification, ×400. Rats of the t1-t3 groups without
SCI and laminectomy, which were used together with SCI rats, were
sacrificed at an identical time to SCI rats from T2-T4, to extract
samples. T1-T4=3, 6, 9 and 12 months following SCI. SCI, spinal
cord injury; Rb1, retinoblastoma 1; qRT-PCR, quantitative reverse
transcription-polymerase chain reaction. |
Overall trends of miR-1949 and Rb1
protein expression
The expression levels of miR-1949 and Rb1 protein in
rat bladders were measured by qRT-PCR and western blotting,
respectively, at 1 day, 14 days and 1 month following SCI, and no
significant difference was detected among the groups (Fig. 5A and B). In order to analyze the
overall trends of the expression of miR-1949 and Rb1 protein, the
data from the T1-T4 time points were used and are indicated as
c4-c7 in Fig. 5C and D,
respectively. The expression of miR-1949 remained stable for 3
months following SCI, and then significantly increased, a trend
that was maintained for at least 9 months (Fig. 5C). The expression of the Rb1
protein did not significantly decrease until c5 (Fig. 5D). If these trends are maintained,
the Rb1 protein levels may decline to a carcinogenic level.
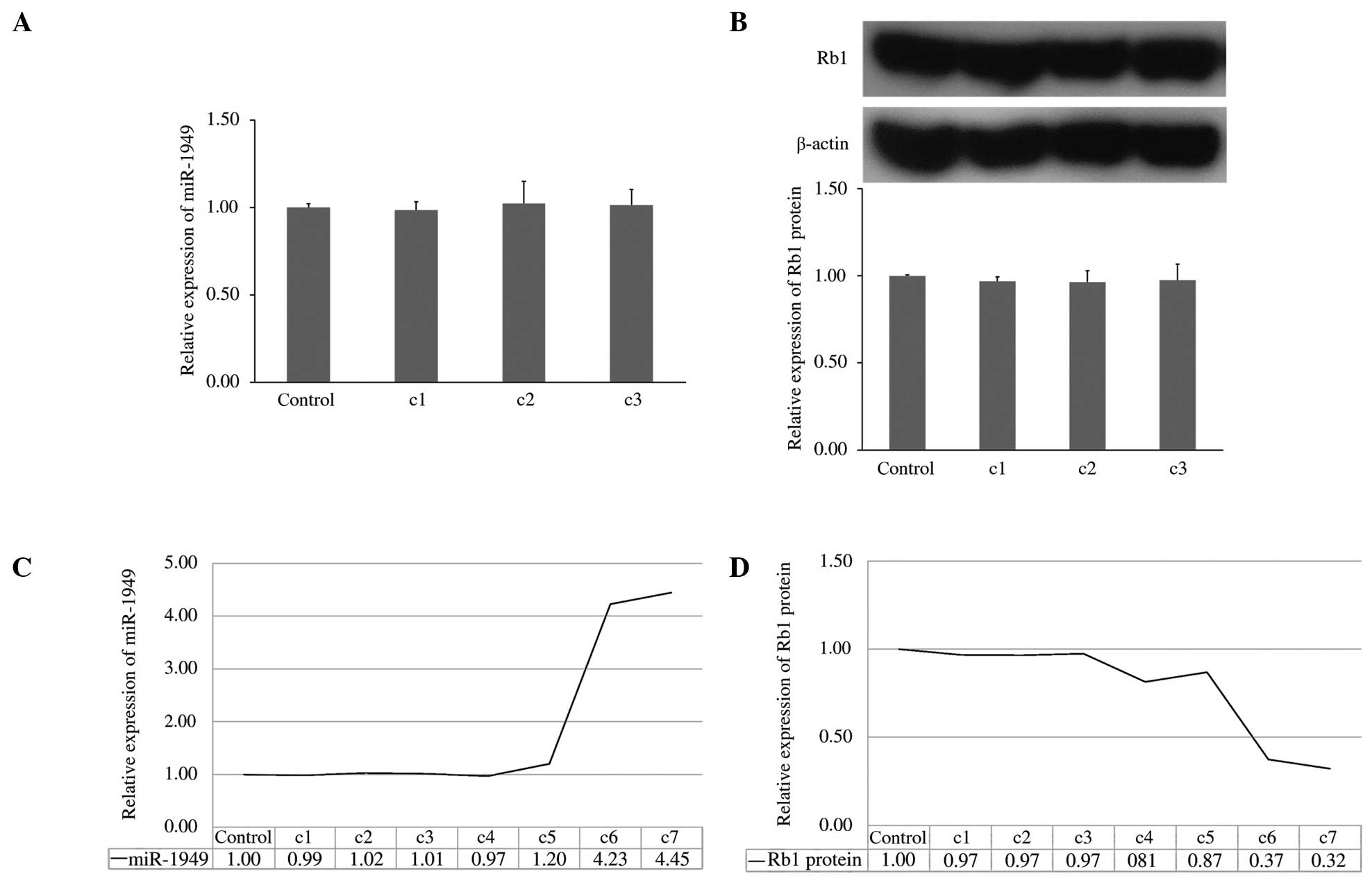 | Figure 5Overall trends of miR-1949 and the
Rb1 protein. (A) Expression level of miR-1949 in rat bladders was
measured by qRT-PCR at 1 day, 14 days and 1 month following SCI.
