Introduction
Ewing's sarcoma (ES) is a highly malignant bone
tumor occurring in children, adolescents and young adults (1), which is a type of primitive neural
ectoderm family of invasive tumor (2). Patients with ES under the age of 15
comprise ~80% of ES cases (3).
Currently, clinical chemotherapy, surgery and radiotherapy,
including traditional methods, are used to treat ES. However, only
60% of the patients with local disease are cured (4,5). The
underlying mechanism of ES remains to be elucidated.
MicroRNAs (miRNAs) are a type of endogenous
non-coding small RNA molecule, ~22 nucleotides in length, which
regulate the expression of target genes predominantly at the
post-transcriptional level (6,7).
Previous studies demonstrated that miRNAs were involved in almost
all the human physiological activities, including cell
proliferation, apoptosis, cell growth and differentiation, and
provided increasing confirmation in these aspects, and at the same
time also suggested that miRNAs are involved in the occurrence of
various types of disease (8,9).
Revealing the regulatory function and mechanism of miRNAs may
assist in better understanding the complex regulatory network of
higher eukaryotes. This may lead to miRNAs being used clinically as
convenient and practical gene therapy treatments.
miRNAs were demonstrated to be important in ES cells
(10,11). The present study used ES cell
lines, A673 and TC252, to investigate the function and mechanism of
miR-199b-5p in vitro. It was demonstrated that miR-199b-5p
was a tumor suppressor in these ES cell lines, which inhibited cell
proliferation and cell invasion, arrested cell cycle progression,
and promoted apoptosis. In addition, it was revealed that
miR-199b-5p directly targeted CCNL1 to perform this function in ES
cells. Taken together, the present study successfully identified
miR-199b-5p as a key regulator in ES cells.
Materials and methods
Cell lines and cell culture
The A673 and TC252 cell lines were maintained in
RPMI-1640 (HyClone Corp., Logan, UT, USA), supplemented with 10%
fetal bovine serum (FBS; PAA, Cölbe, Germany). The human
mesenchymal stem cells (MSCs) as well as the A673 and TC252 cell
lines were obtained from the American Type Culture Collection
(ATCC; Rockville, MD, USA) and were cultured in Iscove's modified
Eagle's medium, supplemented with 10% FBS and platelet-derived
growth factor-BB (10 ng/ml). The 293T cells were obtained from ATCC
and cultured in Dulbecco's modified Eagle's medium, supplemented
with 10% FBS.
Oligonucleotides and transfection
The miRNA-199b-5p mimic and scramble control
molecules were obtained from Dharmacon (Chicago, IL, USA) and were
transfected into the A673 and TC252 cells at a final concentration
of 60 nM. The A673 and TC252 cells were mixed into complete medium
following 6 h culture and were washed with phosphate-buffered
saline (PBS) 48 h following transfection for the subsequent
experiments.
RNA extraction and reverse transcription
quantitative polymerase chain reaction (RT-qPCR)
The total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA), according to the
manufacturer's instructions. To determine the quality and purity of
the RNA, the absorption peak of RNA was detected at 260 nm, 260/280
nm, and 260/230 nm using a NanoDrop (NanoDrop Technologies,. Inc.,
Thermo Fisher Scientific, Wilmington, DE, USA). The cDNA was
generated using M-MLV reverse transcriptase (Invitrogen Life
Technologies), according to the manufacturer's instructions.
RT-qPCR was performed using the SYBR Premix ExTaq
kit (Takara Bio, Inc., Dalian, China) on the ABI PRISM 7500
real-time PCR System (Applied Biosystems, Foster City, CA, USA).
All oligonucleotides were transfected into A673 and TC252 cells
using Dhamafect 1 (Dharmacon). The sequences of the specific
primers used for PCR were: miR-199b-5P, forward: 5′-CAG CCC AGT GTT
TAG ACT ATC-3′ and reverse: 5′-CAG TGC AGG GTC CGA GGT-3′; U6,
forward: 5′-CTC GCT TCG GCA GCA CAT ATACT-3′ and reverse: 5′-ACG
CTT CAC GAA TTT GCG TGTC-3′. The cycling conditions were as
follows: 95°C for 3 min, 95°C for 5 sec, 60°C for 30 sec, 72°C for
30 sec then 72°C for 10 min for 30 cycles. The data were uniformly
normalized to the internal control U6 and the relative expression
levels were evaluated using the 2-ΔΔCt method (12). The mRNA expression levels of U6
were used as endogenous control. All the experiments were performed
three times.
