Introduction
Head and neck squamous cell carcinomas (HNSCC) are
loco-regionally aggressive tumors, which result in debilitating
functional and aesthetic sequelae in patients. Chemotherapeutic
management of patients with HNSCC typically involves treatment with
taxol (docetaxel), platinum compounds (e.g. cisplatin or
carboplatin) and the anti-metabolite 5-fluorouracil (5-FU), either
as single agents or in combination. Preclinical and clinical
studies indicate a potential synergy between the three drugs
(1,2); however, drug resistance accounts for
high rates of loco-regional recurrence (3). The process of drug resistance is
mediated by modulations in multiple molecular pathways, including
drug efflux/metabolism, DNA repair, apoptosis and cell cycle
control (4–6). An improved understanding of the
cellular and molecular mediators of drug resistance may potentially
lead to the identification of candidate genes and pathways, which
can be targeted to improve therapeutic efficacy.
In vitro model systems have been ideal for
delineating the mechanisms contributing towards the phenomenon of
drug resistance and also for identifying novel druggable targets.
Resistant cell lines developed from ovarian and breast cancer cells
have been instrumental in understanding its molecular basis. Copper
transporter genes and P-glycoprotein have been implicated to impart
resistance to cisplatin and cross-resistance to paclitaxel,
respectively (7). The role of
melanoma antigen, G antigen family of genes and ATP-binding
cassette (ABC) transporters were identified using resistant cell
lines (8–13). High-throughput studies comparing
the cisplatin-sensitive/resistant HNSCC cells have indicated the
involvement of multiple pathways (14). Activation of survival signaling and
apoptotic pathways have been demonstrated to result in the
overexpression of rat sarcoma/rapidly accelerated
fibrosarcoma/mitogen-activated protein kinase kinase (15) and other genes, including
Dickkopf-related protein 1, signal transducer and activator of
transcription 3 and Notch 1 (16,17).
Docetaxel resistance in in vitro models has also been
correlated with increased expression levels of multidrug resistance
(MDR)1/multidrug resistance-associated protein 1 (MRP) and an
increase in mitochondrial DNA and reactive oxygen species (18,19).
Di-hydropyrimidine (DPD) and thymidylate synthase (TS), which are
involved in 5-FU metabolism, have been reported to be important in
determining the sensitivity of the head and neck cancer cells to
the drug (20,21). Treatment with a combination of
drugs has been reported to demonstrate a synergistic effect on the
modulation of the cell cycle, angiogenesis and signal transduction,
as observed in cells treated with cisplatin and docetaxel (22). Although multiple pathways have been
implicated in the process of drug resistance, the underlying
mechanisms remain to be elucidated, particularly in the case of
resistance to combinatorial therapy.
The present study aimed to facilitate an
understanding of resistance to the TPF regimen of drugs. Towards
this effort, cell lines resistant to this combination of drugs were
established and their resistance index was determined. The
resistant cell lines were evaluated for the changes in cell cycle
distribution and apoptotic patterns. In addition, the expression
profile of the molecular markers involved in resistance to
TPF-based drug action was compared between the parental and
resistant cells. These markers included drug transporters, such as
MDR1, MRP2 and copper transporter (CTR1), as well as survivin,
which is involved in cell survival, excision repair
cross-complementing rodent repair deficiency, complementation
(ERCC1), which is involved in DNA repair, and TS, which is involved
in nucleotide synthesis/metabolism.
Materials and methods
Reagents, cell lines and culture
The chemotherapeutic drugs cisplatin
[cis-diammineplatinum (II) dichloride], docetaxel, 5-FU and other
reagents, including MTT, propidium iodide (PI) and RNase A, were
purchased from Sigma-Aldrich (St. Louis, MO, USA). The
reconstitution of the drugs was according to the manufacturer’s
instructions. Briefly, cisplatin was dissolved in 0.9% sodium
chloride solution, while docetaxel and 5-FU were reconstituted in
dimethyl sulfoxide (DMSO; HiMedia India Pvt., Ltd., Mumbai, India).
The stock solutions of the drugs were stored in aliquots at −80°C.
The HNSCC cell lines CAL-27, kindly gifted by Dr Aditi Chatterjee
(Institute of Bioinformatics, Bangalore) and Hep-2 (National Centre
for Cell Science, Maharashtra, India) (passage number 28–30), were
used in the present study. The cell lines were cultured in
Dulbecco’s modified Eagle’s medium (Gibco-BRL, Invitrogen Life
Technologies, Carlsbad, CA, USA), supplemented with 10%
heat-inactivated fetal bovine serum and 1X penicillin (100
U/ml)/streptomycin (100 mg/ml) (HiMedia India Pvt., Ltd.). The
cells were grown as monolayer cultures and maintained in a
humidified atmosphere of 5% CO2 at 37°C.
Development of drug-resistant cell
lines
The methodology for developing the resistant cell
lines were based on the methods described previously (23). Briefly, the Hep-2 and
CAL-27-resistant sublines were selected based on constant exposure
of the parental cells to the combination of docetaxel, cisplatin
and 5-FU (TPF) in a stepwise dose incremental strategy. For each
cell line, the half maximal inhibitory concentration
(IC50) of each drug was calculated by MTT or trypan blue
assay. The two cell lines were treated with a sequential increase
in dosage of the three drugs ranging from IC6.25 (Hep-2:
0.42 μM cisplatin, 1.38 nM docetaxel, 10.35 μM 5-FU;
CAL27: 0.43 μM cisplatin, 0.22 nM docetaxel, 0.22 μM
5-FU), IC12.5 (Hep-2: 0.84 μM cisplatin, 2.75 nM
docetaxel, 20.70 μM 5-FU; CAL27: 0.86 μM cisplatin,
0.44 nM docetaxel, 0.44 μM 5-FU), IC25 (Hep-2:
1.68 μM cisplatin, 5.50 nM docetaxel, 41.41 μM 5-FU;
CAL27: 1.71 μM cisplatin, 0.87 nM docetaxel, 0.88 μM
5-FU) to IC50. Individual IC50 values are
presented in Table I. Cells were
incubated for 24 h with each concentration of the drug. Following
each drug treatment, the surviving cells were cultured in drug-free
medium for a period of 3–5 days and following the third cycle of
drug treatment, the resistant cells were cultured in the presence
of drug-containing medium. These cells were used for cytotoxicity
assays to assess the IC50-value post-exposure.
