Introduction
The frequency of endometriosis among infertile women
diagnosed by laparoscopic examination ranges from 20 to 50%
(1). Patients with
endometriosis-related infertility frequently display increased
blood plasma levels of prolactin (1). It has been hypothesized that
hyperprolactinemia may result in endometriosis-related infertility
and that fertility may be restored by prolactin suppression
(1,2). Increased levels of prolactin result
in anovulation, by blocking estrogen receptor function in the
hypothalamus (3). The effect of
raised levels of prolactin on the ovary may also reduce affinity of
LH receptors in the corpus luteum and decrease the biosynthesis of
progesterone, leading to anovulation and suppression of follicular
maturation (3). Furthermore,
prolactin may contribute to the pathogenesis of endometriosis by
supporting angiogenesis (4), which
initiates and enhances endometrial lesions (5).
Estrogen supports the proliferation of anterior
pituitary lactotroph cells, and induces prolactin gene
transcription and protein release from the anterior pituitary gland
(6). By contrast, the hypothalamus
exerts a tonic inhibitory action against prolactin via the
excretion of dopamine from the portal vessels of the pituitary
(6). There are two subfamilies of
dopamine receptors: DRD1, which stimulates adenylyl cyclase
activity; and DRD2, which inhibits the activity of this enzyme
(7). The adenohypophysis primarily
expresses the DRD2 dopamine receptor (7). Dopamine binds to DRD2 in the
pituitary lactotrophs and decreases the level of intracellular
cyclic adenosine monophosphate, which in turn inhibits prolactin
secretion (8).
Data suggests that DRD2 gene variants may
contribute to hyperprolactinemia (9,10).
Furthermore, it has been demonstrated that the DRD2 single
nucleotide polymorphism (SNP), 3438C>T (rs6277), at proline
codon in exon 7, is associated with an increased risk of
moderate/severe peritoneal endometriosis in women with infertility
(11). In order to evaluate
whether DRD2 gene variants are genetic risk factors for
endometri-osis-related infertility in women from a Polish
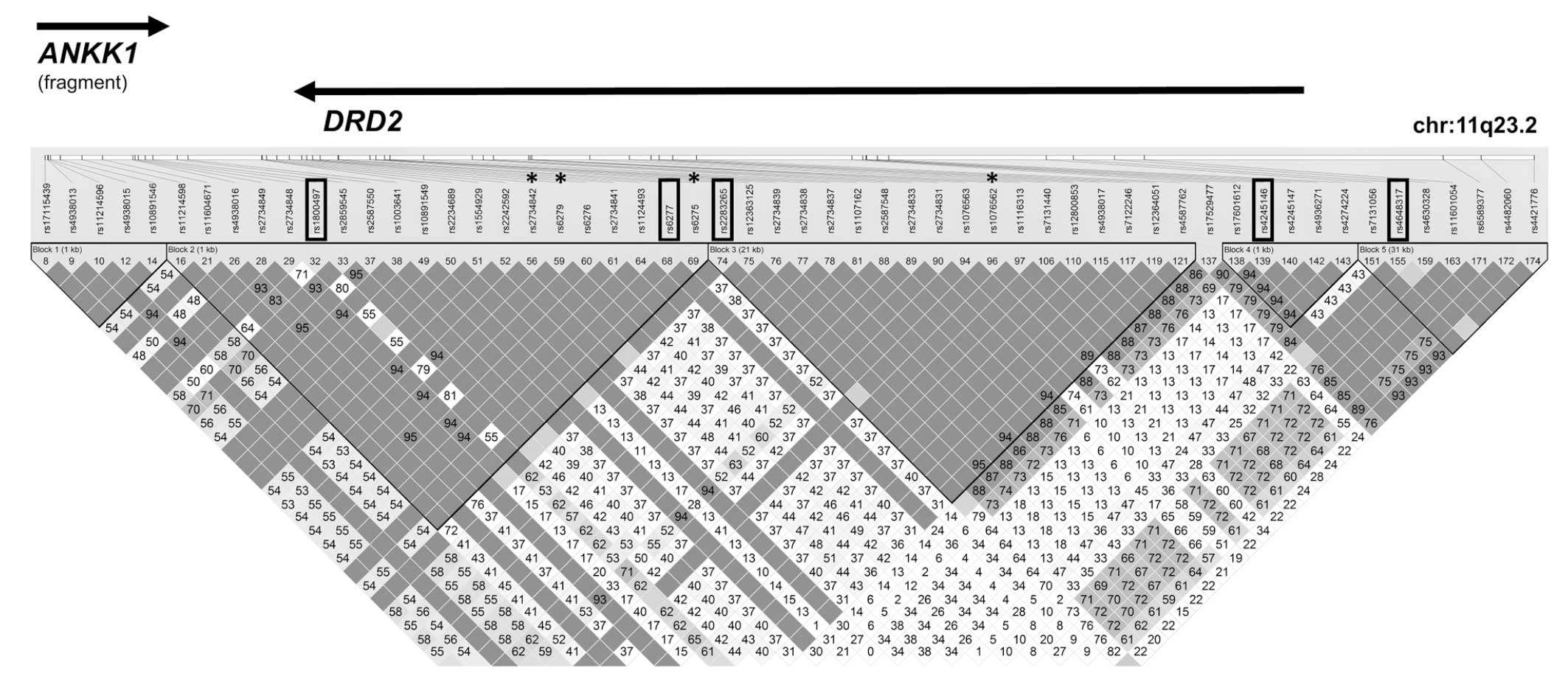
population, the rs1800497, rs6277, rs2283265, rs4245146 and
rs4648317 SNPs, which are located in different blocks of linkage
disequilibrium (LD), were selected for further investigation.
Materials and methods
Patients and controls