Proliferation assay
The cells were seeded (8×103 cells/well)
in 24-well plates and proliferation was determined using a cell
counting kit-8 (CCK8; Dojindo Laboratories, Kumamoto, Japan).
miR-99b-5p mimic was transfected after 16 h. The cells were
collected and digested with 0.25% trypsin, then, suspended in fresh
medium. The proliferative activity was detected at 0, 24, 48 and 72
h with CCK8. A total of 10 µl/well CCK8 was added to the
medium 2 h prior to testing at 37°C. The absorbance in each well
was measured with a microplate reader at 450 and 630 nM. (650 nm
wavelength as reference wavelength; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Cell cycle analysis
The A673 and TC252 cells were cultured in serum-free
medium following 4 h of transfection and were cultured for 24 h,
prior to the addition of complete medium. The A673 and TC252 cells
were washed twice with cold PBS 48 h following transfection and
were subsequently fixed in cold 70% alcohol overnight. The fixed
cells were incubated in propidium iodide and RNase A, and were
detected by flow cytometry using a C6 Flow Cytometer®
Instrument (BD Biosciences, Franklin Lakes, NJ, USA).
Apoptosis assay
The A673 and TC252 cells were labeled with Annexin-V
at early apoptosis and were labeled with 7AAD at late apoptosis or
to indicate cell death. The collected cells were diluted to
5×105 cells/ml 48 h after transfection. The cells were
washed twice with cold PBS and were subsequently incubated with PE
annexin-V and 7AAD (BD Biosciences, Bedford, MA, USA). The data
were analysis by fluorescence-activated cell sorting.
Invasion assays
The A673 and TC252 cells were collected and adjusted
at a concentration of 2×105 cells/ml 24 h after
transfection. Culture medium, containing 10% FBS, was added to the
lower chamber (at the bottom of a 24-well plate) and the cell
suspension was added to the upper chamber. The A673 and TC252 cells
were maintained in culture for 24 h. The cells in the upper chamber
were washed away and stained with 0.1% crystal violet at room
temperature for 15 min. The number of cells were counted in 10
different fields under the microscope (CKX41; Olympus, Tokyo,
Japan) and the total number of cells invading through the Matrigel
were counted in 10 representative fields under a microscope (BD
Biosciences).
Western blot analysis
The A673 and TC252 cells were collected 48 h after
transfection, washed twice with PBS and lysed on ice in cold
modified radioimmunoprecipitation buffer supplemented with protease
inhibitors (Roche, Mannheim, Germany) for 30 min. The protein
concentration was detected using a Bicinchoninic Acid Protein Assay
kit (Beyotime Institute of Biotechnology, Shanghai, China). An
equal quantity of protein (30 µg) was separated by 10%
SDS-PAGE. The protein was transferred onto a nitrocellulose
membrane (Millipore, Billerica, MA, USA) following electrophoresis.
The membrane was blocked for 2 h with 5% non-fat milk and was
incubated with antibodies against CCNL1 (1:200; cat. no. abc-102;
Millipore), c-kit (1:100; cat. no. ab5506; Abcam, Cambridge, MA,
USA) and GAPDH (1:5,000; Cell Signaling Technology, Inc., Danvers,
MA, USA) overnight at 4°C. Following incuabtion with a secondary
antibody (cat. no. ZB-2301; Zhong-Shan JinQuao, Shanghai, China),
detection was performed using an enhanced chemiluminescence system
(Thermo Fisher Scientific, Waltham, MA, USA).
