Introduction
Breast cancer is a common malignant tumor in
females, accounting for a high proportion of cancer-associated
mortality (1). However, the
molecular mechanisms underlying breast cancer remain to be
elucidated. The identification of potential molecular targets for
the treatment of breast cancer appears promising (2).
MicroRNAs (miRNAs) are a class of short (~20 nt)
non-coding RNAs, which bind to the 3′-untranslated region (UTR) of
their target mRNAs, and exert a suppressive effect on protein
translation or induce degradation of the mRNA (3). By negatively regulating the protein
expression of their targets, miRNAs influence various biological
processes, including cell survival, proliferation, differentiation
and motility (4–6). The deregulation of miRNAs has been
demonstrated to be associated with the development and progression
of multiple types of human malignancy, including breast cancer,
indicating that these molecules act as tumor suppressors or
oncogenes (7,8). Among the miRNAs investigated, miR-29a
has been shown to act as a tumor suppressor in several types of
human cancer, including prostate cancer, colorectal cancer, oral
squamous carcinoma and breast cancer (9–12).
Wu et al reported that miR-29a exerted an inhibitory effect
on breast cancer cell growth (9).
However, the precise function of miR-29a in the regulation of cell
migration and invasion in breast cancer cells, and the mechanisms
underlying its effects on these processes remain to be
elucidated.
The secreted Slit glycoproteins and their Roundabout
(Robo) receptors were originally identified as important axon
guidance molecules (13,14). Robo1 is a member of Robo family,
which is expressed in multiple cell types and is important in the
regulation of various biological processes, including cell
proliferation, differentiation and migration (15–17).
A previous study demonstrated the involvement of Robo1 in cancer
cell migration and invasion (18).
In addition, a bioinformatic analysis suggested that Robo1 is a
putative target of miR-29a (19).
However, the underlying mechanisms by which Robo1 mediates breast
cancer remain to be elucidated.
The present study primarily aimed to investigate the
effects of miR-29a and Robo1 on the regulation of cell migration
and invasion in breast cancer cells. The mechanisms underlying
these effects were also examined.
Materials and methods
Tissue specimen collection
The present study was approved by the Ethical
Committee of Jishou University (Jishou, China). Breast cancer
tissues and matched adjacent normal tissues (n=18) were obtained
from the Department of General Surgery, People’s Hospital of
Xiangxi Autonomous Prefecture, Jishou University (Jishou, China).
Written informed consent was obtained from patients with breast
cancer. The tissue samples were frozen in liquid nitrogen following
surgery, until use.
Cell culture
T47D, MCF-7, BCAP-37 and MDA-MB-435S human breast
cancer cell lines, and the MCF-10A normal breast epithelial cell
line, were purchased from ScienCell (San Diego, CA, USA). All cells
were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Life
Technologies, Carlsbad, CA, USA), supplemented with 10% fetal
bovine serum (FBS; Life Technologies) at 37°C with 5%
CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA), according to the
manufacturer’s instructions. For miRNA detection, total RNA was
reverse transcribed using the miScript Reverse Transcription kit
(Qiagen, Valencia, CA, USA), according to the manufacturer’s
instructions. RT-qPCR of the miRNA was then performed using the
miScript SYBR Green PCR kit (Qiagen) on an ABI7500 PCR machine
(Invitrogen Life Technologies). RT-qPCR was conducted at 95°C for
15 min, followed by 40 cycles of 94°C for 15 sec, 55°C for 30 sec
and 70°C for 30 sec. The relative expression of miRNA was
normalized against that of U6. For mRNA detection, the total RNA
was reverse transcribed into cDNA using the RevertAid First-Strand
cDNA Synthesis kit (Fermentas, Carlsbad, CA, USA), according to the
manufacturer’s instructions. RT-qPCR was subsequently performed
using iQTM SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) by
denaturation at 95°C for 15 min, followed by 40 cycles of 94°C for
15 sec, 55°C for 30 sec and 70°C for 30 sec. The specific primers
used were as follows: Forward: 5′-GGCGGTGAAGGAGATGAAC-3′ and
reverse: 5′-TGATGAGGAAATCCACGATAGAG-3′ for Robo1 and forward:
5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse: 5′-GCCATCACGCCACAGTTTC-3′
for GAPDH. The relative mRNA expression of Robo1 was normalized
against that of GAPDH.
