Introduction
As a crucial component of gastrointestinal
homeostasis, the intestinal epithelial barrier is the first line of
defense against numerous adverse factors, including toxins, certain
antigens and pathogenic microorganisms. Dysfunction and destruction
of molecules and functional proteins of the intestinal epithelial
barrier results in the disturbance of the latter, leading to
activation of mucosal immune responses, which are associated with
the pathogenesis of intestinal disorders. Among these, irritable
bowel syndrome (IBS) is one of the most common chronic,
relapsing-remitting inflammatory diseases (1,2).
The pathophysiological mechanisms of IBS are complex
and have not been fully elucidated; however, they are reckoned to
be multifactorial. Of note, the dysregulation of the brain-gut axis
and cross-regulation between the central and the enteric nervous
system has attracted attention in recent studies (3). Three essential factors are required
in the pathogenesis of IBS: Breakdown of intestinal barrier
function, activation of lamina propria immune cells by luminal
contents and, importantly, an overexaggerated immune response
(4).
Tight junctions (TJ) are apical-host adhesive
junctional complexes in epithelial cells, and regulate
proliferation, polarization and differentiation of mammalian gut
cells (5,6). Between the apical and lateral
membrane, a continuous belt-like ring is formed by TJs around
epithelial cells, which regulates the selective/semipermeable
transportation of paracellular ionic solutes and intercellular
signaling responses (7,8). The biological importance of TJs was
initially recognized in the 1960s with the emergence of electron
microscopy (9). TJs are a series
of multiprotein complexes of dynamic function, of which claudins,
occludin, junctional adhesion molecules (JAMs) and tricellulin are
four unique families of transmembrane proteins (9).
According to recent studies, claudins are 20- to
27-kDa integral membrane proteins and consist of two extracellular
loops, four hydrophobic transmembrane domains as well as N- and
C-terminal cytoplasmic domains (7,10–12),
among which two extracellular loops are essential for their
homophilic and heterophilic properties. Moreover, interactions of
TJ proteins form ion-selective channels, facilitating the passage
of iron and selective solutes from the intercellular space and
preventing adverse events caused by toxins or microorganisms
(7).
The regulatory role of claudins in barrier function
has been proved in recent studies on claudin-deficient mice
(12), and Furuse et al
(13) found that
claudin1−/− mice suffered significant transepidermal
water loss and died within the first day following birth. The
present study aimed to investigate the molecular and cellular
mechanisms of TJ dysfunction in IBS, particularly the role of
claudin1.
Materials and methods
Patients
Bowel tissues from 93 IBS patients were collected at
the First Hospital of Zhengzhou University (Zhengzhou, China). The
patients consisted of 58 women and 35 men, and they were diagnosed
according to the Rome III criteria (14). Electron microscopic observation was
performed on specimens from 10 patients with diarrhea-predominant
IBS (mean age, 48.5 years; range, 19–68 years) and 10 patients with
constipation-predominant IBS (mean age, 46.3 years; range, 18–65
years) and bowel tissues from 10 patients unaffected by IBS with
bleeding hemorrhoids (mean age, 50.2 years; range, 17–71 years).
Furthermore, claudin-1 was investigated in specimens from 23
patients with diarrhea-predominant IBS (mean age, 39.7 years;
range, 18–65 years) and 20 patients with constipation-predominant
IBS (mean age, 38.6 years; range, 19–70 years) and bowel tissues
from 20 patients unaffected by IBS with bleeding hemorrhoids (mean
age, 40.1 years; range, 17–71 years).
Specimens
All selected patients were separately subjected to
electronic colonoscopy, and four biopsies were taken from the
terminal ileum and ascending colon mucosa, respectively. Specimens
were at least 0.2×0.2×0.2 cm in size. The use of human tissue was
approved by the Ethics Committee of the First Hospital of Zhengzhou
University (Zhengzhou, China) and all patients gave informed
consent.
Reagents and antibodies
Rabbit anti-claudin-1 (cat. no. 50011919) was
purchased from Zymed Laboratories. Goat anti-rabbit immunoglobulin
(Ig)G antibody labeled with horseradish peroxidase (HRP) (cat. no.
ZB-2301) was purchased from Zhongshan Golden Bridge Biotechnology
Company (Beijing, China). Taurocholic acid (TCA) and glycocholic
acid (GCA) were purchased from Amresco LLC (Solon, OH, USA), and
deoxycholic acid (DCA) was purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). β-actin antibody (cat. no. A5441)
was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Specimen preparation and ultrastructure
observation of intestinal TJs
Electron microscopy (V6458; Pentax, Tokyo, Japan)
using cytochemical techniques with a lanthanum nitrate tracer was
used to evaluate the samples. Sample specimens were cut into
1-mm3 squares and marked regarding their orientation.
Tissue samples were fixed in phosphate-buffered saline (PBS; Xi'an
Chemical Reagent Company, Xi'an, China) containing 3%
glutaraldehyde (Xi'an Chemical Reagent Company), 1.5% (0.1 mol/l)
paraformaldehyde (Xi'an Chemical Reagent Company) and 1% lanthanum
nitrate (pH 7.2; Xi'an Chemical Reagent Company) at 4°C for 2 h,
then immersed in 0.1 mol/l sodium cacodylate buffer for 30 min,
fixed with 1% osmium tetroxide fixative (containing 1% lanthanum
nitrate; pH 7.2; Xi'an Chemical Reagent Company) at 4°C for 1.5 h,
washed with 0.1 mol/l sodium cacodylate buffer (Xi'an Chemical
Reagent Company) for 1 min and dehydrated with a graded series of
ethanol (Xi'an Chemical Reagent Company): 30% ethanol for 5 min,
50% ethanol for 5 min, 70% ethanol for 5 min, 90% ethanol 5 min
twice and pure ethanol for 5 min three times. Samples were then
dehydrated with acetone for 5 min and embedded with Epon618 epoxy
resin (Xi'an Chemical Reagent Company). The embedded tissues was
cut into 90-nm slices. Finally, TJs were observed by using a
Hitachi H-600 projection electron microscope (Hitachi, Tokyo,
Japan) and images were captured.