Peripheral blood samples from females with
endometriosis-related infertility and fertile controls, were
obtained from the Gynecologic and Obstetrical University Hospital,
Division of Reproduction at Poznań University of Medical Sciences
(Poznań, Poland). The studied population was divided into two
groups: Those with endometriosis and infertility (151), and a
fertile control group (381) (Table
I). The following inclusion criteria for infertile women with
endome-triosis were used: No anatomical changes in the reproductive
tract, no hormonal treatment, a minimum 1 year of infertility and a
current desire to achieve conception. The exclusion criteria were
as follows: Male factor infertility, polycystic ovary syndrome
(PCOS), mechanical distortion of the endo-metrial cavity by
fibroids and bilateral tubal occlusion. All patients with
endometriosis received laparoscopic and histological diagnoses of
endometriosis. The stage of endometriosis was evaluated according
to the revised classification of the American Society for
Reproductive Medicine (rASRM) (12).
 | Table IClinical characteristics of
patients. |
Table I
Clinical characteristics of
patients.
| Characteristic | Endometriosis | Controls |
|---|
| Number of
patients | 151 | 381 |
| Age in years
(range) | 32 (21–42) | 32 (20–39) |
| Parity | NA | 1 (1–4) |
| Duration of
infertility | 3 (1–7) | NA |
| in years (range) | | |
| rASRM stage | | |
| Stage I | (n=83) | |
| Stage II | (n=68) | NA |
All fertile women assigned to the control group were
examined for the cause of pelvic pain. However, the laparos-copy
evaluation did not demonstrate any pelvic abnormalities. The
controls were diagnosed by laparoscopy with varicose veins in the
pelvic floor, and exhibited no signs of past or present
inflammation. The following inclusion criteria for the fertile
controls were used: Regular menses, no anatomical changes in the
reproductive tract, no hormonal treatments, and ≥1 child born ≤1
years prior to the laparoscopy (Table
I). The exclusion criteria were as follows: Diagnosis of past
or present inflammation, pelvic abnormalities, endometriotic
lesions and PCOS. Patients and controls were matched by age, and
were all Caucasians of Polish descent (Table I). Written and verbal consent was
obtained from all participating individuals. The study procedures
were approved by the Local Ethical Committee of Poznań University
of Medical Sciences.