(B) Protein level of Rb1 at 1 day, 14 days and 1 month following
SCI. (C) Overall trend of miR-1949, which was stable until c4, and
then increased significantly. (D) Overall trend of the Rb1 protein.
It decreased significantly at c5. c1-7=1 day, 14 days, and 1, 3, 6,
9 and 12 months following SCI. Rb1, retinoblastoma 1; qRT-PCR,
quantitative reverse transcription-polymerase chain reaction; SCI,
spinal cord injury. |
Discussion
miRNAs are small non-coding RNA molecules that
regulate gene expression by interacting with target mRNAs. These
post-transcriptional regulators serve to maintain the
characteristic protein expression of distinct cellular phenotypes
(2,41). Although translation is blocked, the
target mRNAs are thought to be degraded only by those miRNAs with
high complementarity (1,3). The level of Rb1 mRNA was not
significantly influenced by miR-1949 expression, suggesting that
Rb1 expression may be inhibited by miR-1949 primarily through
translational inhibition.
When miRNAs were identified, they were identified as
a class of gene regulators, which were associated with cell growth,
differentiation and apoptosis. Notably, deregulation of these
miRNAs may cause them to function as ‘oncomiRs’, which induce
tumorigenesis and promote tumor development. Therefore, knowledge
of the tissue microRNAome as a potential microenvironment for
inducing tumorigenesis is a prerequisite for an improved
understanding of cancer cell biology, and also extends the number
of existing molecules that may be developed for use as accurate
diagnostic makers and efficient therapeutic targets (4).
Microarray is a powerful high-throughput tool which
is able to monitor the global miRNA expression dynamically within
tens of samples that are processed in parallel during a single
experiment (42). Compared with
investigation of the expression of single miRNAs, microarray
technology makes it possible to study the mechanism of disease and
injury in a systemic and global context.
It has been reported that miRNAs that are expressed
at a low level are unlikely to perform biological functions, and a
minimum expression threshold amount must be achieved in order to
inhibit target mRNAs (4,43,44),
suggesting that the intensity expression values of miRNAs in the
bladder microRNAome have more possibility to execute their
biological functions (3). In the
candidate lists obtained in the present study, miRNAs were
prioritized if they exhibited an RMA normalization signal value in
the experimental group of ≥5, in order to identify the miRNAs with
potential biological function.
The absence of the Rb1 protein is involved in a
number of pathways in bladder cancer, either at its initiation or
during its progression (16,17).
Alteration or deletion of the Rb1 protein is observed in human
bladder cancer, and transfecting the Rb1 gene into bladder cancer
cells suppresses the tumorigenicity of these cells (16). The alteration in Rb1 protein
expression may independently predict recurrence and progression in
bladder cancer with Bacillus Calmette-Guerin administration, and
low expression of Rb1 is a predictor of non-response and cancer
recurrence (45,46).
One of the first studies to show an increased risk
of bladder cancer following SCI was performed by Kawaichi (47). A number of studies have
demonstrated that independent risk factors for the development of
bladder cancer in patients with SCI, include age, long-term
indwelling catheters, recurrent UTIs and bladder stones (12,48,49).
