Introduction
In China, bladder cancer has the highest rate of
incidence out of all malignancies of the urinary system (1). In addition to surgical treatment,
chemotherapy is an important strategy for the therapy of bladder
cancer. Cisplatin-based chemotherapy is widely used in bladder
cancer treatment, and the anti-cancer effect was demonstrated
(2). However, certain patients
exhibit a poor sensitivity to cisplatin, and this resistance to
cisplatin is a problem that should not be overlooked. The low
sensitivity to cisplatin and drug resistance affect the therapeutic
efficacy of bladder cancer treatment (3,4).
Therefore, it is necessary to investigate the mechanism of
resistance of bladder cancer to cisplatin and aim to improve the
sensitivity of bladder cancer cells to this drug.
Vascular endothelial growth factor C (VEGF-C) is a
dimeric glycoprotein of the VEGF family of cytokines. VEGF-C has
been demonstrated to be involved with the majority of aggressive
tumors (5). A number of previous
studies have reported that high levels of VEGF-C promote tumor
invasion and metastasis by binding to its receptor (6,7).
Clinical studies have verified that VEGF-C expression is closely
associated with the invasive phenotype and affects patient survival
in cervical cancer, in addition to accelerating cervical cancer
metastasis by directly driving cancer cell migration and invasion
(8). In brief, high levels of
VEGF-C correlate with poor prognosis for the patient (8). However, few studies investigating
whether high expression levels of VEGF-C are implicated in
chemoresistance have been conducted. One previous study suggested
that the overexpression of VEGF-C induced chemoresistance in acute
myeloid leukemic cells via a cyclooxygenase-2-mediated mechanism.
Cho et al (9) demonstrated
that RhoGDI2-induced VEGF-C expression results in gastric cancer
cell metastasis and cisplatin resistance. Therefore, based on the
previous studies, it was hypothesized that high expression levels
of VEGF-C result in chemoresistance to cisplatin in bladder cancer
cells. Furthermore, it was hypothesized that maspin may mediate the
effects of VEGF-C in regulating chemoresistance. As an inhibitor of
serine protease, accumulating evidence indicates that maspin is
able to inhibit the growth of tumors by inducing apoptosis
(10). In certain types of tumor,
low expression of maspin may induce growth of tumours (11). Induction of apoptosis is a crucial
function of chemotherapeutic drugs, therefore, it is important to
analyze the association between maspin and the effectiveness of
chemotherapy. In a previous study, the elevated expression level of
maspin was observed to be typical for cisplatin-sensitive ovarian
cancer tumors (12). Thus, it was
considered that maspin is associated with the sensitivity of
bladder cancer cells to cisplatin. The current study utilized small
interfering (si)RNA technology to inhibit VEGF-C expression in
BIU87-CisR cells, then observed the alterations in sensitivity to
cisplatin of BIU87-CisR cells, and the alterations in maspin levels
following VEGF-C inhibition.
Materials and methods
Patients and preparation for tissue
specimens
The current study included 32 patients with bladder
cancer (18 males and 14 females; median age, 65.9; range 51–76) who
underwent surgical treatment and cisplatin-based combination
chemotherapy between March 2012 and February 2013 at the Fifth
Affiliated Hospital of Zhengzhou University (Zhengzhou, China). A
total of 20 patients were sensitive to chemotherapy, while 12 were
resistant to it, while no significant correlations were observed
with regard to the demographic information about age, gender, stage
of disease and treatment regimen. Tumor tissue specimens were
obtained at the time of surgical esophageal tissue resection and
stored in liquid nitrogen until further analysis. The current study
was approved by the ethics committee of The Fifth Affiliated
Hospital of Zhengzhou University, with all patients’ informed
consents.
Cell line culture and establishment of
cisplatin-resistant subline
The BIU87 cell line was purchased from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). BIU87 cells were maintained in RPMI-1640 medium (Thermo
Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal
bovine serum at 37°C with 5% CO2. The
cisplatin-resistant subline (BIU87-CisR cell line) was established
as previously described and its resistance to cisplatin
(Sigma-Aldrich, St. Louis, MO, USA) was proven (13). In brief, BIU87-CisR cells were
obtained from parental BIU87 cells through a continuous exposure to
increasing cisplatin over 12 months, with a final concentration of
6 μM cisplatin.
