Introduction
Bone defects are common in the clinic due to
increasing incidences of trauma, tumor excision and other
deformities (1,2). Despite bone tissue possessing the
ability to heal itself, manual intervention is often required to
promote repair processes when the defect is larger than the
critical size (3). With the
development of bone tissue engineering, scaffold-based bone
substitutes have resulted in significant progress, among which the
hydroxyapatite (HA)-based scaffold is the most frequently used
since it best mimics the natural bone composition with an ideal
calcium and phosphorus ratio (4).
Aside from the scaffold, the cell source is also important in
tissue regeneration. Bone marrow-derived mesenchymal stem cells
(BMSCs) are the most frequently used, due to their multilineage
differentiation capacity (5,6).
However, the bone marrow is difficult to obtain in practice,
whereas adipose-derived stem cells (ASCs) are easily accessible,
which is an advantage for tissue engineering (7). It has already been recognized that
ASCs can be used as an alternative to BMSCs for tissue engineering
(8,9) and are considered to be a more
promising source of stem cells (10). However, the osteogenic capability
of ASCs is limited despite being incorporated with an HA scaffold
(11) or other scaffolds (12). Consequently, the osteogenic
capability of ASCs requires improvement (13).
To date, the recombinant adenovirus vector
containing human bone morphogenetic protein 2 (BMP2) has been used
to transfect ASCs prior to incorporation with the scaffold
(13). However, as the BMP2 gene
is exogenous, this may potentially lead to genome modifications.
microRNA (miRNA)-based intervention to improve bone anabolic
metabolism has been previously investigated and has potential for
application in the clinic due to its biosafety and high gene
regulatory efficiency (14,15).
More specifically, miR-26a has been demonstrated to simultaneously
promote osteogenesis and angiogenesis in BMSCs (16). In the present study, it was
hypothesized that miR-26a may also modify the function of ASCs
incorporated with an HA scaffold to promote new bone
generation.
In the present study, primary ASCs were isolated and
expanded from rat peritoneal adipose tissue. The miR-26a-modified
ASCs were analyzed in vitro and the cell loaded HA scaffold
was implanted into tibias with a critical sized defect in order to
observe its ability to repair bone tissue.
Materials and methods
Cell isolation and culture
The animal procedures used in the present study were
approved by the Ethics Committee of the School of Medicine, Ningbo
University (Ningbo, China). The primary ASCs were isolated and
cultured as described previously (17). Specifically, the fresh peritoneal
adipose tissue (~2 cm3) was isolated under sterilized
conditions, washed with phosphate-buffered saline (PBS) and minced,
followed by digestion in 0.1% collagenase (type I; Sigma-Alrich,
St. Louis, MO, USA) for 40 min in an orbital shaker at 37°C. The
digestion was terminated by culture medium containing α-minimum
essential medium (HyClone Laboratories, Inc., Logan, UT, USA), 10%
fetal bovine serum (HyClone Laboratories, Inc.), 100 U/ml
penicillin and 100 mg/ml streptomycin (HyClone Laboratories, Inc.).
The mixture was filtered through a cell strainer (100 µm
pore size; Falcon, BD Biosciences, Franklin Lakes, NJ, USA) and
centrifuged at 120 × g for 5 min. The pellet was then resuspended
and maintained in culture medium. Medium was changed twice a week
and when cells reached 80% confluence, they were detached with
Trypsin (HyClone Laboratories, Inc.) for passage culture. Passages
3–5 were used for further experiments.