However, none of these studies has explored the pathogenesis of
bladder cancer following SCI at the miRNA level. To the best of our
knowledge, the present study is the first to explore the potential
mechanism of the increased incidence of bladder cancer following
SCI in the context of the microRNAome.
Based on the literature pertaining to the duration
of SCI at the time of diagnosis of bladder cancer, and using a
formula to adjust these times to the lifespan of a rat, four time
points (3, 6, 9 and 12 months following SCI) were selected for use
in the present study (8,22,23).
Since the pathological environment of the bladder following SCI
differ from the normal bladder, investigation of the potential
association between miRNA and the protein expression of Rb1 with
the occurrence of bladder cancer following SCI, is required.
However, as a high number of experimental animals
are required to obtain bladder cancer cases from rats with SCI, and
the lifespan of experimental animals is limited, the present study
is restricted to potential underlying mechanisms and the
identification of miRNAs that may be involved in this process
(8,10,22,23).
However, a long-term clinical study with a large sample is also
necessary.
In conclusion, the present study suggests that
miR-1949 is involved in the translational regulation of Rb1, and
also that the upregulation of miR-1949 may promote tumorigenesis
via targeting Rb1. Future clinical studies aiming at understanding
how miRNAs contribute to the development of bladder cancer
following SCI, may help to further elucidate the role of these
small non-coding RNAs.
Acknowledgments
This study was supported by the Medical Science and
Technology Youth Cultivation Project of the Chinese People’s
Liberation Army (grant no. 13QNP017), the Key Program of National
Natural Science Foundation of China (grant no. 81330042), the
General Program of National Natural Science Foundation of China
(grant nos. 81371957 and 81171714), the Special Project between
Ministry of Science and Technology of China and Russia (grant no.
2014DFR31210) and the Cooperation Project between Tianjin Municipal
Science and Technology Commission and Australia (grant no.
13RCGFSY19000).
References
|
1
|
Williams AE: Functional aspects of animal
microRNAs. Cell Mol Life Sci. 65:545–562. 2008. View Article : Google Scholar
|
|
2
|
Nagalla S, Shaw C, Kong X, et al: Platelet
microRNA-mRNA coexpression profiles correlate with platelet
reactivity. Blood. 117:5189–5197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hou J, Lin L, Zhou W, et al:
Identification of miRNomes in human liver and hepatocellular
carcinoma reveals miR-199a/b-3p as therapeutic target for
hepatocellular carcinoma. Cancer Cell. 19:232–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Munding JB, Adai AT, Maghnouj A, et al:
Global microRNA expression profiling of microdissected tissues
identifies miR-135b as a novel biomarker for pancreatic ductal
adenocarcinoma. Int J Cancer. 131:E86–E95. 2012. View Article : Google Scholar
|
|
5
|
Hua R, Shi J, Wang X, et al: Analysis of
the causes and types of traumatic spinal cord injury based on 561
cases in China from 2001 to 2010. Spinal cord. 51:218–221. 2013.
View Article : Google Scholar
|
|
6
|
Bejany DE, Lockhart JL and Rhamy RK:
Malignant vesical tumors following spinal cord injury. J Urol.
138:1390–1392. 1987.PubMed/NCBI
|
|
7
|
Groah SL, Weitzenkamp DA, Lammertse DP,
Whiteneck GG, Lezotte DC and Hamman RF: Excess risk of bladder
cancer in spinal cord injury: Evidence for an association between
indwelling catheter use and bladder cancer. Arch Phys Med Rehabil.
83:346–351. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Subramonian K, Cartwright RA, Harnden P
and Harrison SC: Bladder cancer in patients with spinal cord
injuries. BJU Int. 93:739–743. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalisvaart JF, Katsumi HK, Ronningen LD
and Hovey RM: Bladder cancer in spinal cord injury patients. Spinal
Cord. 48:257–261. 2010. View Article : Google Scholar
|
|
10
|
Kaufman JM, Fam B, Jacobs SC, et al:
Bladder cancer and squamous metaplasia in spinal cord injury
patients. J Urol. 118:967–971. 1977.PubMed/NCBI
|
|
11
|
Geisse LJ and Tweeddale DN: Pre-clinical
cytological diagnosis of bladder cancer. J Urol. 120:51–56.