RNA isolation and quantification of
VEGF-C mRNA expression
Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) was performed to quantify the mRNA expression of
VEGF-C. Total RNA of tumor tissue specimens, BIU87 cells and
BIU87-CisR cells were extracted using TRIzol reagent (Thermo Fisher
Scientific). The purity and concentration of total RNA was verified
by spectrophotometry (Biomate 3; Thermo Fisher Scientific).
Confirmed RNA was reverse-transcribed to cDNA using
PrimeScript® RT reagent (Takara Biotechnology Co., Ltd.,
Dalian, China) according to manufacturer’s instructions. cDNA was
then amplified with SYBR® Premix Ex Taq™ II kit (Takara
Biotechnology Co., Ltd.) using a 7500 Real Time PCR system (Applied
Biosystems Life Technologies, Foster City, CA, USA). The cycling
conditions were set according to the manufacturer’s instructions.
The sequences of the primers of targeted genes and β-actin used
were as follows: Forward: 5′CAA GCA TGG CCT GTA CAA CCT C′3 and
reverse: 5′GGG TTC ACA CAC CAG CAC TC′3 for VEGF-C; and forward:
5′ATC ATG TTT GAG ACC TTC AA′ and reverse: 5′CAT CTC TTG CTC GAA
GTC CA′3 β-actin. The fold changes of target genes were calculated
using the ΔΔ cycle threshold (2−ΔΔCt) method and the
result was normalized to β-actin.
Western blot analysis for VEGF-C protein
expression
Protein samples of tissue, BIU87 cells and
BIU87-CisR cells were prepared using radioimmunoprecipitation assay
buffer combined with 1% protease inhibitor cocktail (Applygen
Technologies, Inc., Beijing, China). Protein samples (30 μg)
were separated by 12% SDS-PAGE (100 V, 1.5 h) and transferred to
polyvinylidene difluoride membranes (150 mA, 1 h; Applygen
Technologies, Inc.). Membranes were blocked with 5% skimmed milk in
phosphate-buffered saline (PBS) for 1 h and incubated at 4°C for 12
h with the primary antibody mous anti-VEGF-C monoclonal (1:1,000;
cat. no. sc-374628; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) and mouse anti-β-actin monolonal (1:5,000; cat. no. sc-130300;
Santa Cruz Biotechnology, Inc.). Membranes were washed by PBS with
Tween 20 buffer and followed an incubation with horseradish
peroxidase-conjugated secondary antibodies. The bands were detected
using an Enhanced Chemiluminescence Detection Reagent kit (Applygen
Technologies, Inc.) and analyzed by Image J software version 1.48
(NIH, Bethesda, MD, USA).
Transfection of VEGF-C siRNA
Downregulation of VEGF-C expression in BIU87-CisR
cells was induced using siRNA. siRNA targeted to human VEGF-C were
designed and synthesized by Shanghai GenePharma Co., Ltd.
(Shanghai, China) and then transfected into cells using
Lipofectamine® 2000 reagent (Life Technologies, Grand
Island, NY, USA). The concentration of siRNA was 3 nM and
BIU87-CisR cells were cultured 48 h following transfection. The
silencing effect was assessed at the mRNA and protein expression
levels in the preliminary experiment and the effect of siRNA
transefection was efficient.
Overexpression of maspin
The pcDNA-maspin recombinant plasmids were
constructed (Data not shown) and were transfected into BIU87-CISR
cells using Lipofectamine 2000 reagent in order to increase the
expression of maspin.
Cell inhibition analysis
Cell inhibition induced by cisplatin was detected by
cell counting kit 8 (CCK-8) kits (Wuhan Boster Biological
Technology, Ltd., Wuhan, China). Normal BIU87 cells and VEGF-C
silenced BIU87-CisR cells were plated at a density of
1×104 cells/well in a 96-well plate and divided into the
following four groups: Control group, BIU87-CisR cells without any
treatment; 3 μM cisplatin-treated group, BIU87-CisR cells
treated with 3 μM cisplatin; 3 μM cisplatin + siRNA
treated group, VEGF-C silenced BIU87-CisR cells treated with 3
μM cisplatin; and the siRNA group, VEGF-C silenced
BIU87-CisR cells without cisplatin treatment. Following 24 h, 10
μl CCK-8 reagent was added and the cells were incubated at
37°C for 4 h, then the optical density of the culture solution in
each plate was measured using a Synergy Mx Microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA) at 450 nm.