Western blot assay
Cells were lysed in radioimmunoprecipitation buffer
(Sigma-Aldrich, St. Louis, MO, USA). The total protein was isolated
and the concentration was determined using the bicinchoninic acid
protein assay kit (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA). For western blot analysis, equal quantities of protein were
boiled and separated using 10% SDS-PAGE gels (Sigma-Aldrich), and
blotted onto polyvinylidene difluoride (PVDF) membrane (Life
Technologies), which was blocked with 5% non-fat dried milk in
phosphate-buffered saline (PBS) for 1.5 h at room temperature. The
PVDF membrane was subsequently incubated with rabbit polyclonal
anti-Robo1 (1:50; cat. no. ab7279) or rabbit polyclonal anti-GAPDH
(1:50; cat. no. ab181602) antibodies (Abcam, Cambridge, MA, USA) at
room temperature for 3 h. The membranes were washed with PBS, and
were then incubated with goat anti-rabbit immunoglobulin G H&L
secondary antibody (1:5,000; cat. no. ab175773; Abcam) for 40 min
at room temperature. Chemiluminescent detection was performed using
an enhanced chemiluminescence kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA).
Transfection
Transfections were performed using lipofectamine
2000 (Invitrogen Life Technologies), according to the
manufacturer’s instructions. For miR-29a functional analysis, the
cells were transfected with scrambled miRNA as a negative control
(NC), miR-29a mimics or an miR-29a inhibitor (Invitrogen Life
Technologies). For Robo1 functional analysis, the cells were
transfected with Robo1-specific small interfering RNA (siRNA) or
Robo1 plasmid (Nlunbio, Changsha, China).
Luciferase reporter assay
A luciferase reporter assay was performed in MCF-7
human breast cancer cells. The wild-type (WT) 3′-UTR of Robo1 mRNA
or the mutant type (MUT) 3′-UTR of Robo1 mRNA were inserted
downstream of the luciferase reporter gene in the pMIR-REPORT
vector (Life Technologies). Subsequently, cells were cotransfected
with miR-29a mimics or scrambled miRNA; pMIR-REPORT vectors
containing the WT or MUT Robo1 3′-UTR; and pRL-SV40 (Promega