Transfection procedure
The cells were plated at a concentration of
2×104 cells/ml in different cell culture plates (96-well
plates for proliferation analysis and 6-well plates for other in
vitro studies) 24 h prior to transfection. The transfection
procedure was performed according to the manufacturer's
instructions of the Lipofectamine 2000 transfection reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). Briefly,
Lipofectamine 2000, the miR-26a mimic (sense,
5′-UUCAAGUAAUCCAGGAUAGGCU-3′ and antisense,
5′-CCUAUCCUGGAUUACUUGAAUU-3′, denoted as Lipo/miR-26a), the
negative control (sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′, denoted as Lipo/NC) or equal volume of
RNase-free water (denoted as Lipo) were diluted in Opti-MEM I
(Gibco-BRL, Carlsbad, CA, USA) and mixed together. The transfection
complex was added directly into the cell culture medium (final
miRNA concentration, 50 nM) and incubated for 6 h. Subsequently,
the medium was changed to normal culture medium to terminate
transfection. The non-treated (NTR) cells were used as a blank
control. To observe the transfection process, Cy5-labeled miR-26a
was used and the cell membrane was stained with
3,3′-dioctadecyloxacarbocyanine perchlorate (Beyotime Institute of
Biotechnology, Haimen, China) at the end of transfection. Images
were captured using an inverted fluorescence microscope (Olympus
IX70; Olympus, Tokyo, Japan).
Morphological observation and cell
proliferation analysis
To observe the morphology of ASCs, cells were
pre-seeded on the cover glass and transfection was performed. Cells
were fixed with 2% glutaraldehyde overnight at 4°C 1 day post
transfection. Following dehydration in a series of graded ethanol,
the specimens were sputter coated with platinum and observed by
scanning electron microscopy (SEM; S-4800; Hitachi, Tokyo, Japan).
For proliferation analysis, the ASCs were seeded into a 96-well
plate and transfected. The cell proliferation was represented by
the cell viability, which was determined using a Cell Counting
kit-8 (CCK-8) kit (Beyotime Institute of Biotechnology)
continuously for 7 days. Briefly, the medium was removed and
replaced with 100 µl reaction solution (CCK-8: medium=1:10).
Following incubation for 3 h, the supernatant was transferred into
a new 96-well plate and the absorbance was read at 465 nm using a
microplate reader (Bio-Tek Instruments, Inc., Winooski, VT,
USA).
Reverse transcription
quantitative-polymerase chain reaction (RT-qPCR) analysis
Following seeding and transfection, the cells were
allowed to proliferate for 3 days in culture medium and then the
medium was replaced with osteogenic medium containing 10 mM
β-glycerophosphate (Sigma-Aldrich), 50 mg/ml ascorbic acid
(Sigma-Aldrich) and 10−7 M dexamethasone (MP
Biomedicals, Santa Ana, CA, USA) for a further 3 and 7 days. Total
RNA was then isolated using TRIzol reagent (Invitrogen Life
Technologies) and reverse transcribed to complementary DNA (cDNA)
using a PrimeScript™ RT reagent kit (Takara Bio, Inc., Shiga,
Japan). Normalized cDNA was used for amplification with an SYBR
Premix Ex™ Taq II RT-PCR kit (Takara Bio, Inc.) in an Applied
Biosystems 7500 Real-Time PCR System (Applied Biosystems, Foster
City, CA, USA). RT-qPCR was performed to measure the
osteogenic-associated genes, including alkaline phosphatase (ALP),
collagen I (COL1), osteocalcin (OCN) and bone morphogenetic protein
2 (BMP2). Glyceraldehyde 3-phosphate dehydrogenase was used as an
endogenous reference. The primers are listed in Table I.