Cell cycle and cell apoptosis
analysis
Cell cycle and cell apoptosis analysis was performed
using flow cytometery. For cell cycle analysis, BIU87-CisR cells
were collected and fixed with pre-cooled 70% ethanol. Subsequent to
fixing for 12 h, 500 μl propidium iodide (Sigma-Aldrich, St.
Louis, MO, USA) was added and cells were incubated for 30 min. Cell
cycle analysis was performed using a BD FACSCalibur flow cytometer
(Beckman Coulter, Brea, CA, USA) for 15 min. For cell apoptosis
analysis, the Annexin V-fluorescein isothiocyanate assay kit
(Sigma-Aldrich) was used according to the manufacturer’s
instructions.
Analysis of maspin expression and its
effect on the sensitivity of BIU87-CisR cells to cisplatin
Maspin expression in normal and VEGF-C silenced
BIU87-CisR cells was detected by RT-qPCR and western blot analysis
according to the above mentioned protocols. The sequences for the
primers for maspin were as follows: Forward: 5′AAC TGA AGA TGG TGG
GGA TT′3 and reverse: 5′TGG GAA GAA GAG CTT CCA AA′3. Futhermore,
the proliferation inhibition of maspin-overexpressing BIU87-CisR
cells treated with 3 μM cisplatin was analyzed using the
CCK-8 kit.
Statistical analysis