 | Table IIC50-values for the
resistant cell lines. |
Table I
IC50-values for the
resistant cell lines.
| Cell line | Drug |
IC50a | SEM | RI |
|---|
| Parental cells | | | | |
| Hep-2 P | Cisplatin | 3.35 | 0.13 | |
| Docetaxel | 11.00 | 2.41 | |
| 5-FU | 82.81 | 10.84 | |
| CAL-27 P | Cisplatin | 3.43 | 0.06 | |
| Docetaxel | 1.56 | 0.55 | |
| 5-FU | 1.76 | 1.20 | |
| TPF cell lines | | | | |
| Hep-2 TPFR | Cisplatin | 16.90 | 0.23 | 5.04 |
| Docetaxel | 53.57 | 0.51 | 4.87 |
| 5-FU | 512.55 | 12.39 | 6.19 |
| CAL-27 TPFR | Cisplatin | 6.75 | 0.15 | 1.97 |
| Docetaxel | 3.17 | 0.10 | 2.02 |
| 5-FU | 14.93 | 5.44 | 8.48 |
Drug sensitivity assay
Briefly, the parental and resistant cells were
plated at a concentration of 1×104 cells/well in 96-well
plates. The cells were incubated overnight in humidified air with
5% CO2 at 37°C. The cells were subsequently treated with
serial dilutions of drugs or vehicle control for 24 h, followed by
further culture in drug-free medium for two days. An MTT assay was
performed, according to the manufacturer’s instructions. Briefly,
20 μl MTT (5 mg/ml; Sigma-Aldrich) was added following the
removal of the culture medium and the cells incubated for 4 h at
37°C. The formazan crystals were dissolved by adding 100 μl
DMSO per well and the plate was read at 570 nm against 690 nm, as
the reference wavelength, using a microplate reader (Model 680;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). Cells without drug
were used as the control. The percentage of viable cells was
calculated using the formula: Mean optical density (OD) of the
experiment / mean OD of the control ×100. All assays were performed
in triplicate. For the cell viability staining assays, the cells
were plated and treated with the different concentrations of the
drug in triplicate and the percentage of viable cells was counted
following staining with trypan blue (Sigma-Aldrich). The
IC50-values in each case were calculated using
regression analysis (Microsoft Excel 7; Microsoft Corporation,
Redmond, WA, USA) and are expressed as an average of triplicate
experiments. The resistance index (RI) was calculated by the ratio
of the IC50 of resistant cell lines over the parental
cell lines. Chemoresistance was defined as an RI of ≥2.
Cell cycle assay
Resistant and parental cells (1×105) were
resuspended in 0.3 ml phosphate-buffered saline (PBS; HiMedia India
Pvt., Ltd.) and fixed in 0.7 ml cold ethanol (70%; Merck Millipore,
Darmstadt, Germany). The cells were incubated on ice for 1 h,
followed by a single wash with ice-cold PBS. The resuspended cell
pellet was incubated at 37°C for 40 min in the presence of 5
μl 10 mg/ml RNase A (Sigma-Aldrich), 5 μl 10 mg/ml PI
(Sigma-Aldrich) and 0.05% Triton X-100 (Sigma-Aldrich). The cells
were stored in the dark at 40°C until analyzed on a FACSCalibur (BD
Biosciences, Franklin Lakes, NJ, USA). The percentage of cells in
each cycle was calculated using Cell Quest Pro version 6 (BD
Biosciences) software. The experiments on each cell line were
performed in triplicate.
Apoptosis assay
An apoptosis assay was performed using annexin
V-fluorescein isothiocyanate (FITC; BD Biosciences) and PI
(Sigma-Aldrich) staining. The parental and resistant cells from
each cell line were treated for 48 h with the IC25 for
all three drug treatments. Following trypsinization,
~1×105 cells were resuspended in 100 μl binding
buffer containing annexin V-FITC (5 μl; 20 μg/ml) and
PI (10 μl; 20 μg/ml) for 15 min at room temperature
in the dark. Following incubation, 400 μl annexin binding
buffer was added and the percentage of apoptotic cells (cells which
were annexin-positive and/or PI-positive) was calculated using a
FACSCalibur (BD Biosciences). Untreated cells and cells incubated
with PI or annexin V alone were used as controls.
Expression profiling
The mRNA expression levels of the multidrug
resistance-associated genes MDR1, MRP2, ERCC1, CTR1, survivin and
TS were determined using a Step One polymerase chain reaction
machine (Applied Biosystems, Foster City, CA, USA). The total RNA
was extracted from 1×106 cells using TRIzol reagent
(Sigma-Aldrich), according to the manufacturer’s instructions. The
RNA was treated with DNase (Thermo Fisher Scientific, Waltham, MA,
USA) and ~1 μg RNA was converted into cDNA using a High
Capacity cDNA conversion kit (Applied Biosystems) according to the
manufacturer’s instructions. The expression of the MDR genes was
calculated using specific primer sets (Amnion Biosciences Pvt.
Ltd., Bengaluru, India; Table
II). PCR conditions were set as follows: Initial denaturation
at 95°C for 10 min and 95°C for 15 sec, followed by 40 cycles at
95°C for 15 sec and 60°C for 1 min. Melting curve conditions were
as follows: 95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec.
Relative quantification was performed using GAPDH as the endogenous
control and the parental cells as the calibrator. The relative
comparison method (ΔΔCt) was used for the quantification of mRNA
expression for all target genes with untreated parental cells as
the calibrator
 | Table IIList of primers used for expression
profiling. |
Table II
List of primers used for expression
profiling.
| Gene | Primer
sequence | Amplicon (bp) |
|---|
| MDR1 | Forward:
5′TGACAGCTACAGCACGGAAG3′ | 134 |
| Reverse:
3′TCTTCACCTCCAGGCTCAGT5′ | |
| MRP2 | Forward:
5′TACCAATCCAAGCCTCTACC3′ | 104 |
| Reverse:
3′AGAATAGGGACAGGAACCAG5′ | |
| CTR1 | Forward:
5′AGGACTCAAGATAGCCCGAGAGA3′ | 78 |
| Reverse:
3′TGGTCCTGGGACAGGCATGG5′ | |
| Survivin | Forward:
5′GAGGCTGGCTTCATCCACTG3′ | 159 |
|
Reverse:5′GCACTTTCTTCGCAGTTTCCTC3′ | |
| ERCC1 | Forward:
5′GGCGACGTAATTCCCGACTA3′ | 60 |
| Reverse:
3′AGTTCTTCCCCAGGCTCTGC5′ | |
| TS | Forward:
5′GGCCTCGGTGTGCCTTT3′ | 63 |
| Reverse:
3′GATGTGCGCAATCATGTACGT5′ | |
| GAPDH | Forward:
5′TCGACAGTCAGCCGCCATCTTCTTT3′ | 105 |
| Reverse:
3′GCCCAATACGACCAAATCCGTTGA5′ | |
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Differences between the parental and resistant cell lines
were analyzed for statistical significance using Student’s t-test.