 | Table IPrimers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer
sequence (5′-3′) | Reverse primer
sequence (5′-3′) |
|---|
| ALP |
AACGTGGCCAAGAACATCATCA |
TGTCCATCTCCAGCCGTGTC |
| COL1 |
GCCTCCCAGAACATCACCTA |
GCAGGGACTTCTTGAGGTTG |
| OCN |
GGTGCAGACCTAGCAGACACCA |
AGGTAGCGCCGGAGTCTATTCA |
| BMP2 |
CAACACCGTGCTCAGCTTCC |
TTCCCACTCATTTCTGAAAGTTCC |
| GAPDH |
GGCACAGTCAAGGCTGAGAATG |
ATGGTGGTGAAGACGCCAGTA |
ALP quantification
The cells were cultured and induced as described
above. ALP quantification was performed 7 days post induction based
on measuring the rate of p-nitrophenol formation from p-nitrophenyl
phosphate (Sigma-Aldrich). Briefly, the cells were rinsed with
Tris-buffered saline and fixed in 3.7% formaldehyde in 90% ethanol
for 30 sec at room temperature. Following removal of fixation, the
reaction substrate solution (1 mg/ml P-nitrophenyl phosphate in 50
mM NaHCO3, at pH 9.6 with 1 mM MgCl2) was
added and incubated at 37°C for 20 min. NaOH was added to terminate
the reaction and the absorbance was measured at 405 nm to represent
the relative ALP expression. ALP activity was expressed as
nanomoles of p-nitrophenyl produced per minute per microgram of
protein.
Osteogenic staining assay
The cells were treated and induced as described for
the RT-qPCR analysis. After 14 days of induction, the medium was
removed and rinsed with PBS followed by fixation in 4%
paraformaldehyde for 15 min. ALP staining was performed with a
BCIP/NBT Alkaline Phosphatase Color Development kit (Beyotime
Institute of Biotechnology) for 30 min. Collagen secretion and
extracellular matrix (ECM) mineralization was stained with Sirius
Red (0.1 wt% in saturated picric acid) and Alizarin Red (40 mM, pH
4.2), respectively. Unbound dye was removed with 0.1 M acetic acid
or distilled water and then images were captured using an inverted
optical microscope (IX70; Olympus).
ASC-loaded HA scaffold implantation
The rat tibial defect model was produced according
to a previous study (18). A total
of 20 male Sprague Dawley rats (6–8 weeks old) were purchased from
the School of Medicine, Ningbo University and maintained in clean
conditions with a normal diet. Animals were anesthetized with
sodium pentobarbital (Sigma-Aldrich). A 3.5 mm diameter cortical
defect was created and implanted with different pretreated
ASC-loaded HA scaffolds (Jiangsu Yenssen Biotech Co., Ltd.,
Jiangyin, China). Animals were sacrificed 12 weeks post
implantation and the tibia was isolated and fixed immediately in 4%
formaldehyde.
SEM
The specimen was dehydrated with ethanol and
freeze-dried. The dried sample was mounted by a double side carbon
tape, sputter coated and observed by SEM (S4800; Hitachi).
Histological evaluation
The tibia specimen was decalcified in 20% EDTA (pH
7.2–7.4) over 2 weeks. Decalcified tissue samples were embedded in
paraffin and sliced into 3 µm thick sections. Histological
evaluation was performed with routine hematoxylin and eosin
(H&E) and Masson's trichrome staining. Images were captured
using an optical microscope (Olympus) following being sealed in
neutral balsam.
Statistical analysis
Three independent biological experiments were
repeated and the quantitative data are presented as the mean ±
standard deviation. One-way analysis of variance accompanied with
Student-Newman-Keuls test were performed to compare the means.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Transfection, morphology and
proliferation
The cells were observed under an inverted
fluorescence microscope 1 day post transfection. Cy5-labeled
miR-26a was able to collocate with cells, which confirmed
successful transfection (Fig. 1A).
The cells spread well with abundant pseudopodia and protuberances
in all groups and no particular morphological alterations were
observed following miR-26a transfection (Fig. 1B). The proliferation curve was
obtained by optical density measurement following incubation with
CCK-8. Cell viability increased in each group and no statistical
differences were observed (Fig.
1C). A partial suppression was observed in the lipofectamine
only group and the NTR cells had the highest viability at each day,
suggesting that the partial suppression in viability in the
transfection groups may be due to lipofectamine cytotoxicity
(Fig. 1C).