Corporation, Sunnyvale, CA, USA), expressing Renilla
luciferase. The cells were cultured for 48 h following transfection
and the luciferase activities were measured using the
Dual-Luciferase Reporter assay system (Promega Corporation).
Cell migration and invasion assay
Cell migration and invasion assays were performed
using transwell chambers (BD Bioscience, Franklin Lakes, NJ, USA).
Cell suspension, containing 5×105 cells/ml, was prepared
in serum-free media. For cell migration assays, 300 μl cell
suspension was added into the upper chamber of the transwells. For
cell invasion assays, 300 μl cells were added into the upper
chamber of transwells that were pre-coated with matrigel (BD
Bioscience). DMEM (500 μl), containing 10% FBS as a
chemo-attractant, was added into the lower chamber of the
transwell. The cells were incubated for 24 h and cells, which
failed to migrate or invade through the pores were carefully
removed using a cotton-tipped swab. The filters were fixed in 90%
alcohol and stained using crystal violet (Sigma-Aldrich). Cell
numbers were determined in five randomly-selected fields under an
inverted microscope (TS100; Nikon Corporatino, Tokyo, Japan).
Statistical analysis
The data are expressed as the mean ± standard
deviation of three independent experiments. SPSS 17.0 statistical
software (SPSS, Inc., Chicago, IL, USA) was used to analyze the
differences between the groups using one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
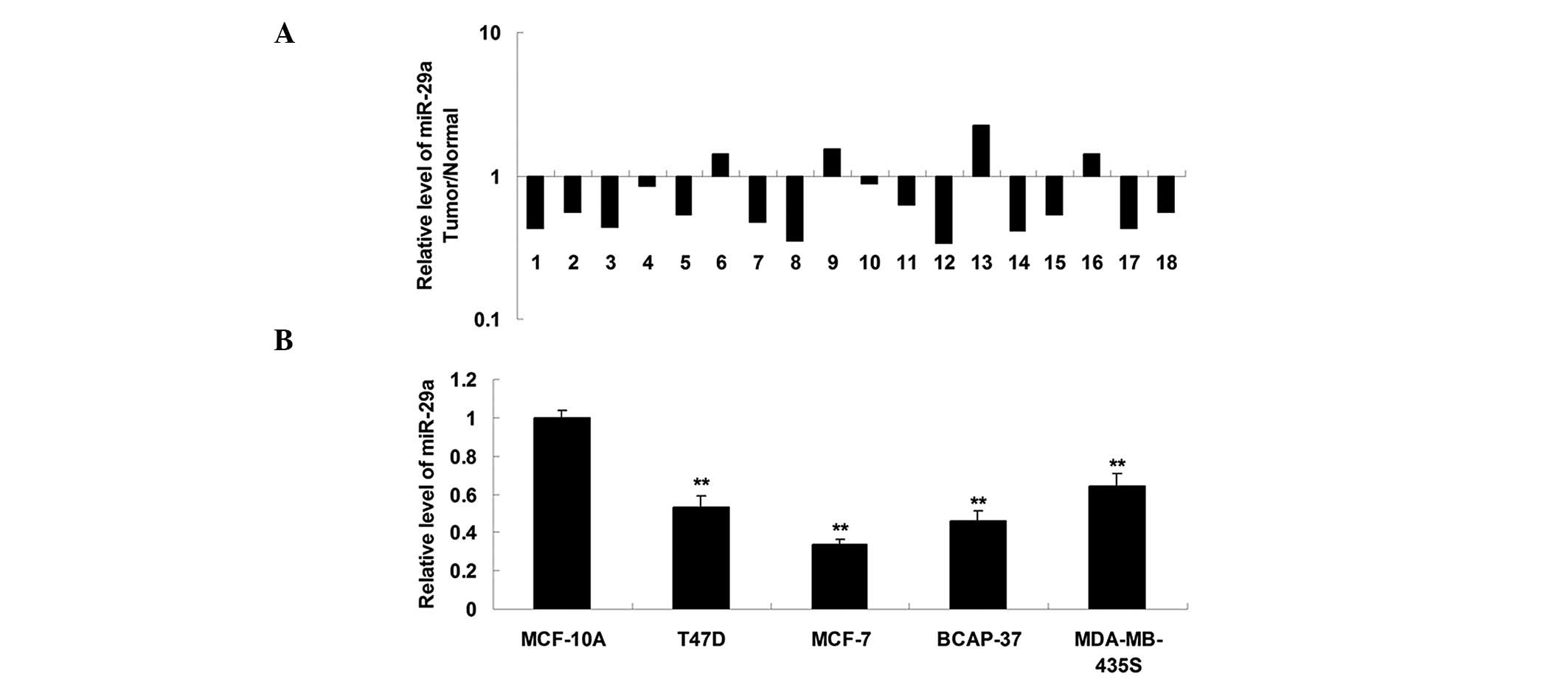
miR-29a is downregulated in breast cancer
tissues and cells
The expression of miR-29a was determined using
RT-qPCR in breast cancer tissues and matched normal adjacent
tissues. As shown in Fig. 1A, the
expression of miR-29a in breast cancer tissues was markedly reduced
compared with that in the normal adjacent tissues. The expression
of miR-29a was determined in four breast cancer cell lines,
including T47D, MCF-7, BCAP-37 and MDA-MB-435S, and in one normal
breast epithelial cell line, MCF-10A. As shown in Fig. 1B, miR-29a was also downregulated in
