Introduction
The unlimited proliferation of tumor cells can cause
hypoxia in tumor tissue. Hypoxia-inducible factor (HIF)-1α is an
important transcription factor that regulates oxygen homeostasis,
and may be associated with the occurrence of gastric cancer
(1,2). HIF-1α is able to interact with
specific signaling pathways that have important roles in adapting
to hypoxia in tumor cells. In gastrointestinal tumors, the
Wnt/β-catenin signaling pathway has been shown to affect cell
proliferation, differentiation, and the regulation of
microenvironment adaptability. HIF-1α competes with Tcf-4 for
binding to β-catenin, and once bound it can activate HIF-1 target
genes (3). Tumor cells generated
in specific microenvironments have increased viability and can
easily adapt to hypoxia (4)
through activation of the canonical Wnt/β-catenin signaling
pathway, which may lead to gastrointestinal tumorigenesis (5,6).
Invasion and metastasis involve numerous proteins
from the matrix metalloproteinase (MMP) enzyme family (7,8).
MMP-7 possesses strong matrix degradation activity, broad substrate
specificity and has been shown to be overexpressed in invasive
digestive cancers (9,10). In addition, the uroki-nase-type
plasminogen activator (uPA) system can mediate cell-surface
plasminogen activation, which can lead to degradation of the
extracellular matrix and indirect activation of MMPs, which results
in further degradation of extracellular matrix components (11). The expression of MMPs is associated
with the micrometastasis of gastric cancer cells and poor
prognosis, due to the important roles of these proteins in invasion
and metastasis (12).
The present study examined the association between
HIF-1α and Wnt/β-catenin in vitro in the SGC-7901 gastric cancer
cell line. Furthermore, the signaling involved in metastasis and
invasion was determined in vitro and in vivo under hypoxic
conditions in SGC-7901 gastric cancer cells. The results of the
present study may help to identify molecular targets for improved
gastric cancer therapeutic strategies.
Materials and methods
Cell culture, plasmids and reagents
The SGC-7901 gastric cancer cell line was obtained
from the Chongqing Medical University Key Laboratory of Neurology
(Chongqing, China). The RNA interference (RNAi) sequences for
pcDNA™6.2-GW/EmGFP-miR-β-catenin (5′-TGAACAAGACGTTGACTTGGA-3′) and
the negative control pcDNA™6.2-GW/EmGFP-miR-β-catenin-n
(5′-AAATGTACTGCGCGTGGAGAC-3′) were constructed by Invitrogen Life
Technologies (Carlsbad, CA, USA). RPMI-l640 medium was purchased
from HyClone Laboratories, Inc. (Logan, UT, USA). Fetal bovine
serum (FBS) was purchased from Hangzhou Sijiqing Biological
Engineering Materials Co., Ltd. (Hangzhou, China). Blasticidin was
purchased from Sigma-Aldrich (St. Louis, MO, USA).
Lipofectamine® 2000 was purchased from Invitrogen Life
Technologies. RNAiso Plus, reverse transcription-polymerase chain
reaction (RT-PCR) amplification, and reverse transcription kits
were all purchased from Takara Biotechnology Co., Ltd. (Dalian,
China).
Anti-HIF-1α (rabbit monoclonal antibody),
anti-β-catenin (rabbit monoclonal antibody), anti-uPA (rabbit
monoclonal antibody) and anti-MMP-7 (rabbit polyclonal antibody)
were purchased from Epitomics (Burlingame, CA, USA)
Anti-GAPDH (rabbit monoclonal antibody), horseradish
peroxidase (HRP)-conjugated goat anti-immunoglobulin G (IgG),
radioimmunoprecipitation lysis buffer, phenylmethyl-sulfonyl
fluoride, Bicinchoninic Acid (BCA) Protein Assay kit, Enhanced
Chemiluminescence (ECL) reagent, goat anti-rabbit IgG and the
immunohistochemistry streptavidin-peroxidase (SP) method chemical
kit were purchased from Jiangsu BiYunTian Bio, Ltd. (Jiangsu,
China).
Cell culture and transfection
SGC-7901 human gastric cancer cells were cultured
under normoxic conditions in RPMI-1640 medium supplemented with 10%
fetal bovine serum (FBS) at 37°C in an atmosphere containing 5% CO2
and 25% O2. SGC-7901 cells were cultured under hypoxic conditions
in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS)
at 37°C, in the presence of 5% CO2, 1% O2 and 94% N2 for 16 h. For
double hypoxic conditions, SGC-7901 cells were cultured with 150
µmol/l CoCl2 (Sigma-Aldrich, St. Louis, MO, USA) (13) for 8 h concurrently with physical
hypoxia for 16 h (2). All of the
treatment and control groups were transfected with
pcDNA™6.2-GW/EmGFP-miR-β-catenin or
pcDNA™6.2-GW/EmGFP-miR-β-catenin-n in RPMI-1640 for 18 h at 37°C. A
total of 1.6 µg/ml blasticidin was used for selection, and
the cells were screened after 6-8 weeks in order to obtain the
stably transfected cell line miR-β-catenin-7901.
Transwell invasion assays
The following five groups were included in the
invasion assays: Control, liposome, negative control, hypoxia and
hypoxia β-catenin knockdown. The cell migration and invasion assays
of the cells were performed as described previously (14), using 8.0-µm pore
polycarbonate membrane Transwell inserts in a 24-well plate. The
control group was cultured under normoxic conditions. The liposome
group was cultured under normoxic conditions with the addition of
10 µl liposome culture (Life Technologies, Carlsbad, CA,
USA). The negative control group was cultured under normoxic
conditions and transfected with the negative control plasmid
pcDNA™6.2-GW/EmGFP-miR-β-catenin-n. The hypoxia group was cultured
under physical hypoxic conditions. The hypoxia β-catenin knockdown
group consisted of the stably transfected miR-β-catenin-7901 cell
line cultured under physical hypoxic conditions.
To prepare the artificial basement membrane,
Matrigel (40 µl; Life Technologies) was evenly spread on a
Boyden chamber membrane (Life Technologies), and placed into
12-well plates. SGC-7901 cells (2×105) were seeded into
the wells and cultured in RPMI-1640 supplemented with 5% FBS. The
control, liposome and negative control groups were cultured for 24
h under normoxic conditions, whereas the hypoxic and hypoxic
β-catenin knockdown groups were cultured for 8 h under normoxic
conditions, and then cultured for 16 h under hypoxic conditions.