*P<0.05 was considered to indicate a statistically
significant difference between values. Statistical analysis and
graphical illustration of datasets was performed using the GraphPad
Prism 6.00 statistical software (GraphPad Software, La Jolla,
CA).
Results
Resistance characteristics of the cell
lines
The IC50 concentration of each drug for
the two cell lines was assessed by exposing them to increasing
concentrations of the drug and then evaluating the cell viability.
The IC50 values of the parental cells are presented in
Table I. The cell lines were
subsequently exposed to increasing concentrations
(IC6.25, IC12.5, IC25 and
IC50) of each drug in combination for developing
resistance (Fig. 1A–F). The
drug-free period between each concentration was decided based on
the recovery period required for each cell line-drug combination.
The cells were continuously maintained in drug-containing medium
prior to performing the assays. The resistant cell lines Hep-2 TPF
resistant (TPFR) and CAL-27 TPFR were initially assessed for their
resistance characteristics by cell viability assays.
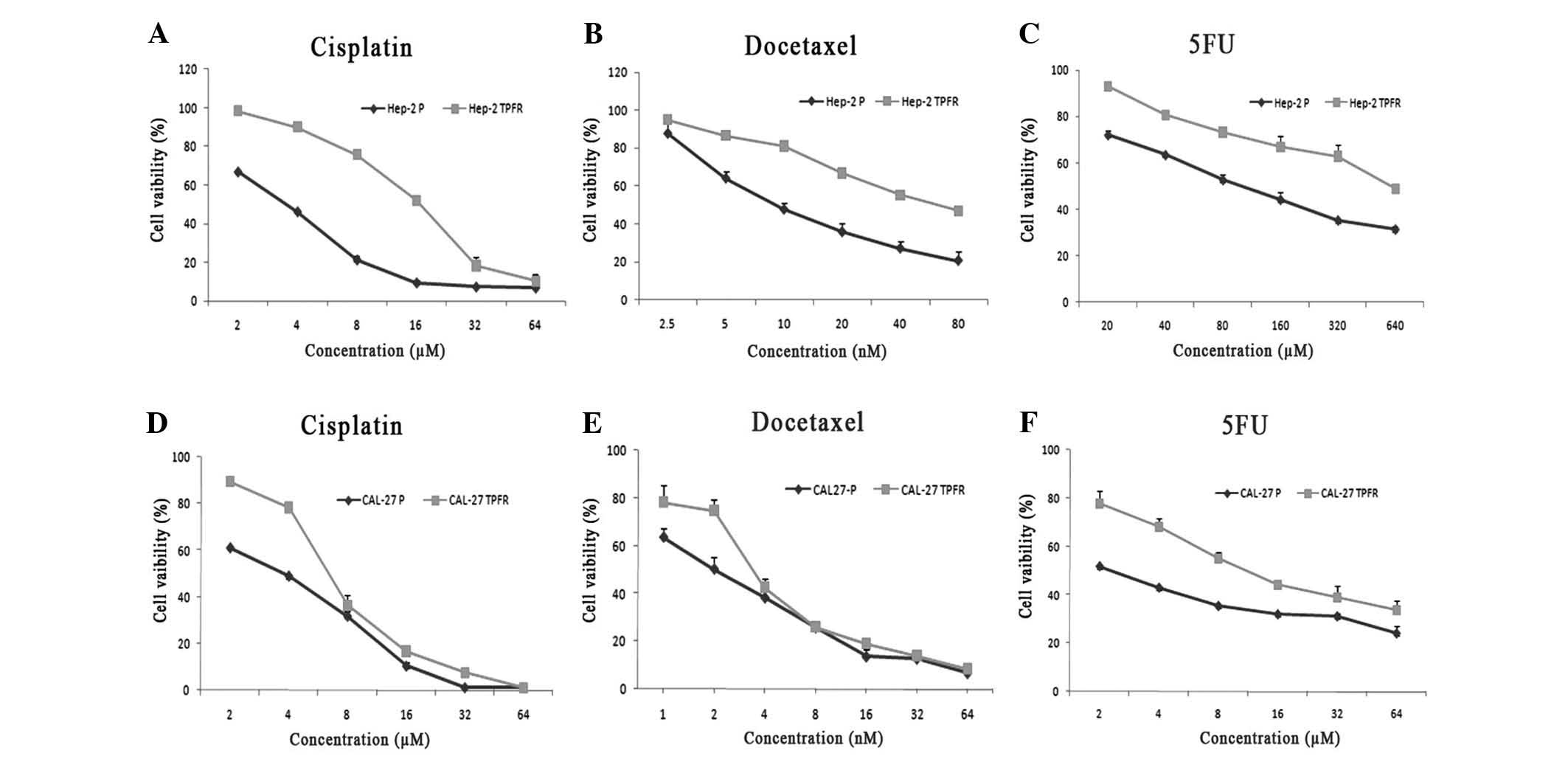 | Figure 1Tumor cells treated with TPF
demonstrated an increased resistance to the three drugs.
IC50 values of the TPF-resistant cells generated from
(A-C) Hep-2 and (D-F) CAL-27 cells following sequential treatment
with the combination of the drugs, were assessed by MTT assays. The
viability of these cells was evaluated against treatment with (A
and D) cisplatin, (B and E) docetaxel and (C and F) 5-FU. The
resistant cell lines, Hep-2 TPFR and CAL-27 TPFR, demonstrated a
significant increase in the IC50 values as analyzed by
GraphPad Prism software. Experiments were performed in triplicate
(P<0.05, as compared with the parental cells). 5-FU,
5-fluorouracil; TPFR, combination of docetaxel, cisplatin and 5-FU;
IC50, half maximal inhibitory concentration; R,
resistant; P, parental. |
The Hep-2 TPFR cells demonstrated an increase in the
IC50 compared with that of the parental cell line
(RI=2–9), with the exception of the resistance to cisplatin in
CAL-27 TPFR cells (RI=1.97; Fig.