breast cancer cell lines compared with the MCF-10A cell line. These
findings suggested that miR-29a is involved in breast cancer
progression. MCF-7 cells exhibited the greatest downregulation in
the expression of miR-29a (Fig.
1B) and were therefore used for subsequent analysis.
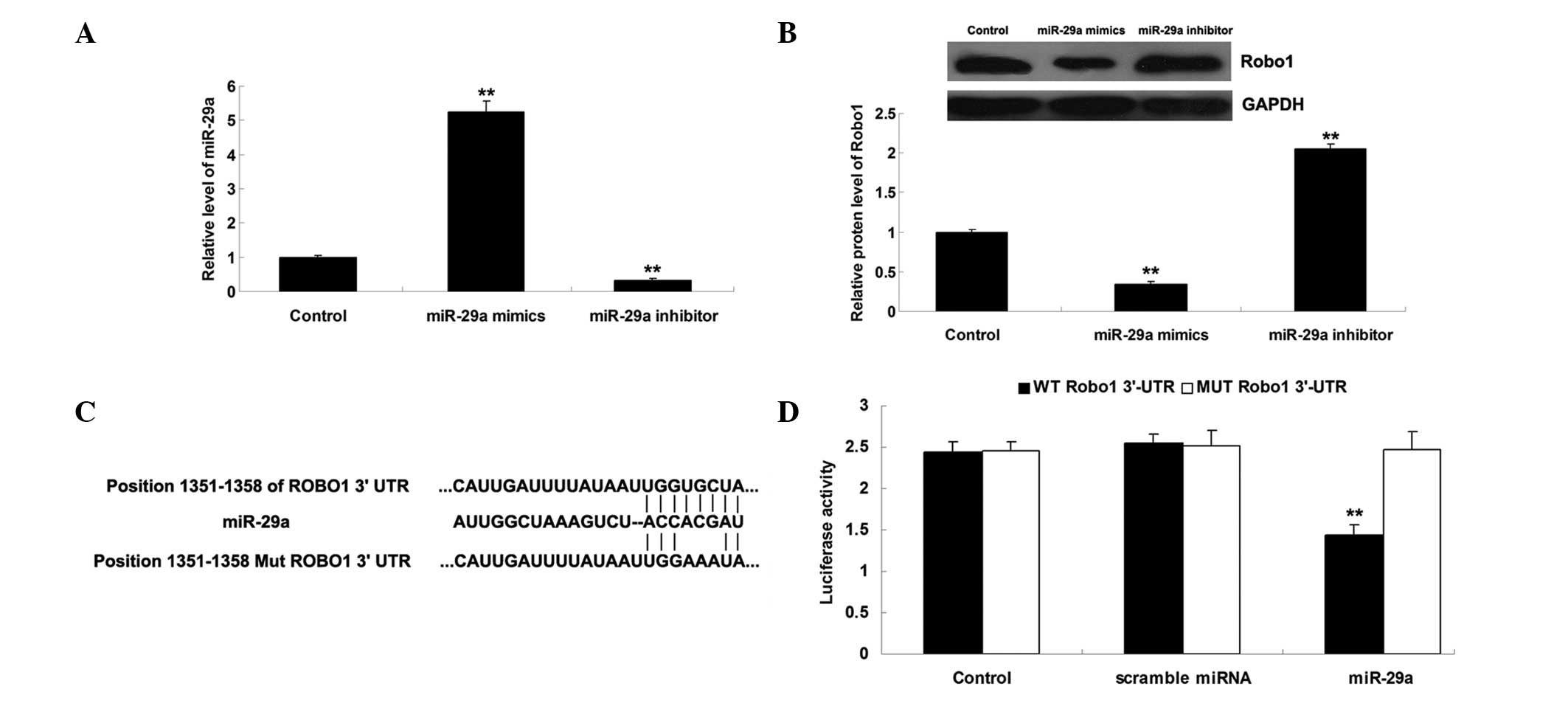
miR-29a negatively regulates the protein
expression of its target, Robo1, in MCF-7 cells
The effect of miR-29a on the regulation of the
expression of Robo1 was assessed in MCF-7 breast cancer cells.
Following transfection with miR-29a mimics and an miR-29a
inhibitor, the expression of miR-29a in MCF-7 cells was measured.
As shown in Fig. 2A, transfection
with miR-29a mimics caused an increase in the expression of
miR-29a, while transfection with an miR-29a inhibitor led to
downregulation of miR-29a, indicating that the transfection was
successful. Subsequently, western blot analysis was performed to
determine the protein expression levels of Robo1 in each group. As
shown in Fig. 2B, upregulation of
miR-29a led to a reduction in the expression of the Robo1 protein,
whereas downregulation of miR-29a resulted in an increase in the
expression of the Robo1 protein, indicating that the expression of
Robo1 is negatively regulated by miR-29a in MCF-7 breast cancer
cells. A luciferase reporter assay was conducted in order to
confirm whether miR-29a directly binds to the 3′ UTR of Robo1 mRNA.
As shown in Fig. 2C, a wild-type
(WT-Robo1) and a mutant (MUT-Robo1) type of the Robo1 3′ UTR was
generated. MCF-7 cells were cotransfected with the WT-Robo1 vector
or the MUT-Robo1 vector, and the miR-29a mimics or a scrambled RNA.
As demonstrated in Fig. 2D, the
luciferase activity was significantly reduced compared with the
control group, only in MCF-7 cells cotransfected with the WT-Robo1
vector and the miR-29a mimics. This repressive effect was abrogated
in MCF-7 cells cotransfected with the MUT-Robo1 vector or the
miR-29a mimics. These results suggested that miR-29a inhibits
expression of the Robo1 protein via an interaction with the 3′-UTR
of Robo1.