All data are expressed as the mean ± standard
deviation. All calculations were performed using SPSS software,
version 18.0 (SPSS, Inc., Chicago, IL, USA). A one-way analysis of
variance followed by a least significant difference test was used
to determine the statistical significance among four groups and
between each group. P<0.05 was considered to indicate a
statistically significant difference.
Results
High VEGF-C expression levels were
detected in the tumor tissue of chemotherapy-resistant patients and
BIU87-CisR cells
As presented in Fig.
1, the mRNA levels of VEGF-C were higher (1.6±0.03 fold) in
chemoresistant patients (n=20), compared with chemosensitive
patients (n=12, P<0.05). In addition, a higher (1.7±0.06 fold)
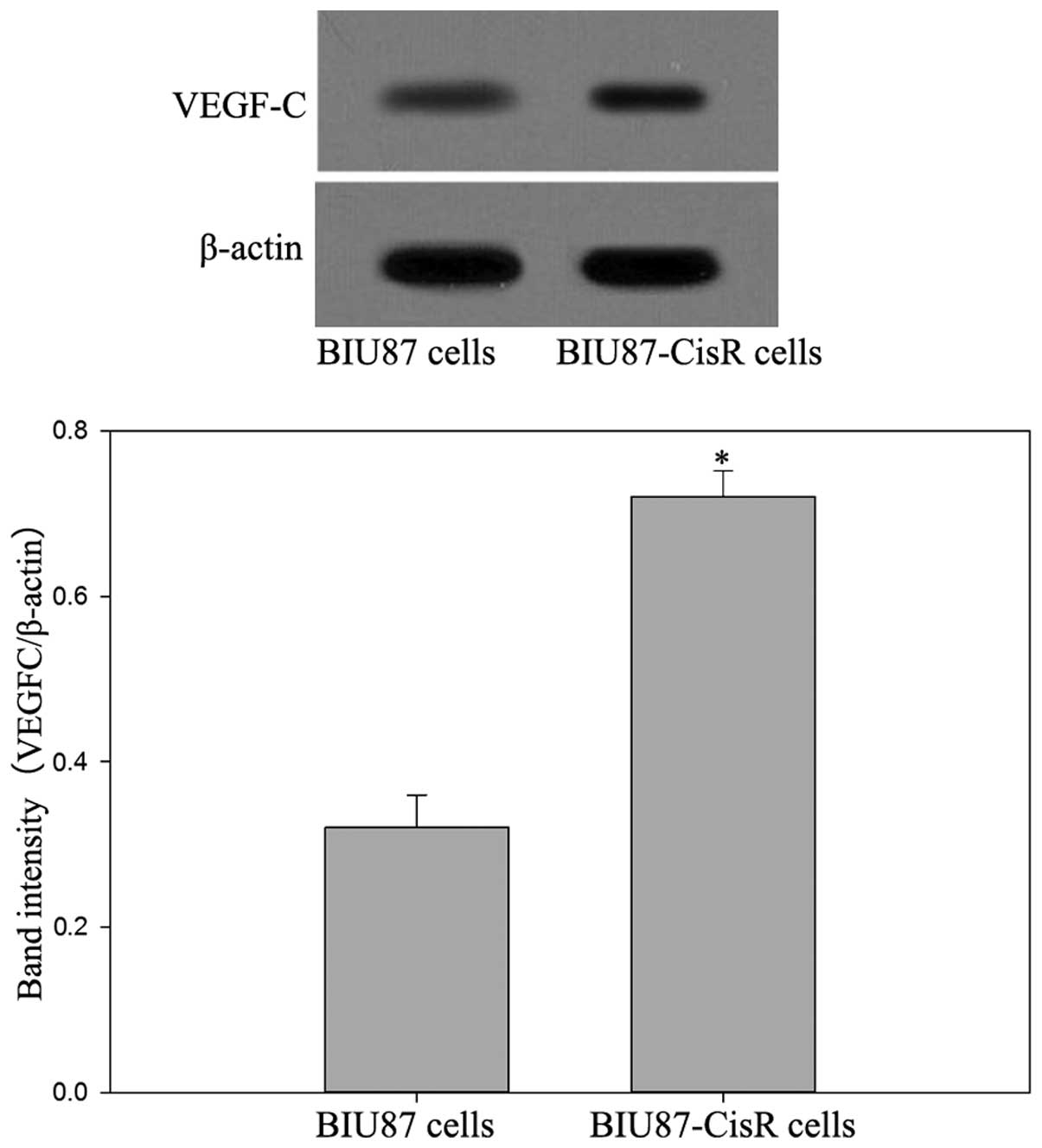
mRNA level of VEGF-C was observed in BIU87-CisR cells (Fig. 2), compared with the parental BIU87
cells (P<0.05).
Western blot analysis confirmed the higher protein
expression levels of VEGF-C in chemoresistant patients and
BIU87-CisR cells (Figs. 3 and
4).
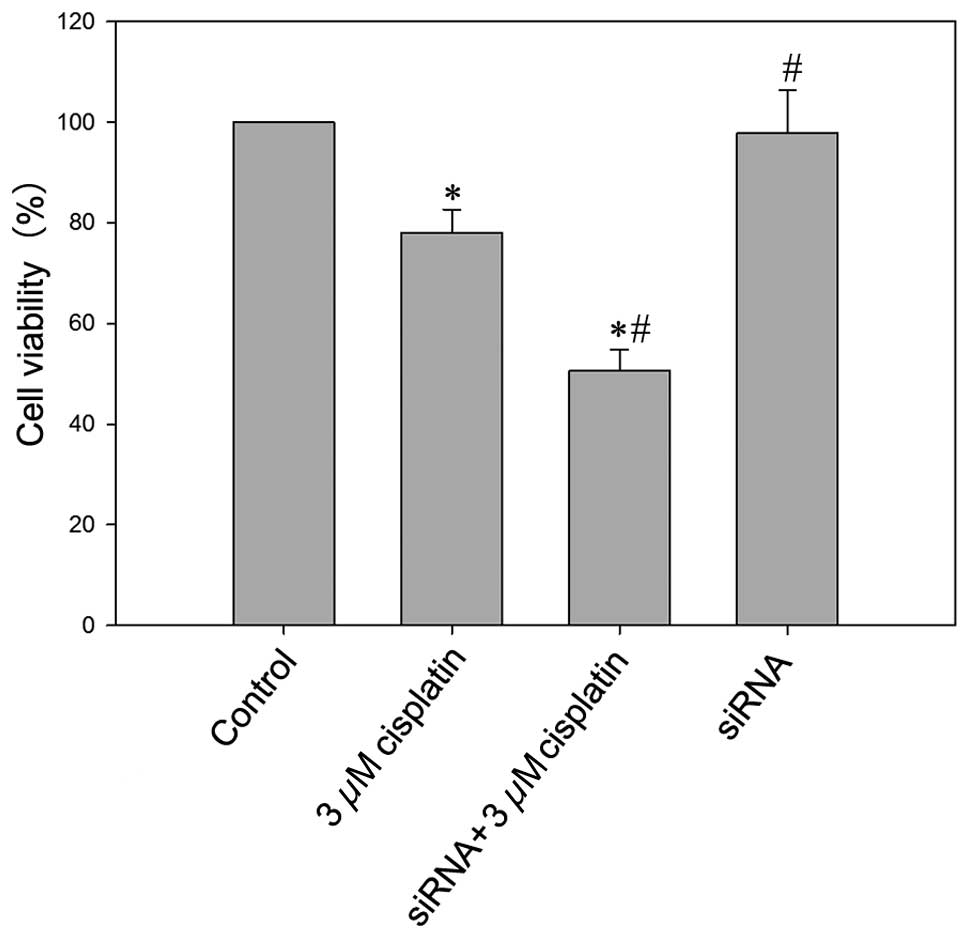
Knock-down of VEGF-C may enhance the
proliferation inhibition effect of cisplatin
As presented in Fig.
