Introduction
When cardiomyocytes suffer from ischemic or hypoxic
insults, the metabolic balance between glucose and fatty acid
shifts to fatty acid oxidation, which aggravates the oxygen
deficiency, as fatty acid consumes 10% more oxygen than glucose
when an equal amount of adenosine triphosphate (ATP) is produced.
Furthermore, increased fatty acid oxidation has been reported to
induce mitochondrial uncoupling and increase oxidative stress
(1,2). Normalizing metabolic imbalances,
which are underlying causes of metabolic disorders, has been one of
the targets for the treatment of ischemic heart diseases (3).
Glucagon-like peptide-1 (GLP-1), an incretion
hormone, has been confirmed to potently promote insulin secretion
and down-regulate glucose levels, which gives it a high potential
to be used for the treatment of diabetes patients (4–7).
However, its half life time is too short for it to be used in
clinic (8). Therefore, analogues
with significantly longer half-lives, such as exendine-4, have been
developed and used in the clinic (9). Over the last decade, a growing body
of evidence has demonstrated that GLP-1 and its analogues can exert
protective effects on cardiomyocytes with ischemic or hypoxic
damage (10–13); however, the exact mechanism is
still elusive. Studies have reported that GLP-1 and its analogues
increased the glucose uptake and helped to preserve the cardiac
function in animal experiments and clinical trials (13,14).
However, whether exendine-4 can contribute to restore the metabolic
balance between glucose oxygen and fatty acid oxidation and
therefore ameliorate the energy imbalance of hypoxia-induced
cardiomyocytes has not been fully elucidated.
The aim of the present study was to determine
whether exendin-4 is capable of reducing hypoxia/reoxygenation
(H/R)-induced injury by normalizing the energy imbalance in
cardiomyocytes. For this, a model of hypoxia/reoxygenation
(H/R)-induced injury in H9c2 cardiomyocyte cells was established to
assess the effects of exendin-4 on glucose uptake. The possible
mechanism involved in this process was also investigated by
assessing the activation of the p38 mitogen-activated protein
kinase (MAPK) signaling pathway.
Materials and methods
Cell culture and hypoxia/reoxygenation
treatment
H9c2 cells (Chinese Academy of Medical Sciences,
Shanghai, China) were cultured in Dulbecco’s modified Eagle’s
medium: Nutrient Mixture F-12 (DMEM/F12; Thermo Fisher Scientific,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Invitrogen Life Technologies, Carlsbad, CA, USA), in
50-cm2 flasks in a humidified atmosphere of 5%
CO2 at 37°C. The hypoxia/reoxygenation (H/R) model was
established according to the methods described in previous studies
with certain modifications (15).
Briefly, when cells were cultured to 80% confluence in appropriate
culture, they were subjected to hypoxia using DMEM/F12 without FBS
and glucose in a hypoxia chamber (Forma 370; Thermo Fisher
Scientifiic, Waltham, MA, USA) saturated with a gas mixture (95%
N2 and 5% CO2) at 37°C. Following hypoxia
treatment, the culture medium was replaced with fresh normal medium
and the plate was placed in the humidified atmosphere of 5%
CO2 to receive reoxygenation treatment. Different
treatment times of hypoxia and reoxygenation were used to determine
the optimal time for establishing the H/R model. Exendine-4 (Eli
Lilly, Indianapolis, IN, USA), a GLP-1 analogue, was added to the
culture medium for 30 min before they were subjected to hypoxia. In
certain cases, inhibitors of p38MAPK, BIRB796 (1 μM) and
SB203580 (5 μM) (Santa Cruz Biotechnology Inc., Dallas, TX,
USA), were added to the culture medium 10 min prior to treatment
with exendine-4.
Cell counting kit (CCK)-8 assay
Cell viability was assessed using the CCK-8
(Beyotime Institute of Biotechnology, Haimen, China) as described
previously with certain modifications (16). The H9c2 cells (1×104)
were seeded in 96-well microplates. Folowing H/R treatment (4/2,
6/3, 12/4, 14/5, 16/6 and 22/10 h) with or without exendine-4 (0,
50, 100, 200 and 300 nM, respectively), cells were cultured in
fresh medium and 10 μl CCK-8 solution. The plates were then
incubated in the humidified atmosphere of 5% CO2 at 37°C
for 2 h. Finally, the optical density (OD) values at 470 nm were
measured using a microplate reader (Multiskan MK33; Thermolab
systems, Helsinki, Finland).
Measurement of myocardial glucose
uptake
Myocardial glucose uptake was measured as the levels
of intracellular
2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglu-cose
(2-NBDG). 2-NBDG is a fluorescent labeled glucose analogue, which
can be transported into the cytoplasm from the culture medium but
cannot be metabolized further (17). Following incubation for 30 min,
cells were rinsed with phosphate-buffered saline (PBS) and the
fluorescent density was measured by a microplate reader (721D;
Pudong Shanghai Physical Optical Instrument Factory, Shanghai,
China) at an excitation wavelength (Ex.) of 488 nm/and an emission
wavelength (Em.) of 520 nm. The total fluorescent density of every
well was adjusted by the OD values of cell viability from the CCK-8
cell counting kit assay, which was performed as soon as the
measurement of the fluorescent density was finished. The actual
intracellular glucose levels were defined as the ratio of
fluorescent density (arbitrary unit, a.u.) to the OD values of cell
viability.
Colorimetry
The levels of lactate dehydrogenase (LDH) in the
culture medium, as well as the activity of phosphofructoki-nase-1
(PFK-1) and carnitine palmitoyltransferase-1 (CPT-1) in the H9c2
cells were determined by colorimetry. The experiment was performed
using commercially available kits according to the manufacturer’s
instructions. The LDH Activty Colorimetric assay was purchased from
Jiancheng Bioengineering Institute (Nanjing, China). The PFK-1 and
CPT-1 Activty Colorimetric assays were purchased from Sigma-Aldrich
(St. Louis, MO, USA). Briefly, culture medium was separated by
centrifugation at 1,600 x g for 10 min at 4°C and then used for the
measurement of the LDH levels. The H9c2 cells were collected and
lysed with cell lysis buffer (Beyotime Institute of Biotechnology,
Haimen, China). The cell lysates were then centrifuged at 1,600 x g
for 10 min at 4°C and the supernatants of were collected for the
detection of PFK-1 and CPT-1 activity. Following incubation with
the reagents included in the kits, the absorbance values at 340 and
420 nm were measured continuously using a spectrophotometer
(Multiskan MK33, Thermolab systems, Helsinki, Finland). The LDH
levels was expressed as U/l. The activity of PFK-1 and CPT-1 was
defined as the fold-change in enzyme activity relative to that in
the control group. The protein concentration was determined using
the bicinchoninic acid (BCA) protein assay (Beyotime Institute of
Biotechnology) (18).
ELISA assays
The levels of creatine kinase-MB (CK-MB) in the
culture medium were measured using a CK-MB ELISA assay kit (R&D
Systems, Minneapolis, MN, USA) according to the manufacturer’s
instructions. Following the indicated treatments as mentioned
above, the culture medium were collected and centrifuged at 1,600 x
g for 10 min at 4°C. The supernatants were collected for the
detection of CK-MB. The supernatants were then incubated with the
reagents included in the kits. Finally, the absorbance values were
measured using a microplate reader (Multiskan MK33; Thermolab
systems, Helsinki, Finland) at 450 nm. The CK-MB levels were
expressed as U/l.
Flow cytometry
Cell apoptosis was examined using flow cytometry.
H9c2 cells (2×104/100 μl) were seeded in six-well
plates for 72 h. After the indicated treatments mentioned above,
cells were collected, washed with cold PBS and resuspended at a
density of 1×106/ml. Cells (500 μl) were mixed
with 5 μl Annexin V-fluorescein isothiocyanate (Beyotime
Institute of Biotechnology) and 10 μl propidium iodide (PI,
20 mg/ml; Beyotime Institute of Biotechnology) and incubated for 20
min in the dark at room temperature. Flow cytometric analysis (Ex.
488 nm/Em. 530 nm) was performed with a FACSCalibur cell sorter (BD
Biosciences, Franklin Lakes, NJ, USA). Flow cytometric data were
analyzed using CellQuest™ version 4.5 software (BD
Biosciences).
ATP measurement
Cellular ATP content was measured using the ATP
bioluminescent assay kit (Beyotime Institute of Biotechnology)
according to the manufacturer’s instructions. For each experiment,
cells were briefly washed two times with ice-cold PBS, resuspended
in 100 μl Tris-EDTA buffer (100 mM Tris-HCl and 4 mM EDTA,
pH 7.55; Beyotime Institute of Biotechnology) and then incubated
for 3 min at 100°C. Following centrifugation at 10,000 x g for 2
min, supernatants were extracted and 10 μl of them plus 40
μl ATP assay buffer was added into the wells of a
microplate, which had each been filled with 50 μl ATP
reaction mix (ATP assay buffer, 48.5 μl; ATP probe, 0.2
μl; ATP converter, 2.0 μl; and Development mix, 2.0
μl). The microplate was covered with aluminium foil and
incubated at 37°C for 30 min prior to measuring the OD at Ex. 535
nm/Em. 587 nm using a microplate reader (721D; Pudong Shanghai
Physical Optical Instrument Factory).