Specimen preparation and ultrastructural
observation of intestinal epithelial cells
Samples were cut into 1-mm3 small squares
and immediately placed in 0.1 M PBS containing 2.5% glutaraldehyde
and 4% paraformaldehyde at 4°C for 2 h, then immersed in 0.1 M PBS
for 30 min, fixed with 1% osmium tetroxide in 0.1 M PBS at 4°C for
2 h, washed by 0.1 M PBS for 1 min, and then dehydrated in a graded
series of ethanol: 30% ethanol for 10 min, 50% ethanol for 10 min,
70% ethanol for 10 min, and 70% ethanol; 90% ethanol 10 min twice
and pure ethanol for 10 min three times. Samples were the incubated
in propylene oxide for 10 min, then dehydrated with acetone for 5
min and embedded in Epon812 epoxy resin. After polymerization, half
ultrathin sections of 1–2 µm were cut and positioned under a
light microscope after methylene blue staining (Xi'an Chemical
Reagent Company) for 30 sec at room temperature. Ultrathin sections
of 50-70 nm were cut using an LKB-V ultramicrotome machine
(LKB-8800; Lanka Electricity Company, Bromma, Sweden). After uranyl
acetate and lead citrate staining, tissues were observed using the
Hitachi H-600 projection electron microscope and images were
captured.
Fluorescence quantitative polymerase
chain reaction (FQ-PCR) analysis of intestinal claudin-1 mRNA
expression
Extraction of total RNA samples
The tissue sample was placed in a glass of
homogenizer, TRIzol (50 mg/ml) was added according to the
manufacturer's instructions, and tissue was homogenized over 5 min.
Following centrifugation at 17,000 x g for 5 min at 4°C, the pellet
was discarded. Chloroform was added at a ratio of 1:5 with regard
to TRIzol followed by ultrasonication for 15 min at room
temperature. Following centrifugation at 17,000 x g for 10 min, the
supernatant was discarded by adding 1 ml water and transferring the
sample to a fresh centrifuge tube. Isopropanol was added at a ratio
of 1:2 with regard to TRIzol followed by incubation for 10 min at
room temperature. The supernatant was discarded after
centrifugation at 4°C and 9,000 x g for 5 min. 1 ml 75% ethanol was
then added to suspend the precipitate, followed by centrifugation
at 4°C and 9,000 x g for 5 min, and the supernatant was discarded.
20 µl diethylpyrocarbonate (DEPC)-treated water was added to
dissolve the precipitate, followed by incubation at 60°C for 5 min.
The optical density (OD) value was measured using a DNM-9606
Microplate reader (PuLang, Nanjing, China) to determine the
concentration and purity of the mRNA.
Synthesis of cDNA
Oligo (dT) 18 primer (Huada, Shenzhen, China) (1
µl) was added to 0.5 µg template RNA, and
DEPC-treated distilled water (Xi'an Chemical Reagent Company) to a
final volume of 12 µl. The mixture was gently agitated and
centrifuged at 17,000 × g for 3–5 sec. The mixture was incubated at
70°C for 5 min, cooled on ice and 5X reaction buffer (4 µl),
RiboLock™ ribonuclease inhibitor (20 U/µl; 1 µl), 10
mM deoxyribonucleotide triphosphate mix (2 µl) were added
(all from Thermo Fisher Scientific, Pittsburgh, PA, USA), followed
by incubation at 37°C for 5 min. Subsequently 1 µl
RevertAid™ Moloney murine leukemia virus reverse phase
transcriptase (200 U/µl; Thermo Fisher Scientific) was
added, followed by incubation at 42°C for 60 min. Following
incubation at 70°C for 10 min, the reaction was terminated by
rapidly placing the sample on ice, followed by preservation at
−20°C.
FQ-PCR detection
According to the target gene in GenBank (www.ncbi.nlm.nih.gov/genbank/), primers
were designed using Oligo 6.64 software (Molecular Biology
Insights, Inc., Colorado Springs, CO, USA), and are shown in
Table I. A 25-µl reaction
system containing SYBR Primix (12.5 µl), ROX deference dye
(0.5 µl), upstream and downstream primer (20 pmol,
respectively) and template cDNA (50 ng) was used (all from
Invitrogen Life Technologies). A real time PCR instrument was used
with a three-step PCR reaction program (ABI 7000; Applied
Biosystems, Life Technologies, Thermo Fisher Scientific, Waltham,
MA, USA). The reaction conditions were as follows: Denaturation at
50°C for 2 min and 95°C for 2 min, 1 cycle; denaturation at 95°C
for 15 sec, annealing at 55°C for 30 sec, extension at 72°C for 30
sec, over 45 cycles. The dissolution profile of the PCR reaction
solution was determined for each sample. The CT value of each
sample was calculated using ABI prime 7000 SDS software (Applied
Biosystems), and the relative gene expression in each group was
calculated according to the 2−ΔΔCT method, with
ΔΔCT=(CTTarget−CTGAPDH)Time
x−(CTTarget−CTGAPDH)Time 0,
where Time x represented the expression of the target gene at the
time point of interest and Time 0 represented the target gene
1-fold expression normalized to GAPDH.
 | Table IPrimers used for polymerase chain
reaction. |
Table I
Primers used for polymerase chain
reaction.
| Gene | Upstream primer | Downstream
primer | Length | Genbank number |
|---|
| GAPDH |
TGAACGGGAAGCTCACTGG |
TCCACCACCCTGTTGCTGTA | 307 | NM_008084 |
| Claudin-1 |
ATGGTATGGCAATAGAATCGT |
GCCTTGGTGTTGGGTAAGAG | 179 | NM_021101 |
Western blot analysis of intestinal
claudin-1 protein
Total intestinal protein extracted using the
radioimmunoprecipitation assay (RIPA) total protein extraction kit
(990 µl RIPA buffer and 10 µl
phenylmethanesulfonylfluoride; Amresco). For blotting, 10%
separating gel (10 m) and 5% stacking gel (5 ml) (Amresco) were
used. Sample and loading buffer were mixed at a ratio of 4:1,
heated for denaturation at 100°C for 5 min, and 15 µl were
loaded onto the gel following instantaneous centrifugation.