5, 3 μM cisplatin
treatment may significantly induce cell death with a cell viability
rate of 78.1±3.6% (P<0.05 vs. the control group). In
VEGF-C-silenced BIU87-CisR cells, 3 μM cisplatin resulted in
a lower cell viability rate (50.6±4.6%) and the difference was
significant compared with other groups (P<0.05).
Knock-down of VEGF-C contributed to
G2/M phase arrest of BIU87-CisR cells induced by
cisplatin
Cisplatin was able to arrest BIU87-CisR cells at the
G2/M phase, as presented in Fig. 6. In the control group, the cell
population percentages were 68.34±7.45% in the
G0/G1 phase and 10.5±0.9% in the
G2/M phase. Following treatment with 3 μM
cisplatin, the proportion of G0/G1 phase
cells was reduced to 53.9±5.3%, and the proportion of cells in the
G2/M phase increased to 31.3±4.1%. In addition, VEGF-C
silencing suppressed the proportion of G0/G1
phase cells (35.5±3.5%) and elevated the percentage of cells in the
G2/M phase (42.3±3.1%). The differences between each
group were statistically significant (P<0.05).
Apoptotic rate of BIU87-CisR cells
resulting from cisplatin increase due to inhibition of VEGF-C
In agreement with the results of the cell cycle
analysis, the silencing of VEGF-C was observed to enhance the
apoptosis-promoting effect of cisplatin. As presented in Fig. 7, which is a representative image,
the apoptotic rate in the control group was 5.3±0.4%, whereas
following treatment with 3 μM cisplatin, the apoptotic rate
was increased to 17.1±3.1%. For the VEGF-C-silenced BIU87-CisR
cells, the apoptotic rate with cisplatin was increased to
29.3±4.5%. The differences between each group were statistically
significant (P<0.05).
Inhibition of VEGF-C increases the
expression levels of maspin, which may improve the sensitivity of
BIU87-CisR cells to cisplatin
In the VEGF-C-silenced BIU87-CisR cells, high mRNA
and protein expression levels of maspin were observed, and a small
increase in maspin expression was observed in the cisplatin-treated
group compared with the control (Figs.
8 and 9). As presented in
Fig. 10, following treatment with
3 μM cisplatin, maspin-overexpressing BIU87-CisR cells
exhibited a lower cell viability compared with normal BIU87-CisR
cells (65.1±3.9% vs. 75.3±5.6%; P<0.05). This suggests that
inhibition of VEGF-C improves the sensitivity of bladder cancer
cells to cisplatin via the upregulation of maspin expression.
Discussion
The current study supports a crucial role for VEGF-C
expression in modulating the resistance of bladder cancer cells to
cisplatin, VEGF-C inhibition is suggested to lead to
chemosensitization through inducing maspin expression. Cisplatin is
an effective broad-spectrum anticancer drug, however, extensive
previous studies have reported cisplatin resistance in human cancer
cells in vivo and in vitro (12,14).
Cisplatin sensitivity and resistance is complex and alterations can
occur in almost every mechanism influencing cell growth,
developmental pathways, apoptosis, DNA repair, drug metabolism and
drug transporters (15). Previous
studies aiming to elucidate the underlying mechanism have
identified that the level of several of expression of genes
contributes to the chemoresistance, and these levels are often
abnormal in patients with cancer (13). These alterations in gene expression
can suppress or promote tumor cell growth and apoptosis.