1978.PubMed/NCBI
|
|
12
|
Pannek J: Transitional cell carcinoma in
patients with spinal cord injury: A high risk malignancy? Urology.
59:240–244. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
West DA, Cummings JM, Longo WE, Virgo KS,
Johnson FE and Parra RO: Role of chronic catheterization in the
development of bladder cancer in patients with spinal cord injury.
Urology. 53:292–297. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lau P, Verrier JD, Nielsen JA, Johnson KR,
Notterpek L and Hudson LD: Identification of dynamically regulated
microRNA and mRNA networks in developing oligodendrocytes. J
Neurosci. 28:11720–11730. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sparkes RS, Sparkes MC, Wilson MG, et al:
Regional assignment of genes for human esterase D and
retinoblastoma to chromosome band 13q14. Science. 208:1042–1044.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miyamoto H, Shuin T, Torigoe S, Iwasaki Y
and Kubota Y: Retinoblastoma gene mutations in primary human
bladder cancer. Br J Cancer. 71:831–835. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mitra AP, Hansel DE and Cote RJ:
Prognostic value of cell-cycle regulation biomarkers in bladder
cancer. Semin Oncol. 39:524–533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Workman P, Aboagye EO, Balkwill F, et al:
Guidelines for the welfare and use of animals in cancer research.
Br J Cancer. 102:1555–1577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ban DX, Kong XH, Feng SQ, Ning GZ, Chen JT
and Guo SF: Intraspinal cord graft of autologous activated Schwann
cells efficiently promotes axonal regeneration and functional
recovery after rat’s spinal cord injury. Brain Res. 1256:149–161.
2009. View Article : Google Scholar
|
|
20
|
Zhou HX, Li XY, Li FY, et al: Targeting
RPTPsigma with lentiviral shRNA promotes neurites outgrowth of
cortical neurons and improves functional recovery in a rat spinal
cord contusion model. Brain research. 1586:46–63. 2014. View Article : Google Scholar
|
|
21
|
Liu Y, Wang CY, Kong XH, et al: Novel
multifunctional polyethylene glycoltransactivating-transduction
protein-modified liposomes cross the blood-spinal cord barrier
after spinal cord injury. J Drug Target. 18:420–429. 2010.
View Article : Google Scholar
|
|
22
|
Andreollo NA, Santos EF, Araújo MR and
Lopes LR: Rat’s age versus human’s age: What is the relationship?
Arq Bras Cir Dig. 25:49–51. 2012.In English, Portuguese. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Quinn R: Comparing rat’s to human’s age:
How old is my rat in people years? Nutrition. 21:775–777. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee H, Jun SY, Lee YS, Lee HJ, Lee WS and
Park CS: Expression of miRNAs and ZEB1 and ZEB2 correlates with
histopathological grade in papillary urothelial tumors of the
urinary bladder. Virchows Arch. 464:213–220. 2014. View Article : Google Scholar
|
|
25
|
Ratert N, Meyer HA, Jung M, et al: miRNA
profiling identifies candidate mirnas for bladder cancer diagnosis
and clinical outcome. J Mol Diagn. 15:695–705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tran MN, Choi W, Wszolek MF, et al: The
p63 protein isoform ΔNp63α inhibits epithelial-mesenchymal
transition in human bladder cancer cells: Role of MIR-205. J Biol
Chem. 288:3275–3288. 2013. View Article : Google Scholar :
|
|
27
|
Dip N, Reis ST, Timoszczuk LS, et al:
Stage, grade and behavior of bladder urothelial carcinoma defined
by the microRNA expression profile. J Urol. 188:1951–1956. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang G, Chan ES, Kwan BC, et al:
Expression of microRNAs in the urine of patients with bladder
cancer. Clin Genitourin Cancer. 10:106–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wiklund ED, Bramsen JB, Hulf T, et al:
Coordinated epigenetic repression of the miR-200 family and miR-205
in invasive bladder cancer. Int J Cancer. 128:1327–1334. 2011.