Statistical analysis
The data were analyzed by the Student's t-test
(two-tailed). P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of miR-199b-5p is
downregulated in ES cells
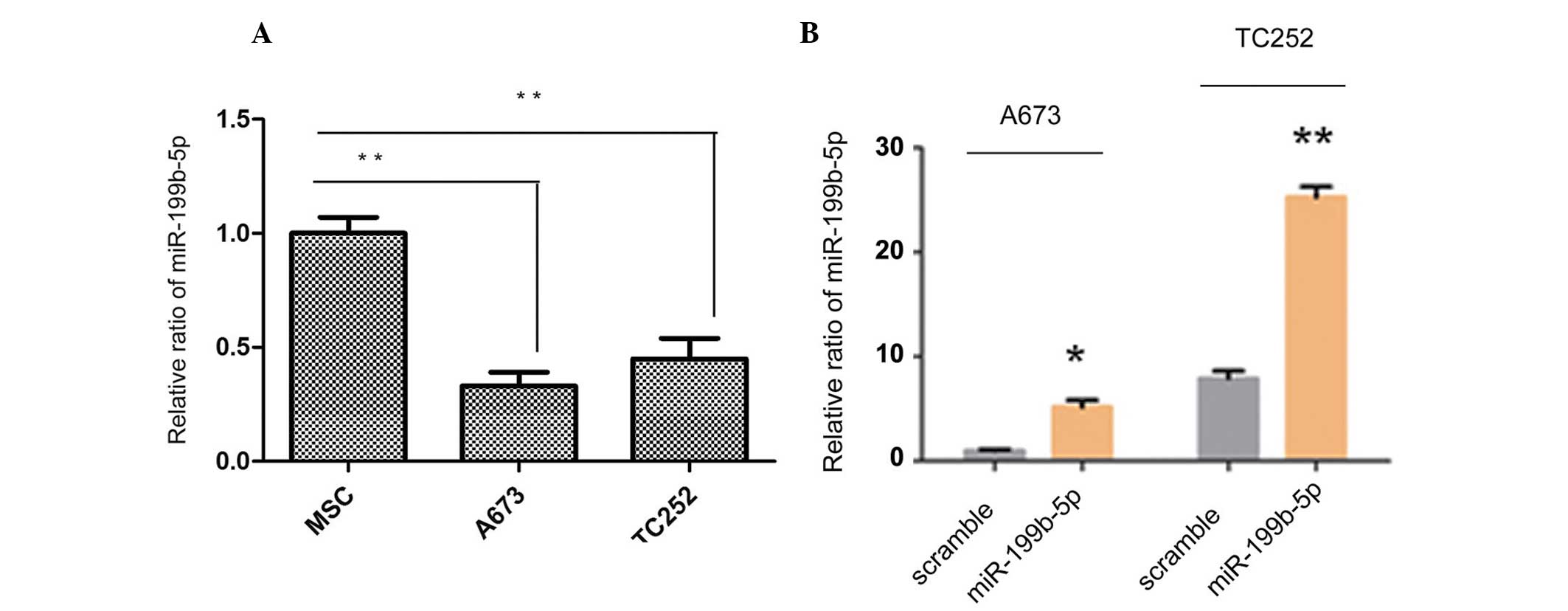
RT-qPCR revealed that the expression levels of
miR-199b-5p were downregulated in the ES cell line compared with
the human MSCs, as shown in Fig.
1A. The MSC bone marrow mesenchymal stem cells were used as
normal scramble control ES cells. A mature miR-199b-5p mimic and
scramble mimic were constructed and transfected into the cells
in vitro to overexpress the levels of miR-199b-5p in the
A673 and TC252 cells. The expression levels of the miR-199b-5p
mimic were subsequently detected in the A673 and TC252 cells. As
shown in Fig. 1B, the
overexpression was considered significantly different, compared
with the scramble control. Taken together, these findings suggested
that miR-199b-5p may act as a negative modulator in ES cells.