Western blot analysis
Membrane proteins of H9c2 cells were extracted from
cell lysates using a membrane protein extraction kit (Beyotime
Institute of Biotechnology, Haimen, China). The protein
concentration was determined using the BCA assay (Beyotime
Institute of Biotechnology). Protein samples (40 or 20 μg)
were mixed with 2X SDS sample loading buffer (Beyotime Institute of
Biotechnology) and then separated on a 12% polyacrylamide gel and
blotted on a nitrocellulose membrane (Beyotime Institute of
Biotechnology). Blots were blocked with 5% skimmed milk, followed
by incubation with antibodies specific to p38MAPKα (1:100; cat. no.
sc-398305; Santa Cruz Biotechnology Inc.), p38MAPKγ (1:100; cat.
no. sc-366013; Santa Cruz Biotechnology Inc.), GLUT-1 (1:100; cat.
no. ab652; Abcam Trading Company Ltd., Shanghai, China), GLUT-4
(1:100; cat. no. 7796-3; Epitomics Biotechnology Inc., Burlingame,
CA, USA) or β-actin (1:1,000; cat. no. sc-130656; Santa Cruz
Biotechnology Inc,). Blots were then incubated at room temperature
for 30 min with secondary antibody (1:1,000; cat. no. zm0441;
Zhongshan Goldenbridge Biotechnology Corporation) and an enhanced
chemiluminescence detection system (Bio-Rad, Hercules, CA, USA) was
used for visualization. The grey value was measured using Quantity
One version 4.5 software (Bio-Rad).
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Values are expressed as the mean
± standard deviation. Differences between groups were determined by
one-way analysis of variance followed Dunnett’s post-hoc test and
P<0.05 was considered to indicate a statistically significant
difference between values.
Results
Exentin-4 increases the viability of H9c2
cells subjected to H/R
Following H/R for various times (4/2, 6/3, 12/4,
14/5, 16/6 and 22/10 h), H9c2 cell viability was assessed using a
CCK-8 kit. As shown in Fig. 1A,
cell viability decreased in an H/R time-dependent manner. Cell
viability after 4/2 h and 6/3 h H/R decreased to 95.21 and 86.90%,
respectively, compared with that in the control group (P<0.05),
while 12/4, 14/5, 16/6 and 22/10 h H/R further decreased the cell
viability to 64.99, 61.44, 53.65 and 35.52% of the control,
respectively (P<0.05). As 12/4 h was the shortest H/R time that
caused a significant difference in cell viability (P<0.05),
these conditions were then selected to investigate the potential
effects of exentin-4 on cardiomyocyte protection.
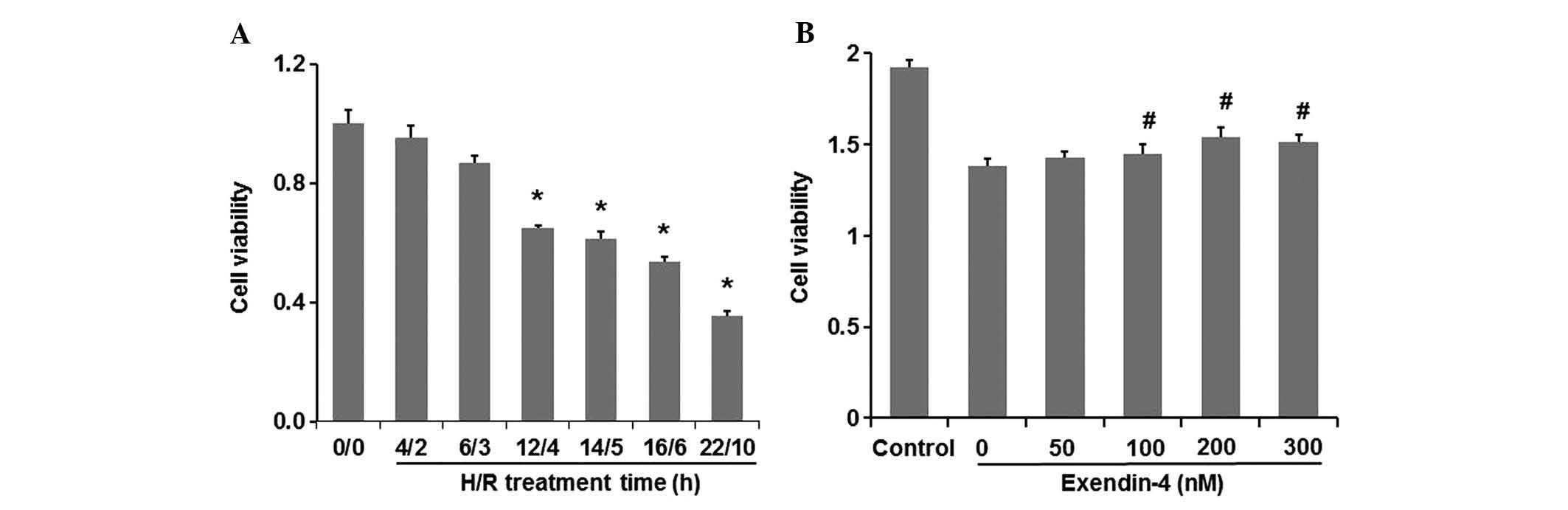 | Figure 1Effects of H/R on cell viability of
H9c2 cells and the protective effects of exendin-4 on H/R-induced
injury. (A) H9c2 cells were exposed to H/R conditions for different
time periods (4/2, 6/3, 12/4, 14/5, 16/6 and 22/10 h). (B) H9c2
cells were pre-treated with exendin-4 (0, 50, 100, 200 and 300 nM,
respectively) for 30 min prior to treatment of 12 h hypoxia and
followed by 4 h reoxygenation. After the H/R treatment, cell
viability was assessed using cell counting kit-8. Values are
expressed as the percentage of the control and presented as the
mean ± standard deviation (n=6). *P<0.05 versus
control group (0/0); #P<0.05 versus the 0 group. H/R,
hypoxia/reoxygenation. |
H9c2 cells were pre-treated with exentin-4 (0, 50,
100, 200 and 300 nM, respectively) for 30 min prior to H/R
treatment (12/4 h). Fig. 1B shows
that treatment with exendin-4 increased H9c2 cell viability even at
the lowest concentration of 50 nM. The percentage of cells
surviving the H/R insult was increased by exendin-4 in a
dose-dependent manner between 0 and 200 nM, and the cell viability
reached a peak in the presence of 200 nM exendin-4. When the
concentration of exendin-4 was further increased to 300 nM, the
percentage of surviving cells did not increase correspondingly, but
was slightly decreased; however, this change was not significant.
These results strongly suggested that exendin-4 exerted a
protective effect against H/R injury of H9c2 cardiomyocyte cells.
Exendin-4 achieved the best efficiency to protect cell viability at
a concentration of 200 nM. Therefore, the concentration of 200 nM
was selected for the treatment of H9c2 cells in the following
experiment.
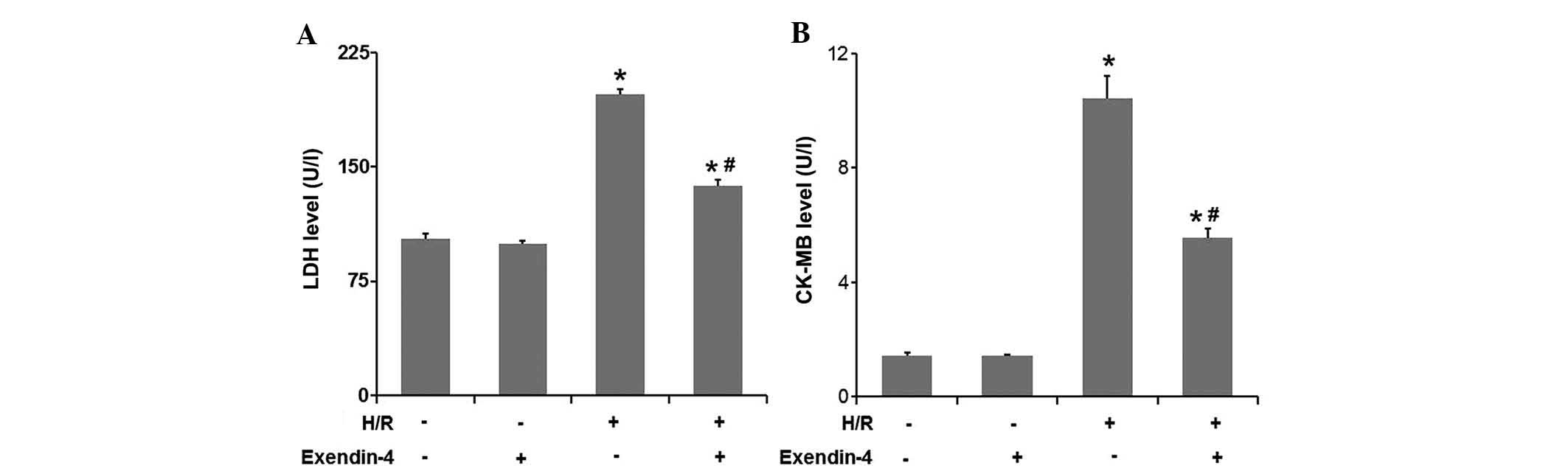
Exentin-4 reduces LDH and CK-MB release
in H9c2 cells subjected to H/R
As LDH and CK-MB release are two acknowledged
markers for cardiomyocyte injury, these proteins were examined in
the culture medium (Fig. 2A and
B). LDH and CK-MB release significantly increased in the H/R
group compared to that in the control group (P<0.05), while
pre-treatment with 200 nM exendin-4 significantly decreased LDH and
CK-MB release induced by H/R (P<0.05). These results strongly
suggested that exendin-4 exerted a protective effect against the
H/R injury of H9c2 cardiomyocyte cells.