View Article : Google Scholar
|
|
30
|
Neely LA, Rieger-Christ KM, Neto BS, et
al: A microRNA expression ratio defining the invasive phenotype in
bladder tumors. Urol Oncol. 28:39–48. 2010. View Article : Google Scholar
|
|
31
|
Gottardo F, Liu CG, Ferracin M, et al:
Micro-RNA profiling in kidney and bladder cancers. Urol Oncol.
25:387–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Friedman JM, Liang G, Liu CC, et al: The
putative tumor suppressor microRNA-101 modulates the cancer
epigenome by repressing the polycomb group protein EZH2. Cancer
Res. 69:2623–2629. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han Y, Chen J, Zhao X, et al: MicroRNA
expression signatures of bladder cancer revealed by deep
sequencing. PLoS One. 6:e182862011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamada Y, Enokida H, Kojima S, et al:
MiR-96 and miR-183 detection in urine serve as potential tumor
markers of urothelial carcinoma: Correlation with stage and grade,
and comparison with urinary cytology. Cancer Sci. 102:522–529.
2011. View Article : Google Scholar
|
|
35
|
Pignot G, Cizeron-Clairac G, Vacher S, et
al: microRNA expression profile in a large series of bladder
tumors: Identification of a 3-miRNA signature associated with
aggressiveness of muscle-invasive bladder cancer. Int J Cancer.
132:2479–2491. 2013. View Article : Google Scholar
|
|
36
|
Yoshino H, Seki N, Itesako T, Chiyomaru T,
Nakagawa M and Enokida H: Aberrant expression of microRNAs in
bladder cancer. Nat Rev Urol. 10:396–404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang QH, Sun HM, Zheng RZ, et al:
Meta-analysis of microRNA-183 family expression in human cancer
studies comparing cancer tissues with noncancerous tissues. Gene.
527:26–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu Q, Seeger FH, Castillo J, et al:
Micro-RNA-34a contributes to the impaired function of bone
marrow-derived mononuclear cells from patients with cardiovascular
disease. J Am Coll Cardiol. 59:2107–2117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ito T, Yagi S and Yamakuchi M:
MicroRNA-34a regulation of endothelial senescence. Biochem Biophys
Res Commun. 398:735–740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nishino J, Kim I, Chada K and Morrison SJ:
Hmga2 promotes neural stem cell self-renewal in young but not old
mice by reducing p16Ink4a and p19Arf Expression. Cell. 135:227–239.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li S, Zhu J, Zhang W, et al: Signature
microRNA expression profile of essential hypertension and its novel
link to human cytomegalovirus infection. Circulation. 124:175–184.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu CG, Calin GA, Volinia S and Croce CM:
MicroRNA expression profiling using microarrays. Nat Protoc.
3:563–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Brown BD, Gentner B, Cantore A, et al:
Endogenous microRNA can be broadly exploited to regulate transgene
expression according to tissue, lineage and differentiation state.
Nat Biotechnol. 25:1457–1467. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sarasin-Filipowicz M, Krol J, Markiewicz
I, Heim MH and Filipowicz W: Decreased levels of microRNA miR-122
in individuals with hepatitis C responding poorly to interferon
therapy. Nat Med. 15:31–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Esuvaranathan K, Chiong E, Thamboo TP, et
al: Predictive value of p53 and pRb expression in superficial
bladder cancer patients treated with BCG and interferon-alpha.
Cancer. 109:1097–1105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lu X, Wu L, Liu Z, Xie L and Wang S:
Peripheral blood mono-nuclear cells inhibit proliferation and
promote apoptosis of HeLa cells following stimulation with Bacillus
Calmette-Guerin. Exp Ther Med. 5:561–566. 2013.PubMed/NCBI
|
|
47
|
Kawaichi S: Cancer of bladder os spinal
cord injury patients. Proceed Ninth Ann Clin Cord Injury Conf; pp.
104–105. 1961
|
|
48
|
Silverman DT, Hartge P, Morrison AS and
Devesa SS: Epidemiology of bladder cancer. Hematol Oncol Clin North
Am. 6:1–30. 1992.PubMed/NCBI
|
|
49
|
Burch JD, Rohan TE, Howe GR, et al: Risk
of bladder cancer by source and type of tobacco exposure: A
case-control study. Int J Cancer. 44:622–628. 1989. View Article : Google Scholar : PubMed/NCBI
|



