The Boyden chamber was then removed, and the cells that remained on
the upper surface were collected using a cotton swab. The cells
remaining on the filter were fixed with 4% paraformaldehyde (Life
Technologies) for 15 min, dried at room temperature, and stained
with hematoxylin and eosin (Life Technologies). Images of the cells
that had successfully migrated through the membrane were captured
by microscopy (CKX41; Olympus, Tokyo, Japan) using a Canon camera
(Canon, Japan). At least five different fields (magnification,
×200) were counted for each experiment, and the results of three
independent experiments were averaged.
RT-PCR detection of HIF-1α, β-catenin,
uPA and MMP-7 mRNA expression levels
SGC-7901 cells were divided into the following
groups: Control (48 h normoxia), hypoxia (32 h normoxia, physical
hypoxia 16 h), and double hypoxia (24 h normoxia, concurrent 8 h
chemical hypoxia and 16 h physical hypoxia). In addition,
miR-β-catenin-7901 cells were divided into the following groups:
Control β-catenin knockdown group, hypoxia β-catenin knockdown
group and double hypoxia β-catenin knockdown group, all of which
were cultured under the same conditions as listed above. Total RNA
was extracted from the cells using RNAiso Plus according to the
manufacturer's instructions. Reverse transcription to generate cDNA
and PCR amplification were performed using a 7300 Real-Time PCR
system (Applied Biosystems, Life Technologies, Thermo Fisher
Scientific, Waltham, MA, USA). The cycle parameters were set as
follows: 94°C for 5 min, then 94°C for 30 sec, 56°C (internal
reference, β-actin), 55°C (HIF-1α), 57°C (β-catenin), 56°C (uPA)
and 57°C (MMP-7) for 35 sec, and 72°C extension for 1 min for 30
cycles; and a final extension at 72°C for 5 min. The primer
sequences are shown in Table I.
PCR products were separated by 2.5% agarose gel electrophoresis and
quantified using Quantity One 4.62 software (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) to analyze the gray value of the PCR
products.
 | Table IPrimers used for polymerase chain
reaction amplification. |
Table I
Primers used for polymerase chain
reaction amplification.
| Gene | Primer sequence | Product size
(bp) |
|---|
| β-catenin | R:
5-GCCATTACAACTCTCCACAACC-3
F: 5-GACAGATAGCACCTTCAGCACTC-3 | 285 |
| HIF-1α | R:
5-CCAACAGTAACCAACCTCAGTG-3
F: 5-CAACCCAGACATATCCACCTCT-3 | 345 |
| uPA | R:
5-GCTTGCTCACCACAACGACA-3
F: 5-CCTTGGAGGGAACAGACGAG-3 | 262 |
| MMP-7 | R:
5-GAAGCCAAACTCAAGGAGATGC-3
F: 5-GTCAGCAGTTCCCCATACAACT-3 | 294 |
| β-actin | R:
5-GACCCAGATCATGTTTGAGACC-3
F: 5-ATCTCCTTCTGCATCCTGTCG-3 | 594 |
Western blot analysis of HIF-1α,
β-catenin, uPA and MMP-7 protein expression levels
Total protein was extracted from the six groups of
cells, and the protein concentration was measured using the BCA
method. Equal amounts of protein (50 µg) were separated by
SDS-PAGE (Life Technologies) and transferred to polyvinylidene
fluoride membranes. The membranes were primarily blocked with 5%
skimmed milk and then incubated with the primary antibodies
targeting HIF-1α (1:5,000; rabbit monoclonal), β-catenin (1:10,000;
rabbit monoclonal), uPA (1:3,000; rabbit monoclonal), MMP-7
(1:10,000; rabbit monoclonal) and GAPDH (1:1,000; rabbit
monoclonal). All antibodies were purchased from Epitomics
(Burlingame, CA, USA). The membranes were then incubated with
HRP-conjugated secondary antibodies and visualized using the ECL
reagent. The film was scanned using a gel imager (Bio-Rad
Laboratories, Inc.) and Quantity One 4.62 software was used to
analyze the gray values of the protein bands.
Tumorigenicity assay and
immunohistochemical staining in nude mice
BALB/c nude mice (n=5; 3 male, 2 female; 4–6 weeks
old) were obtained from the Department of Laboratory Animal
Science, Chongqing University Health Science Center (Chongqing,
China). All animals were housed in standard conditions at 26–28°C,
with 10 h light/14 h dull light and were given free access to food
and water. All of the experiments were performed according to the
animal protocol approved by the Institutional Animal Care and Use
Committee of Chongqing Medical University (Chongqing, China).
SGC-7901 cells were divided into the following groups: Control (48
h normoxia) and hypoxia (32 h normoxia, 16 h physical hypoxia). The
miR-β-catenin-7901 cells were divided into a control β-catenin
knockdown group and a hypoxia β-catenin knockdown group, which were
cultured in the same manner as the SGC-7901 groups. The cells from
each group were trypsinized, washed in phosphate-buffered saline
(Life Technologies) and re-suspended in saline solution (Life
Technologies). The nude mice were subcutaneously injected with
5×106 cells per 0.2 ml. Mice were divided into the control,
hypoxia, interference and hypoxia interference groups, with five
mice in each group. Tumor size was measured every third day. Tumor
volume was calculated according to the following formula:
V=(axb2)/2, where a was the largest superficial diameter and b the
smallest superficial diameter. After four weeks, the mice were
sacrificed by cervical dislocation, and the tumors were harvested
and images were captured using a Canon IXUS 245 camera (Canon).
For the immunohistochemistry experiments, the tumors
were harvested, fixed in formalin and embedded in paraffin (Life
Technologies), and conventional immunohistochemistry sections were
prepared. Detection was performed using the SP method in accordance
with standard procedures (15).
Positive expression was regarded as the presence of yellow-brown
particles in cytoplasm or nucleus.
Western blot analysis of protein
expression levels in tumor tissues
Fresh tumor tissue from each group was harvested,
and total protein was extracted using a protein extraction kit,
according to the manufacturer's instructions. Western blots were
performed as described above. Blots were quantified using grey
scale analysis.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. The significance of differences between the groups was
determined using Student's t-test and one-way analysis of variance.