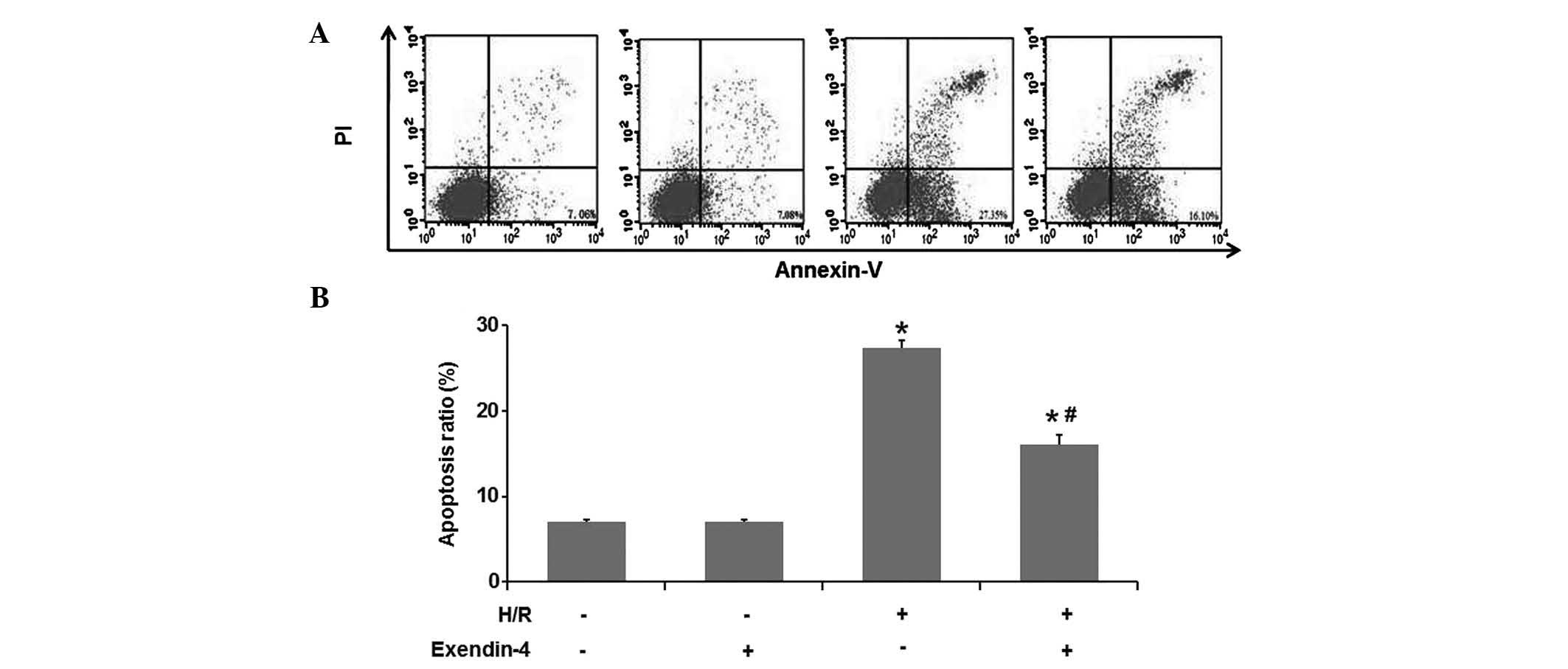
Exendin-4 attenuates H/R-induced
apoptosis of H9c2 cells
The present study investigated the effect of
exendin-4 on the H/R-induced apoptosis in cultured H9c2 cells using
flow cytometry (Fig. 3A and B).
The results of the flow cytometric analysis suggested that the
number of apoptotic cells in the H/R treatment group was higher
than that in the control group (P<0.05). Pre-treatment with 200
nM exendin-4 decreased the amount of apoptotic cells in comparison
to that in the H/R group (P<0.05), which suggested that
exendin-4 attenuated H/R-induced apoptosis of H9c2 cells.
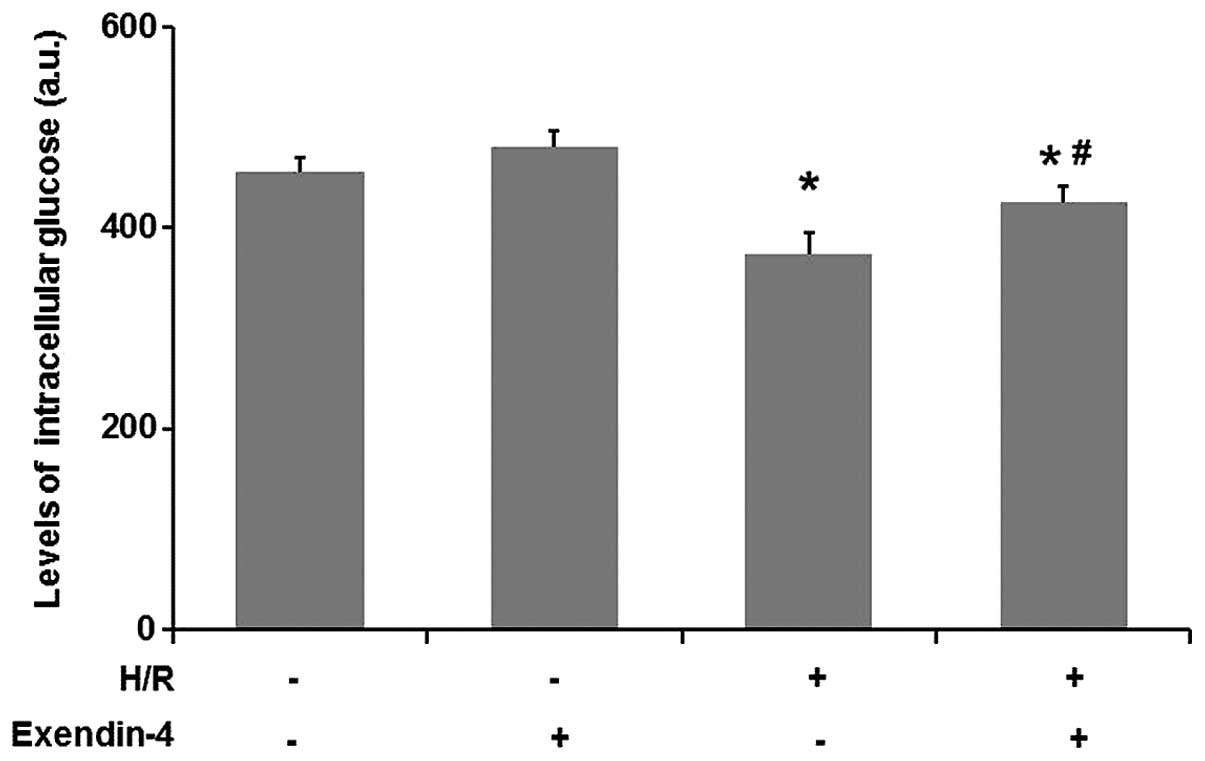
Exendin-4 enhances glucose uptake in
H/R-injured H9c2 cells
An increase of glucose uptake in cardiomyocytes is
beneficial in protecting the heart against ischemic injury
(19). To explore the possible
mechanisms involved in the protection of cardiomyocytes by
exendin-4, intracellular glucose levels were determined. As
Fig. 4 shows, the glucose uptake
was significantly decreased in the H/R group compared with that in
the control group (P<0.05). As compared with the H/R group,
pre-treatment with exendin-4 (200 nM) increased the glucose uptake
of cells (P<0.05). These results indicated that exendin-4
enhanced glucose uptake in H/R-injured H9c2 cells.