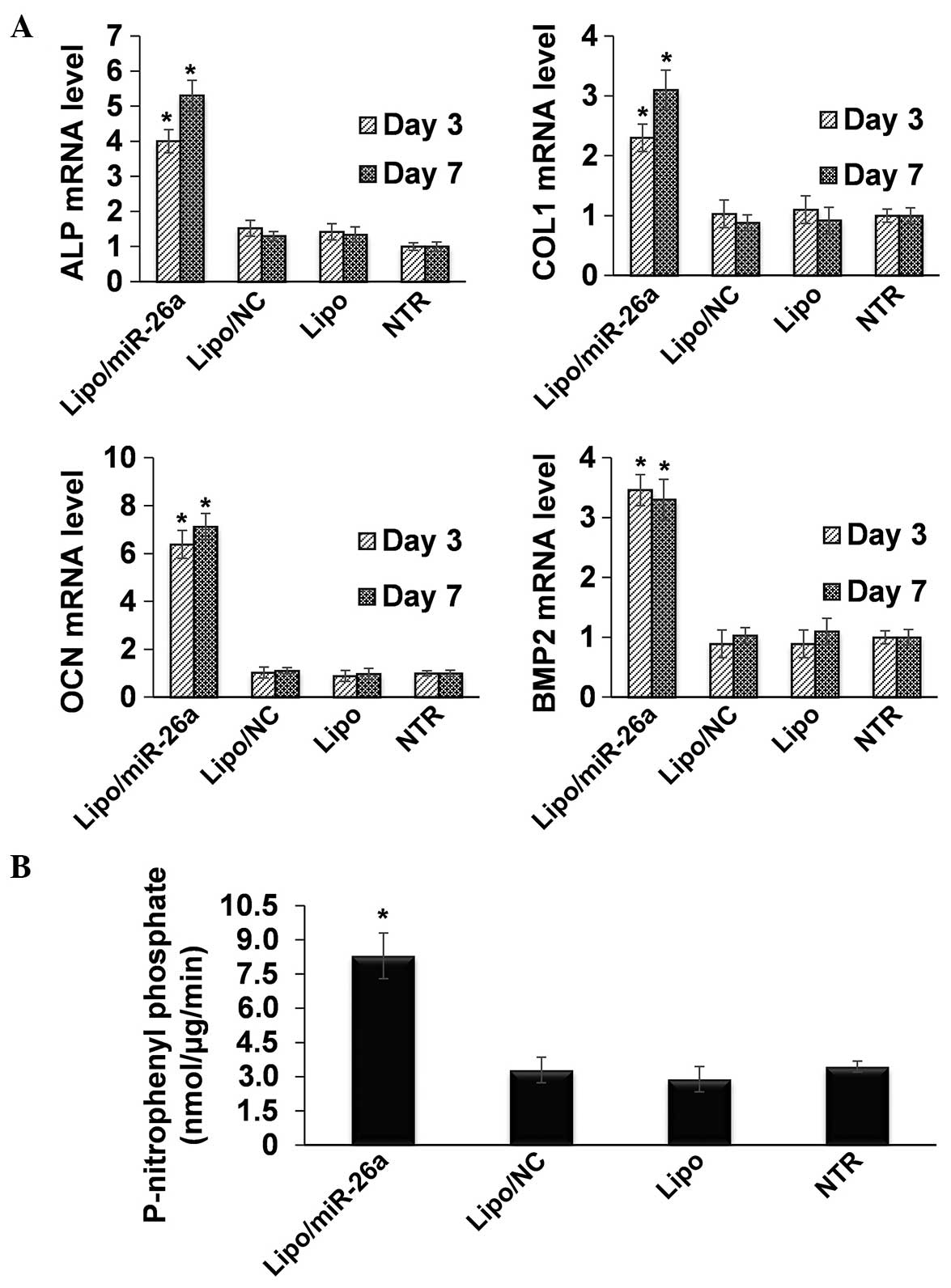
RT-qPCR and ALP quantification
In order to analyze the promotion of osteogenic
differentiation following transfection of miR-26a, RT-qPCR was
performed to measure the expression of osteogenic-associated genes.