Genotyping
Genomic DNA was obtained from peripheral blood
leukocytes using salt extraction. The DNA samples were subsequently
genotyped for the 5 SNPs in DRD2 (Table II and Fig. 1). SNPs were selected using the
genome browser of the International HapMap Consortium (http://www.hapmap.org/index.html.en), UCSC
(http://genome.ucsc.edu) and dbSNP database
(http://www.ncbi.nlm.nih.gov/projects/SNP/). SNPs were
selected based on functional significance, location in the distinct
LD blocks and minor allele frequency (MAF) of >0.1 in the
Caucasian population.
 | Table IICharacteristics of polymorphisms
genotyped in the data set. |
Table II
Characteristics of polymorphisms
genotyped in the data set.
| Gene symbol | Chromosome
location | rs no. | SNP function | Allelesa | MAFb | Primers for PCR
amplification (5′-3′) | Annealin temp.
(°C) | PCR product length
(bp) | HRM Melt. temp.
(°C) | RFLP conditions
|
|---|
| RE | RFL (bp) |
|---|
| ANKK1 | chr11:113270828 | rs1800497 | Missense
p.Glu713Lys | C/T | 0.18 | F:
CCATCCTCAAAGTGCTGGTC
R: ATCTCGGCTCCTGGCTTAGA | 61.0 | 172 | NA | TaqI | C=153+19
T=172 |
| DRD2 | chr11:113283459 | rs6277 | cds-synon
p.Pro319Pro | C/T | 0.46 | F:
TCTCTGGTTTGGCGGGGCTCTCc
R: GGAACTTGTCCGGCTTTACC | 65.0 | 213 | NA | DdeI | C=213
T=193+20 |
| DRD2 | chr11:113285536 | rs2283265 | intron | G/T | 0.14 | F:
CACACTCACGTCCCTTCTCA
R: GGGCTAGACGCATCAGGTT | 61.0 | 171 | 75–90 | NA | NA |
| DRD2 |
chr11:113317973 | rs4245146 | intron | C/T | 0.44 | F:
CTAGCATGTCATAGCCCTTGC
R: ACATCACGGAGCCTGAGC | 61.0 | 193 | 81–96 | NA | NA |
| DRD2 |
chr11:113331532 | rs4648317 | intron | C/T | 0.18 | F:
CTCCCACCAGGATTATGGAC
R: CATTGGGCCTTCACTACCTC | 61.0 | 170 | 80–95 | NA | NA |
Genotyping of DRD2 rs2283265, rs4245146 and
rs4648317 was conducted by high-resolution melting (HRM) using the
HOT FIREPol EvaGreen HRM mix (Solis BioDyne, Tartu, Estonia), on
the LightCycler 480 system (Roche Diagnostics, Mannheim, Germany).
Evaluation frequency of DRD2 rs1800497 and rs6277 was
performed using polymerase chain reaction (PCR), followed by
digestion by the appropriate restriction enzyme (PCR-RFLP),
according to the manufacturer’s instructions (Fermentas, Vilnius,
Lithuania), and 3% agarose separations (Serva, Heidelberg,
Germany). The primary sequences and conditions for HRM and PCR-RFLP
analyses are presented in Table
II. Genotyping quality was evaluated by repeated genotyping of
a random selection of 10% of the study population.
Statistical analysis
For each SNP, the Hardy-Weinberg equilibrium (HWE)
was assessed using Pearson’s goodness-of-fit χ2
statistic. The differences in the allele and genotype frequencies
between cases and controls were determined using standard
χ2 or Fisher tests. Odds ratios (OR) and the associated
95% confidence intervals (95% CI) were also calculated. The data
was analyzed under recessive and dominant inheritance models. For
the additive inheritance model, SNPs were tested for association
with endometriosis using the Cochran-Armitage trend test (13). In order to adjust for the multiple
testing, the Bonferroni correction was employed. A haplotype-based
association analysis was performed using the Haploview software
(http://www.broadinstitute.org/mpg/haploview; Broad
Institute, Cambridge, MA, USA). P-values for both global and
individual tests of haplotype distribution between cases and
controls were calculated. Statistical significance was assessed
using the 1,000-fold permutation testing with a cut-off of
<0.05.