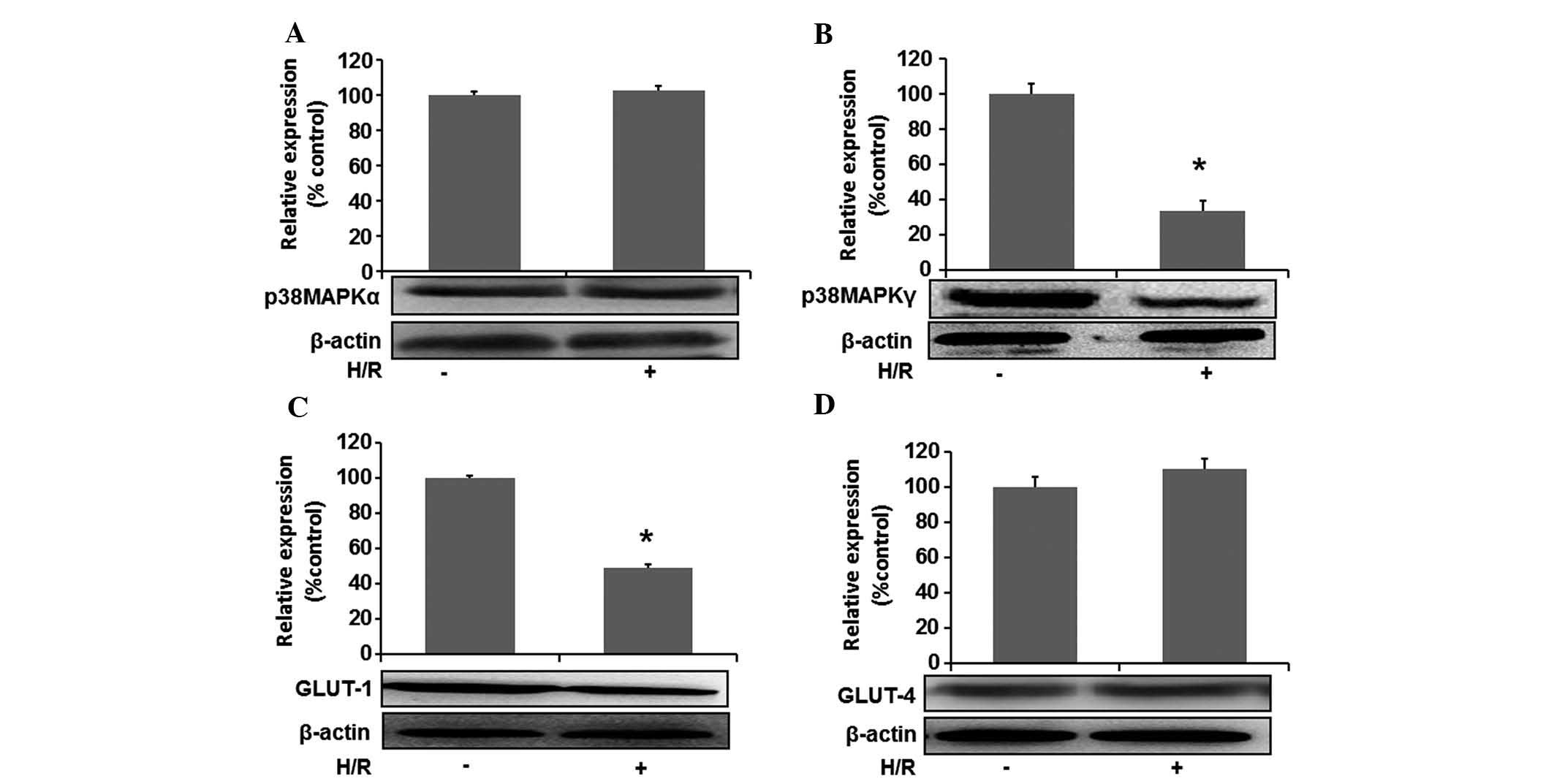
H/R decreases intracellular expression of
p38MAPKγ and translocation of GLUT1 in H9c2 cells
To study the role of p38MAPK and GLUT in H/R-induced
injury of cardiomyocytes, changes in the protein expression of
p38MAPKα and p38MAPKγ as well as translocation of GLUT1 and GLUT4
were detected by western blot analysis. Fig. 5A and B shows that the expression of
p38MAPKγ in the H/R group was lower than that in the control group
(P<0.05), while no significant changes in p38MAPKα levels were
found (P>0.05).
With regard to the effects of H/R on GLUT, it was
demonstrated that H/R reduced GLUT-1 translocation from the
cytoplasm to the membrane as compared with that in the control
group (P<0.05), while the translocation of GLUT-4 was not
significantly decreased (P>0.05) (Fig. 5C and D). The results indicated that
p38MAPKγ and GLUT-1 may have an important protective role in
H/R-injured cardiomyocytes.
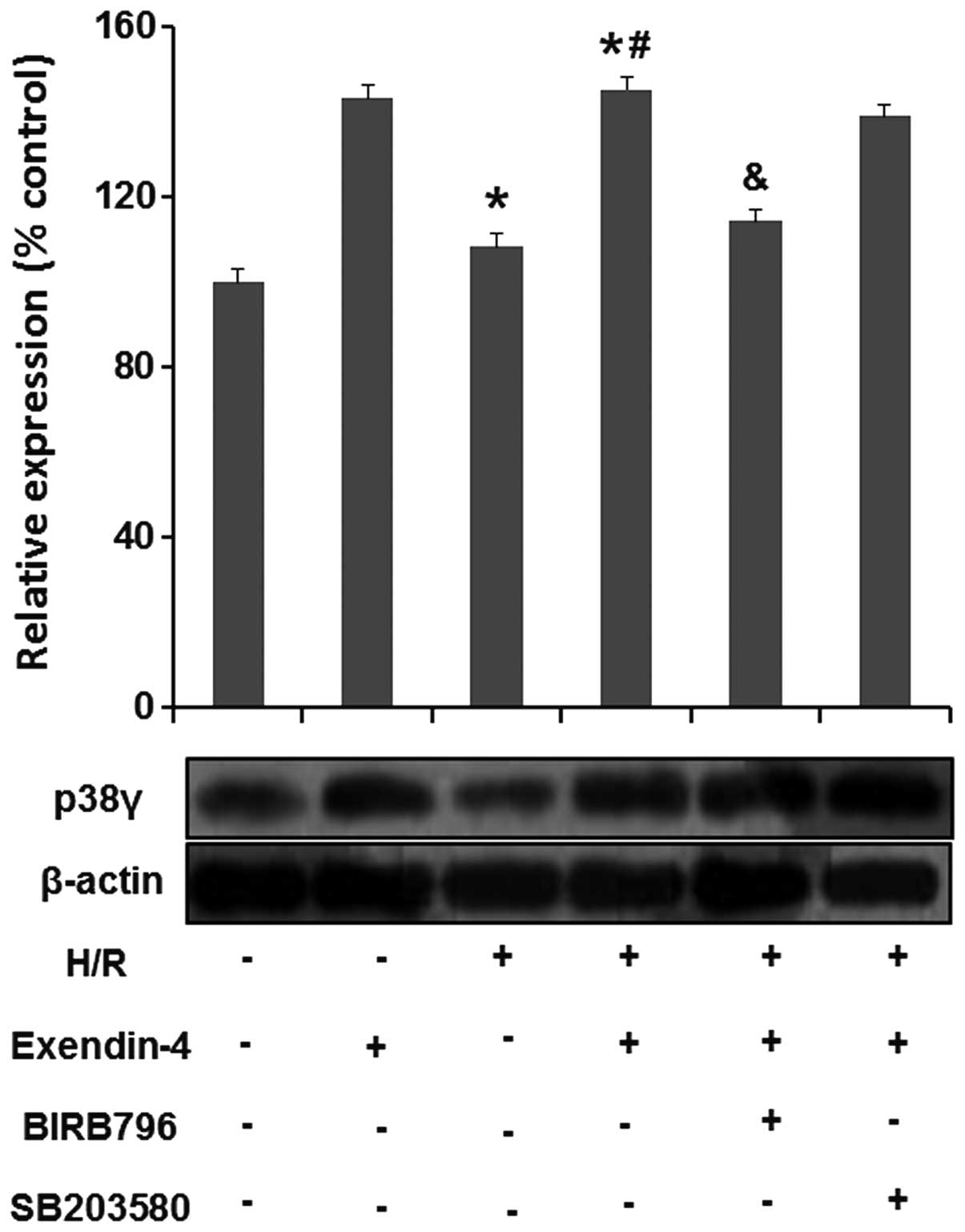
Exendin-4 increases the expression of
p38MAPKγ in H9c2-cells subjected to H/R
To determine the effects of exendin-4 on p38MAPKγ in
H/R-injured cardiomyocytes, the p38MAPKγ expression in H9c2 cells
was assessed using western blot analysis (Fig. 6). p38MAPKγ expression was
significantly reduced in the H/R group compared with that in the
control group (P<0.05), while it was markedly enhanced following
pre-treatment with 200 nM exendin-4 (P<0.05). To further study
the role of p38MAPKγ in the effects of exendin-4 on p38MAPK in
H/R-injured cardiomyocytes, two different p38MAPK inhibitors,
BIRB796 and SB203580, were used. As shown in Fig. 6, the effects of exendin-4 on
p38MAPKγ were inhibited by BIRB796 (P<0.05), while SB203580 did
not show any such effect (P>0.05). These results suggested that
p38MAPKγ may have an important role in exendin-4 mediated
protection of cardiomyocytes against H/R injury.