Overexpression of miR-199b-5p inhibits
proliferation and invasion, inhibits cell cycle progression, and
induces apoptosis in ES cells
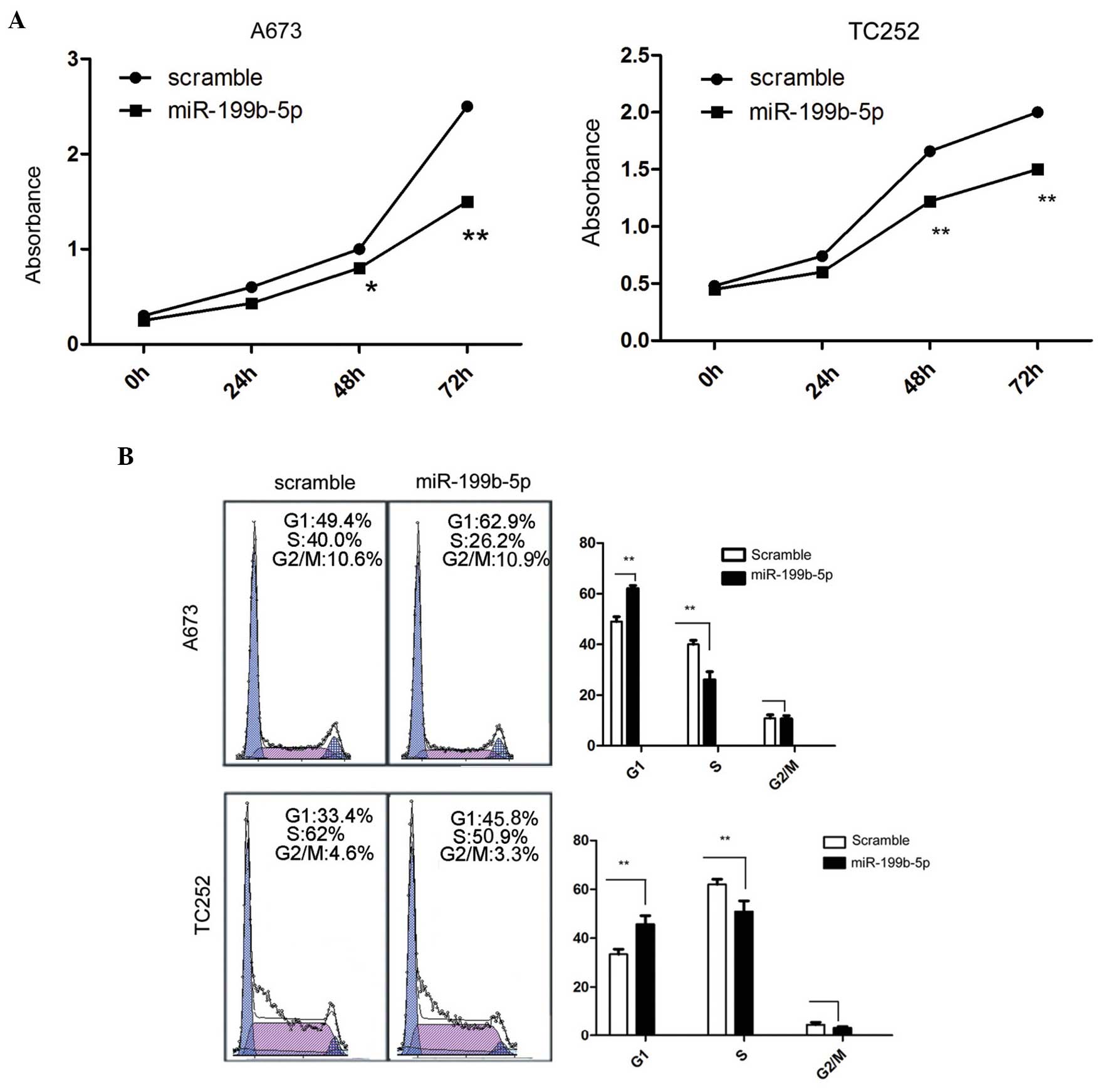
The A673 and TC252 cells were harvested separately
following transfection with miR-199b-5p mimic and scramble control
at 0, 24, 48 and 72 h. The activity of A673 cells was subsequently
assessed at different time points. The overexpression of
miR-199b-5p significantly inhibited the cell proliferation compared
with the scramble control in vitro, as shown in Fig. 2A.