Proteins were separated using 10% SDS-PAGE at 80 V for 90 min,
until bromophenol blue (Amresco) reached the bottom of the
resolving gel. The gel was then immersed with Coomassie blue dye
(Amresco) and the blot was transferred onto a polyvinylidene
difluoride (PVDF; Amresco) membrane at 25 V for 2.5 h. the PVDF
membrane was stained with Ponceau S (Amresco) for 5 min. The marker
position was located using India Ink (Amresco). The membrane was
washed with distilled water several times until completely clear
and then blocked with 8% skimmed milk. Following agitation in
Tris-buffered saline with Tween 20 (TBST; Sigma-Aldrich) for 60
min, the membrane was rinsed with TBST three times. Then antibody
(rabbit anti-human claudin-1 polyclonal antibody at a dilution of
1:120) was added, followed by incubation with agitation at 37°C for
2 h and subsequent incubation at 4°C overnight. The membrane was
rinsed with TBST three times, followed by addition of the secondary
antibody [goat anti-rabbit IgG-HRP conjugate at a dilution of
1:500] and gentle agitation for 3 h. The membrane was rinsed in
TBST three times for a total of 30 min, and an enhanced
chemiluminescence kit (BioTeke Corporation, Beijing, China) was
used for antibody visualization. The blots were exposed to X-ray
film (Kodak, Tokyo, Japan) and a gel imaging acquisition system
(HQ-320XT Film Washing Machine; Huqiu Imaging Technologies (Suzhou)
Co., Ltd., Suzhou, China) was applied to analyze the results.
Immunohistochemical analysis of
intestinal claudin-1 protein
The tissue sections were dewaxed and hydrated with
xylene and graded alcohol, then were incubated with 3%
H2O2 for 10 min. The tissue sections were
fixed with citrate buffer in the microwave (high-power for 3 min,
and low-power for 10 min; Galanz Enterprise Group Co., Ltd.,
Guangdong, China). Primary antibody (rabbit anti-human claudin-1
polyclonal antibody; 1:150 dilution) was added followed by
incubation at 4°C overnight. Samples were washed with PBS three
times for 3 min each. Goat anti-rabbit-HRP IgG was added as the
secondary antibody, followed by incubation at 37°C for 30 min.
Samples were washed with PBS three times for 3 min each and
antibodies were visualized with diaminobenzidine. Samples were
washed with distilled water, stained with hematoxylin, dehydrated
with alcohol, washed with xylene, sealed with flavor sealing
tablets and images were captured using a microscope (IX50; Olympus,
Tokyo, Japan). Images were quantitatively evaluated using Image Pro
Plus software, version 5.1 (Media Cybernetics, Rockville, MD,
USA).
Statistical analysis
All values are presented as the mean ± standard
error and all statistical analysis was conducted using SPSS
software, version 19.0 (IBM SPSS, Armonk, NY, USA). Data were
analyzed using Student's t-test for two independent groups or
one-way analysis of variance followed by Fisher's paired
least-significant difference or Scheffe's F test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference between values.
Results
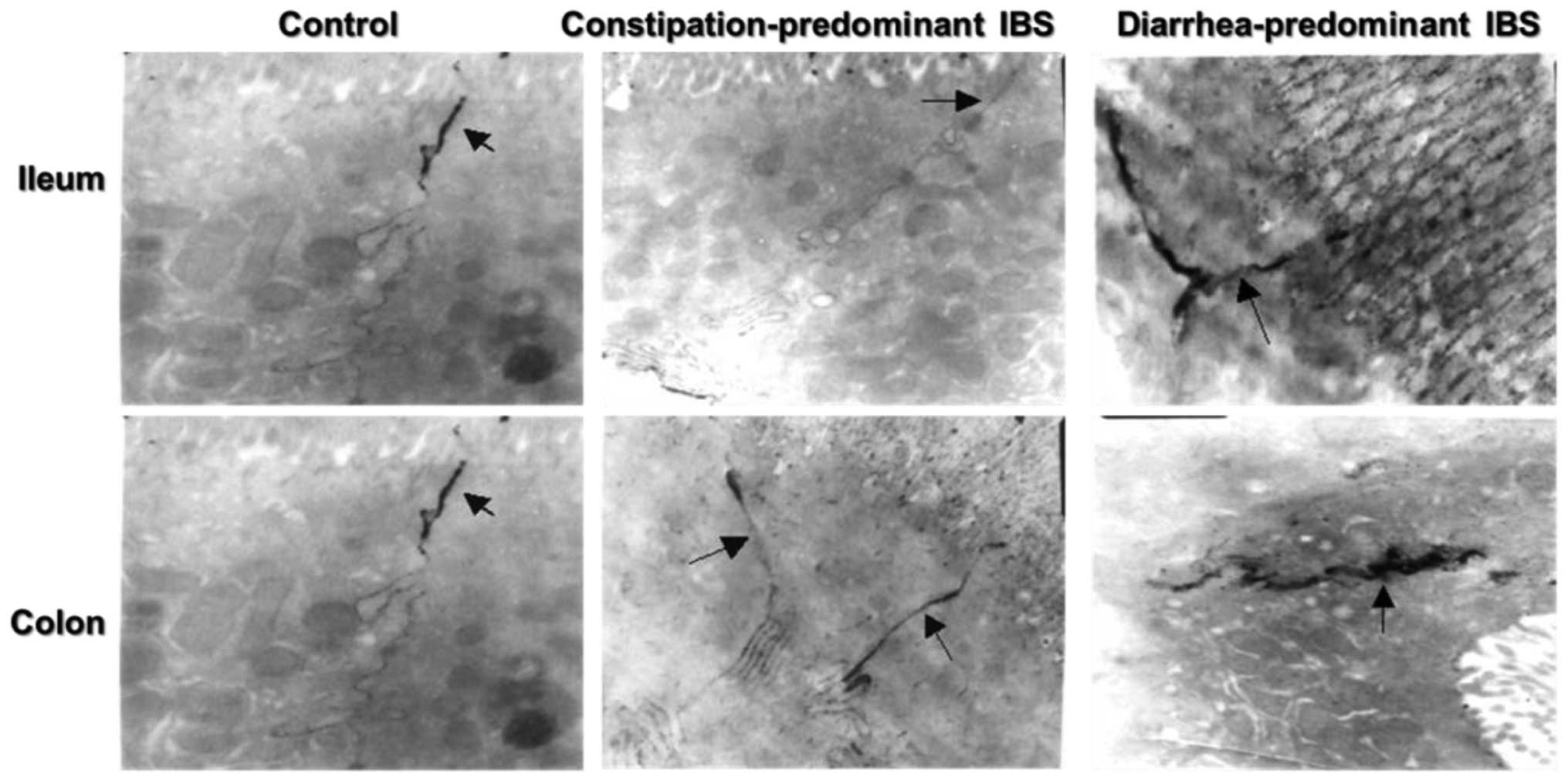
Ultrastructural observation of intestinal
TJs
Electron microscopic observation showed that the
terminal ileum and ascending colon mucosa of
constipation-predominant IBS was as normal as that of the control
group (Fig. 1); intercellular TJ
structures were not widened and no tracer extravasation phenomenon
was observed. Among the 10 diarrhea-predominant IBS cases, gaps
between TJs were widened in the ileum mucosa in seven cases and in
the ascending colonic mucosa in eight cases, and varying degrees of
tracer extravasation phenomenon were observed. Among four patients
with a history of infectious diarrhea in addition to IBS, the
terminal ileum and ascending colon mucosa of three cases displayed
these changes.