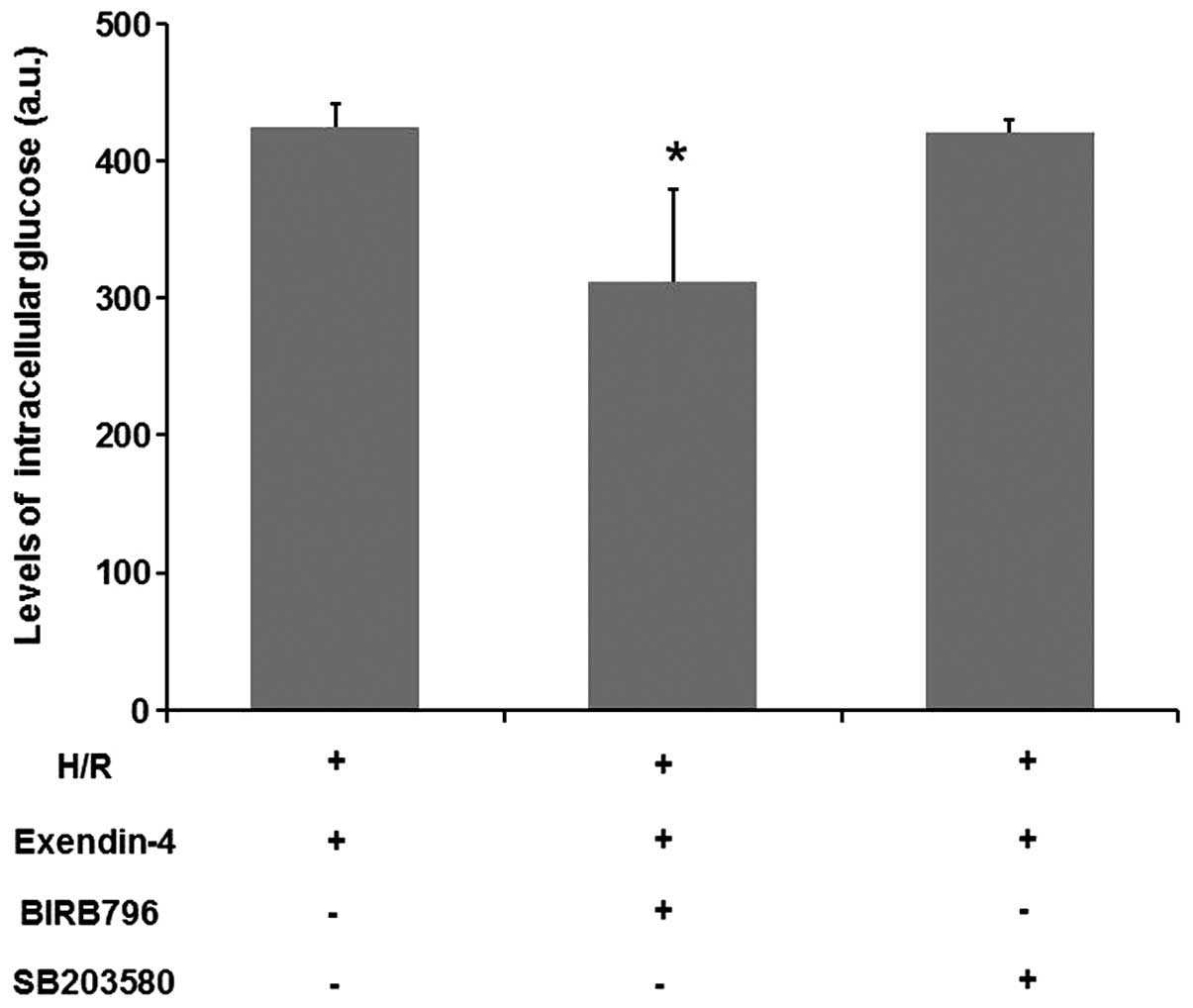
p38MAPK inhibitor BIRB796 abolishes the
effect of exendin-4 on glucose uptake in H9c2 cells
To further confirm the role of p38MAPKγ in the
effect of exendin-4 on cardiomyocytes, the effect of the p38MAPK
inhibitors BIRB796 and SB203580 on the glucose uptake in H9c2 cells
was assessed. As shown in Fig. 7,
the effects of exendin-4 on the glucose uptake were inhibited by
BIRB796 (P<0.05), while SB203580 did not show any such
inhibitory function (P>0.05). These results suggested that
p38MAPKγ may have an important role in exendin-4-mediated glucose
uptake.
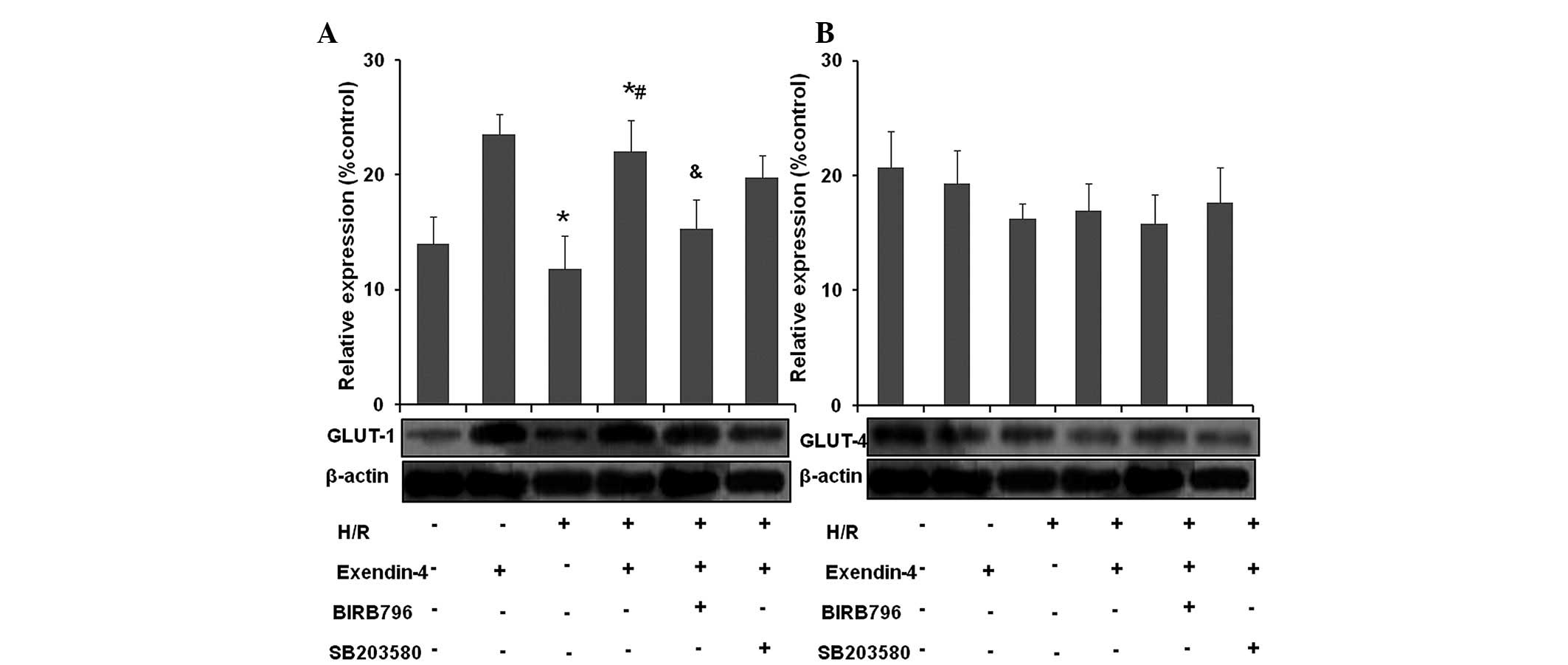
Exendin-4 increases the translocation of
GLUT-1 in H9c2 cells subjected to H/R
H/R reduced GLUT-1 translocation from the cytoplasm
to the membrane as compared with that in the control group
(Fig. 8A and B; P<0.05), while
the translocation of GLUT-4 was not significantly decreased
(P>0.05). Pre-treatment with 200 nM exendin-4 increased GLUT-1
translocation from the cytoplasm to the membrane (P<0.05) in
comparison to that in the H/R group, while the translocation of
GLUT-4 was not significantly decreased (P>0.05). These results
indicated that exendin-4 increased the translocation of GLUT-1 but
not GLUT-4 in H/R-injured cardiomyocytes.
By contrast, translocation of GLUT-1 in the presence
of exendin-4 was not significantly affected by SB203580 following
H/R (P>0.05), while GLUT-1 translocation was abolished by
BIRB796 compared with that in the exendin-4 + H/R group
(P<0.05). No significant effect of SB203580 or BIRB796 on GLUT-4
translocation was identified (P>0.05). These results suggested
that p38MAPKγ may have an important role in exendin-4-mediated
GLUT-1 trans-location in H9c2 cells subjected to H/R.