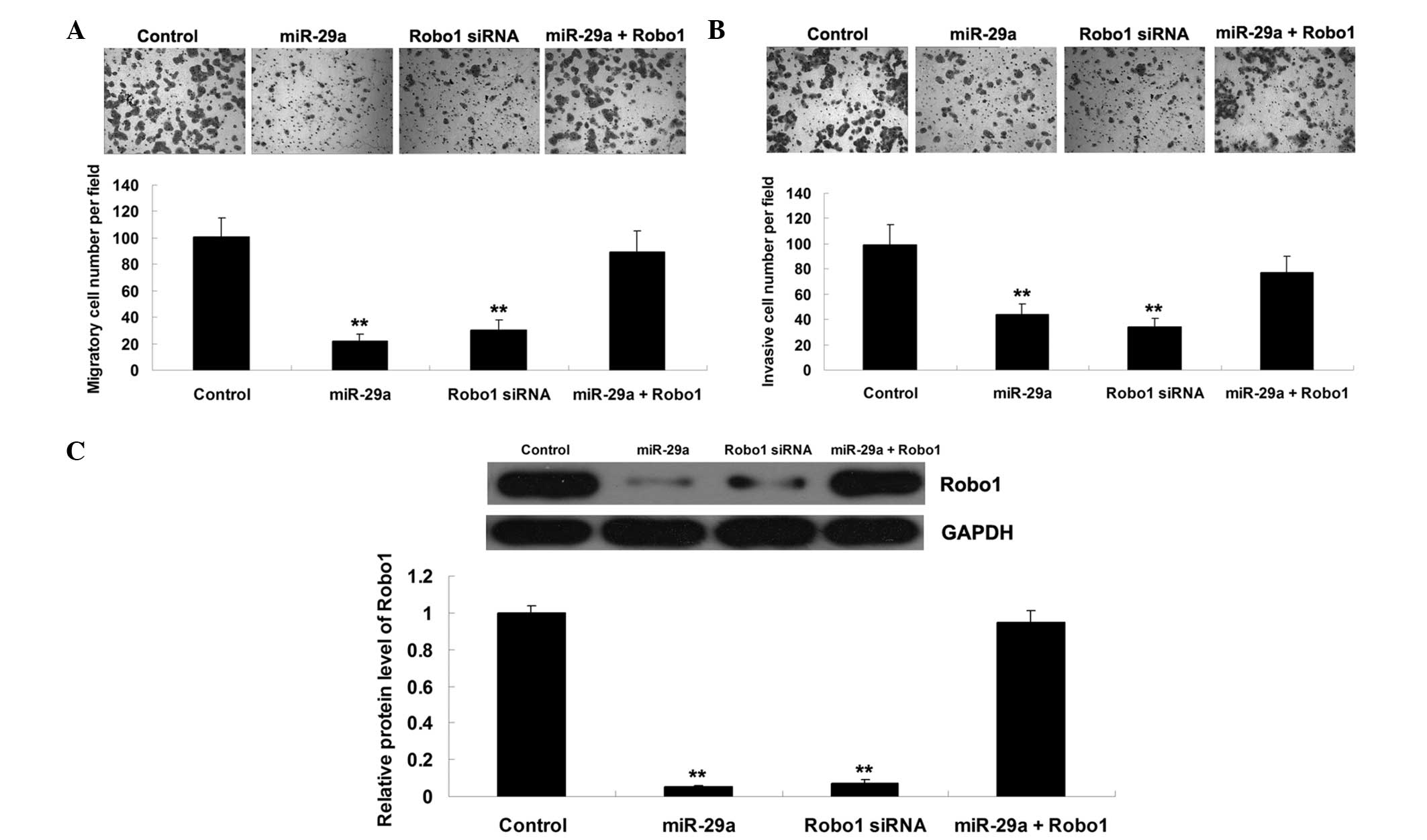
miR-29a suppresses cell migration and
invasion by targeting Robo1 in MCF-7 cells
The functions of miR-29a and Robo1, and their
association in the regulation of cell migration and invasion were
further assessed in MCF-7 breast cancer cells. MCF-7 cells were
transfected with miR-29a mimics or Robo1-specific siRNA, or
cotransfected with miR-29a mimics and Robo1 plasmid. As shown in
Fig. 3A and B, upregulation of
miR-29a or downregulation of Robo1 significantly suppressed MCF-7
cell migration and invasion. However, the suppressive effects of
miR-29a upregulation on MCF-7 cell migration and invasion were
abrogated by overexpression of Robo1. Furthermore, western blotting
was performed to determine the protein expression level of Robo1 in
each group. As shown in Fig. 3C,
the protein expression level of Robo1 was associated with the
migratory and invasive capacities of MCF-7 cells in each group.
These data suggested that miR-29a exerts a suppressive role in the
regulation of cell migration and invasion, at least in part,
through the direct inhibition of Robo1 expression in breast cancer
cells.