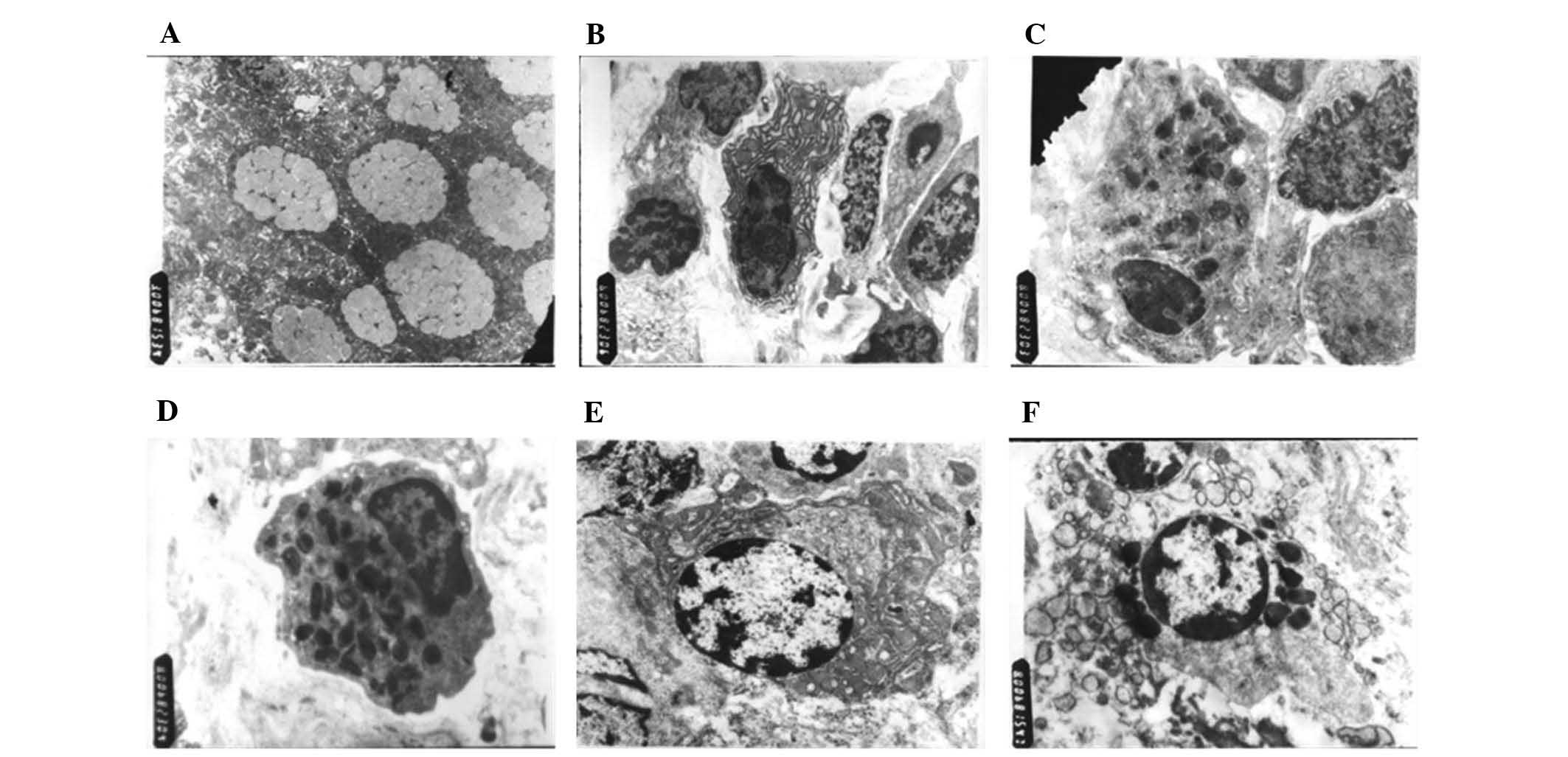
Ultrastructural observation of intestinal
epithelial cells
Electron microscopic observation (Fig. 2) showed that compared with the
control group, the diarrhea-predominant IBS and
constipation-predominant IBS groups displayed significantly
increased mucus secretion and mucus bubble fusion in goblet cells
of the colon and small intestinal epithelium, which were in a state
of exuberant secretion (Fig. 2A).
Plasma cells of the above mentioned four IBS patients with a
history of infectious diarrhea were increased, of which the rough
endoplasmic reticula were dilated and mitochondria were
significantly increased (Fig. 2B).