Since cell proliferation was directly associated
with the cell cycle, the effect of the miR-199b-5p mimic on the
cell cycle was analysed. The quantity of cells in the G1 phase
increased significantly following the forced expression of
miR-199b-5p (Fig. 2B). By
contrast, the percentage of cells in S phase decreased in the A673
and TC252 cells (Fig. 2B).
Therefore, the G1- to S-phase transition was inhibited by the
overexpression of miR-199b-5p.
Cell apoptosis may be the cause of the change in
cell growth and proliferation in the ES cells. The number of early
apoptosis cells following transfection with the miR-199b-5p mimic
was then assessed. The ratio of early apoptotic cells markedly
increased, as detected by PE-Annexin V staining following
transfection with the miR-199b-5p mimic compared with the scramble
(Fig. 2C).
In addition, the cell invasive ability was
determined following the forced expression of miR-199b-5p in ES
cells. The Matrigel invasion chamber assays demonstrated that the
invasive ability of cells with overexpression of miR-199b-5p
significantly decreased (Fig. 2D).
These results suggested that miR-199b-5p markedly inhibited cell
proliferation, inhibited the cell cycle transition, induced cell
apoptosis and suppressed the invasion of ES cells.
miR-199b-5p represses CCNL1 to regulate
ES cells
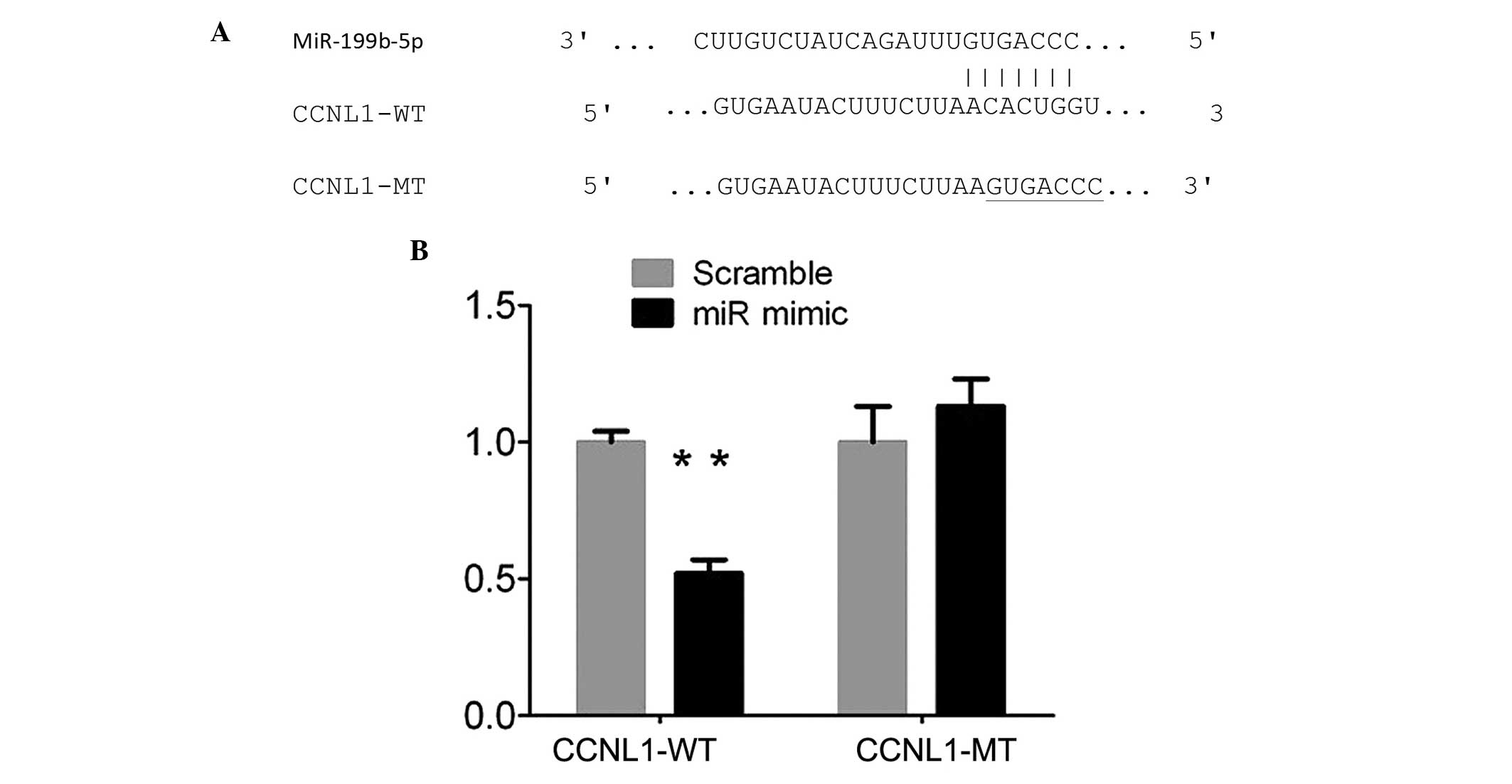
miRNAs regulate mRNA targets by inhibiting protein
translation or directly degrading the mRNA. Therefore, the present
study determined potential target genes of miR-199b-5p using
Targetscan (www.targetscan.org) and PicTar
(www.picTar.mdc-berlin.de). CCNL1 was