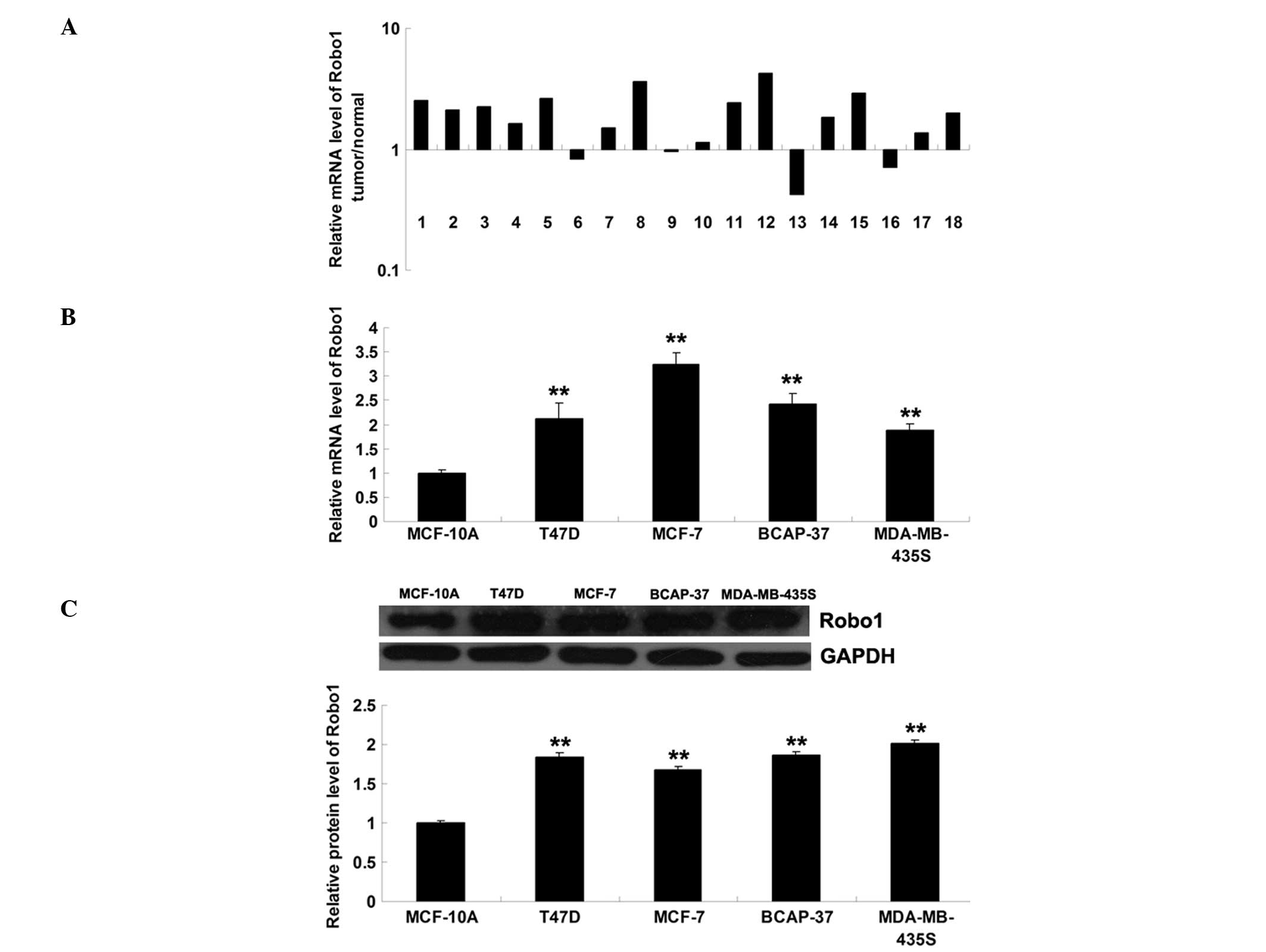
Expression of Robo1 is increased in
breast cancer tissues and cells
The level of Robo1 mRNA was subsequently determined
in breast cancer tissues and matched normal adjacent tissues, using
RT-qPCR. As shown in Fig. 4A, the
mRNA level of Robo1 was significantly increased in breast cancer
tissues compared with the normal adjacent tissues. In addition, the
mRNA and protein expression levels of Robo1 were assessed in the
T47D, MCF-7, BCAP-37 and MDA-MB-435S breast cancer cell lines, and
in MCF-10A normal breast epithelial cells. As shown in Fig. 4B and C, Robo1 was upregulated in
these four breast cancer cell lines compared with MCF-10A
cells.