The structural changes of constipation-predominant IBS were not
manifested. Neuroendocrine cells were present in the two types of
IBS patient, which were cone-shaped, big in the upper end and small
in the other, with nuclei located at the upper end and the
cytoplasm at the lower end. The cytoplasm of neuroendocrine cells
was filled with a high density of endocrine granules, several of
which were vacuole-like, while in the normal control samples, fewer
neuroendocrine cells were observed in the small intestine and colon
mucosa, the endocrine particles of which were fewer, and no
significant changes in vacuoles were present (Fig. 2C and D). In addition, certain
vacuoles were fully filled with high-density particles in the mast
cell cytoplasm in the two types of IBS patients, which were in the
active degranulation state, while a low density of particles and no
vacuoles were observed in the mast cells of the colonic mucosa and
small intestine of the healthy control samples (Fig. 2E).
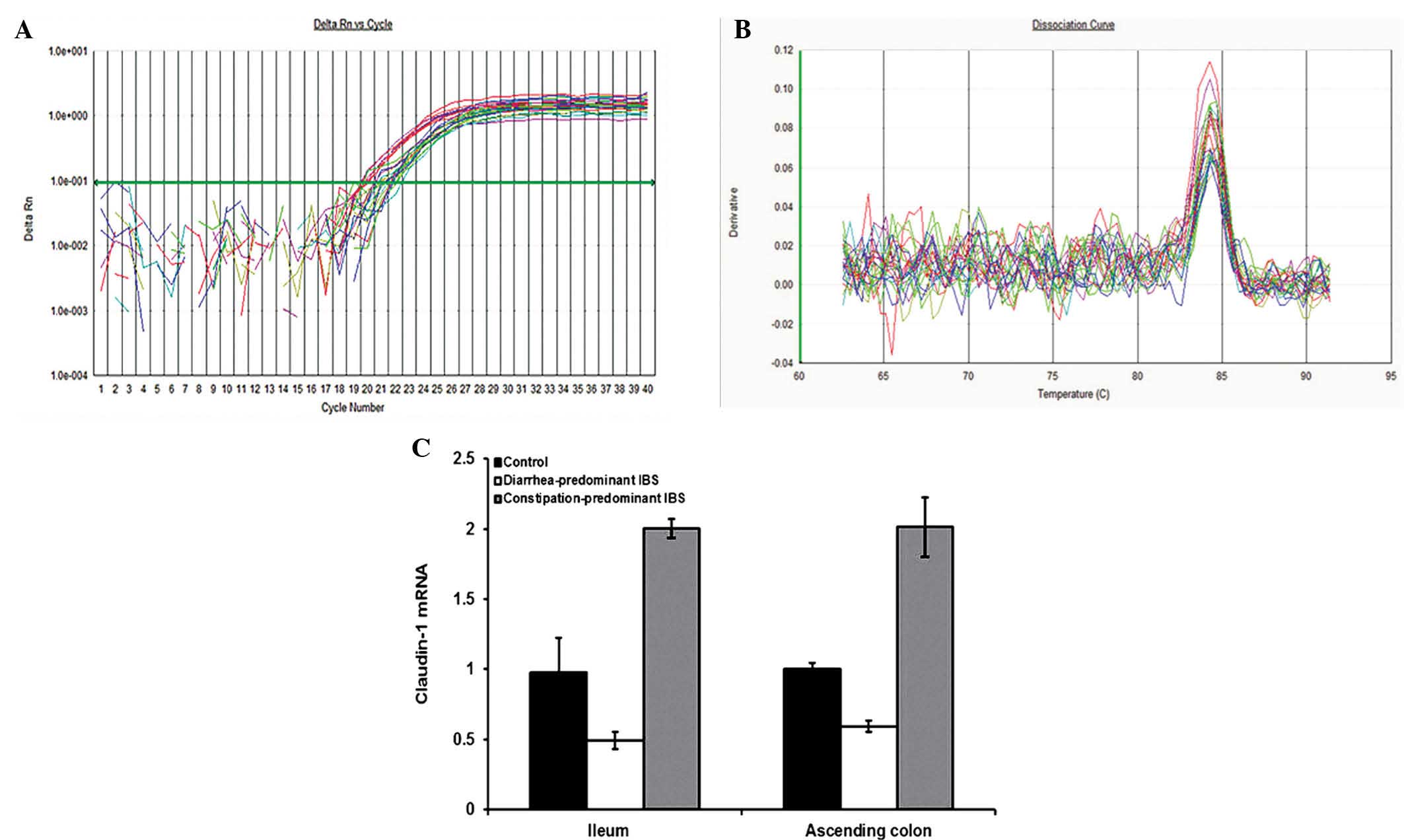
Claudin-1 is upregulated in
constipation-predominant IBS and downregulated in
diarrhea-predominant IBS
Compared with claudin-1 mRNA expression in the small
intestine as well as the colonic mucosa of the control group, it
was significantly decreased in diarrhea-predominant IBS
(P<0.05), while it was significantly increased in
constipation-predominant IBS (P<0.05) (Fig. 3). Accordingly, there was a
significant difference between the diarrhea-predominant group and
the constipation-predominant group (P<0.05).