Results
Prevalence of the DRD2 rs1800497, rs6277,
rs2283265, rs4245146 and rs4648317 SNPs in patients with
endometriosis-related infertility
The distribution of the DRD2 rs1800497,
rs6277, rs2283265, rs4245146 and rs4648317 SNP genotypes did not
display deviation from the HWE in either the patient or control
groups (P>0.05). The number of genotypes, in addition to the ORs
and 95% CI intervals for these SNPs are stated in Table III. DRD2 rs1800497,
rs6277, rs2283265, rs4245146 and rs4648317 SNP association was
observed in neither the dominant nor recessive inheritance models
of endometriosis-related infertility. The lowest P-values of the
trend test were observed for DRD2 rs6277 in women with
endometriosis-related infertility (ptrend=0.435).
 | Table IIIAssociation of polymorphic variants
of the DRD2 gene region with the risk of endometriosis. |
Table III
Association of polymorphic variants
of the DRD2 gene region with the risk of endometriosis.
| Gene | rs no. | Allelesa | Genotypes
casesb | Genotypes
controlsb | Ptrend
value |
Pgenotypic value | Pallelic
value |
ORdominant (95% CI)c; P-value |
ORrecessive(95% CI)d ; P-value |
|---|
| ANKK1 | rs1800497 | C/T | 99/46/6 | 263/100/18 | 0.619 | 0.599 | 0.605 | 1.171
(0.785–1.746); 0.440 | 0.835
(0.325–2.145); 0.707 |
| DRD2 | rs6277 | C/T | 37/76/38 | 95/208/78 | 0.435 | 0.480 | 0.451 | 1.023
(0.661–1.585); 0.917 | 1.306
(0.838–2.036); 0.237 |
| DRD2 | rs2283265 | G/T | 102/45/4 | 260/103/18 | 0.797 | 0.489 | 0.791 | 1.032
(0.690–1.545); 0.877 | 0.549
(0.183–1.649); 0.342e |
| DRD2 | rs4245146 | C/T | 52/74/25 | 116/205/60 | 0.622 | 0.587 | 0.636 | 0.833
(0.558–1.244); 0.372 | 1.062
(0.637–1.768); 0.819 |
| DRD2 | rs4648317 | C/T | 110/32/9 | 265/100/16 | 0.778 | 0.369 | 0.765 | 0.852
(0.560–1.296); 0.453 | 1.446
(0.625–3.348); 0.387 |
Association of DRD2 haplotypes with
endometriosis-related infertility
Haplotype analysis of the DRD2 rs1800497,
rs6277, rs2283265, rs4245146 and rs4648317 SNPs did not reveal
these polymorphisms to be risk factors for endometriosis-related
infertility (Table IV). The
lowest global P=0.070, pcorr=0.207, refers to haplotypes
comprising DRD2 rs2283265, rs4245146 and rs4648317 (Table IV).
 | Table IVHaplotype analysis of SNPs genotyped
in the DRD2 gene region. |
Table IV
Haplotype analysis of SNPs genotyped
in the DRD2 gene region.