Discussion
Deregulation of miRNAs has been demonstrated to be
involved in the progression of breast cancer (20). In the present study, the expression
level of miR-29a was shown to be significantly reduced in breast
cancer tissues and cell lines. Additionally, Robo1 was identified
as a novel target of miR-29a and its expression level was markedly
upregulated in breast cancer tissues and cell lines. It was shown
that the protein expression of Robo1 was negatively regulated by
miR-29a and that miR-29a suppressed cell migration and invasion, at
least in part, by directly targeting Robo1 in MCF-7 breast cancer
cells.
Deregulation of miR-29a has been shown to contribute
to multiple types of human malignancy. Yu et al (21) reported that downregulation of
miR-29a contributes to cisplatin resistance in ovarian cancer
cells. Zhao et al (22)
demonstrated that miR-29a inhibited glioma tumor growth and
invasion by targeting heat shock protein 47. The present study
demonstrated that miR-29a was significantly downregulated in breast
cancer tissues compared with normal adjacent tissues. In addition,
the expression level of miR-29a was also reduced in breast cancer
cell lines compared with normal MCF-10A breast epithelial cells.
miR-29a has been previously reported to be upregulated in serum,
but downregulated in breast milk, in patients with breast cancer
(23,24). Furthermore, Wu et al
(9) suggested an inhibitory role
of miR-29a in breast cancer cells. The authors demonstrated that
the overexpression of miR-29a significantly suppressed breast
cancer cell proliferation and led to a higher percentage of cells
in G0/G1 phase. In addition, the novel target, B-Myb, an important
transcription factor associated with tumorigenesis, was identified
(9). The present study revealed
that miR-29a exerts an inhibitory function in the regulation of
cell migration and invasion of MCF-7 breast cancer cells. Based on
previous studies and the results of the present study, it is
hypothesized that miR-29a acts as a key tumor suppressor in breast
cancer.
Since a single miRNA may have different targets in
different cancer cells, the present study aimed to identify a novel
target that is involved in the progression of breast cancer. Robo1
was identified as a target of miR-29a in MCF-7 breast cancer cells.
Robo1 was shown to be involved in tumorigenesis and the expression
of Robo1 is known to be increased in several types of cancer
(25). Alajez et al
(26) demonstrated that Robo1 was
upregulated in nasopharyngeal carcinoma, and its overexpression was
significantly associated with a reduced level of overall and nodal
relapse-free survival. In addition, Robo1 was also shown to be
regulated by Src and Abl, and to promote tumor cell migration
(27). Previously, Robo1 was shown
to be involved in the regulation of cell migration and invasion in
breast cancer cells (28). Yang
et al (28) demonstrated
that as one of the receptors for Slit, Robo1 is involved in
miR-218-mediated inhibition of migration and invasion in breast
cancer cells. The present study demonstrated a similar molecular
mechanism, in which miR-29a inhibited cell migration and invasion
by directly inhibiting the protein expression of Robo1 in breast
cancer cells. Therefore, Robo1 is a common target for miR-29a and
miR-218, each of which exert similar inhibitory effect on breast
cancer cell migration and invasion. In addition, the oncogenic role
of Robo1 in the regulation of cancer cell migration and invasion
has been reported in other types of cancer. For instance, Tie et
al (29) reported that Robo1
was negatively regulated by miR-218, and that this promoted the
invasion and metastasis of gastric cancer (29).
In conclusion, the present study identified Robo1 as
a direct target of miR-29a, and suggested that miR-29a exerts an
inhibitory function in the regulation of cell migration and
invasion, at least in part by suppressing the protein expression
levels of Robo1 in breast cancer cells. Therefore, the results of
the present study suggest that miR-29a and Robo1 may serve as
potential targets for the treatment of metastatic breast
cancer.
References
|
1
|
Murawa P, Murawa D, Adamczyk B and Polom
K: Breast cancer: Actual methods of treatment and future trends.
Rep Pract Oncol Radiother. 19:165–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Serpico D, Molino L and Di Cosimo S:
microRNAs in breast cancer development and treatment. Cancer Treat
Rev. 40:595–604. 2014. View Article : Google Scholar
|
|
3
|
Brower J, Clark PA, Lyon W and Kuo JS:
MicroRNAs in cancer: Glioblastoma and glioblastoma cancer stem
cells. Neurochem Int. 77:68–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ishiguro H, Kimura M and Takeyama H: Role
of microRNAs in gastric cancer. World J Gastroenterol.
20:5694–5699. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bouyssou JM, Manier S, Huynh D, Issa S,
Roccaro AM and Ghobrial IM: Regulation of microRNAs in cancer
metastasis. Biochim Biophys Acta. 1845:255–265. 2014.PubMed/NCBI
|
|
6
|
Kotaja N: MicroRNAs and spermatogenesis.