Appropriate post hoc tests were used when comparing multiple
parameters. All analysis was performed using SPSS 17.0 software
(IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
β-catenin knockdown decreases the
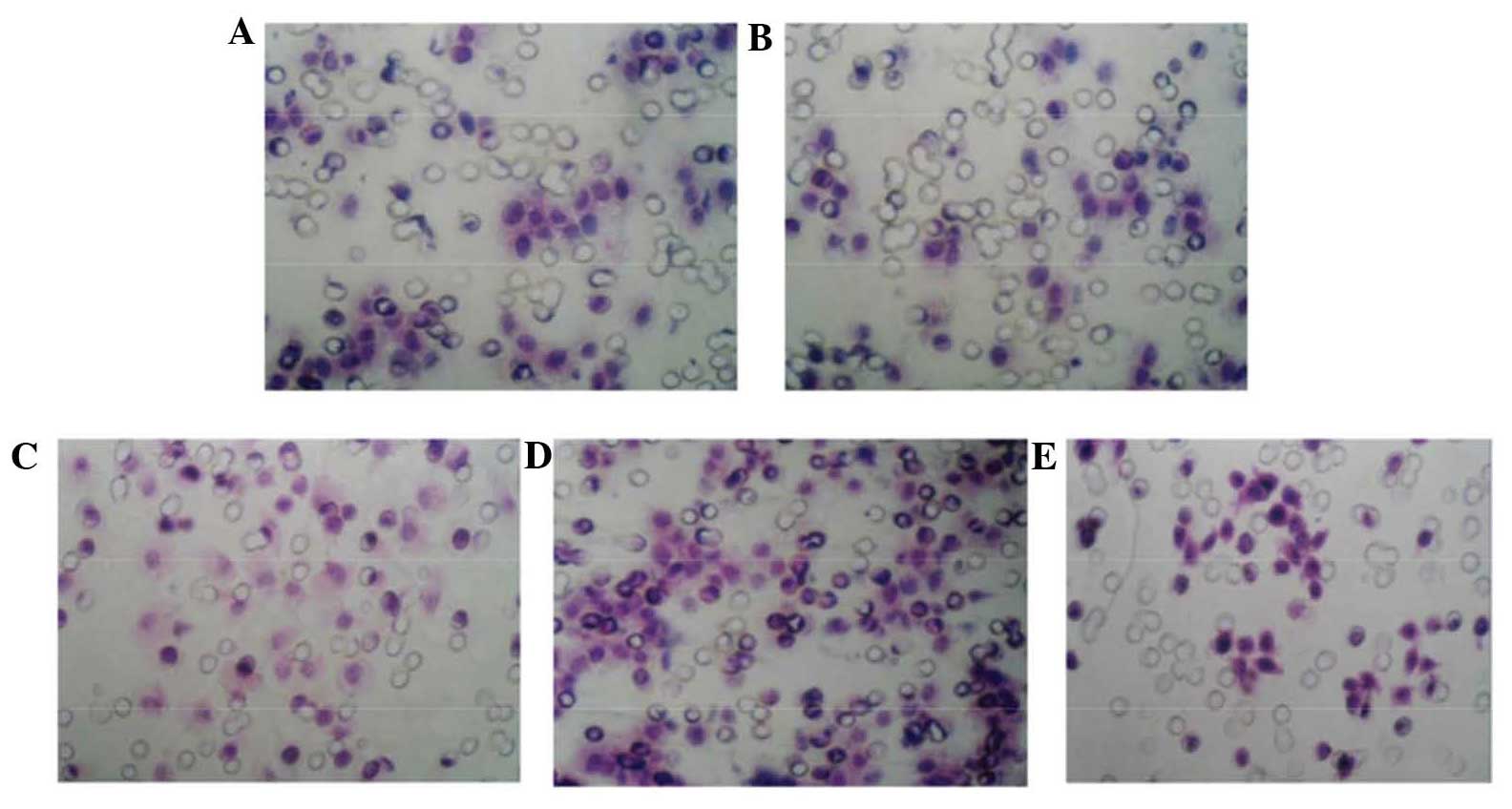
invasiveness of gastric cancer cells under hypoxia
The invasion assay demonstrated that the number of
invaded cells was not significantly different between the control,
negative control and liposome groups. However, the number of
invaded cells in the hypoxia group was increased as compared with
that the control group, while the number in the hypoxia
interference group was significantly decreased as compared with
that in the hypoxia group (Table
II and Fig. 1).
 | Table IIInvasive capacity of various groups of
SGC-7901 gastric cancer cells. |
Table II
Invasive capacity of various groups of
SGC-7901 gastric cancer cells.
| Group | Invaded cells
(n) |
|---|
| Control | 66±3 |
| Liposome | 62±5 |
| Negative control | 65±3 |
| Hypoxia | 118±4a |
| Hypoxic
interference | 50±2b |
Effects of β-catenin knockdown and
hypoxia on HIF-1α, β-catenin, uPA and MMP-7 mRNA and protein
expression levels
RT-PCR and western blot analyses demonstrated that
the mRNA and protein expression levels of β-catenin, HIF-1α, uPA
and MMP-7 were low in the SGC-7901 control group, while they were
significantly enhanced in the hypoxia group, and in the double
hypoxia to an even greater extent (P<0.05) (Tables III and IV, Figs.
2 and 3). The control
β-catenin knockdown (interference) group expressed significantly
lower levels of β-catenin, HIF-1α, uPA and MMP-7 mRNA and protein
as compared with those in the control group. In addition, the
hypoxia β-catenin knockdown group expressed significantly lower
levels of β-catenin, HIF-1α, uPA and MMP-7 mRNA and protein as
compared with those in the hypoxia and control groups (P<0.05).
The hypoxia β-catenin knockdown group expressed significantly lower
levels of β-catenin, HIF-1α, uPA and MMP-7 mRNA and protein as
compared with those in the double hypoxia interference group.