1A–F; Table I). These results
indicated the development of a drug-resistant phenotype in these
cell lines.
Cell cycle and apoptosis analysis
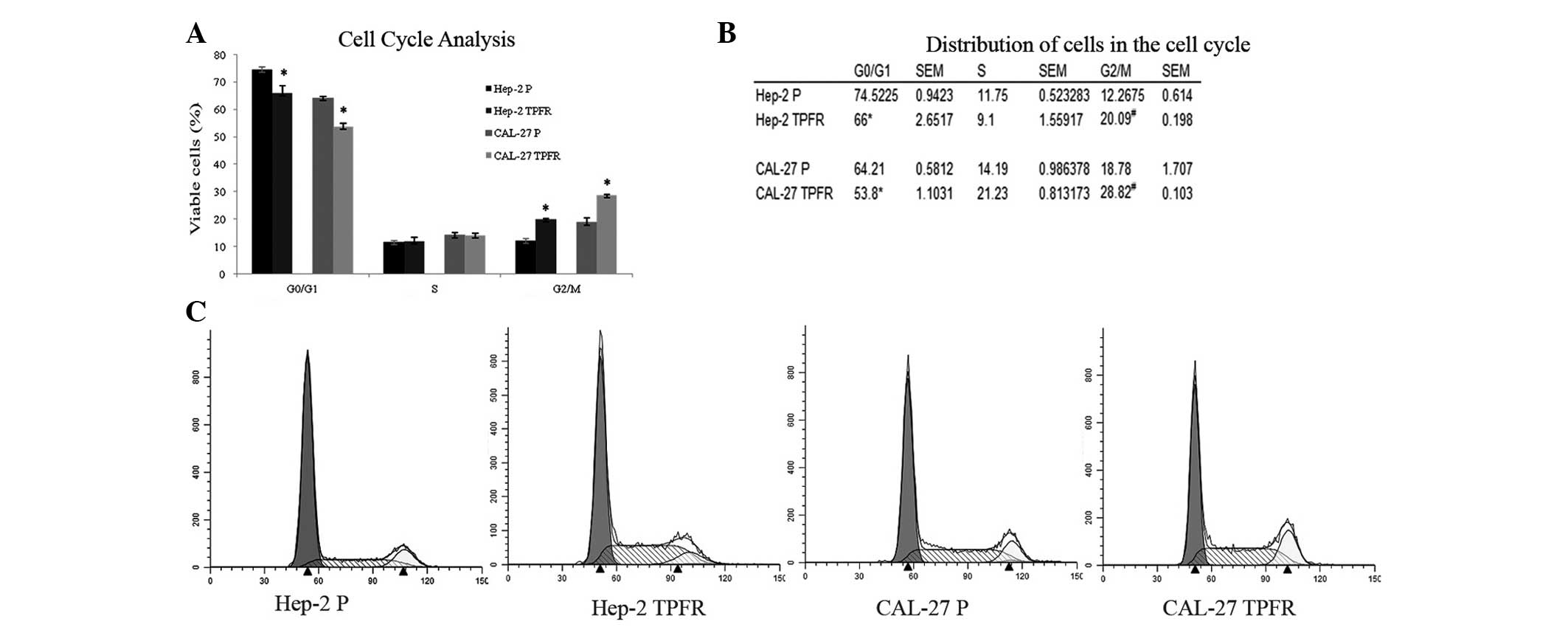
Analysis of the cell cycle pattern using PI staining
demonstrated a decreased accumulation of cells in G0/G1 phase in
the resistant cell lines (Hep-2 TPFR, 74 vs 66%, P=0.017; CAL-27
TPFR, 64 vs 53%, P=0.002; Fig.
2A–C). At basal levels, this decrease in the G0/G1 phase
population was accompanied by a corresponding increase in the
accumulation of cells in G2/M phase (Hep-2 TPFR, 12 vs 20%,
P=0.001; CAL-27 TPFR, 18 vs 28%; P=0.02).
Annexin V assays using the resistant sublines
revealed a decreased apoptotic rate of the TPFR cell lines
following incubation with the drugs. Hep-2 TPFR cells demonstrated
a significant reduction in the percentage of apoptotic cells (TPFR,
20%; parental, 33%; P=0.003). The apoptotic rate of CAL-27 TPFR
cells was also decreased; however, differences were not significant
(TPFR, 9.7%; parental, 18%; P=0.2; Fig. 3A and B).