Fertil Steril. 101:1552–1562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shah NR and Chen H: MicroRNAs in
pathogenesis of breast cancer: Implications in diagnosis and
treatment. World J Clin Oncol. 5:48–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu J, Zheng Z, Wang J, et al: Different
miRNA expression profiles between human breast cancer tumors and
serum. Front Genet. 5:1492014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu Z, Huang X, Zou Q and Guo Y: The
inhibitory role of Mir-29 in growth of breast cancer cells. J Exp
Clin Cancer Res. 32:982013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishikawa R, Goto Y, Kojima S, et al:
Tumor-suppressive microRNA-29 s inhibit cancer cell migration and
invasion via targeting LAMC1 in prostate cancer. Int J Oncol.
45:401–410. 2014.PubMed/NCBI
|
|
11
|
Yang Y, Gu X, Zhou M, Xiang J and Chen Z:
Serum microRNAs: A new diagnostic method for colorectal cancer.
Biomed Rep. 1:495–498. 2013.
|
|
12
|
Lu L, Xue X, Lan J, et al: MicroRNA-29a
upregulates MMP2 in oral squamous cell carcinoma to promote cancer
invasion and anti-apoptosis. Biomed Pharmacother. 68:13–19. 2014.
View Article : Google Scholar
|
|
13
|
Chaturvedi S and Robinson LA: Slit2-Robo
signaling in inflammation and kidney injury. Pediatr Nephrol.
30:561–566. 2015. View Article : Google Scholar
|
|
14
|
Yang YH, Manning Fox JE, Zhang KL,
MacDonald PE and Johnson JD: Intraislet SLIT-ROBO signaling is
required for beta-cell survival and potentiates insulin secretion.
Proc Natl Acad Sci USA. 110:16480–16485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuen DA and Robinson LA: Slit2-Robo
signaling: a novel regulator of vascular injury. Curr Opin Nephrol
Hypertens. 22:445–451. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cornide-Petronio ME and Barreiro-Iglesias
A: Role of Slit and Robo proteins in the development of
dopaminergic neurons. Dev Neurosci. 35:285–292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dickinson RE and Duncan WC: The SLIT-ROBO
pathway: a regulator of cell function with implications for the
reproductive system. Reproduction. 139:697–704. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ballard MS and Hinck L: A roundabout way
to cancer. Adv Cancer Res. 114:187–235. 2012.PubMed/NCBI
|
|
19
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhong L, Zhu K, Jin N, et al: A systematic
analysis of miRNA-mRNA paired variations reveals widespread miRNA
misregulation in breast cancer. Biomed Res Int. 2014:2912802014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu PN, Yan MD, Lai HC, et al:
Downregulation of miR-29 contributes to cisplatin resistance of
ovarian cancer cells. Int J Cancer. 134:542–551. 2014. View Article : Google Scholar
|
|
22
|
Zhao D, Jiang X, Yao C, et al: Heat shock
protein 47 regulated by miR-29a to enhance glioma tumor growth and
invasion. J Neurooncol. 118:39–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu Q, Wang C, Lu Z, Guo L and Ge Q:
Analysis of serum genome-wide microRNAs for breast cancer
detection. Clin Chim Acta. 413:1058–1065. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gu YQ, Gong G, Xu ZL, et al: miRNA
profiling reveals a potential role of milk stasis in breast
carcinogenesis. Int J Mol Med. 33:1243–1249. 2014.PubMed/NCBI
|
|
25
|
Dontula R, Dinasarapu A, Chetty C, et al:
MicroRNA 203 modulates glioma cell migration via Robo1/ERK/MMP-9
signaling. Genes Cancer. 4:285–296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alajez NM, Lenarduzzi M, Ito E, et al:
MiR-218 suppresses nasopharyngeal cancer progression through
downregulation of survivin and the SLIT2-ROBO1 pathway. Cancer Res.
71:2381–2391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Khusial PR, Vadla B, Krishnan H, et al:
Src activates Abl to augment Robo1 expression in order to promote
tumor cell migration. Oncotarget. 1:198–209. 2010.
|
|
28
|
Yang L, Li Q, Wang Q, Jiang Z and Zhang L:
Silencing of miRNA-218 promotes migration and invasion of breast
cancer via Slit2-Robo1 pathway. Biomed Pharmacother. 66:535–540.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tie J, Pan Y, Zhao L, et al: MiR-218
inhibits invasion and metastasis of gastric cancer by targeting the
Robo1 receptor. PLoS Genet. 6:e10008792010. View Article : Google Scholar : PubMed/NCBI
|