| Polymorphism | Haplotype | Frequency
| χ2 | P-value | p valuea corr |
|---|
| All
individuals | Case, control |
|---|
|
rs1800497_rs6277 | CT | 0.484 | 0.473, 0.488 | 0.199 | 0.656 | 0.949 |
| CC | 0.333 | 0.333, 0.333 | 0.000 | 0.993 | 1.000 |
| TC | 0.152 | 0.170, 0.145 | 1.032 | 0.310 | 0.635 |
| TT | 0.031 | 0.024, 0.033 | 0.638 | 0.425 | 0.791 |
|
rs6277_rs2283265 | TG | 0.492 | 0.487, 0.495 | 0.055 | 0.815 | 0.994 |
| CG | 0.327 | 0.336, 0.324 | 0.143 | 0.706 | 0.971 |
| CT | 0.158 | 0.167, 0.155 | 0.247 | 0.619 | 0.945 |
| TT | 0.023 | 0.011, 0.027 | 2.643 | 0.104 | 0.265 |
|
rs2283265_rs4245146 | GC | 0.450 | 0.448, 0.450 | 0.006 | 0.937 | 1.000 |
| GT | 0.370 | 0.376, 0.367 | 0.074 | 0.786 | 0.984 |
| TC | 0.129 | 0.143, 0.123 | 0.737 | 0.391 | 0.742 |
| TT | 0.052 | 0.033, 0.059 | 2.918 | 0.088 | 0.193 |
|
rs4245146_rs4648317 | CC | 0.426 | 0.442, 0.419 | 0.482 | 0.488 | 0.861 |
| TC | 0.403 | 0.392, 0.408 | 0.221 | 0.639 | 0.951 |
| CT | 0.152 | 0.147, 0.153 | 0.066 | 0.797 | 0.986 |
| TT | 0.019 | 0.018, 0.020 | 0.022 | 0.882 | 0.999 |
|
rs1800497_rs6277_rs2283265 | CTG | 0.479 | 0.473, 0.482 | 0.071 | 0.790 | 1.000 |
| CCG | 0.323 | 0.328, 0.321 | 0.049 | 0.824 | 1.000 |
| TCT | 0.153 | 0.173, 0.145 | 1.366 | 0.243 | 0.616 |
| TTT | 0.014 | 0.005, 0.018 | 2.638 | 0.104 | 0.324 |
| TTG | 0.014 | 0.016, 0.013 | 0.147 | 0.701 | 0.998 |
|
rs6277_rs2283265_rs4245146 | TGC | 0.261 | 0.249, 0.265 | 0.296 | 0.587 | 0.993 |
| TGT | 0.231 | 0.238, 0.229 | 0.105 | 0.746 | 0.998 |
| CGC | 0.188 | 0.198, 0.185 | 0.243 | 0.622 | 0.995 |
| CGT | 0.139 | 0.138, 0.139 | 0.002 | 0.964 | 1.000 |
| CTC | 0.119 | 0.139, 0.112 | 1.546 | 0.214 | 0.579 |
| CTT | 0.039 | 0.028, 0.043 | 1.332 | 0.248 | 0.649 |
| TTT | 0.013 | 0.005, 0.016 | 2.027 | 0.155 | 0.416 |
|
rs2283265_rs4245146_rs4648317 | GTC | 0.358 | 0.365, 0.355 | 0.102 | 0.750 | 1.000 |
| GCC | 0.311 | 0.318, 0.309 | 0.093 | 0.760 | 1.000 |
| GCT | 0.137 | 0.129, 0.140 | 0.241 | 0.624 | 0.999 |
| TCC | 0.113 | 0.123, 0.109 | 0.414 | 0.520 | 0.979 |
| TTC | 0.046 | 0.028, 0.053 | 3.296 | 0.070 | 0.207 |
| TCT | 0.016 | 0.020, 0.015 | 0.347 | 0.556 | 0.987 |
| GTT | 0.013 | 0.012, 0.014 | 0.085 | 0.771 | 1.000 |
|
rs1800497_rs6277_rs2283265_rs4245146 | CTGC | 0.257 | 0.245, 0.261 | 0.278 | 0.598 | 0.996 |
| CTGT | 0.223 | 0.228, 0.221 | 0.067 | 0.795 | 1.000 |
| CCGC | 0.188 | 0.197, 0.185 | 0.197 | 0.657 | 0.998 |
| CCGT | 0.135 | 0.131, 0.136 | 0.056 | 0.813 | 1.000 |
| TCTC | 0.116 | 0.141, 0.106 | 2.588 | 0.108 | 0.252 |
| TCTT | 0.037 | 0.033, 0.039 | 0.186 | 0.666 | 0.998 |
|
rs6277_rs2283265_rs4245146_rs4648317 | TGTC | 0.219 | 0.230, 0.214 | 0.296 | 0.586 | 1.000 |