revealed as a possible target gene of miR-199b-5p in ES cells using
a luciferase activity assay. Sequence analysis demonstrated that
the 3′UTR of CCNL1 contained the miR-199b-5p binding sites
(Fig. 3A). The 3′UTR of CCNL1 was
cloned downstream of the pMIR- report gene, which formed CCNL1-WT
and similarly, the CCNL1-MT was generated. The results revealed
that the relative luciferase activity of the CCNL1-WT was
significantly inhibited following transfection with the miR-199b-5p
mimic compared with scramble control. However, luciferase activity
of the CCNL1-MT remained unchanged (Fig. 3B). Additionally, miR-199b-5p may
act as a tumor suppressor by repressing the expression of CCNL1 in
ES cells.
CCNL1 is the target gene of
miR-199b-5p
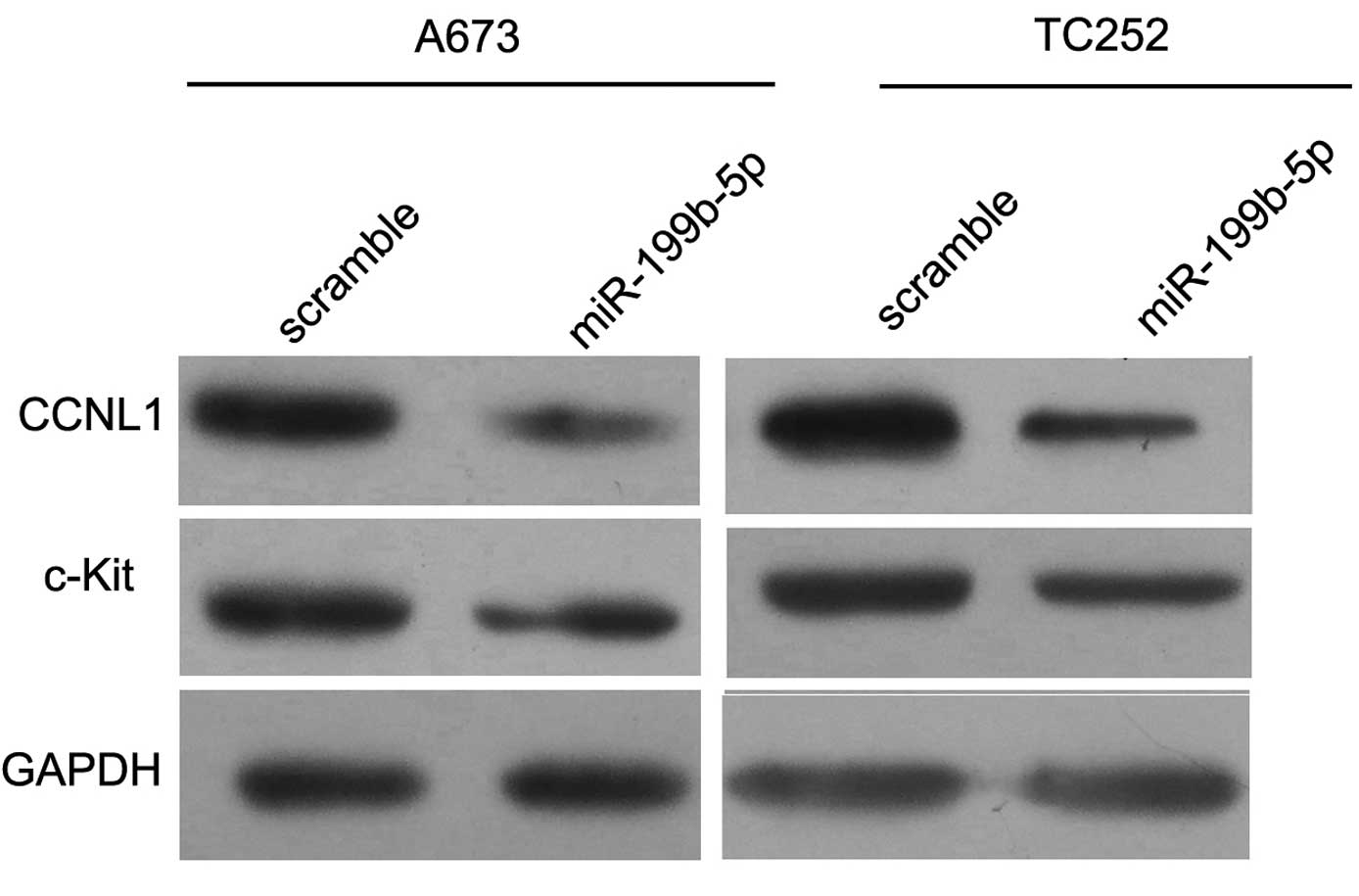
To confirm that CCNL1 was a target gene of
miR-199b-5p, the A673 and TC252 cells were collected and the total
protein was extracted following the overexpression of miR-199b-5p
at 72 h. Western blotting revealed that the protein expression
levels of CCNL1 markedly decreased (Fig. 4A). Therefore, miR-199b-5p may
inhibit ES cells by targeting CCNL1 in vitro.
Discussion
miRNAs are important in the progression of tumor
cells (13). The expression of
miR-199b-5p was downregulated in a wide variety of tumor types,
including ovarian cancer, breast cancer, thyroid cancer and
osteosarcoma. miR-199b-5p may be downregulated by activation of the
JAG1-Notch1 signaling pathways in ovarian cancer (14). miR-199b-5p has been demonstrated to
inhibit cancer cell migration and colony formation in breast cancer
(15) and reduces the
proliferation of thyroid follicular cancer cells (16). Won et al (17) revealed that miR-199b-5p is involved