As expected, all the genes measured in the present study were
markedly upregulated following transfection of miR-26a (Fig. 2A). Specifically, ALP increased
~4-fold on day 3 and >5-fold on day 7 (Fig. 2A). COL1 increased >2-fold on day
3 and ~3-fold on day 7 (Fig. 2A).
The expression of OCN increased by >6-fold on day 3 and
>7-fold on day 7, while the mRNA expression of BMP2 increased
>3-fold at the two time points (Fig. 2A). Quantification of ALP protein
expression was further performed based on the enzyme catalytic
reaction. The NTR sample expressed ALP ~3 nmol/µg/min after
7 days of induction in osteogenic medium, which was significantly
increased to ~8 nmol/µg/min following miR-26a transfection
(Fig. 2B).
Osteogenic staining analysis
ALP production, collagen secretion and ECM
mineralization were analyzed. In accordance with the RT-qPCR
results, ALP production and collagen secretion was abundantly
detected in the miR-26a transfection group (Fig. 3). In addition, the calcium nodules
in the miR-26a group were also more prominent compared with the
control groups (Fig. 3).
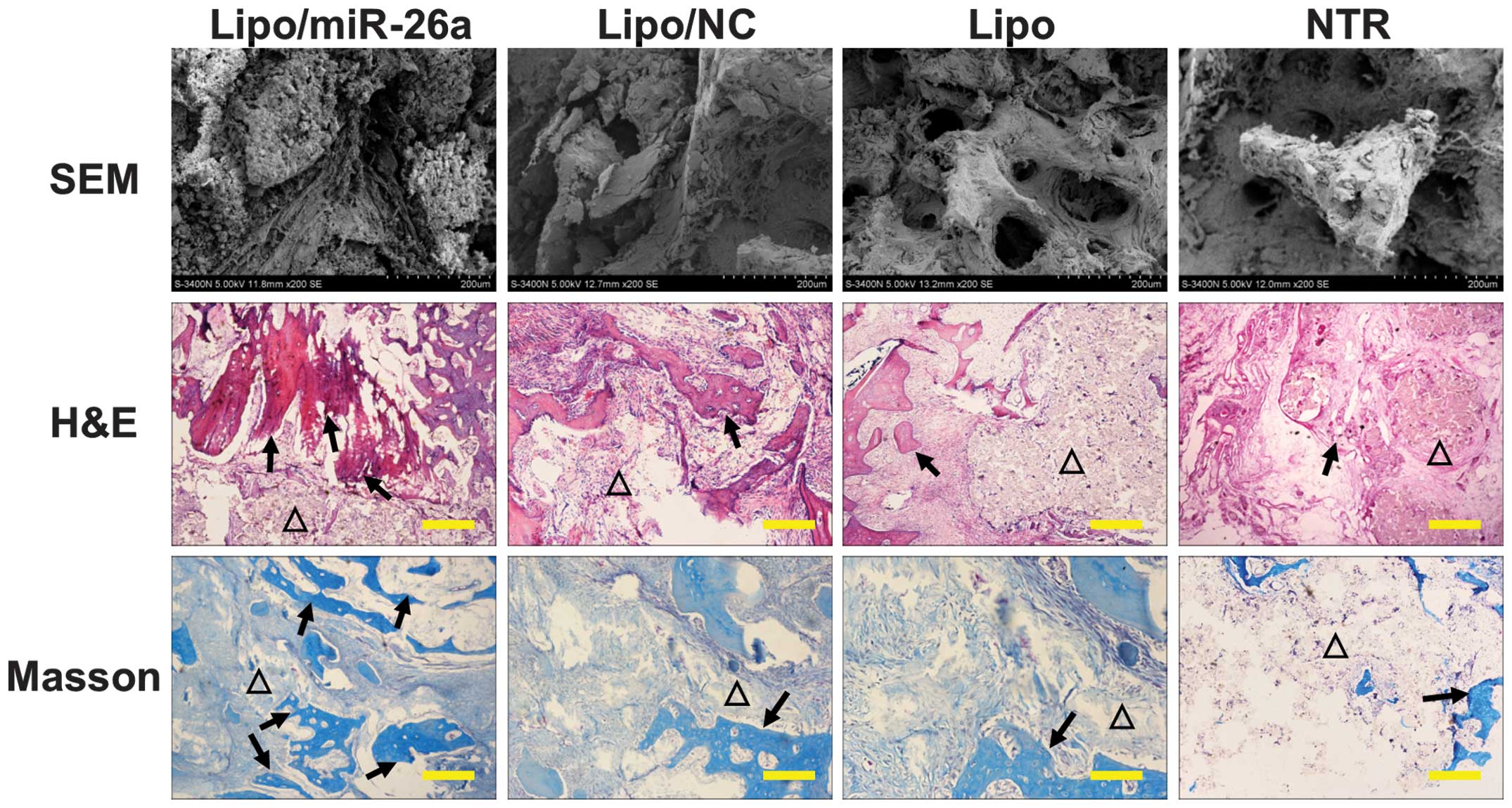
SEM observation and histological
evaluation
The scaffold was collected 12 weeks post
implantation and the residual tissue on the scaffold was observed
by SEM. The scaffold pores were filled with abundant ECM,
particularly in the Lipo/miR-26a group in which the surface was
covered with large collagen fibers and the pores were not visible
(Fig. 4). The pores could still be
detected in the Lipo/NC, Lipo and NTR group (Fig. 4). The in vivo bone anabolic
ability was analyzed by H&E and Masson's trichrome staining to
visualize new bone regeneration. All the groups healed well without
apparent inflammatory granuloma (Fig.
4). In line with the in vitro observation, a larger area
and notable new bone formation was observed in the
Lipo/miR-26a-transfected group under H&E staining (black
arrows) and Masson's trichrome staining (black arrows) compared