| TGCC | 0.215 | 0.213, 0.216 | 0.012 | 0.911 | 1.000 |
| CGTC | 0.138 | 0.133, 0.140 | 0.086 | 0.769 | 1.000 |
| CTCC | 0.105 | 0.120, 0.100 | 0.935 | 0.333 | 0.927 |
| CGCC | 0.099 | 0.107, 0.096 | 0.263 | 0.608 | 1.000 |
| CGCT | 0.084 | 0.087, 0.082 | 0.073 | 0.787 | 1.000 |
| TGCT | 0.051 | 0.041, 0.055 | 0.792 | 0.374 | 0.951 |
| CTTC | 0.036 | 0.025, 0.040 | 1.405 | 0.236 | 0.785 |
| CTCT | 0.014 | 0.017, 0.013 | 0.309 | 0.579 | 1.000 |
| TTTC | 0.012 | 0.004, 0.015 | 1.961 | 0.161 | 0.609 |
|
rs1800497_rs6277_rs2283265 | CTGCC | 0.213 | 0.211, 0.214 | 0.013 | 0.910 | 1.000 |
|
_rs4245146_rs4648317 | CTGTC | 0.211 | 0.218, 0.208 | 0.125 | 0.724 | 1.000 |
| CCGTC | 0.133 | 0.124, 0.136 | 0.263 | 0.608 | 1.000 |
| TCTCC | 0.103 | 0.122, 0.096 | 1.619 | 0.203 | 0.706 |
| CCGCC | 0.101 | 0.107, 0.099 | 0.159 | 0.691 | 1.000 |
| CCGCT | 0.083 | 0.086, 0.081 | 0.073 | 0.788 | 1.000 |
| CTGCT | 0.050 | 0.042, 0.053 | 0.544 | 0.461 | 0.994 |
| TCTTC | 0.034 | 0.030, 0.036 | 0.264 | 0.608 | 1.000 |
| TCTCT | 0.014 | 0.018, 0.013 | 0.537 | 0.464 | 0.994 |
Discussion
Dopamine receptors are members of the G
protein-coupled receptors and contain seven transmembrane domains.
The DRD2 gene is situated on chromosome 11q and encodes the
D2 subtype of the dopamine receptor. Previously, a number of
genetic studies have demonstrated the significance of SNPs located
in the DRD2 gene in various neurological and psychiatric
disorders, including severe alcoholism, schizophrenia, migraine,
post-traumatic stress disorder and addictive disorders (14,15).
Furthermore, Hansen et al (10) showed that DRD2 gene rs6275
was a genetic risk factor for hyperprolactinemia.
Recently, Bilibio et al (11) demonstrated an association between
DRD2 rs6277 and endometriosis in infertile women from the
Brazilian population (11). The
authors also suggested that this polymorphism may lead to a defect
in post-receptor signaling, causing a mild upregulation of
prolactin serum levels. Thus, prolactin may promote angiogenesis of
ectopic endometrial implants (11). However, in the present study, no
association between DRD2 rs1800497, rs6277, rs2283265,
rs4245146 and rs4648317 SNPs and endometriosis-related infertility
was observed. The differences in the effect of DRD2
polymorphisms on the development of endometriosis-related
infertility in the current study may be due to racial
heterogeneity, the small study population, or distinct
environmental factors.
To date, the genetic variants of DRD2 have
been shown to be involved in the pharmacokinetics and
pharmacodynamics of antipsychotic drugs, which may produce varying
effects on prolactin secretion (9,16–23).