in the Notch signaling pathway in osteosarcoma and suggested the
inhibitor of miR-199b-5p may be a potential treatment strategy to
prevent osteosarcoma metastasis. Garzia et al (18) demonstrated that the expression of
miR-199b-5p correlated with metastasis in medulloblastoma tumor and
indicated that miR-199b-5p may be combined with radiation and
chemotherapy as an auxiliary treatment to improve the antitumor
effect and life quality of patients. These studies provided to
suggest the benefit in identifying the role of miR-199b-5p in ES
cells.
The present study assessed the expression levels of
miR-199b-5p in ES A673 cells. The expression of mature miR-199b-5p
in the A673 cells was similar to the result in TC252 cells. In
addition, in A673 and TC252 cells the expression of miR-199b-5p was
downregulated compared with the levels in human MSCs, indicating
that miR-199b-5p may be involved in ES. Functional experiments
indicated that the forced expression of miR-199b-5p suppressed cell
proliferation rate, cell invasion, arrested the cell cycle and
induced cell apoptosis in each ES cell line. Bioinformatic
prediction revealed CCNL1 as a predicted target gene of
miR-199b-5p.
Notably, CCNL1 was demonstrated as a direct target
gene of miR-199b-5p by measuring luciferase activity and protein
expression levels. CCNL1, a cell cycle regulatory protein and a
potential oncogene, is localized in the 3q25 region and associated
with the survival rate of patients with head and neck squamous cell
carcinoma. For instance, CCNL1 was expressed and amplified in human
head and neck squamous cell carcinoma, and was suggested as an
oncogene (19,20).