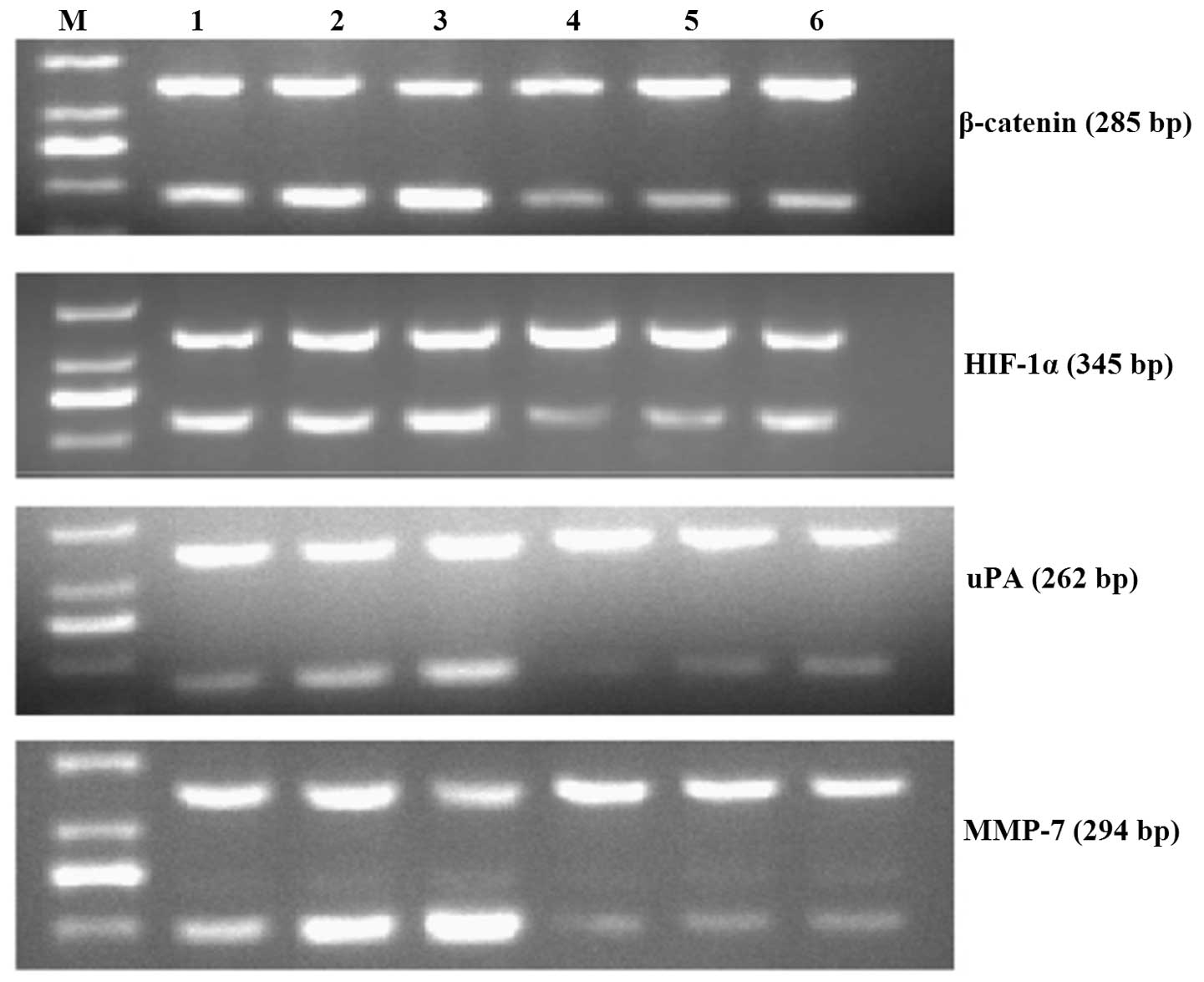 | Figure 2Representative images of HIF-1α,
β-catenin, u-PA and MMP-7 mRNA expression in each of the SGC-7901
gastric cancer cell groups. M, DNA marker DL1,000; 1, control
group; 2, hypoxia group; 3, double hypoxia group; 4, interference
group; 5, hypoxia interference group; 6, double hypoxia
interference group. HIF-1α, hypoxia-inducible factor-1α; uPA,
urokinase-type plasminogen activator; MMP-7, matrix
metalloproteinase-7. |
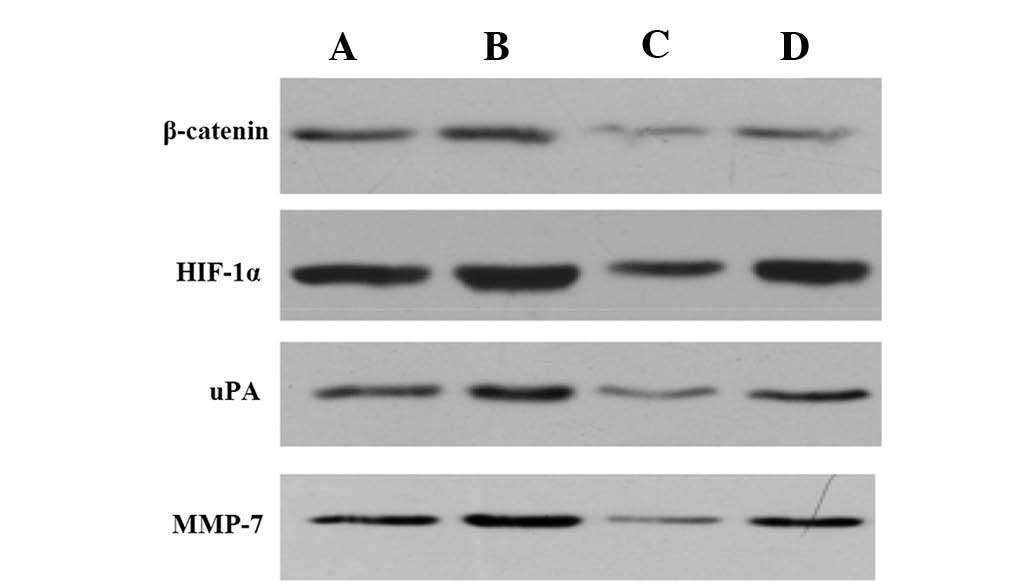
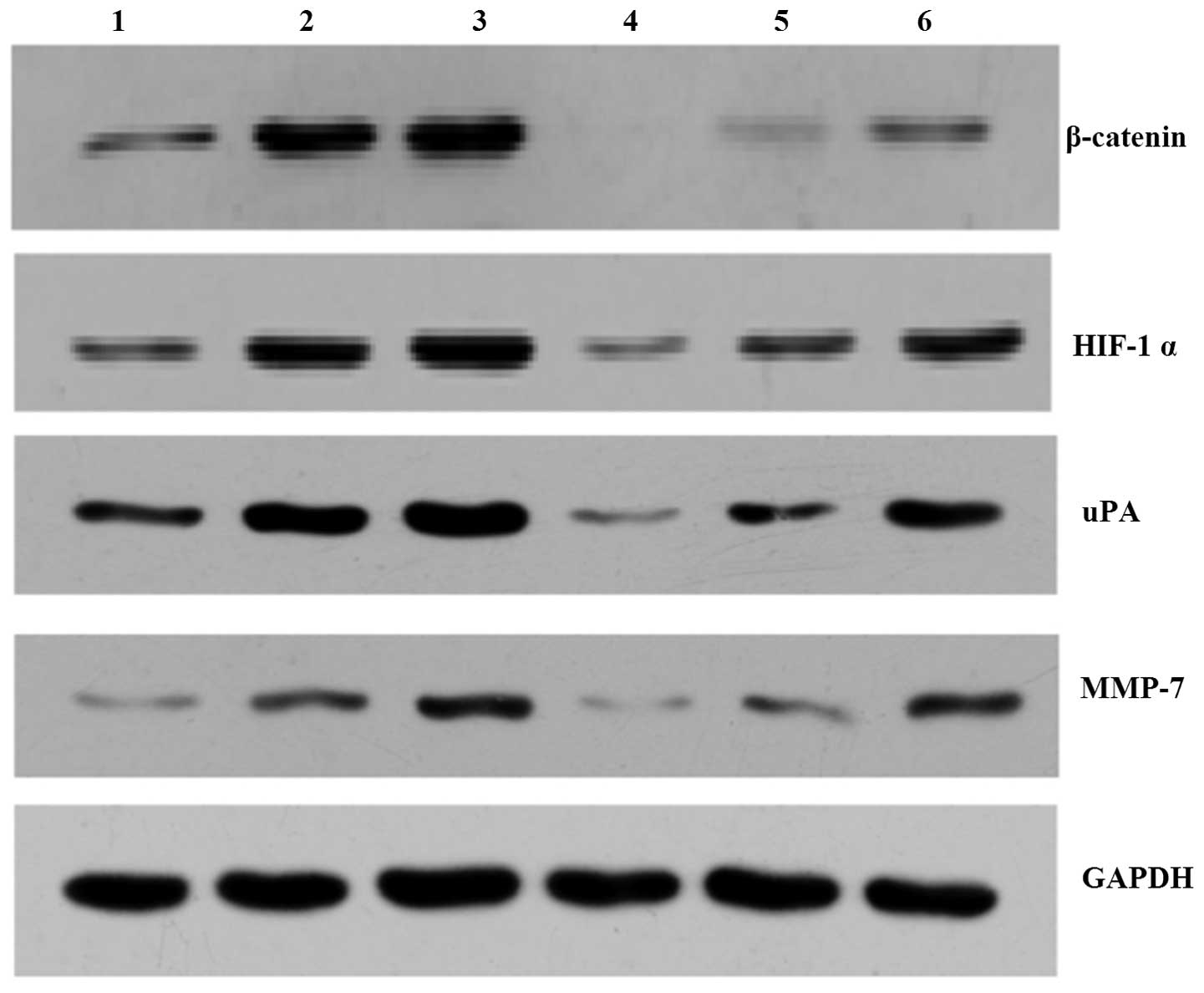 | Figure 3Representative images of HIF-1α,
β-catenin, u-PA and MMP-7 protein expression in each of the
SGC-7901 gastric cancer cell groups. 1, control group; 2, hypoxia
group; 3, double hypoxia group; 4, interference group; 5, hypoxia
interference group; 6, double hypoxia interference group. HIF-1α,
hypoxia-inducible factor-1α; uPA, urokinase-type plasminogen
activator; MMP-7, matrix metalloproteinase-7. |
 | Table IIImRNA expression levels of HIF-1α,
β-catenin, uPA and MMP-7 in each of the SGC-7901 gastric cancer
cell groups. |
Table III
mRNA expression levels of HIF-1α,
β-catenin, uPA and MMP-7 in each of the SGC-7901 gastric cancer
cell groups.
| Group | β-catenin | HIF-1α | uPA | MMP-7 |
|---|
| Control | 0.49±0.03 | 0.51±0.05 | 0.50±0.03 | 0.75±0.04 |
| Hypoxia | 0.76±0.06a | 0.74±0.07a | 0.69±0.05a | 1.15±0.14a |