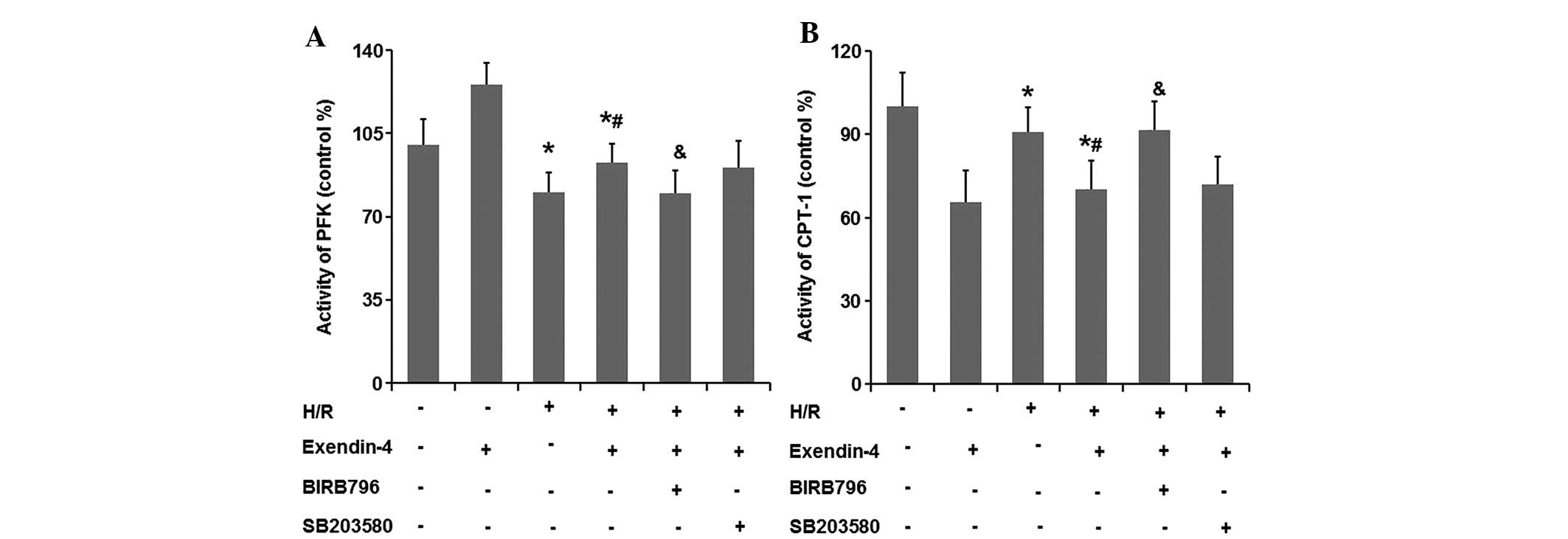
Exendin-4 enhances the activity of PFK-1
and attenuates that of CPT-1 in H/R-damaged H9c2 cells
To investigate the effects of exendin-4 on the
metabolic balance between glucose oxygen and fatty acid oxidation,
the activity of PFK-1 and CPT-1 was examined. As shown in Fig. 9A and B, the activity of PFK-1, the
key regulator of glycolysis, was significantly decreased in the H/R
group compared with that in the control group (P<0.05).
Furthermore, the activity of the rate-limiting enzyme of fatty acid
oxidation, CPT-1, was significantly increased in the H/R group
compared with that in the control group (P<0.05). Pre-treatment
with 200 nM exendin-4 increased the activity of PFK-1 and decreased
that of CPT-1 in comparison to that in the H/R group (P<0.05),
which suggested that exendin-4 enhanced glycolysis in H9c2 cells
subjected to H/R.
The effects of exendin-4 on the activities of PKF-1
and CPT-1 were not significantly affected by SB203580 (P>0.05);
however, they were abolished by the use of BIRB796 (P<0.05).
These results suggested that p38MAPKγ may have an important role in
exendin-4-mediated glycolysis in H9c2 cells subjected to H/R.
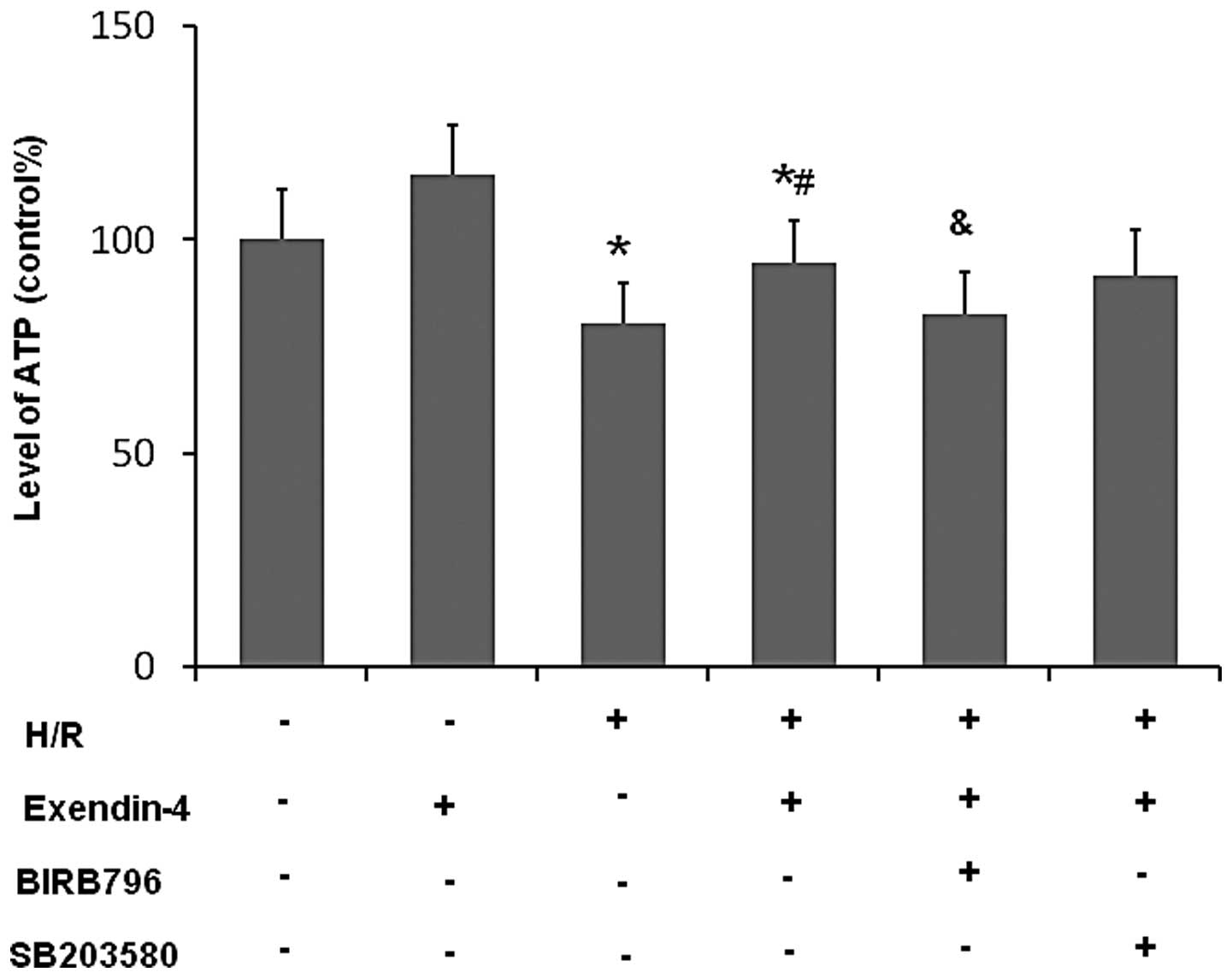
Exendin-4 increases ATP production in
H/R-damaged H9c2 cells
As shown in Fig.
10, the levels of ATP were significantly decreased in the H/R
group compared with those in the control group (P<0.05), while
the levels of ATP in the exendin-4 group were significantly
increased compared with those in the H/R group (P<0.05). This
result suggested that exendin-4 enhanced the production of ATP in
H9c2 cells subjected to H/R. However, BIRB796 treatment
significantly inhibited the effect of exendine-4 on ATP levels in
H9c2 cells following H/R (P<0.05), while SB203580 did not have
any significant effect. These results indicated that
exendin-4-induced production of ATP in H9c2 cells subjected to H/R
may be mediated via p38MAPKγ.
Discussion
The main finding of the present study was that the
GLP-1 analogue exendin-4 reduced H/R-induced cell injury and
enhanced glucose uptake as well as glycolysis of cardiomyocytes by
activating the p38MAPK signaling pathway in a H9c2 cell model.
Importantly, p38MAPKγ, one subunit of p38MAPK, may have the most
significant role in this process.
Effects of GLP-1 and its analogues on ischemic
cardiomyocytes in animal experiments or clinic trials have been
reported in previous studies, most of which supported its
beneficial effects (10,20,21).
In line with previous studies, the present study found that
exendin-4 reduced H/R-induced cell injury, as evidenced by
increases in cell viability, decreases in levels of LDH and CK-MB,
and a reduction in cardiomyocyte apoptosis.