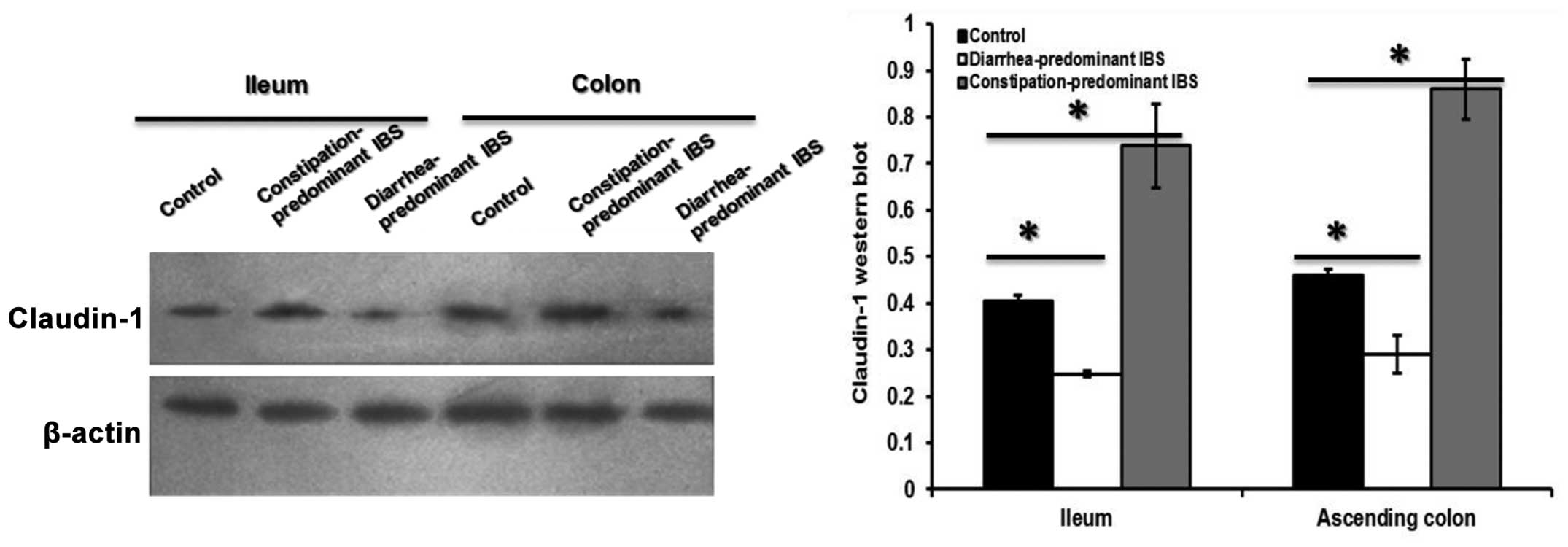
Compared with those in the control group, claudin-1
protein levels in the small intestine as well as in the colon
mucosa in the constipation-predominant IBS group were significantly
increased (P<0.05), while the expression was decreased in the
diarrhea-predominant IBS (P<0.05) (Fig. 4). Changes in the protein levels of
claudin were therefore in accordance with the changes in mRNA
expression as determined by PCR.
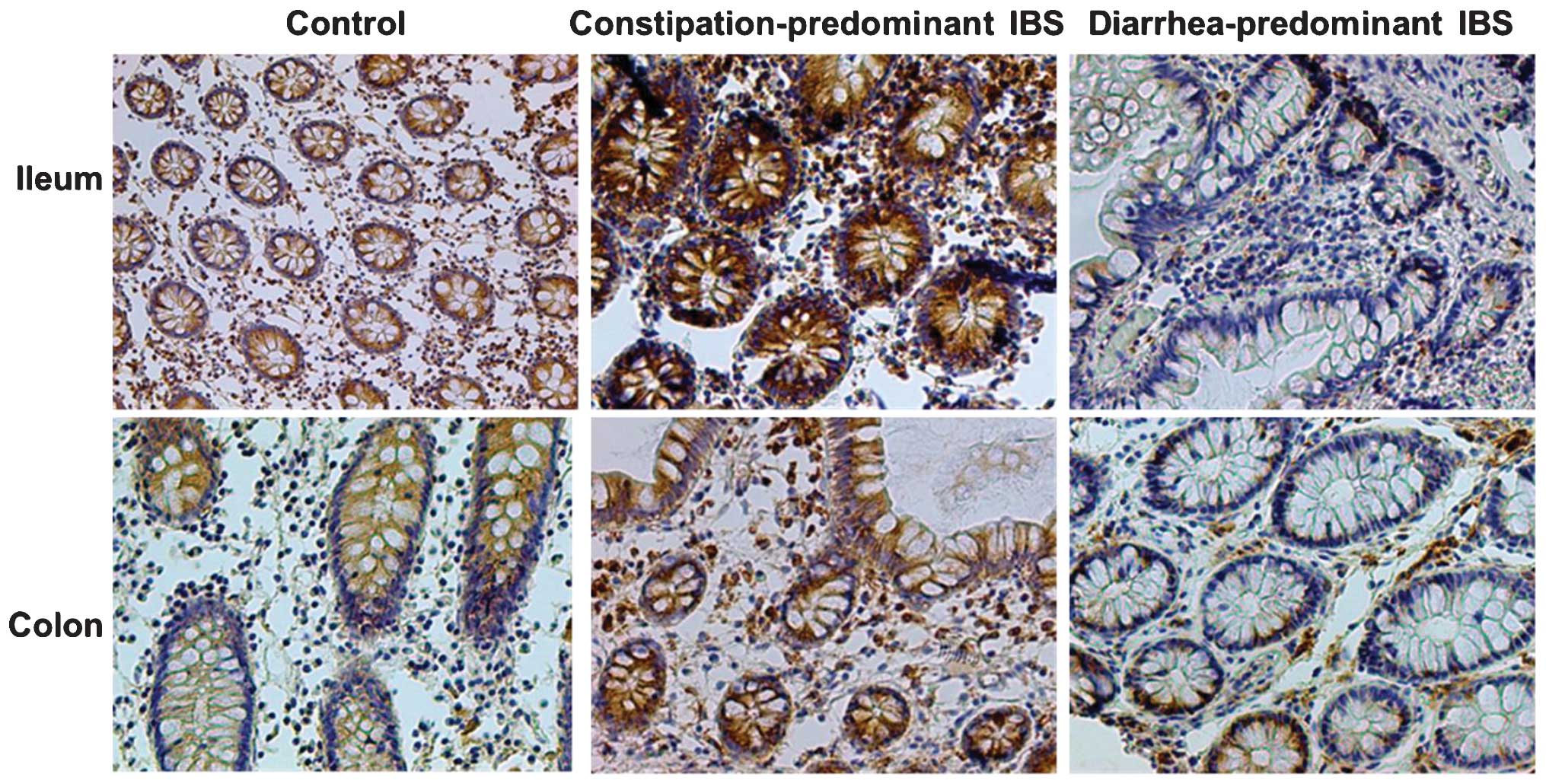
Immunohistochemical analysis of
intestinal claudin-1 protein
Claudin-1 levels were determined based on the OD
values calculated by the image analysis software. In analogy with
the results of western blot and PCR analyses, it was found that,
compared with the control group, expression of small intestinal as
well as colonic mucosal claudin-1 protein in the
diarrhea-predominant group was significantly decreased (P<0.05),
while that in the constipation-predominant group was significantly
increased (P<0.05), and the differences between the two IBS
groups were significant (P<0.05), as shown in Table II and Fig. 5. Furthermore, no significant
changes in the distribution of small intestinal as well as colonic
mucosal claudin-1 protein in each group were found, with location
mainly in the cell membrane, the outer membrane and the cytoplasmic
membrane. Brown staining indicated positivity for claudin-1, and no
expression in the nuclei and nuclear membrane was present (Fig. 5).