| Double hypoxia | 1.25±0.09b,a | 0.99±0.08b,a | 0.80±0.07b,a | 1.70±0.04b,a |
| Interference | 0.23±0.04a | 0.25±0.04a | 0.36±0.03a | 0.38±0.08a |
| Hypoxia
interference | 0.31±0.02b,d | 0.41±0.03b,d | 0.45±0.04b,d | 0.56±0.05b,d |
| Double hypoxia
interference | 0.42±0.05b,c,d | 0.78±0.59b,c,d | 0.54±0.08b,c,d | 0.64±0.04b,c,d |
 | Table IVProtein expression levels of HIF-1α,
β-catenin, uPA and MMP-7 protein in the SGC-7901 gastric cancer
cell groups. |
Table IV
Protein expression levels of HIF-1α,
β-catenin, uPA and MMP-7 protein in the SGC-7901 gastric cancer
cell groups.
| Group | β-catenin | HIF-1α | uPA | MMP-7 |
|---|
| Control | 0.60±0.06 | 0.50±0.06 | 0.27±0.12 | 0.64±0.14 |
| Hypoxia | 1.09±0.18a | 0.90±0.22a | 0.51±0.05a | 1.49±0.20a |
| Double hypoxia | 1.89±0.21 | 1.59±0.36 | 0.88±0.11b,a | 1.98±0.49b,a |
| Interference | 0.11±0.03 | 0.10±0.01 | 0.07±0.05a | 0.15±0.04a |
| Hypoxia
interference | 0.33±0.05 | 0.48±0.13 | 0.33±0.05b,d | 0.58±0.04b,d |
| Double hypoxia
interference | 0.59±0.01 | 1.00±0.25 | 0.56±0.08b,c,d | 1.02±0.11b,c,d |
The mRNA and protein expression levels of β-catenin,
HIF-1a, uPA and MMP-7 were increased in the hypoxia β-catenin
knockdown group and the double hypoxia β-catenin knockdown group,
as compared with those in the control β-catenin knockdown group
(P<0.05) (Tables III and
IV, Figs. 2 and 3).