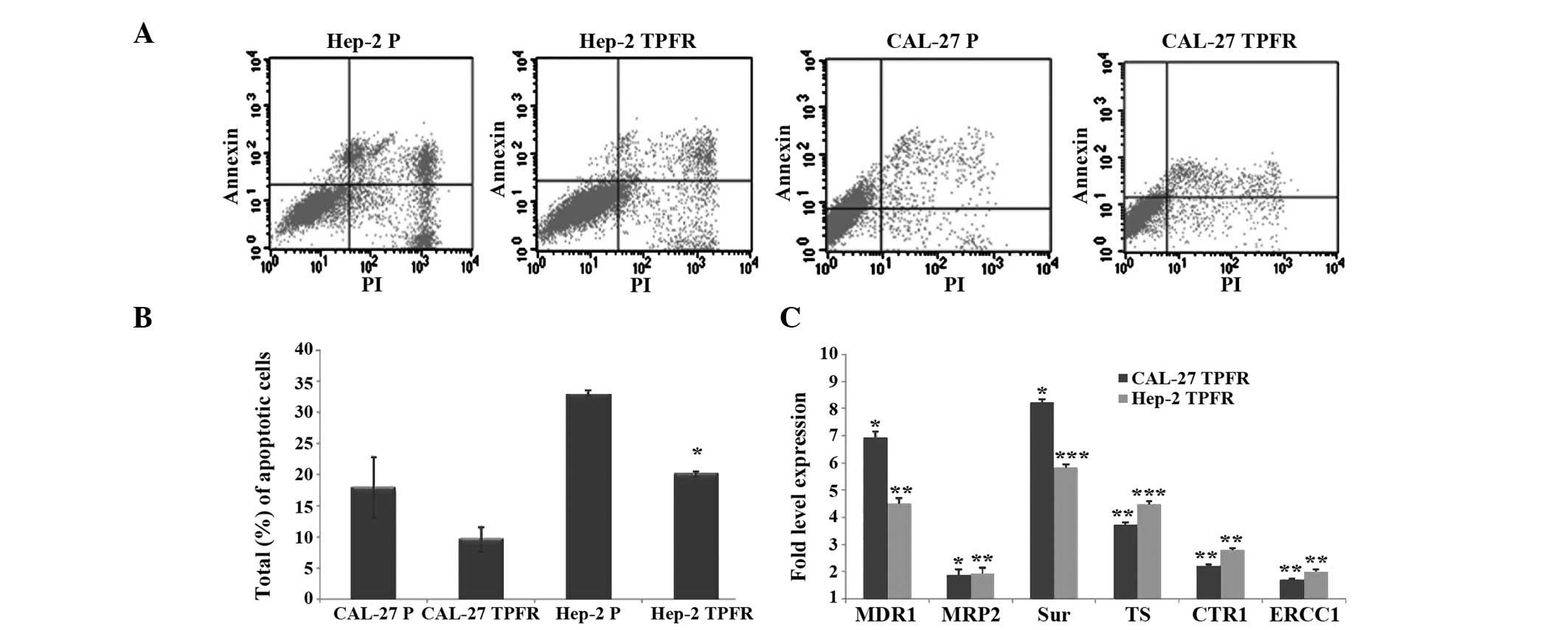 | Figure 3Apoptotic rate and expression
profiles of resistance-associated genes. (A and B) The resistant
cell lines demonstrated decreased level of apoptosis. The TPFR
cells were analyzed by annexin-V staining to assess the apoptotic
pattern. (A) Representative dot plots indicated the total apoptotic
patterns for the parental Hep-2 and CAL-27 cells and their
resistant counterparts. (B) The resistant cells revealed a
decreased population (Hep-2 TPFR, 33 to 20%, P=0.003; CAL-27 TPFR,
18 to 9.7%) in the apoptotic quadrants (PI-positive, annexin
V-positive and PI + annexin V positive). Values are expressed as
the mean ± standard error of the mean of three independent
experiments. (C) The expression of resistance genes, MDR1, MRP2,
Sur, Ts, CTR1 and ERCC1, were increased in the TPFR cell lines. All
the genes were upregulated in the two TPFR cell lines compared with
levels in the parental untreated cells. *P<0.05;
**P<0.005; ***P<0.0005, as compared with the
parental untreated cells. TPF, combination of docetaxel, cisplatin
and 5-fluorouracil; TPFR, TPF resistant; P, parental; PI, propidium
iodide; MDR, multidrug resistance; Sur, survivin; CTR, copper
transporter; ERCC, excision repair cross-complementing rodent
repair deficiency, complementation; TS, thymidylate synthase. |
Expression of MDR genes
The cell lines were profiled for the expression
levels of the MDR genes, including MDR1, MRP2 (ABCC2), survivin,
ERCC1, CTR1 and TS, in order to determine whether they were
involved in the resistance of the three drugs under investigation.
Quantitative profiling of the transcript levels indicated a
concomitant increase in the expression levels of all these markers
in the TPF-treated cell lines (Hep-2 TPFR: Median increase,
3.6-fold and range, 2–5.8; CAL-27 TPFR: Median increase, 3-fold and
range, 1.7–8.2; P<0.05; Fig.
3C). The upregulation of survivin, CTR1, TS and ERCC1 was
significant in the Hep-2 TPFR cells as compared with levels in the
parental cells (P<0.005), with survivin and CTR1 demonstrating a
highly significant upregulation (P<0.0005). The CAL-27 TPFR cell
line demonstrated a significant upregulation of CTR1, ERCC1 and TS
(P≤0.005).
Discussion
Drug-resistant cell lines are essential
in-vitro model systems, as they can facilitate an
understanding of the underlying mechanisms of clinical anti-cancer
drug resistance. Cell line models with acquired resistance to a
broad range of anti-cancer drugs have been generated and
investigated in various types of cancer, including HNSCC (14,22,24–30).
The present study generated two HNSCC cell lines resistant to a
combination of three drugs, docetaxel, cisplatin and 5-FU,
routinely utilized in the clinical treatment of patients with
HNSCC. Sequential treatment of the two cell lines (Hep-2 and
CAL-27) with an intermittent drug-free period led to the successful
development of the drug-resistant phenotype characterized by
RI≥2.
The three chemotherapeutic agents used in the
present study vary in their mechanism of action. Cisplatin is an
alkylating agent, which binds to DNA and forms intrastrand
crosslinks and DNA adducts, which ultimately lead to apoptosis
(31). Docetaxel is a
semisynthetic taxane, which inhibits microtubule depolymerization
leading to mitotic catastrophe and cell death (32). 5-FU is an anti-metabolite, which
exerts its anti-neoplastic activity by inhibiting thymidylate
synthase and mis-incorporation of fluoronucleotides into RNA and
DNA (33). Preclinical and
clinical studies have previously demonstrated that the combination
of these drugs results in a synergistic increase in anti-tumor
activity. The combination of TPF has been demonstrated to increase
the survival rate in patients with locally advanced HNSCC (34). However, 30–40% of the patients
treated with the TPF regimen do not respond to these therapies
(35–37). In HNSCC, cisplatin in combination
with docetaxel demonstrated a response rate of ~88% (38), whereas in combination with
paclitaxel it demonstrated a response rate of 40% in recurrent
cancer (39). This property of
multi-drug resistance is primarily responsible for the low response
rates in this subset of patients. Cell lines resistant to all three
drugs may serve as an important model to assess the underlying
mechanisms.
The concept underlying combination therapy is the
synergistic benefit due to multiple drug action. However, the
different mechanisms involved may also be responsible for inducing
drug resistance. Cell cycle-mediated drug resistance to combination
chemotherapy is currently being investigated. One of the primary
effects of cytotoxic drug action is a reduction in the G0/G1 phase
population and an arrest in G2/M phase of the cell cycle, of which
the latter is known to guide the damaged cells to the apoptotic
pathway. Previous studies have demonstrated that G2/M phase arrest
increases the cytotoxicity of agents in gastric cancer, prostate
tumor and neuronal cells in vitro (40–42).