 | Table IIImmunohistochemical analysis of
intestinal claudin-1 protein (mean ± standard deviation). |
Table II
Immunohistochemical analysis of
intestinal claudin-1 protein (mean ± standard deviation).
| Site | Group | Number | Claudin-1 |
|---|
| Small intestine | Control | 20 | 38.726±2.880 |
| Diarrhea-predominant
group | 23 | 31.376±1.971a,b |
|
Constipation-predominant group | 20 | 45.084±2.230a |
| Colon | Control | 20 | 37.459±3.343 |
| Diarrhea-predominant
group | 23 | 22.580±0.945a,b |
|
Constipation-predominant group | 20 | 53.472±1.767a |
Discussion
A previous study has reported that, among multiple
molecular mechanisms, altered distribution of TJ protein expression
as well as increased epithelial apoptosis affect intestinal
permeability in patients with IBS (15). Furthermore, previous studies have
indicated that the dysfunction of the intestinal epithelial barrier
is associated with a significant disruption of occludin and
claudin-1 expression (16–18).
The present study showed that intercellular TJ
structures of the terminal ileum and ascending colon mucosa of
constipation-predominant IBS patients were as normal as those in
the control group, and no widened gaps or the tracer extravasation
phenomenon were observed. In patients with diarrhea-predominant
IBS, TJ gaps of the ileum mucosa and ascending colonic mucosa were
widened, and varying degrees of tracer extravasation phenomenon
were observed. Additionally, diarrhea-predominant IBS and
constipation-predominant IBS had significantly increased mucus
secretion and mucus bubble fusion in goblet cells of colon and
small intestinal epithelium, which were in a state of exuberant
secretion. Plasma cells in four patients with IBS and a history of
infectious diarrhea were increased, the rough endoplasmic reticula
of which were dilated and mitochondria were significantly
increased. By contrast, structural changes of
constipation-predominant IBS were not manifested. Neuroendocrine
cells were observed in the two types of IBS patients, and the
cytoplasm of neuroendocrine cells was filled with a high density of
endocrine granules, several of which were vacuole-like, while in
the normal control group, fewer neuroendocrine cells were observed
in the small intestine and colon mucosa, the endocrine particles of
which were fewer, and no significant changes in vacuoles were
present. In addition, several vacuoles were fully filled with
high-density particles in the mast cell cytoplasm in the two types
of patients with IBS, which were in an active degranulation state,
while a low density of particles and no vacuoles were present in
mast cells of the colonic mucosa and small intestine of the normal
controls.
Of note, Barbara et al (19) reported that the number of
tryptase-positive cells in patients with IBS was three times that
in normal individuals, and provided evidence of mast cell
degranulation (19). Another study
suggested that in patients with bowel symptoms, the number of
endocrine cells in the colon increased at the in initial stage,
while it was reduced in the chronic phase (20). These two studies are consistent
with the results of the present studies.
In addition, ultrastructural analysis of samples
from patients with IBS showed that goblet cells, mast cells, plasma
cells and neuroendocrine cells had a highly active function and
strong secretion, indicating that the nervous-immune-endocrine
system may have an important role in the pathogenesis of IBS.
Mast cells have an important role in the innate
immune system and are common residents of the intestine,
contributing to the modulation of numerous pathophysiological
processes in the gastrointestinal tract (21). Mast cells have been widely studied
in IBS and results showed that their number was enhanced and that
they were effectively activated, leading to the generation of
visceral hypersensitivity and acute abdominal pain (22–24).
Further studies reported the proximity of mast cells to enteric
nerves in the colonic mucosa of IBS patients, which indicated that
there was a communication between the central nervous system and
the gastrointestinal tract (25,26).
Therefore, intestinal function and inflammation are influenced by
stress apart from other factors, including luminal bacteria. In
fact, mast cells release inflammatory factors, including
granulocytes, tumor necrosis factor alpha, interleukins 3–6,
prostaglandins, platelet-activating factor, leukotriens, monocyte
colony-stimulating factor and even certain specific proteases, such
as carboxypeptidase-A and tryptase, to modulate paracellular
permeability, which has a certain association with TJ proteins
(27).
The expression and distribution of claudins is
associated with TJ permeability. According to the results of the
current study, compared with those in the control group, claudin-1
protein levels in the small intestine and colon mucosa in
constipation-predominant IBS group were significantly increased
(P<0.05), while expression was decreased in the
diarrhea-predominant IBS group (P<0.05), the results of which
were consistent among FQ-PCR, western blot and immunohistochemical
analyses.
The results of the present study led to the
conclusion that claudin-1 has an important role in changes in TJs
in the intestinal mucosa in patients with IBS, which was not
associated with the distributional changes of protein, but with TJ
protein expression. The pathology of IBS patients with symptoms
including changes in bowel movement traits and habits is not only
associated with sensory and motoric disorders of the gut, but also
with a variety of changes in intestinal TJ proteins. Increased
expression of claudin-1 led to decreased intestinal TJ
permeability, obstructing water and electrolyte leakage and
resulting in constipation; on the contrary, reduced expression of
claudin-1 leaded to enhanced intestinal TJ permeability, which
increased penetration of water and electrolytes, resulting in
diarrhea.