The DRD2/ankyrin repeat and kinase domain containing 1
(ANKK1) Taq1A polymorphism (rs1800497) is situated in the
ANKK1 gene, which is downstream from DRD2 and creates
two allelic variants, A1 and A2 (17,18).
The DRD2/ANKK1 rs1800497 A1 allele, is linked to a
reduced density of DRD2 in the striatum (17,18).
Patients with the A1 allele, who were currently receiving
antipsychotics, displayed hyperprolactinemia, compared with
individuals without this allele (9,19,20).
Aklillu et al (21)
observed that carriers of the A1/A1 genotype exhibited an increase
in prolactin level at 2 h following treatment with an
antipsy-chotic drug. The DRD2 Taq1A SNP also produced an
effect on prolactin levels, when induced by atypical antipsychotic
drugs in healthy volunteers (22).
In addition to these findings, clinical trials have also shown that
the DRD2 rs2734842, rs1076562, rs6275 and rs6279 SNPs are
associated with hyper-prolactinemia during antipsychotic treatment
(23,24).
Despite the association of DRD2 SNPs with
hyper-prolactinemia and infertility, the present study failed to
demonstrate an association between the selected SNPs and
endometriosis-related infertility. In conclusion, the current study
requires replication in a larger study population, with varying
ethnicity and environmental exposures, for example to different
pollutants and toxins, in order to confirm or refute the
association between these SNPs and endometriosis-related
infertility.
Acknowledgments
The present study was supported by Poznań University
of Medical Sciences (grant no. 502–01–01124182–07474).
References
|
1
|
Gregoriou G, Bakas P, Vitoratos N,
Papadias K, Goumas K, Chryssicopoulos A and Creatsas G: Evaluation
serum prolactin levels in patients with endometriosis and
infertility. Gynecol Obstet Invest. 48:48–51. 1999. View Article : Google Scholar
|
|
2
|
Panidis D, Vavilis D, Rousso D, Panidou E
and Kalogeropoulos A: Provocative tests of prolactin before, during
and after long-term danazol treatment in patients with
endometriosis. Gynecol Endocrinol. 6:19–24. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang H, Gorpudolo N and Behr B: The role
of prolactin- and endometriosis-associated infertility. Obstet
Gynecol Surv. 64:542–547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reese J, Binart N, Brown N, Ma WG, Paria
BC, Das SK, Kelly PA and Dey SK: Implantation and decidualization
defects in prolactin receptor (PRLR)-deficient mice are mediated by
ovarian but not uterine PRLR. Endocrinology. 141:1872–1881.
2000.PubMed/NCBI
|
|
5
|
Novella-Maestre E, Carda C, Noguera I,
Ruiz-Saurí A, García-Velasco JA, Simón C and Pellicer A: Dopamine
agonist administration causes a reduction in endometrial implants
through modulation of angiogenesis in experimentally induced
endometriosis. Hum Reprod. 24:1025–1035. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ben-Jonathan N, LaPensee CR and LaPensee
EW: What can we learn from rodents about prolactin in humans?
Endocr Rev. 29:1–41. 2008. View Article : Google Scholar
|
|
7
|
Missale C, Nash SR, Robinson SW, Jaber M
and Caron MG: Dopamine receptors: From structure to function.
Physiol Rev. 78:189–225. 1998.PubMed/NCBI
|
|
8
|
Nilsson C and Eriksson E: Partial dopamine
D2 receptor agonists antagonize prolactin-regulating D2 receptors
in a transfected clonal cell line (GH4ZR7). Eur J Pharmacol.