Under normal physiological conditions,
cardiomyocytes prefer fatty acid as the substrate of metabolism to
maintain their function (19).
However, the reduced availability of oxygen during low-flow
ischemia makes fatty acid oxidation unfavorable, as it aggravates
oxygen deficiency due to larger stochiometric amounts of oxygen
required for ATP production compared with glycolysis (22), another pathway for ATP production
in cardiomyocytes. A study has demonstrated that enhancement of
glycolysis through diverse mechanisms or pharmacological
interventions was able to delay and prevent ischemic damage
(3). Therefore, reducing fatty
acid oxidation and shifting the metabolic balance to glycolysis has
been a focus in the field of ischemic heart disease treatment
(23). In the present study, it
was found that exendin-4 optimized the metabolism in H9c2 cells by
enhancing glucose uptake, increasing PFK-1 activity and decreasing
CPT-1 activity. PFK-1 and CPT-1 are the rate-limiting enzymes in
the biological processes of glycolysis and fatty acid β-oxidation,
respectively, and changes in their activity therefore reflect the
changes of the two main pathways of energy production (24,25).
In addition, the present study found that exendin-4 treatment
significantly increased the levels of ATP in H9c2 cells subjected
to H/R. These results strongly indicated that exendin-4 adjusted
the metabolic imbalance in H9c2 cells subjected to H/R.
A recent in vivo study reported that
exendin-4 failed to increase glucose uptake and glucose oxidation
in rat hearts (26); however, the
heart metabolism is profoundly different from that of
cardiomyocytes in vitro, which may explain why the results
contradicted those of the present study. According to previous
studies, the effects of GLP-1 and its analogues on glucose uptake
were more definite in ischemic cardiac myocytes than those in
non-ischemic ones (27). In
addition, whether glucose metabolism disorders exist at baseline,
may also affect the modification of glucose metabolism by receptor
agonists, such as GLP-1 (21,28).
MAPKs are key signal transmitters in animals and
humans and p38MAPK is one of their sub-families (29). The p38MAPK signaling pathway has an
important role in apoptosis, secretion of cytokines, transcription
regulation and resistance to ischemic damage (30–32).
The role of p38MAPK in the action of GLP-1 receptor agonists and
modification of glucose transportation has been rarely reported,
particularly in models of H/R-induced injury, while there are
discrepancies between the available studies with regards to the
role of p38MAPK and its subunits in the function of GLP-1 and its
analogues (27,33,34).
Further study of the role of p38MAPK and its subunits in the
function of GLP-1 and its analogues is required. In the present
study, H/R was found to decrease intracellular expression of
p38MAPKγ and translocation of GLUT-1 in H9c2 cells. The results
indicated that p38MAPK and GLUT may have an important protective
role in H/R-injured cardiomyocytes.
It has been reported that the use of BIRB796 could
decrease the activity of the four P38MAPK subunits by almost 100%,
while SB203582 failed to exert an obvious effect on the activities
of γ and δ (35,36). Furthermore, the amount of p38MAPKβ
and p38MAPKδ was only 10.6% and 0.08% of that of p38MAPKα (37). Previous studies as well as the
present study have only focused on the α and γ subunits of p38MAPK.
The present study found that BIRB796 treatment inhibited the
effects of exendin-4, including the enhancement of the glucose
uptake, increasing the production of ATP, increasing PFK-1 activity
and decreasing CPT-1 activity, while SB203580 treatment did not
exert any inhibitory effects. Considering the fact that the
distribution of the β and δ subunit significantly lower than that
of the α subunit (only accounting for 10.6 and 0.08% of that of α,
respectively, while γ is similar to α (37), it is assumed that the function of
exendin-4 in H/R-injured cells is mainly mediated via the p38MAPKγ
subunit. These results clearly demonstrated that the p38MAPK
signaling pathway, particularly p38MAPKγ, may have an important
role in exendin-4-mediated glycolysis in H9c2 cells subjected to
H/R. The results of the present study differed from those of
Bhashyam et al (34), which
indicated that GLP-1 increases myocardial glucose uptake cia
p38MAPK in conscious dogs with dilated cardiomyopathy. Zhao et
al (27) reported that the
total expression of p38MAPK was increased in normal and
postischemic isolated rat hearts after treatment with GLP-1;
however, information on changes in the levels of p38MAPK subunits
were not available. The results of the present study contributed to
the knowledge in the field of p38MAPK involvement in the
modification of glucose uptake following H/R; however, as previous
studies using various non-uniform models and drugs have produced
conflicting results, this mechanism requires further
elucidation.
GLUT-1 and GLUT-4 have a critical role in glucose
uptake in cardiomyocytes. Enhanced myocardial glucose uptake by
upregulation of GLUT-1 and GLUT-4 may be one of the underlying
mechanisms to explain the beneficial effect of GLP-1 and its
analogues on reducing myocardial injury (16,38).
In the present study, in accordance with the identified glucose
uptake enhancement in H9c2 cells subjected to H/R, if was found
that exendin-4 increased the translocation of GLUT-1 but not that
of GLUT-4, which was abolished by BIRB796. These results indicated
that exendin-4 enhanced glucose uptake by upregulation of GLUT-1.
The p38MAPK signaling pathway, in particular the p38MAPKγ subunit,
may have the most important role in this process.
The role of GLUTs in the action of GLP-1 and its
analogues has also been reported in several studies, but the
results were contradictory among those reports (27,39–41).
Arnés et al (41) found
that exendin-4 increased the expression of GLUT-4 in rat muscles
and that of GLUT-2 in rat livers. However, in this type 2 diabetes
mellitus model, an increase of GLUT-4 was not observed. Another
study using a myocardial infarction-induced heart failure model
showed that GLP-1 and exenatides analogue AC3174 exerted a
cardioprotective function; however, this was not associated with
the translocation of GLUT-1 or GLUT-4 (40). Furthermore, Zhao et al
(27) reported that GLP-1
increased GLUT-1 and GLUT-4 translocation following ischemic
treatment, while Bhashyam et al (34) found that only GLUT-1 translocation
was involved in a dog model of dilated cardiomyopathy, which was
consistent with the results of the present study. Based on all of
these findings, it remains difficult to draw a solid conclusion
with regard to the role of GLUTs in the action of GLP-1 and its
analogues. However, it can be concluded that the modification of
GLUTs by GLP-1 or its analogues is different to that by
insulin.
In conclusion, the present study demonstrated for
the first time, to the best of our knowledge, that GLP-1 analogue
exendin-4 improved the energy metabolism of cardiomyocytes by
activating the p38MAPK signaling pathway in H9c2 cells subjected to
H/R treatment. Importantly, p38MAPKγ, one subunit of p38MAPK, was
indicated to have an important role in this process. Of course,
further studies in vivo are required to fully evaluate the
cardioprotective effects of exendin-4 and to determine the exact
underlying molecular mechanism.
Acknowledgments
This work was supported by the National Natural
Science Fund (grant no. 81100196), the Natural Science Foundation
Project of Chongqing Science & Technology Commission (grant no.
CSTC, 2011BB5133), Chongqing Municipal Health Bureau fund (grant
nos. 2010-1-07, 2012-2-125 and ZY20132124) and the National Key
Clinical Specialties Construction Program of China (grant no.
2011-170). The authors would like to thank Mr. Jianyong Wu and Mr.
Dezhang Zhao (Institute of Life Sciences, Chongqing Medical
University) for their excellent technical support with the flow
cytometric analysis.
References
|
1
|
Hütter JF and Soboll S: Role of fatty acid
metabolites in the development of myocardial ischemic damage. Int J
Biochem. 24:399–403. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gambert S, Vergely C, Filomenko R, Moreau
D, Bettaieb A, Opie LH and Rochette L: Adverse effects of free
fatty acid associated with increased oxidative stress in
postischemic isolated rat hearts. Mol Cell Biochem. 283:147–152.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grynberg A: Effectors of fatty acid
oxidation reduction: Promising new anti-ischaemic agents. Curr
Pharm Des. 11:489–509. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Avogaro A: Cardioprotective effects of
glucagon-like peptide-1: Preclinical and clinical data. G Ital
Cardiol (Rome). 12(Suppl 2): 17–24. 2011.In Italian.
|
|
5
|
Otto-Buczkowska E: Glucagon and
glucagon-like peptides the role in control glucose homeostasis.
Part I. Pediatr Endocrinol Diabetes Metab. 17:215–221. 2011.In
Polish.
|
|
6
|
Gallwitz B: Anorexigenic effects of GLP-1
and its analogues. Handb Exp Pharmacol. 209:185–207.
2012.PubMed/NCBI
|
|
7
|
Quintanilla-García C and Zúñiga-Guajardo
S: The incretin effect and type 2 diabetes. Rev Med Inst Mex Seguro
Soc. 48:509–520. 2010.In Spanish.
|
|
8
|
Lindamood CA and Taylor JR: Emerging new
therapies for the treatment of type 2 diabetes mellitus:
Glucagon-like peptide-1 receptor agonists. Clin Ther. Feb
3–2015.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harris KB and McCarty DJ: Efficacy and
tolerability of glucagon-like peptide-1 receptor agonists in
patients with type 2 diabetes mellitus. Ther Adv Endocrinol Metab.