In contrast, a prolonged arrest in this phase of the cell cycle is
also known to be one of the mechanisms used to escape apoptosis by
enabling repair of the damaged DNA and thereby rendering themselves
resistant to the drugs. Abrogation of this G2/M checkpoint is known
to render cells sensitive to apoptosis (43), to agents, including mitomycin C, in
human colon carcinoma cells (44)
and to radiation in breast cancer cells (45). The present study indicated a
significant G2/M phase arrest in each of the resistant cell lines.
This arrest may be due to the combined effect of cisplatin, which
induces arrest in the early G2/M phase, and docetaxel, which
induces mitotic arrest, with this fraction of cells contributing to
the resistance phenotype. An arrest in G2 phase by activation of
cell cycle checkpoints was also reported to be the mechanism
adopted by cancer stem-like cells to evade apoptosis (46); the relevance of this concept in
these resistant cells remains to be elucidated.
As reported by other studies, the accumulation of
cells in G2/M phase observed in the present study is also
accompanied by a corresponding upregulation of the survivin gene, a
member of the inhibitor of apoptosis family, known to inhibit
apoptosis and thereby induce resistance in several types of cancer
(47,48). The overexpression of this gene in
each of the resistant cell lines suggested its role in the
induction of drug resistance in the HNSCC cell lines investigated.
The ABC transporters, MDR1 and MRP2, were the other class of genes
upregulated in the resistant cell lines generated in the present
study. In vitro assessment indicated their role in
resistance to cisplatin and other cytotoxic drugs (29,30).
Previous studies correlating their expression levels to patient
outcome suggested the downregulation of these markers to be
predictive of disease-free and overall survival (49,50).
ERCC1 and CTR1 are molecules associated with
cisplatin resistance in several solid types of tumor (51,52).
An increase in the expression of the CTR1 gene, the copper influx
transporter, increases the intake of cisplatin in the cells
(53,54), thereby increasing sensitivity.
However, a study using ovarian cancer cells demonstrated that an
increase in CTR1 was not accompanied by an increase in
susceptibility to the drug, possibly due to a lack of access to its
targets (54). The present study
demonstrated the overexpression of ERCC1 and a marginal
upregulation of CTR1 in the TPFR cells, suggesting that they have a
role in the resistance of the cells to TPF. Previous studies on
HNSCC have also revealed an increased expression of CTR1 in
resistant patients, indicating that this may be an effect of
exposure to the drug treatment. Induction of TS is one of the
mechanisms underlying 5-FU resistance (55,56)
and an increased expression of this gene was also observed in the
resistant cell lines established in the present study. In the
present study, expression profiling of these multi-drug resistance
genes indicated a synergistic action in the TPF-resistant cell
lines, whereby all these markers were upregulated.
Multimodal chemotherapy has been conceived with the
concept of combining chemotherapeutic drugs to increase the
cytotoxic effect on the cells. With the increase in the
understanding of individual drug action, effects on the cell cycle
and the processes of acquired drug resistance, it is clear that
there is a requirement for refining the concept to determine
improved results. An in vitro study on the effects of
cisplatin, docetaxel and 5-FU provided evidence towards the inverse
association observed between resistance to cisplatin and docetaxel
in cell lines (57). It was also
suggested that platinum- and taxol-resistant cell lines exhibited
cross resistance with the molecular background being of prime
importance. The overexpression of the MRP2 gene is known to mediate
docetaxel resistance in cisplatin-resistant cell lines (7). The consistent upregulation of
MDR1/MRP2 in the cell lines suggested their role in multidrug
resistance to the majority of chemotherapeutic drugs. Sequential
administration of the drugs, which complements the cellular and
molecular effects of various drugs is now being considered an
option. An in vitro study indicated that docetaxel treatment
is known to downregulate the expression levels of TS and DPD, which
in turn render the cells sensitive to 5-FU (58). The sequential treatment of cells
with docetaxel, followed by 5-FU, therefore increased the
cytotoxicity compared with that of the individual or combined
treatments. Further investigations into the molecular and cell
cycle effects of these drugs may enable an improved insight into
the optimal methods of performing combination chemotherapy.
The present study described the establishment of
triple drug-resistant cell lines and also provided valuable insight
into the mechanism of resistance in a multidrug-resistant
phenotype. Further evaluations of these resistant sublines may
provide valuable inputs into the cellular and molecular methods
adopted for acquiring drug resistance. The global differences in
the gene expression profiles of these cells and the possible role
of stem cells in the process of acquiring drug resistance is
another area of interest currently under investigation.
Acknowledgments
The authors would like to thank Dr Aditi Chatterjee
(Institute of Bioinformatics, Bangalore) for her kind gift of the
CAL-27 cells. The present study was supported by the Department of
Biotechnology, India (no. BT/PR15027/GBD/27/286/2010) and the
fellowship for SVG from the Indian Council of Medical Research
(ICMR) is also acknowledged.
References
|
1
|
Kogashiwa Y, Sakurai H, Kimura T and Kohno
N: Docetaxel suppresses invasiveness of head and neck cancer cells
in vitro. Cancer Sci. 101:1382–1386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pignon JP, Syz N, Posner M, et al:
Adjusting for patient selection suggests the addition of docetaxel
to 5-fluorouracil-cisplatin induction therapy may offer survival
benefit in squamous cell cancer of the head and neck. Anticancer
Drugs. 15:331–340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salama JK, Seiwert TY and Vokes EE:
Chemoradiotherapy for locally advanced head and neck cancer. J Clin
Oncol. 25:4118–4126. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Posner MR, Hershock DM, Blajman CR, et al:
Cisplatin and fluorouracil alone or with docetaxel in head and neck
cancer. N Engl J Med. 357:1705–1715. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bourhis J, Le Maître A, Baujat B, Audry H
and Pignon JP: Individual patients’ data meta-analyses in head and
neck cancer. Curr Opin Oncol. 19:188–194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brockstein B, Haraf DJ, Rademaker AW, et
al: Patterns of failure, prognostic factors and survival in
loco-regionally advanced head and neck cancer treated with
concomitant chemoradiotherapy: a 9-year, 337-patient,
multi-institutional experience. Ann Oncol. 15:1179–1186. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stordal B, Hamon M, McEneaney V, et al:
Resistance to paclitaxel in a cisplatin-resistant ovarian cancer
cell line is mediated by P-glycoprotein. PLoS One. 7:e407172012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duan Z, Ames RY, Ryan M, Hornicek FJ,
Mankin H and Seiden MV: CDDO-Me, a synthetic triterpenoid, inhibits
expression of IL-6 and Stat3 phosphorylation in multi-drug
resistant ovarian cancer cells. Cancer Chemother Pharmacol.