The present study investigated the molecular and
cellular mechanisms of TJ dysfunction in IBS. Based on
ultrastructural observation of intestinal TJs and epithelial cells,
it was found that small intestine and colon samples of patients
with constipation-predominant IBS and diarrhea-predominant IBS
exhibited changes compared with those from normal controls, with
changes of goblet cells, mast cells, plasma cells and
neuroendocrine cells, which exhibited a highly active function and
strong secretion. Furthermore, using various methods, it was found
that claudin-1 protein levels in the small intestine and colon
mucosa in constipation-predominant IBS were significantly increased
compared with those in normal controls, while expression was
decreased in samples from patients with diarrhea-predominant
IBS.
In conclusion, the present study indicated that
cellular changes as well as claudin-1 levels were associated with
Tjs in IBS. Further studies are required to investigate the
association between claudin-1 protein levels and different types of
IBS.
Acknowledgments
The present study was supported in part by the
Medical Science and Technology research projects in Henan Province
(no. 201303014).
References
|
1
|
Garrett WS, Gordon JI and Glimcher LH:
Homeostasis and inflammation in the intestine. Cell. 140:859–870.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Turner JR: Intestinal mucosal barrier
function in health and disease. Nat Rev Immunol. 9:799–809. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mayer EA and Tillisch K: The brain-gut
axis in abdominal pain syndromes. Annu Rev Med. 62:381–396. 2011.
View Article : Google Scholar
|
|
4
|
Clayburgh DR, Shen L and Turner JR: A
porous defense: the leaky epithelial barrier in intestinal disease.
Lab Invest. 84:282–291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsukita S, Furuse M and Itoh M:
Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol.
2:285–293. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Förster C: Tight junctions and the
modulation of barrier function in disease. Histochem Cell Biol.
130:55–70. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harhaj NS and Antonetti DA: Regulation of
tight junctions and loss of barrier function in pathophysiology.
Int J Biochem Cell Biol. 36:1206–1237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsukita S and Furuse M: The structure and
function of claudins, cell adhesion molecules at tight junctions.
Ann N Y Acad Sci. 915:129–135. 2000. View Article : Google Scholar
|
|
9
|
Groschwitz KR and Hogan SP: Intestinal
barrier function: molecular regulation and disease pathogenesis. J
Allergy Clin Immunol. 124:3–20; quiz 21–22. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Van Itallie CM and Anderson JM: Claudins
and epithelial para-cellular transport. Annu Rev Physiol.
68:403–429. 2006. View Article : Google Scholar
|
|
11
|
Schneeberger EE and Lynch RD: The tight
junction: a multifunctional complex. Am J Physiol Cell Physiol.
286:C1213–1228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Turksen K and Troy TC: Barriers built on
claudins. J Cell Sci. 117:2435–2447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saitou M, Furuse M, Sasaki H, Schulzke JD,
Fromm M, Takano H, Noda T and Tsukita S: Complex phenotype of mice
lacking occludin, a component of tight junction strands. Mol Biol
Cell. 11:4131–4142. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dang J, Ardila-Hani A, Amichai MM, Chua K
and Pimentel M: Systematic review of diagnostic criteria for IBS
demonstrates poor validity and utilization of Rome III.
Neurogastroenterol Motil. 24:853–e397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gitter AH, Bendfeldt K, Schulzke JD and
Fromm M: Leaks in the epithelial barrier caused by spontaneous and
TNF-alpha-induced single-cell apoptosis. FASEB J. 14:1749–1753.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeissig S, Burgel N, Günzel D, et al:
Changes in expression and distribution of claudin 2, 5 and 8 lead
to discontinuous tight junctions and barrier dysfunction in active
Crohn's disease. Gut. 56:61–72. 2007. View Article : Google Scholar
|
|
17
|
Yuan B, Zhou S, Lu Y, Liu J, Jin X, Wan H
and Wang F: Changes in the expression and distribution of claudins,
increased epithelial apoptosis, and a mannan-binding
lectin-associated immune response lead to barrier dysfunction in
dextran sodium sulfate-induced rat colitis. Gut Liver. Feb
26–2015.Epub ahead of print. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kyoko OO, Kono H, Ishimaru K, Miyake K,
Kubota T, Ogawa H, Okumura K, Shibata S and Nakao A: Expressions of
tight junction proteins Occludin and Claudin-1 are under the
circadian control in the mouse large intestine: Implications in
intestinal permeability and susceptibility to colitis. PLoS One.
9:e980162014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barbara G, Zecchi L, Barbaro R, et al:
Mucosal permeability and immune activation as potential therapeutic
targets of probiotics in irritable bowel syndrome. J Clin
Gastroenterol. 46(Suppl): S52–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barbara G: Mucosal barrier defects in
irritable bowel syndrome. Who left the door open? Am J
Gastroenterol. 101:1295–1298. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Santos J, Guilarte M, Alonso C and
Malagelada JR: Pathogenesis of irritable bowel syndrome: the mast
cell connection. Scand J Gastroenterol. 40:129–140. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guilarte M, Santos J, de Torres I, et al:
Diarrhoea-predominant IBS patients show mast cell activation and
hyperplasia in the jejunum. Gut. 56:203–209. 2007. View Article : Google Scholar
|
|
23
|
Cenac N, Andrews CN, Holzhausen M, et al:
Role for protease activity in visceral pain in irritable bowel
syndrome. J Clin Invest. 117:636–647. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barbara G, Wang B, Stanghellini V, et al:
Mast cell-dependent excitation of visceral-nociceptive sensory
neurons in irritable bowel syndrome. Gastroenterology. 132:26–37.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iweala OI and Nagler CR: Immune privilege
in the gut: the establishment and maintenance of non-responsiveness
to dietary antigens and commensal flora. Immunol Rev. 213:82–100.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wisner DM, Harris LR III, Green CL and
Poritz LS: Opposing regulation of the tight junction protein
claudin-2 by interferon-gamma and interleukin-4. J Surg Res.
144:1–7. 2008. View Article : Google Scholar
|
|
27
|
Jacob C, Yang PC, Darmoul D, et al: Mast
cell tryptase controls paracellular permeability of the intestine.
Role of protease-activated receptor 2 and beta-arrestins. J Biol
Chem. 280:31936–31948. 2005. View Article : Google Scholar : PubMed/NCBI
|