Jayachandran et al (16)
observed that the induction of NPRL2 expression by plasmid vectors
containing human NPRL2 cDNA were able to overcome cisplatin
resistance in non-small cell lung cancer cells. Furthermore, NPRL2
is an accepted tumor suppressor gene (17,18).
Hour et al (19) indicated
that expression of the CCAAT/enhancer binding protein Δ (CEBPD)
gene was specifically elevated in a cisplatin resistant subline.
CEBPD was able to antagonize reactive oxygen species and apoptosis
via inducing the expression of Cu/Zn-superoxide dismutase (20). In addition to the above studies,
the classical tumor suppressor gene, p53, is also important. A
larger number of studies have examined the association between p53
and cisplatin-resistance, for example, Gutekunst et al
(20) suggested that
siRNA-mediated silencing of p53 abrogated hypersensitivity to
cisplatin. According to this line of reasoning, it is hypothesized
that any tumor suppressor or pro-oncogenic gene should be taken
into consideration when investigating the resistance to cisplantin
in carcinoma cells. Thus, the expression of VEGF-C was investigated
due to the following reasons: As a tumor-promoter, it induces
metastasis through enhancing angiogenesis, lymphangiogenesis and
cancer cell invasion; the presence of high levels of VEGF-C is an
accepted risk factor for poor prognosis; and its effect on
promoting tumor growth has been confirmed in several studies
(21,22). In the current study, tissue
specimens of patients with bladder cancer were analyzed, and the
results indicated that expression of VEGF-C was significantly
higher in chemoresistant patients compared with chemosensitive
patients. In addition, high expression levels of VEGF-C were
detected in the BIU87-CisR cell line, but not in the normal BIU87
cell line. Subsequent to VEGF-C inhibition, cisplatin-treated
BIU87-CisR cells exhibited increased cell death, cell cycle arrest
and apoptosis. These results supported the theory that high levels
of VEGF-C result in resistance to cisplatin. With regard to the
downstream mechanism, Hua et al (23) identified that in acute myeloid
leukemic cells, VEGF-C induced cyclooxygenase-2-mediated resistance
to chemotherapy through the induction of endothelin 1 expression
(23). An additional study
demonstrated that inhibition of the expression of VEGF-C may
reverse drug resistance by regulating the activity of mTOR complex
1 (24). However, it was suggested
that the expression of maspin was involved in induction of
resistance to cisplatin by VEGF-C. This was hypothesized for two
reasons: Firstly, maspin has been demonstrated to exhibit
tumor-suppressing activities by inducing apoptosis (25), in addition it has been reported to
be expressed in normal mammary epithelial cells but absent in
mammary carcinoma cell lines, with a tendancy for the level of
maspin to fall in line with tumor promotion and progression in
humans (26). In addition, it has
been previously reported that low levels of maspin maintain cancer
cell growth and survival. For example, maspin is downregulated in
breast and prostate cancer, which results in reduced cell motility
(11,27). In non-small cell lung carcinoma
cells, high-maspin expressing cells were significantly less
invasive and apoptotic than low-maspin expressing cells (28,29).
Secondly, there is an association between the expression of maspin
and the chemotherapeutic response. Surowiak et al (30) demonstrated that ovarian cancer cell
lines expressing maspin in the cytoplasm were more sensitive to
cisplatin, and for ovarian cancer, maspin expression is associated
with a longer survival rate. In the current study, the expression
of maspin was detected in bladder cancer tissue and in the
cisplatin-resistant BIU87 subline. The results suggest that
chemoresistant patients exhibit lower levels of maspin expression
when compared with chemosensitive patients. In addition, low
expression levels of maspin expression were observed in the
BIU87-CisR cell line. Subsequent to treatment with VEGF-C-targeted
siRNA, the maspin expression levels of BIU87-CisR cells were
significantly increased. Subsequently, overexpression of maspin in
BIU87-CisR cells induced by a recombinant plasmid enhanced the
proliferation inhibition effect of cisplatin. These results suggest
that VEGF-C inhibition reverses the resistance of bladder cancer
cells to cisplatin via upregulating maspin. However, there are
limitations in the current study as follows: The maspin protein is
regulated by methylation of the gene promoter, however,
investigation of whether VEGF-C is associated with DNA methylation
was not conducted. In addition, maspin expression should also be
subjected to investigation as a potential surrogate marker for
cisplatin sensitivity of individual patients in vivo.