63:681–689. 2009. View Article : Google Scholar
|
|
9
|
Wahl H, Tan L, Griffith K, Choi M and Liu
JR: Curcumin enhances Apo2L/TRAIL-induced apoptosis in
chemoresistant ovarian cancer cells. Gynecol Oncol. 105:104–112.
2007. View Article : Google Scholar
|
|
10
|
Duan Z, Duan Y, Lamendola DE, et al:
Overexpression of MAGE/GAGE genes in
paclitaxel/doxorubicin-resistant human cancer cell lines. Clin
Cancer Res. 9:2778–2785. 2003.PubMed/NCBI
|
|
11
|
Husain A, He G, Venkatraman ES and Spriggs
DR: BRCA1 up-regulation is associated with repair-mediated
resistance to cis-diamminedichloroplatinum(II). Cancer Res.
58:1120–1123. 1998.PubMed/NCBI
|
|
12
|
Hill BT, Whelan RD, Shellard SA, McClean S
and Hosking LK: Differential cytotoxic effects of docetaxel in a
range of mammalian tumor cell lines and certain drug resistant
sublines in vitro. Invest New Drugs. 12:169–182. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Plumb JA, Luo W and Kerr DJ: Effect of
polyunsaturated fatty acids on the drug sensitivity of human tumour
cell lines resistant to either cisplatin or doxorubicin. Br J
Cancer. 67:728–733. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamano Y, Uzawa K, Saito K, et al:
Identification of cisplatin-resistance related genes in head and
neck squamous cell carcinoma. Int J Cancer. 126:437–449. 2010.
View Article : Google Scholar
|
|
15
|
Aoki K, Ogawa T, Ito Y and Nakashima S:
Cisplatin activates survival signals in UM-SCC-23 squamous cell
carcinoma and these signal pathways are amplified in
cisplatin-resistant squamous cell carcinoma. Oncol Rep. 11:375–379.
2004.PubMed/NCBI
|
|
16
|
Gosepath EM, Eckstein N, Hamacher A, et
al: Acquired cisplatin resistance in the head-neck cancer cell line
Cal27 is associated with decreased DKK1 expression and can
partially be reversed by overexpression of DKK1. Int J Cancer.
123:2013–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gu F, Ma Y, Zhang Z, et al: Expression of
Stat3 and Notch1 is associated with cisplatin resistance in head
and neck squamous cell carcinoma. Oncol Rep. 23:671–676.
2010.PubMed/NCBI
|
|
18
|
Li L, Jiang AC, Dong P, Wan Y and Yu ZW:
The characteristics of Hep-2 cell with multiple drug resistance
induced by Taxol. Otolaryngol Head Neck Surg. 137:659–664. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mizumachi T, Suzuki S, Naito A, et al:
Increased mitochondrial DNA induces acquired docetaxel resistance
in head and neck cancer cells. Oncogene. 27:831–838. 2008.
View Article : Google Scholar :
|
|
20
|
Yasumatsu R, Nakashima T, Uryu H, et al:
The role of dihydropyrimidine dehydrogenase expression in
resistance to 5-fluorouracil in head and neck squamous cell
carcinoma cells. Oral Oncol. 45:141–147. 2009. View Article : Google Scholar
|
|
21
|
Beck A, Etienne MC, Chéradame S, et al: A
role for dihydropyrimidine dehydrogenase and thymidylate synthase
in tumour sensitivity to fluorouracil. Eur J Cancer. 30A:1517–1522.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoo GH, Lin HS, Iskander AJ, et al:
Docetaxel associated pathways in cisplatin resistant head and neck
squamous cell carcinoma: a pilot study. Laryngoscope.
115:1938–1946. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Coley HM: Development of drug-resistant
models. Methods Mol Med. 88:267–273. 2004.
|
|
24
|
Murakami H, Ito S, Tanaka H, Kondo E,
Kodera Y and Nakanishi H: Establishment of new intraperitoneal
paclitaxel-resistant gastric cancer cell lines and comprehensive
gene expression analysis. Anticancer Res. 33:4299–4307.
2013.PubMed/NCBI
|
|
25
|
Rao GH, Liu HM, Li BW, et al:
Establishment of a human colorectal cancer cell line P6C with stem
cell properties and resistance to chemotherapeutic drugs. Acta
Pharmacol Sin. 34:793–804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loh YN, Hedditch EL, Baker LA, Jary E,
Ward RL and Ford CE: The Wnt signalling pathway is upregulated in
an in vitro model of acquired tamoxifen resistant breast cancer.
BMC Cancer. 13:1742013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takahashi K, Tanaka M, Inagaki A, et al:
Establishment of a 5-fluorouracil-resistant triple-negative breast
cancer cell line. Int J Oncol. 43:1985–1991. 2013.PubMed/NCBI
|
|
28
|
Maseki S, Ijichi K, Tanaka H, et al:
Acquisition of EMT phenotype in the gefitinib-resistant cells of a
head and neck squamous cell carcinoma cell line through
Akt/GSK-3β/snail signalling pathway. Br J Cancer. 106:1196–1204.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Negoro K, Yamano Y, Fushimi K, et al:
Establishment and characterization of a cisplatin-resistant cell
line, KB-R, derived from oral carcinoma cell line, KB. Int J Oncol.
30:1325–1332. 2007.PubMed/NCBI
|
|
30
|
Nakamura M, Nakatani K, Uzawa K, et al:
Establishment and characterization of a cisplatin-resistant oral
squamous cell carcinoma cell line, H-1R. Oncol Rep. 14:1281–1286.
2005.PubMed/NCBI
|
|
31
|
Pinto AL and Lippard SJ: Binding of the
antitumor drug cis-diamminedichloroplatinum(II) (cisplatin) to DNA.
Biochim Biophys Acta. 780:167–180. 1985.PubMed/NCBI
|
|
32
|
Owellen RJ, Hartke CA, Dickerson RM and
Hains FO: Inhibition of tubulin-microtubule polymerization by drugs
of the Vinca alkaloid class. Cancer Res. 36:1499–1502.