6:3–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bao W, Aravindhan K, Alsaid H, Chendrimada
T, Szapacs M, Citerone DR, Harpel MR, Willette RN, Lepore JJ and
Jucker BM: Albiglutide, a long lasting glucagon-like peptide-1
analog, protects the rat heart against ischemia/reperfusion injury:
Evidence for improving cardiac metabolic efficiency. PLoS One.
6:e235702011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bose AK, Mocanu MM, Carr RD, Brand CL and
Yellon DM: Glucagon-like peptide 1 can directly protect the heart
against ischemia/reperfusion injury. Diabetes. 54:146–151. 2005.
View Article : Google Scholar
|
|
12
|
Bose AK, Mocanu MM, Carr RD and Yellon DM:
Glucagon like peptide-1 is protective against myocardial
ischemia/reperfusion injury when given either as a preconditioning
mimetic or at reperfusion in an isolated rat heart model.
Cardiovasc Drugs Ther. 19:9–11. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Read PA, Khan FZ and Dutka DP:
Cardioprotection against ischaemia induced by dobutamine stress
using glucagon-like peptide-1 in patients with coronary artery
disease. Heart. 98:408–413. 2012. View Article : Google Scholar
|
|
14
|
Read PA, Hoole SP, White PA, Khan FZ,
O’Sullivan M, West NE and Dutka DP: A pilot study to assess whether
glucagon-like peptide-1 protects the heart from ischemic
dysfunction and attenuates stunning after coronary balloon
occlusion in humans. Circ Cardiovasc Interv. 4:266–272. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Phong WY, Lin W, Rao SP, Dick T, Alonso S
and Pethe K: Characterization of phosphofructokinase activity in
Mycobacterium tuberculosis reveals that a functional glycolytic
carbon flow is necessary to limit the accumulation of toxic
metabolic intermediates under hypoxia. PLoS One. 8:e560372013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Idrovo JP, Yang WL, Nicastro J, Coppa GF
and Wang P: Stimulation of carnitine palmitoyltransferase 1
improves renal function and attenuates tissue damage after
ischemia/reperfusion. J Surg Res. 177:157–164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rajaram N, Frees AE, Fontanella AN, Zhong
J, Hansen K, Dewhirst MW and Ramanujam N: Delivery rate affects
uptake of a fluorescent glucose analog in murine metastatic breast
cancer. PLoS One. 8:e765242013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee J and Chang JH: Facile and
high-efficient immobilization of histidinetagged multimeric protein
G on magnetic nanoparticles. Nanoscale Res Lett. 9:6642014.
View Article : Google Scholar
|
|
19
|
Ji L, Zhang X, Liu W, et al:
AMPK-regulated and Akt-dependent enhancement of glucose uptake is
essential in ischemic preconditioning-alleviated reperfusion
injury. PLoS One. 8:e699102013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moberly SP, Berwick ZC, Kohr M, Svendsen
M, Mather KJ and Tune JD: Intracoronary glucagon-like peptide 1
preferentially augments glucose uptake in ischemic myocardium
independent of changes in coronary flow. Exp Biol Med (Maywood).
237:334–342. 2012. View Article : Google Scholar
|
|
21
|
Gejl M, Søndergaard HM, Stecher C, Bibby
BM, Møller N, Bøtker HE, Hansen SB, Gjedde A, Rungby J and Brock B:
Exenatide alters myocardial glucose transport and uptake depending
on insulin resistance and increases myocardial blood flow in
patients with type 2 diabetes. J Clin Endocrinol Metab.
97:E1165–E1169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tani M and Neely JR: Role of intracellular
Na+ in Ca2+ overload and depressed recovery
of ventricular function of reperfused ischemic rat hearts. Possible
involvement of H+-Na+ and
Na+-Ca2+ exchange. Circ Res. 65:1045–1056.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferrari R, Pepi P, Ferrari F, et al:
Metabolic derangement in ischemic heart disease and its therapeutic
control. Am J Cardiol. 82:2K–13K. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mor I, Cheung EC and Vousden KH: Control
of glycolysis through regulation of PFK1: Old friends and recent
additions. Cold Spring Harb Symp Quant Biol. 76:211–216. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aoi W, Naito Y and Yoshikawa T: Potential
role of oxidative protein modification in energy metabolism in
exercise. Subcell Biochem. 77:175–187. 2014.PubMed/NCBI
|
|
26
|
Nguyen TD, Shingu Y, Amorim PA, Schwarzer
M and Doenst T: Glucagon-like peptide-1 reduces contractile
function and fails to boost glucose utilization in normal hearts in
the presence of fatty acids. Int J Cardiol. 168:4085–4092. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao T, Parikh P, Bhashyam S, Bolukoglu H,
Poornima I, Shen YT and Shannon RP: Direct effects of glucagon-like
peptide-1 on myocardial contractility and glucose uptake in normal
and postischemic isolated rat hearts. J Pharmacol Exp Ther.
317:1106–1113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moberly SP, Mather KJ, Berwick ZC, Owen
MK, Goodwill AG, Casalini ED, Hutchins GD, Green MA, Ng Y,
Considine RV, et al: Impaired cardiometabolic responses to
glucagon-like peptide 1 in obesity and type 2 diabetes mellitus.
Basic Res Cardiol. 108:3652013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cuenda A and Rousseau S: p38 MAP-kinases
pathway regulation, function and role in human diseases. Biochim
Biophys Acta. 1773:1358–1375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Erdogdu O, Eriksson L, Xu H, Sjöholm A,
Zhang Q and Nyström T: Exendin-4 protects endothelial cells from
lipoapoptosis by PKA, PI3K, eNOS, p38 MAPK, and JNK pathways. J Mol
Endocrinol. 50:229–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu H, Li HL, Niu ZY, Li GZ, Cao J and
Jiang YD: Involvement of p38 MAPK pathway in GLP-1-induced
inhibition of apoptosis in human umbilical vein endothelial cells.
Sheng Li Xue Bao. 64:444–448. 2012.In Chinese. PubMed/NCBI
|
|
32
|
Kawasaki Y, Harashima S, Sasaki M, Mukai
E, Nakamura Y, Harada N, Toyoda K, Hamasaki A, Yamane S, Yamada C,
et al: Exendin-4 protects pancreatic beta cells from the cytotoxic
effect of rapamycin by inhibiting JNK and p38 phosphorylation. Horm
Metab Res. 42:311–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ho RC, Alcazar O, Fujii N, Hirshman MF and
Goodyear LJ: p38gamma MAPK regulation of glucose transporter
expression and glucose uptake in L6 myotubes and mouse skeletal
muscle. Am J Physiol Regul Integr Comp Physiol. 286:R342–R349.
2004. View Article : Google Scholar
|
|
34
|
Bhashyam S, Fields AV, Patterson B,
Testani JM, Chen L, Shen YT and Shannon RP: Glucagon-like peptide-1
increases myocardial glucose uptake via p38alpha MAP
kinasemediated, nitric oxide-dependent mechanisms in conscious dogs
with dilated cardiomyopathy. Circ Heart Fail. 3:512–521. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kuma Y, Sabio G, Bain J, Shpiro N, Márquez
R and Cuenda A: BIRB796 inhibits all p38 MAPK isoforms in vitro and
in vivo. J Biol Chem. 280:19472–19479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Caverzasio J and Manen D: Essential role
of Wnt3a-mediated activation of mitogen-activated protein kinase
p38 for the stimulation of alkaline phosphatase activity and matrix
mineralization in C3H10T1/2 mesenchymal cells. Endocrinology.
148:5323–5330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dingar D, Merlen C, Grandy S, Gillis MA,
Villeneuve LR, Mamarbachi AM, Fiset C and Allen BG: Effect of
pressure overload-induced hypertrophy on the expression and
localization of p38 MAP kinase isoforms in the mouse heart. Cell
Signal. 22:1634–1644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huisamen B, Genade S and Lochner A:
Signalling pathways activated by glucagon-like peptide-1 (7–36)
amide in the rat heart and their role in protection against
ischaemia. Cardiovasc J Afr. 19:77–83. 2008.PubMed/NCBI
|
|
39
|
Vyas AK, Yang KC, Woo D, Tzekov A, Kovacs
A, Jay PY and Hruz PW: Exenatide improves glucose homeostasis and
prolongs survival in a murine model of dilated cardiomyopathy. PLoS
One. 6:e171782011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Q, Anderson C, Broyde A, Polizzi C,
Fernandez R, Baron A and Parkes DG: Glucagon-like peptide-1 and the
exenatide analogue AC3174 improve cardiac function, cardiac
remodeling, and survival in rats with chronic heart failure.
Cardiovasc Diabetol. 9:762010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Arnés L, Moreno P, Nuche-Berenguer B,
Valverde I and Villanueva-Peñacarrillo ML: Effect of exendin-4
treatment upon glucose uptake parameters in rat liver and muscle,
in normal and type 2 diabetic state. Regul Pept. 153:88–92. 2009.
View Article : Google Scholar
|