Compared to the double hypoxia β-catenin knockdown
group to the hypoxia β-catenin knockdown group, uPA and MMP-7 mRNA
and protein expression levels were significantly increased
(P<0.05) (Table V).
 | Table VmRNA expression levels of uPA and
MMP-7 in each of the SGC-7901 gastric cancer cell groups. |
Table V
mRNA expression levels of uPA and
MMP-7 in each of the SGC-7901 gastric cancer cell groups.
| Group | uPA | MMP-7 |
|---|
| Hypoxia
interference group-interference group | 0.09±0.03 | 0.10±0.08 |
| Double hypoxia
interference group-hypoxia interference group | 0.15±0.04a | 0.18±0.06a |
Hypoxia accelerates growth of gastric
xenograft tumors
Ten days following inoculation of the cells into
nude mice, the cells from the hypoxia group began to form tumors.
After 14 days, the cells from the control group also began to form
tumors. Finally, after 16 days, the control and hypoxia β-catenin
knockdown groups began to form tumors. Following inoculation,
time-dependent increases in tumor volume were observed, which were
accelerated after 22 days. According to the tumor volume and speed
of growth, the tumorigenicity of the hypoxia group was higher as
compared with that in the control group and the β-catenin knockdown
group (Table VI, Fig. 4).
 | Table VIAverage volume and weight of
xenograft tumors in four groups. |
Table VI
Average volume and weight of
xenograft tumors in four groups.
| Group | Volume
(m3) | Weight (g) |
|---|
| Control | 1232±56 | 0.61±0.03 |
| Hypoxia | 1273±48a | 1.20±0.07a |
| Interference | 334±16a | 0.37±0.05a |
| Hypoxia
interference | 683±39b,c | 0.82±0.03b,c |
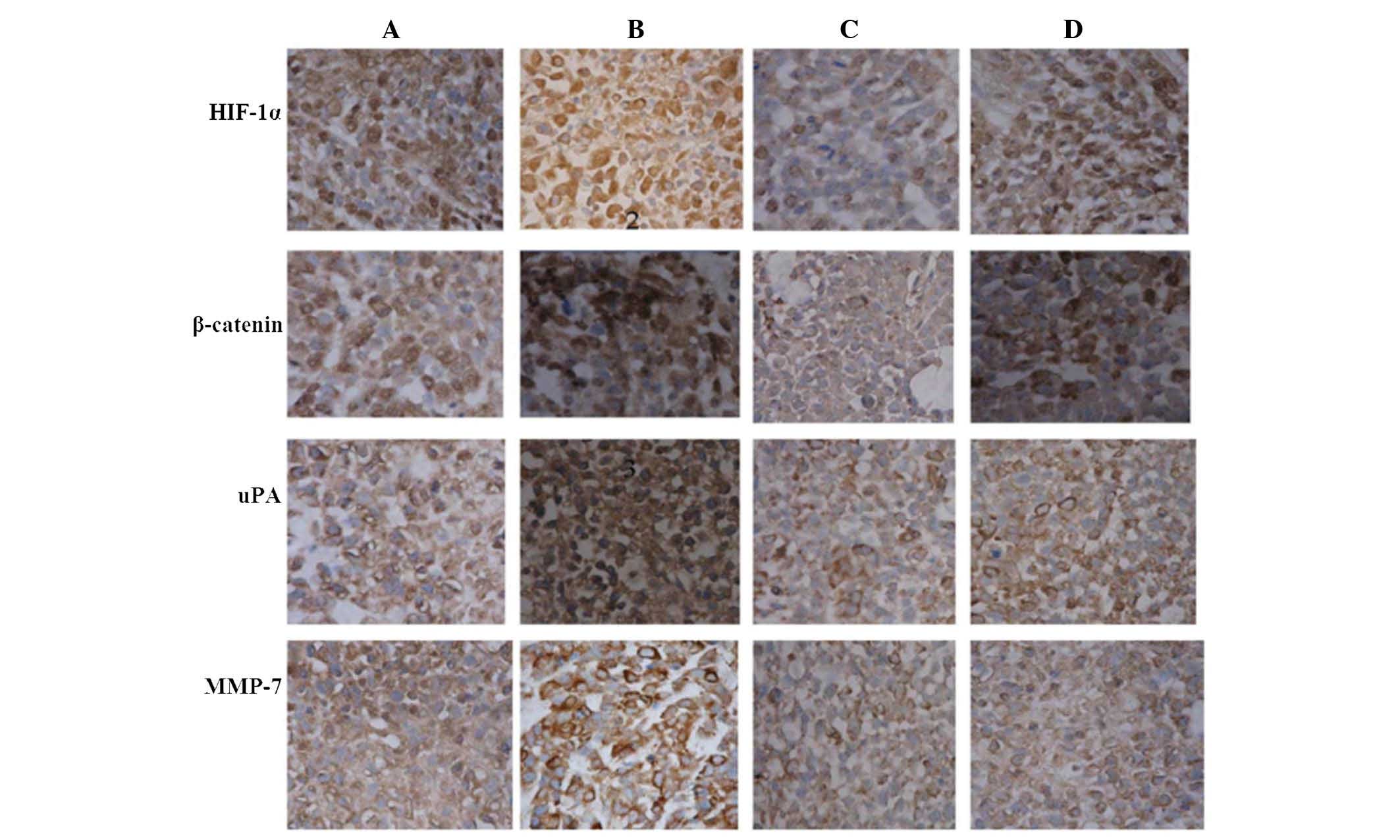
Immunohistochemical analysis of HIF-1α,
β-catenin, uPA and MMP-7 expression in nude mouse xenografts
Four weeks after inoculation, the nude mice were
sacrificed. The tumors were embedded in paraffin and
immunohistochemistry detected HIF-1α, β-catenin and uPA expression
predominantly in the nucleus, while MMP-7 expression was primarily
located in the nucleus and the cytoplasm (Fig. 5). A lower protein expression levels
was observed in the interference group compared with the control
group.