218:205–211. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calarge CA, Ellingrod VL, Acion L, Miller
DD, Moline J, Tansey MJ and Schlechte JA: Variants of the dopamine
D2 receptor gene and risperidone-induced hyperprolactinemia in
children and adolescents. Pharmacogenet Genomics. 19:373–382. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hansen KA, Zhang Y, Colver R, Tho SP,
Plouffe L Jr and McDonough PG: The dopamine receptor D2 genotype is
associated with hyperprolactinemia. Fertil Steril. 84:711–718.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bilibio JP, Matte U, de Conto E, Genro VK,
Souza CA and Cunha-Filho JS: Dopamine receptor D2 genotype (3438)
is associated with moderate/severe endometriosis in infertile women
in Brazil. Fertil Steril. 99:1340–1345. 2013. View Article : Google Scholar
|
|
12
|
Canis M, Donnez JG, Guzick DS, Halme JK,
Rock JA, Schenken RS and Vernon MW: Revised American Society for
Reproductive Medicine classification of endometriosis: 1996. Fertil
Steril. 67:817–821. 1997. View Article : Google Scholar
|
|
13
|
Sasieni PD: From genotypes to genes:
Doubling the sample size. Biometrics. 53:1253–1261. 1997.
View Article : Google Scholar
|
|
14
|
Comings DE and Blum K: Reward deficiency
syndrome: Genetic aspects of behavioral disorders. Prog Brain Res.
126:325–341. 2000.PubMed/NCBI
|
|
15
|
Noble EP: D2 dopamine receptor gene in
psychiatric and neurologic disorders and its phenotypes. Am J Med
Genet B Neuropsychiatr Genet. 116B:103–125. 2003. View Article : Google Scholar
|
|
16
|
Caccavelli L, Cussac D, Pellegrini I,
Audinot V, Jaquet P and Enjalbert A: D2 dopaminergic receptors:
Normal and abnormal transduction mechanisms. Horm Res. 38:78–83.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thompson J, Thomas N, Singleton A, Piggott
M, Lloyd S, Perry EK, Morris CM, Perry RH, Ferrier IN and Court JA:
D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: Reduced
dopamine D2 receptor binding in the human striatum associated with
the A1 allele. Pharmacogenetics. 7:479–484. 1997. View Article : Google Scholar
|
|
18
|
Noble EP, Blum K, Ritchie T, Montgomery A
and Sheridan PJ: Allelic association of the D2 dopamine receptor
gene with receptor-binding characteristics in alcoholism. Arch Gen
Psychiatry. 48:648–654. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Young RM, Lawford BR, Barnes M, Burton SC,
Ritchie T, Ward WK and Noble EP: Prolactin levels in antipsychotic
treatment of patients with schizophrenia carrying the DRD2*A1
allele. Br J Psychiatry. 185:147–151. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mihara K, Suzuki A, Kondo T,
Yasui-Furukori N, Ono S, Otani K, Kaneko S and Inoue Y:
Relationship between Taq1 A dopamine D2 receptor (DRD2)
polymorphism and prolactin response to bromperidol. Am J Med Genet.
105:271–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aklillu E, Kalow W, Endrenyi L, Harper P,
Miura J and Ozdemir V: CYP2D6 and DRD2 genes differentially impact
pharmacodynamic sensitivity and time course of prolactin response
to perphenazine. Pharmacogenet Genomics. 17:989–993. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
López-Rodríguez R, Román M, Novalbos J,
Pelegrina ML, Ochoa D and Abad-Santos F: DRD2 Taq1A polymorphism
modulates prolactin secretion induced by atypical antipsy-chotics
in healthy volunteers. J Clin Psychopharmacol. 31:555–562. 2011.
View Article : Google Scholar
|
|
23
|
Houston JP, Fijal B, Heinloth AN and Adams
DH: Genetic associations of prolactin increase in
olanzapine/fluoxetine combination-treated patients. Psychiatry Res.
175:171–172. 2010. View Article : Google Scholar
|
|
24
|
Houston J, Dharia S, Bishop JR, Ellingrod
VL, Fijal B, Jacobson JG and Hoffmann VP: Association of DRD2 and
ANKK1 polymorphisms with prolactin increase in olanzapine-treated
women. Psychiatry Res. 187:74–79. 2011. View Article : Google Scholar
|