In conclusion, the present study has demonstrated
that miR-199b-5p acted as a tumor suppressor by targeting CCNL1 in
ES cell lines. These findings may provide a novel insight into the
molecular mechanism underlying human ES. Furthermore, miR-199b-5p
may be a novel diagnostic marker or therapeutic target for the
treatment of human ES in the future.
Acknowledgments
This study was supported by the National Nature
Science Foundation of China (no. 51375142) and the Ministry of
Health Nature Science Foundation of China (nos. W2013ZT134 and
W2013ZT135).
References
|
1
|
Windsor R, Strauss S, Seddon B, et al:
Experimental therapies in Ewing's sarcoma. Expert Opin Invest
Drugs. 18:143–159. 2009. View Article : Google Scholar
|
|
2
|
Teicher BA, Bagley RG, Rouleau C, et al:
Characteristics of human Ewing/PNET sarcoma models. Ann Saudi Med.
31:174–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang ZZ, Huang L, Yu ZM, et al: Let-7a
Functions as a tumor suppressor in Ewing's sarcoma cell lines
partly by targeting cyclin-dependent kinase 6. DNA Cell Biol.
33:136–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borinstein SC, Beeler N, Block JJ, et al:
A decade in banking ewing sarcoma: a report from the children's
oncology group. Front Oncol. 3:572013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dylla L, Moore C and Jedlicka P: MicroRNAs
in Ewing sarcoma. Front Oncol. 3:652013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lujambio A and Esteller M: How epigenetics
can explain human metastasis: a new role for microRNAs. Cell Cycle.
8:377–382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Castilla-Llorente V, Nicastro G and Ramos
A: Terminal loop-mediated regulation of miRNA: selectivity and
mechanisms. Biochem Soc Trans. 41:861–865. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, You T and Jing J: MiR-125b inhibits
cell biological progression of Ewing's sarcoma by suppressing the
PI3K/Akt signalling pathway. Cell Prolif. 47:152–160. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nugent M: MicroRNA function and
dysregulation in bone tumors: the evidence to date. Cancer Manag
Res. 6:15–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu H, Huang L, Zhang Z, et al: LIM
mineralization protein-1 inhibits the malignant phenotypes of human
osteosarcoma cells. Int J Mol Sci. 15:7037–7048. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu MX, Siu MK, Liu SS, et al: Epigenetic
silencing of microRNA-199b-5p is associated with acquired
chemoresistance via activation of JAG1-Notch1 signaling in ovarian
cancer. Oncotarget. 5:944–958. 2014.PubMed/NCBI
|
|
15
|
Fang C, Zhao Y and Guo B: MiR-199b-5p
targets HER2 in breast cancer cells. J Cell Biochem. 114:1457–1463.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rossing M, Borup R, Henao R, et al:
Down-regulation of microRNAs controlling tumourigenic factors in
follicular thyroid carcinoma. J Mol Endocrinol. 48:11–23. 2012.
View Article : Google Scholar
|
|
17
|
Won KY, Kim YW, Kim HS, Lee SK, Jung WW
and Park YK: MicroRNA-199b-5p is involved in the Notch signaling
pathway in osteosarcoma. Hum Pathol. 44:1648–1655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garzia L, Andolfo I, Cusanelli E, Marino
N, Petrosino G, De Martino D, Esposito V, et al: MicroRNA-199b-5p
impairs cancer stem cells through negative regulation of HES1 in
medulloblastoma. PLoS One. 4:e49982009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Redon R, Hussenet T, Bour G, et al:
Amplicon mapping and transcriptional analysis pinpoint cyclin L as
a candidate oncogene in head and neck cancer. Cancer Res.
62:6211–6217. 2002.PubMed/NCBI
|
|
20
|
Sticht C, Hofele C, Flechtenmacher C, et
al: Amplification of Cyclin L1 is associated with lymph node
metastases in head and neck squamous cell carcinoma (HNSCC). Br J
Cancer. 92:770–704. 2005. View Article : Google Scholar : PubMed/NCBI
|