Western blot analysis of protein
expression levels in tumor tissue
HIF-1α, β-catenin, uPA and MMP-7 protein expression
levels were highest in the tumor tissue from the hypoxia group and
lowest in the interference group. Expression levels of these
proteins were significantly reduced in the hypoxia interference
group, as compared with those in the hypoxia group (P<0.05)
(Fig. 6, Table VII).
 | Table VIIProtein levels of HIF-1α, β-catenin,
uPa and MMP-7 in nude mouse xenografts. |
Table VII
Protein levels of HIF-1α, β-catenin,
uPa and MMP-7 in nude mouse xenografts.
| Group | β-catenin | HIF-1α | uPA | MMP-7 |
|---|
| Control | 1.61±0.03 | 2.82±0.09 | 1.86±0.05 | 1.94±0.03 |
| Hypoxia | 2.06±0.09a | 3.68±0.09a | 2.92±0.06a | 2.87±0.07a |
| Interference | 0.57±0.06a | 1.86±0.11a | 1.12±0.06a | 0.81±0.07a |
| Hypoxia
interference | 1.10±0.08b,c | 2.07±0.15c | 1.97±0.04b,c | 1.8±0.06b,c |
Discussion
The carcinogenic activity of the Wnt/β-catenin
pathway relies on the accumulation of cytosolic β-catenin. The Wnt
signaling pathway is regulated by the levels of β-catenin in the
cell; when the levels of β-catenin are elevated, the Wnt pathway is
activated (16). HIF-1α
stabilization depends on the phosphoinositide 3-kinase (PI3K)/Akt
pathways, which are involved in the transcriptional activity of the
extracellular signal-regulated kinase (ERK) pathway (17). The Wnt/β-catenin pathway
communicates with the PI3K/Akt pathway (18), and β-catenin is also involved in
activation of the ERK pathway (19). These observations suggested that
increased HIF-1 expression and activity may be associated with the
Wnt/β-catenin signaling pathway.
Following suppression of β-catenin expression using
RNAi, β-catenin and HIF-1α expression levels were significantly
reduced, indicating that HIF-1α levels dropped due to the decrease
in β-catenin. These results are concordant with the findings of
Kaidi et al (20) and Lee
et al (21). The results of
the present study suggested that HIF-1α may function downstream of
Wnt/β-catenin and may be regulated by the Wnt/β-catenin signaling
pathway. Conversely, in the double hypoxia and hypoxia groups,
HIF-1α and β-catenin expression levels were increased as compared
with those in the control group. Furthermore, following suppression
of β-catenin expression, the levels of β-catenin did not remain
constant in the stably transfected cell line. The hypoxia β-catenin
knockdown group and the double hypoxia β-catenin knockdown group
had increased expression levels of β-catenin, as compared with
those in the control β-catenin knockdown group. It may therefore be
hypothesized that hypoxia can stimulate an increase in HIF-1α, and
that HIF-1α can activate β-catenin, stimulating the Wnt/β-catenin
signaling pathway and activating downstream genes. Jiang et
al (22) previously
demonstrated, by western blot and RT-PCR analyses, that in prostate
cancer cells, HIF-1α regulated the expression levels of β-catenin.
Furthermore, Lim et al (23) suggested that HIF-1α may interact
with hARD1 to regulate β-catenin.
A previous study by our group indicated that hypoxia
is able to increase HIF-1α expression, which upregulates MMP-9 and
uPA receptor expression in gastric cancer cells, resulting in
increased adhesion, migration and invasion (2). In the present study, western blot and
RT-PCR analyses demonstrated that in the hypoxia and double hypoxia
groups, uPA and MMP-7 mRNA and protein expression levels gradually
increased. Following knockdown of β-catenin expression, the hypoxia
β-catenin knockdown group, as compared with the hypoxia group, as
well as the double hypoxia β-catenin knockdown group, as compared
with the double hypoxia group, had significantly decreased
expression levels of uPA and MMP-7 mRNA and protein. Under hypoxic
conditions and following treatment with cobalt chloride in order to
increase the effectiveness of hypoxia, followed by β-catenin
knockdown, the double hypoxia β-catenin knockdown group and the
hypoxia β-catenin knockdown group had significantly elevated
expression levels of uPA and MMP-7, as compared with those in the
hypoxia β-catenin knockdown group and the β-catenin knockdown
group. This suggested that suppression of β-catenin expression
influenced MMP-7 and uPA expression levels, and under hypoxic
conditions, HIF-1α was capable of regulating the expression of uPA
and MMP-7, based on the magnitude of the enhanced hypoxia.
According to the in vitro invasion assay, the number
of cells transgressing the membrane was increased in the hypoxia
group, and invasion of the gastric cancer cells under hypoxic
conditions was enhanced. Following knockdown of β-catenin
expression, invasiveness was reduced under hypoxia. These results
indicated that the absence of oxygen in the environment promotes
the expression of HIF-1α, which increases β-catenin expression and
activate the Wnt/β-catenin signaling pathway in gastric cancer
cells, resulting in increased invasiveness.
In the nude mouse xenograft experiment, tumors from
the hypoxia group were significantly larger as compared with those
from the control group and the hypoxia β-catenin knockdown group,
indicating that tumor growth was enhanced for the hypoxia group, as
compared with that in the control group. The immunohistochemistry
and western blotting results demonstrated that in the xenograft
tumors from the hypoxia group, HIF-1α, β-catenin, uPA and MMP-7
protein expression levels were higher as compared with those in the
control group and the hypoxia β-catenin knockdown group.
In conclusion, the in vitro transwell invasion assay
capacity of gastric cancer cells is enhanced under hypoxic
conditions when HIF-1α expression is increased by activating the
Wnt/β-catenin signaling pathway, subsequently inducing the
expression of uPA and MMP-7, which act on the extracellular matrix,
promoting cell invasion and the development of gastric cancer.
In the present study, RT-PCR and western blot
analyses demonstrated that a regulatory interaction exists between
the Wnt/β-catenin pathway and HIF-1α. Concurrently, inhibiting
β-catenin expression under hypoxic conditions reduced the size of
the xenograft tumor as well as uPA and MMP-7 protein expression
levels. These results suggested that the ability to enhance gastric
cancer cell invasion under hypoxic conditions may be due to an
increase in HIF-1α expression, which is real-ized through the
activation of Wnt/β-catenin signaling, which can promote uPA and
MMP-7 expression. uPA and MMP-7 may then degrade the extracellular
matrix in order to promote tumor invasion, thereby contributing to
gastric cancer invasiveness.