In conclusion, the resistance of bladder cancer
cells to cisplatin may be induced by upregulation of VEGF-C, since
inhibition of VEGF-C reverses resistance by increasing the
expression levels of maspin.
References
|
1
|
Xu W, Wang F, Ying L and Wang HH:
Association between glutathione S-transferase M1 null variant and
risk of bladder cancer in Chinese Han population. Tumour Biol.
35:773–777. 2014. View Article : Google Scholar
|
|
2
|
Costantini C and Millard F: Update on
chemotherapy in the treatment of urothelial carcinoma. Scientific
World Journal. 11:1981–1994. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shirato A, Kikugawa T, Miura N, Tanji N,
Takemori N, Higashiyama S and Yokoyama M: Cisplatin resistance by
induction of aldo-keto reductase family 1 member C2 in human
bladder cancer cells. Oncol Lett. 7:674–678. 2014.PubMed/NCBI
|
|
4
|
Galluzzi L, Vitale I, Michels J, Brenner
C, Szabadkai G, Harel-Bellan A, Castedo M and Kroemer G: Systems
biology of cisplatin resistance: Past, present and future. Cell
Death Dis. 5:e12572014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ding M, Fu X, Tan H, Wang R, Chen Z and
Ding S: The effect of vascular endothelial growth factor C
expression in tumor-associated macrophages on lymphangiogenesis and
lymphatic metastasis in breast cancer. Mol Med Rep. 6:1023–1029.
2012.PubMed/NCBI
|
|
6
|
Ciobanu M, Eremia IA, Crăiţoiu S,
Mărgăritescu CL, Stepan A, Pătraşcu V, Georgescu CC, Cernea D and
Dumitrescu D: Lymphatic microvessels density, VEGF-C and VEGFR-3
expression in 25 cases of breast invasive lobular carcinoma. Rom J
Morphol Embryol. 54:925–934. 2013.
|
|
7
|
Liu J, Cheng Y, He M and Yao S: Vascular
endothelial growth factor C enhances cervical cancer cell
invasiveness via upregulation of galectin-3 protein. Gynecol
Endocrinol. 30:461–465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma DM, Xu YP and Zhu L: Expression of
vascular endothelial growth factor C correlates with a poor
prognosis based on analysis of prognostic factors in patients with
cervical carcinomas. J Obstet Gynaecol Res. 37:1519–1524. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cho HJ, Kim IK, Park SM, et al: VEGF-C
mediates RhoGDI2-induced gastric cancer cell metastasis and
cisplatin resistance. Int J Cancer. 135:1553–1563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Snoeren N, Emmink BL, Koerkamp MJ, et al:
Maspin is a marker for early recurrence in primary stage III and IV
colorectal cancer. Br J Cancer. 109:1636–1647. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Machowska M, Wachowicz K, Sopel M and
Rzepecki R: Nuclear location of tumor suppressor protein maspin
inhibits proliferation of breast cancer cells without affecting
proliferation of normal epithelial cells. BMC Cancer. 14:1422014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lopez-Ayllon BD, Moncho-Amor V, Abarrategi
A, Ibañez de Cáceres I, Castro-Carpeño J, Belda-Iniesta C, Perona R
and Sastre L: Cancer stem cells and cisplatin-resistant cells
isolated from non-small-lung cancer cell lines constitute related
cell populations. Cancer Med. 3:1099–1111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou BH, Huang JN, Zuo YL, Li BJ, Guo Q,
Cui BC, Shao WY, Du J and Bu XZ: 2a, a novel curcumin analog,
sensitizes cisplatin-resistant A549 cells to cisplatin by
inhibiting thioredoxin reductase concomitant oxidative stress
damage. Eur J Pharmacol. 707:130–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ajani JA, Wang X, Song S, et al: ALDH-1
expression levels predict response or resistance to preoperative
chemoradiation in resectable esophageal cancer patients. Mol Oncol.