1976.PubMed/NCBI
|
|
33
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Loehrer PJ Sr, Einhorn LH, Williams SD,
Hui SL, Estes NC and Pennington K: Cisplatin plus 5-FU for the
treatment of adenocarcinoma of the colon. Cancer Treat Rep.
69:1359–1363. 1985.PubMed/NCBI
|
|
35
|
Qin H, Luo J, Zhu YP, Xie HL, Yang WQ and
Lei WB: Combination of taxanes, cisplatin and fluorouracil as
induction chemotherapy for locally advanced head and neck cancer: a
meta-analysis. PLoS One. 7:e515262012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hitt R, Paz-Ares L, Brandariz A, et al:
Induction chemotherapy with paclitaxel, cisplatin and
5-fluorouracil for squamous cell carcinoma of the head and neck:
long-term results of a phase II trial. Ann Oncol. 13:1665–1673.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hussain M, Salwen W, Kucuk O and Ensley J:
Paclitaxel, cisplatin, and 5-fluorouracil in patients with advanced
or recurrent squamous cell carcinoma of the head and neck: a
preliminary report. Semin Oncol. 24:S19–43. S19–45. 1997.
|
|
38
|
Choi YJ, Chung J, Shin HJ, et al:
Induction chemotherapy of docetaxel and Cisplatin for the elderly
patients with squamous cell carcinoma of the head and neck. Cancer
Res Treat. 39:1–5. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Adamo V, Ferraro G, Pergolizzi S, et al:
Paclitaxel and cisplatin in patients with recurrent and metastatic
head and neck squamous cell carcinoma. Oral Oncol. 40:525–531.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu YL, Zhang GQ, Yang Y, Zhang CY, Fu RX
and Yang YM: Genistein induces G2/M arrest in gastric cancer cells
by increasing the tumor suppressor PTEN expression. Nutr Cancer.
65:1034–1041. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ismail IA, Kang KS, Lee HA, Kim JW and
Sohn YK: Genistein-induced neuronal apoptosis and G2/M cell cycle
arrest is associated with MDC1 up-regulation and PLK1
down-regulation. Eur J Pharmacol. 575:12–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nawab A, Thakur VS, Yunus M, Ali Mahdi A
and Gupta S: Selective cell cycle arrest and induction of apoptosis
in human prostate cancer cells by a polyphenol-rich extract of
Solanum nigrum. Int J Mol Med. 29:277–284. 2012.
|
|
43
|
Wang WZ, Cheng J, Luo J and Zhuang SM:
Abrogation of G2/M arrest sensitizes curcumin-resistant hepatoma
cells to apoptosis. FEBS Lett. 582:2689–2695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang Q, Fan S, Eastman A, Worland PJ,
Sausville EA and O’Connor PM: UCN-01: a potent abrogator of G2
checkpoint function in cancer cells with disrupted p53. J Natl
Cancer Inst. 88:956–965. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sun XC, Cheng HY, Deng YX, Shao RG and Ma
J: Tetrandrine: a potent abrogator of G2 checkpoint function in
tumor cells and its mechanism. Biomed Environ Sci. 20:495–501.
2007.
|
|
46
|
Chappell J and Dalton S: Altered cell
cycle regulation helps stem-like carcinoma cells resist apoptosis.
BMC Biol. 8:632010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pratt MA, Niu MY and Renart LI: Regulation
of survivin by retinoic acid and its role in paclitaxel-mediated
cytotoxicity in MCF-7 breast cancer cells. Apoptosis. 11:589–605.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nestal de Moraes G, Silva KL, Vasconcelos
FC and Maia RC: Survivin overexpression correlates with an
apoptosis-resistant phenotype in chronic myeloid leukemia cells.
Oncol Rep. 25:1613–1619. 2011.PubMed/NCBI
|
|
49
|
Ohishi Y, Oda Y, Uchiumi T, et al:
ATP-binding cassette super-family transporter gene expression in
human primary ovarian carcinoma. Clin Cancer Res. 8:3767–3775.
2002.PubMed/NCBI
|
|
50
|
Lee J, Jiffar T and Kupferman ME: A novel
role for BDNF-TrkB in the regulation of chemotherapy resistance in
head and neck squamous cell carcinoma. PLoS One. 7:e302462012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Köberle B, Ditz C, Kausch I, Wollenberg B,
Ferris RL and Albers AE: Metastases of squamous cell carcinoma of
the head and neck show increased levels of nucleotide excision
repair protein XPF in vivo that correlate with increased
chemoresistance ex vivo. Int J Oncol. 36:1277–1284. 2010.PubMed/NCBI
|
|
52
|
Hayes M, Lan C, Yan J, et al: ERCC1
expression and outcomes in head and neck cancer treated with
concurrent cisplatin and radiation. Anticancer Res. 31:4135–4139.
2011.PubMed/NCBI
|
|
53
|
Song IS, Savaraj N, Siddik ZH, et al: Role
of human copper transporter Ctr1 in the transport of platinum-based
antitumor agents in cisplatin-sensitive and cisplatin-resistant
cells. Mol Cancer Ther. 3:1543–1549. 2004.
|
|
54
|
Holzer AK, Samimi G, Katano K, et al: The
copper influx transporter human copper transport protein 1
regulates the uptake of cisplatin in human ovarian carcinoma cells.
Mol Pharmacol. 66:817–823. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Peters GJ, Backus HH, Freemantle S, et al:
Induction of thymidylate synthase as a 5-fluorouracil resistance
mechanism. Biochim Biophys Acta. 1587:194–205. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wong NA, Brett L, Stewart M, et al:
Nuclear thymidylate synthase expression, p53 expression and 5-FU
response in colorectal carcinoma. Br J Cancer. 85:1937–1943. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Saiki Y, Ogawa T, Shiga K, Sunamura M,
Kobayashi T and Horii A: A Human Head and Neck Squamous Cell
Carcinoma Cell Line with Acquired
cis-Diamminedichloroplatinum-Resistance Shows Remarkable
Upregulation of BRCA1 and Hypersensitivity to Taxane. Int J
Otolaryngol. 2011:5218522011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tamatani T, Ferdous T, Takamaru N, et al:
Antitumor efficacy of sequential treatment with docetaxel and
5-fluorouracil against human oral cancer cells. Int J Oncol.
41:1148–1156. 2012.PubMed/NCBI
|