The present study suggested that in gastric cancer
cells, HIF-1α has a role both upstream and downstream of the
Wnt/β-catenin signaling pathway, and that these pathways have a
reciprocal regulative function. Under hypoxic conditions, HIF-1α
can facilitate the increased expression of uPA (24) and MMP-7 (25–27)
through the Wnt/β-catenin pathway, which can promote the
invasiveness of gastric cancer cells. These results may provide a
basis for gastric cancer treatment at the molecular level.
References
|
1
|
Zhong H, De Marzo AM, Laughner E, et al:
Overexprcssion of hypoxia-inducible factor 1alpha in common human
cancers and their metastases. Cancer Res. 59:5830–5835. 1999.In
Chinese. PubMed/NCBI
|
|
2
|
Deng T and Zhag JW: Relationship of
hypoxia-inducible factor-1α and invasion of gastric cancer cells in
hypoxia. Chin J Biologicals. 23:696–699. 2010.
|
|
3
|
Kolligs FT, Bommer G and Göke B:
Wnt/beta-catenin/tcf signaling: A critical pathway in
gastrointestinal tumorigenesis. Digestion. 66:131–144. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dou J: Tumor stem cells. Southeast
University Press; pp. 56–57. 2009
|
|
5
|
Singh T and Katiyar SK: Honokiol inhibits
non-small cell lung cancer cell migration by targeting
PGE2-mediated activation of β-catenin signaling. PLoS One.
8:e607492013. View Article : Google Scholar
|
|
6
|
Panza A, Pazienza V, Ripoli M, et al:
Interplay between SOX9, β-catenin and PPARγ activation in
colorectal cancer. Biochim Biophys Acta. 1833:1853–1865. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao XL, Sun T, Che N, et al: Promotion of
hepatocellular carcinoma metastasis through matrix
metalloproteinase activation by epithelial-mesenchymal transition
regulator Twist1. J Cell Mol Med. 15:691–700. 2011. View Article : Google Scholar
|
|
8
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar
|
|
9
|
Zhao H, Yang Z, Wang X, et al: Triptolide
inhibits ovarian cancer cell invasion by repression of matrix
metalloproteinase 7 and 19 and upregulation of E-cadherin. Exp Mol
Med. 30:633–641. 2012. View Article : Google Scholar
|
|
10
|
Zhang JL, Chen GW, Liu YC, et al: Secreted
protein acidic and rich in cysteine (SPARC) suppresses angiogenesis
by down-regulating the expression of VEGF and MMP-7 in gastric
cancer. PLos One. 7:e446182012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sroka IC, Sandoval CP, Chopra H, et al:
Macrophage-dependent cleavage of the laminin receptor α6β1 in
prostate cancer. Mol Cancer Res. 9:1319–1328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ding Y, Zhang H, Zhong M, et al: Clinical
significance of the uPA system in gastric cancer with peritoneal
metastasis. Eur J Med Res. 18:282013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan Y, Hilliard G, Ferguson T and
Millhorn DE: Cobalt inhibits the interaction between
hypoxia-inducible factor-alpha and von Hippel-Lindau protein by
direct binding to hypoxia-inducible factor-alpha. J Biol Chem.
278:15911–15916. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee JW, Bae SH, Jeong JW, et al:
Hypoxia-inducible factor (HIF-1) alpha: Its protein stability and
biological function. Exp Mol Med. 36:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fukumasu H, Cordeiro YG, Rochetti AL,
Barra CN, Sámora TS, Strefezzi RF and Dagli ML: Expression of NR1I3
in mouse lung tumors induced by the tobacco-specific nitrosamine
4-(methylnitrosamino)-4-(3-pyridyl)-1-butanone. Braz J Med Biol
Res. 48:240–244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miller JR, Hocking AM, Brown JD and Moon
RT: Mechanism and function of signal transduction by the
Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene. 18:7860–7872.
1999. View Article : Google Scholar
|
|
17
|
Minet E, Michel G, Mottet D, et al:
Transduction pathways involved in Hypoxia-Inducible Factor-1
phosphorylation and activation. Free Radic Biol Med. 31:847–855.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lau MT, Klausen C and Leung PC: E-cadherin
inhibits tumor cell growth by suppressing PI3K/Akt signaling via
β-catenin-Egr1-mediated PTEN expression. Oncogene. 30:2753–2766.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ji H, Wang J, Nika H, et al: EGF-induced
ERK activation promotes CK2-mediated disassociation of
alpha-Catenin from beta-Catenin and transactivation of
beta-Catenin. Mol Cell. 36:547–559. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaidi A, Williams AC and Paraskeva C:
Interaction between beta-catenin and HIF-1 promotes cellular
adaptation to hypoxia. Nat Cell Biol. 9:210–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee SH, Kim MH and Han HJ: Arachidonic
acid potentiates hypoxia-induced VEGF expression in mouse embryonic
stem cells: involvement of Notch, Wnt and HIF-1alpha. Am J Physiol
CellPhysiol. 297:C207–C216. 2009. View Article : Google Scholar
|
|
22
|
Jiang YG, Luo Y, He DL, et al: Role of
Wnt/beta-catenin signaling pathway in epithelial-mesenchymal
transition of human prostate cancer induced by hypoxia-inducible
factor-lalpha. Int J Urol. 14:1034–1039. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lim JH, Chun YS and Park JW:
Hypoxia-inducible factor-1alpha obstructs a Wnt signaling pathway
by inhibiting the hARD1-mediated activation of beta-catenin. Cancer
Res. 68:5177–5184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moreau M, Mourah S and Dosquet C:
β-Catenin and NF-κB cooperate to regulate the uPA/uPAR system in
cancer cells. Int J Cancer. 128:1280–1292. 2011. View Article : Google Scholar
|
|
25
|
Brabletz T, Jung A, Dag S, et al:
beta-catenin regulates the expression of the matrix
metalloproteinase-7 in human colorectal cancer. Am J Pathol.
155:1033–1038. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Crawford HC, Fingleton B, Gustavson MD, et
al: The PEA3 subfamily of Ets transcription factors synergizes with
beta-catenin-LEF-1 to activate matrilysin transcription in
intestinal tumors. Mol Cell Biol. 21:1370–1383. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang YJ, Park HJ, Chung HJ, et al:
Wnt/β-catenin signaling mediates the antitumor activity of magnolol
in colorectal cancer cells. Mol Pharmacol. 82:168–177. 2012.
View Article : Google Scholar : PubMed/NCBI
|