with the control groups (Fig.
4).
Discussion
The ideal effect of bone defect repair is to induce
autologous bone regeneration instead of exogenous replacement. The
development of stem cell technology has made it possible for
multilineage MSCs to differentiate into osteoblasts and osteocytes
to formulate the new bone tissue. However, bone regeneration often
requires a large number of MSCs and despite MSCs being universally
expressed in numerous tissues, in the majority of cases they are
difficult to isolate. Although in vitro expansion can
increase the cell number, long term proliferation and continuous
passage have adverse effects on the normal function of BMSCs
(19). By contrast, ASCs are
derived from adipose tissue and can be easily obtained in a large
number. Therefore, ASCs are considered to be an ideal alternative
source of cells to BMSCs for tissue engineering.
In addition, to improve the function of ASCs in bone
tissue engineering, the osteogenic capability of ASCs requires
improvement. A previous study demonstrated that ASCs are
transfected by a recombinant adenovirus vector containing human
BMP2 (13). However, as the BMP2
gene is exogenous, this may potentially lead to genome
modifications. miRNAs represent powerful endogenous therapeutic
molecules that can regulate multiple target genes. It is
hypothesized that their application in vivo may better mimic
natural regulatory pathways as they are endogenous regulators in
cells (20). In addition, the
differentiation of stem cells may even be accelerated by miRNA
treatment (21). Hence, miRNA is a
promising molecule for improving the function of ASCs. In the
present study, miR-26a, a novel osteogenic-angiogenic promoting
molecule, was transfected into ASCs. The results demonstrated that
the transfection of miR-26a markedly increased the osteogenic
differentiation of ASCs in vitro without apparent effects on
morphology or viability. The in vivo evaluation also
demonstrated new bone formation following miR-26a transfection.