8:142–149. 2014. View Article : Google Scholar :
|
|
15
|
Shen DW, Pouliot LM, Hall MD and Gottesman
MM: Cisplatin resistance: a cellular self-defense mechanism
resulting from multiple epigenetic and genetic changes. Pharmacol
Rev. 64:706–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jayachandran G, Ueda K, Wang B, Roth JA
and Ji L: NPRL2 sensitizes human non-small cell lung cancer (NSCLC)
cells to cisplatin treatment by regulating key components in the
DNA repair pathway. PLoS One. 5:e119942010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ling KS, Chen GD, Tsai HG, Lee MS, Wang PH
and Liu FS: Mechanisms involved in chemoresistance in ovarian
cancer. Taiwan J Obstet Gyne. 44:209–217. 2005. View Article : Google Scholar
|
|
18
|
Patel NP, Pattni BS, Abouzeid AH and
Torchilin VP: Nanopreparations to overcome multidrug resistance in
cancer. Adv Drug Deliver Rev. 65:1748–1762. 2013. View Article : Google Scholar
|
|
19
|
Hour TC, Lai YL, Kuan CI, et al:
Transcriptional up-regulation of SOD1 by CEBPD: a potential target
for cisplatin resistant human urothelial carcinoma cells. Biochem
Pharmacol. 80:325–334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gutekunst M, Mueller T, Weilbacher A,
Dengler MA, Bedke J, Kruck S, Oren M, Aulitzky WE and van der Kuip
H: Cisplatin hypersensitivity of testicular germ cell tumors is
determined by high constitutive Noxa levels mediated by Oct-4.
Cancer Res. 73:1460–1469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Zhu Z, Sun Z, Sun X, Wang Z and
Xu H: Survivin gene expression increases gastric cancer cell
lymphatic metastasis by upregulating vascular endothelial growth
factor-C expression levels. Mol Med Rep. 9:600–606. 2014.
|
|
22
|
Li D, Xie K, Ding GT, Li J, Chen K, Li H,
Qian J, Jiang C and Fang J: Tumor resistance to anti-VEGF therapy
through up-regulation of VEGF-C expression. Cancer Lett. 346:45–52.
2014. View Article : Google Scholar
|
|
23
|
Hua KT, Lee WJ, Yang SF, Chen CK, Hsiao M,
Ku CC, Wei LH, Kuo ML and Chien MH: Vascular endothelial growth
factor-C modulates proliferation and chemoresistance in acute
myeloid leukemic cells through an endothelin-1-dependent induction
of cyclooxygenase-2. Biochim Biophys Acta. 1843:387–397. 2014.
View Article : Google Scholar
|
|
24
|
Stanton MJ, Dutta S, Zhang H, Polavaram
NS, Leontovich AA, Hönscheid P, Sinicrope FA, Tindall DJ, Muders MH
and Datta K: Autophagy control by the VEGF-C/NRP-2 axis in cancer
and its implication for treatment resistance. Cancer Res.
73:160–171. 2013. View Article : Google Scholar :
|
|
25
|
Zou Z, Anisowicz A, Hendrix MJ, Thor A,
Neveu M, Sheng S, Rafidi K, Seftor E and Sager R: Maspin, a serpin
with tumor-suppressing activity in human mammary epithelial cells.
Science. 263:526–529. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taskiran C, Erdem O, Onan A, Vural C,
Arisoy O, Yildiz S and Guner H: Maspin expression in endometrial
hyperplasia and carcinoma and its relation with angiogenesis. Eur J
Gynaecol Oncol. 35:134–139. 2014.
|
|
27
|
Berardi R, Morgese F, Onofri A, et al:
Role of maspin in cancer. Clin Transl Med. 2:82013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hirai K, Koizumi K, Haraguchi S, et al:
Prognostic significance of the tumor suppressor gene maspin in
non-small cell lung cancer. Ann Thorac Surg. 79:248–253. 2005.
View Article : Google Scholar
|
|
29
|
Takanami I, Abiko T and Koizumi S:
Expression of maspin in non-small-cell lung cancer: Correlation
with clinical features. Clin Lung Cancer. 9:361–366. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Surowiak P, Materna V, Drag-Zalesinska M,
Wojnar A, Kaplenko I, Spaczyński M, Dietel M, Zabel M and Lage H:
Maspin expression is characteristic for cisplatin-sensitive ovarian
cancer cells and for ovarian cancer cases of longer survival rates.
Int J Gynecol Pathol. 25:131–139. 2006. View Article : Google Scholar : PubMed/NCBI
|