Taken together, the results suggest that miR-26a can be applied to
enhance the osteogenic capacity of ASCs.
Scaffolds are another critical element for bone
regeneration. The ideal scaffold is able to support the defective
area, allow the cells to penetrate, provide calcium and phosphorus
elements and eventually degrade. Porous structures with an adequate
pore diameter are beneficial for the penetration of cells and the
circulation of nutrients (22). An
accurate calcium phosphorus ratio is also important for new bone
mineralization (23,24). HA, a frequently used scaffold for
bone substitutes, is similar to the natural bone composition and
has been developed numerous times (25–27).
Consequently, the HA scaffold was selected in the present study to
ensure that the results best demonstrate the capabilities of
transfected ASCs.
In conclusion, the present study demonstrated that
miR-26a can markedly increase the osteogenic differentiation
ability of ASCs without apparent cytotoxicity in vitro. The
combination of miR-26a-enhanced ASCs and an HA scaffold can
significantly improve new bone formation, and thus may be used as a
bone substitute for repairing bone defects.
References
|
1
|
Wiese A and Pape HC: Bone defects caused
by high-energy injuries, bone loss, infected nonunions and
nonunions. Orthop Clin North Am. 41:1–4. 2010. View Article : Google Scholar
|
|
2
|
Nishida J and Shimamura T: Methods of
reconstruction for bone defect after tumor excision: A review of
alternatives. Med Sci Monit. 14:RA107–RA113. 2008.PubMed/NCBI
|
|
3
|
Spicer PP, Kretlow JD, Young S, Jansen JA,
Kasper FK and Mikos AG: Evaluation of bone regeneration using the
rat critical size calvarial defect. Nat Protoc. 7:1918–1929. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kundu B, Soundrapandian C, Nandi SK, et
al: Development of new localized drug delivery system based on
ceftriaxone-sulbactam composite drug impregnated porous
hydroxyapatite: A systematic approach for in vitro and in vivo
animal trial. Pharm Res. 27:1659–1676. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tian HT, Zhang B, Tian Q, Liu Y, Yang SH
and Shao ZW: Construction of self-assembled cartilage tissue from
bone marrow mesenchymal stem cells induced by hypoxia combined with
GDF-5. J Huazhong Univ Sci Technolog Med Sci. 33:700–706. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao J, Yang C, Su C, et al:
Reconstruction of orbital defects by implantation of antigen-free
bovine cancellous bone scaffold combined with bone marrow
mesenchymal stem cells in rats. Graefes Arch Clin Exp Ophthalmol.
251:1325–1333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fraser J, Zhu M, Wulur I and Alfonso Z:
Adipose-derived stem cells. Mesenchymal Stem Cells. Prockop D,
Bunnell B and Phinney D: 449. Human Press; pp. 59–67. 2008,
View Article : Google Scholar
|
|
8
|
Gómez-Lechón M and Tolosa L: Hepatogenic
differentiation: Comparison between adipose tissue-derived stem
cells and bone marrow mesenchymal stem cells. Stem Cells and Cancer
Stem Cells. Hayat MA: 10. Springer; Netherlands: pp. 45–57.
2013
|
|
9
|
Marappagounder D, Somasundaram I, Dorairaj
S and Sankaran R: Differentiation of mesenchymal stem cells derived
from human bone marrow and subcutaneous adipose tissue into
pancreatic islet-like clusters in vitro. Cell Mol Biol Lett.
18:75–88. 2013. View Article : Google Scholar
|
|
10
|
Zhu X, Shi W, Tai W and Liu F: The
comparition of biological characteristics and multilineage
differentiation of bone marrow and adipose derived Mesenchymal stem
cells. Cell Tissue Res. 350:277–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arrigoni E, de Girolamo L, Di Giancamillo
A, et al: Adipose-derived stem cells and rabbit bone regeneration:
Histomorphometric, immunohistochemical and mechanical
characterization. J Orthop Sci. 18:331–339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mohan BG, Suresh Babu S, Varma HK and John
A: In vitro evaluation of bioactive strontium-based ceramic with
rabbit adipose-derived stem cells for bone tissue regeneration. J
Mater Sci Mater Med. 24:2831–2844. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hao W, Dong J, Jiang M, Wu J, Cui F and
Zhou D: Enhanced bone formation in large segmental radial defects
by combining adipose-derived stem cells expressing bone
morphogenetic protein 2 with nHA/RHLC/PLA scaffold. Int Orthop.
34:1341–1349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song W, Wu K, Yan J, Zhang Y and Zhao L:
MiR-148b laden titanium implant promoting osteogenic
differentiation of rat bone marrow mesenchymal stem cells. RSC
Advances. 3:11292–11300. 2013. View Article : Google Scholar
|
|
15
|
Wu K, Song W, Zhao L, et al: MicroRNA
functionalized microporous titanium oxide surface by lyophilization
with enhanced osteogenic activity. ACS Appl Mater Interfaces.
5:2733–2744. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Fan L, Liu S, et al: The promotion
of bone regeneration through positive regulation of
angiogenic-osteogenic coupling using microRNA-26a. Biomaterials.
34:5048–5058. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pieri F, Lucarelli E, Corinaldesi G, et
al: Dose-dependent effect of adipose-derived adult stem cells on
vertical bone regeneration in rabbit calvarium. Biomaterials.
31:3527–3535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu W, Ganz C, Weber U, et al: Evaluation
of injectable silica-embedded nanohydroxyapatite bone substitute in
a rat tibia defect model. Int J Nanomedicine. 6:1543–1552. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Liu C, Li S, et al: Effects of
continuous passage on immunomodulatory properties of human
adipose-derived stem cells. Cell Tissue Bank. 16:143–150. 2015.
View Article : Google Scholar
|
|
20
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yau WW, Rujitanaroj PO, Lam L and Chew SY:
Directing stem cell fate by controlled RNA interference.
Biomaterials. 33:2608–2628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rose FR, Cyster LA, Grant DM, Scotchford
CA, Howdle SM and Shakesheff KM: In vitro assessment of cell
penetration into porous hydroxyapatite scaffolds with a central
aligned channel. Biomaterials. 25:5507–5514. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Masuyama R, Nakaya Y, Katsumata S, et al:
Dietary calcium and phosphorus ratio regulates bone mineralization
and turnover in vitamin D receptor knockout mice by affecting
intestinal calcium and phosphorus absorption. J Bone Miner Res.
18:1217–1226. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koshihara M, Masuyama R, Uehara M and
Suzuki K: Effect of dietary calcium: Phosphorus ratio on bone
mineralization and intestinal calcium absorption in ovariectomized
rats. Biofactors. 22:39–42. 2004. View Article : Google Scholar
|
|
25
|
D'Antonio JA, Capello WN and Jaffe WL:
Hydroxylapatite-coated hip implants. Multicenter three-year
clinical and roentgenographic results. Clin Orthop Relat Res.
285:102–115. 1992.PubMed/NCBI
|
|
26
|
Ghanaati SM, Thimm BW, Unger RE, et al:
Collagen-embedded hydroxylapatite-beta-tricalcium phosphate-silicon
dioxide bone substitute granules assist rapid vascularization and
promote cell growth. Biomed Mater. 5:250042010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lima PA, Resende CX, Soares GD, Anselme K
and Almeida LE: Preparation, characterization and biological test
of 3D-scaffolds based on chitosan, fibroin and hydroxyapatite for
bone tissue engineering. Mater Sci Eng C Mater Biol Appl.
33:3389–3395. 2013. View Article : Google Scholar : PubMed/NCBI
|