Introduction
The migration of cancer cells is a key step in tumor
metastasis, which is associated with high mortality rates in cancer
(1). The metastatic process
involves the movement of cancer cells through the extracellular
matrix (ECM) around the region of the primary tumor and requires
adhesion of cancer cells to the ECM as well as ECM degradation
(2). Focal adhesion kinase (FAK)
and matrix metalloproteinases (MMPs) have been regarded as critical
molecules in this process and previous studies indicated that the
FAK pathway indirectly influences MMP activity as well as cell-ECM
interactions (3–5).
Doxycycline, a member of the tetracycline group of
antibiotics, is commonly used to treat a variety of infections
(6). Numerous studies have
demonstrated that doxycycline induced tumor apoptosis and
suppressed tumor cell migration (7–9).
Furthermore, the role of doxycycline as a non-specific MMP
inhibitor has been established (10). Previous studies have demonstrated
that doxycycline inhibited solid tumor metastasis via
downregulation of FAK (11,12).
However, it remains to be elucidated whether doxycycline has an
analogous effect on leukemia cells.
Acute myelogenous leukemia (AML) is a hematological
malignancy, which may be regarded as a prototype of metastatic
cancer. AML is characterized by the premature egress of leukemic
blasts from the bone marrow and their dissemination into peripheral
tissues (13). Increased
gelatinase (MMP-2 and MMP-9) expression by AML blasts has been
implicated in the invasive phenotype of AML (14). Expression of FAK in AML has been
associated with enhanced blast migration, increased cellularity and
poor prognosis (15).
The current study aimed to investigate the potential
of doxycycline to attenuate the migration of leukemic cells through
inhibiting leukemic cell expression of the gelatinases MMP-2 and
MMP-9 via the FAK pathway. This study may therefore provide novel
insights into the importance of doxycycline as a candidate for the
treatment of leukemia patients.
Materials and methods
Chemical reagents
Doxycycline was purchased from Sigma-Aldrich (St.
Louis, MO, USA). A stock solution was prepared at 10 mg/ml in
phosphate buffered saline (PBS; Dingguo Biotech Corp., Guangzhou,
China) and stored at −20°C.
Cell culture
The human leukemia cell lines KG1a (acute
myelogenous leukemia) and K562 (chronic myelogenous leukemia) were
obtained from the Institute of Hematology and Hospital of Blood
Diseases, Chinese Academy of Medical Sciences (Tianjin, China).
Cells were cultured in RPMI 1640 medium (Gibco-BRL, Paisley, UK)
supplemented with 10% fetal bovine serum (FBS; Gibco-BRL) and
antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin;
Gibco-BRL). Cells were maintained at 37°C in humidified atmosphere
containing 5% CO2.
In vitro invasion assay
The invasion capacity of leukemic cells was
evaluated using a Matrigel®-coated Transwell®
chamber system. RPMI 1640 medium (500 µl supplemented with
10% FBS) containing 100 ng/ml stromal cell-derived factor-1α (Wako
Pure Chemical Industries, Ltd., Tokyo, Japan), a chemoattractant,
was added to the lower chambers in the 24-well
Transwell® plates (Corning, Inc., Corning, NY, USA).
Membrane filters (Merck Millipore, Billerica, MA, USA; diameter,
6.5 mm; pore size, 8 µm) were coated with 50 µg
Matrigel® (BD Biosciences, San Jose, CA, USA), providing
a composition similar to that of human basement membranes. Leukemic
cell lines treated with doxycycline (1 µg/ml) or
anti-β1-integrin antibodies (100 ng/ml; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA,) were added to the upper chamber
(2.0×105 cells/well in 200 µl RPMI 1640), as
previously described (16).
Untreated cells were included as controls. Transwell®
plates were incubated for 24 h at 37°C in a CO2
incubator. Subsequently, cells that had crossed the
Matrigel® and migrated to the lower surface of the
filter were fixed, stained with 0.1% crystal violet (Dingguo
Biotech Corp.) and enumerated in ten randomly selected fields per
filter under a light microscope (Olympus CKX41-A32RC inverted
microscope; magnification, ×200; Olympus Corp., Tokyo, Japan). The
invasive cells on the lower surface of the membrane were stained by
dipping inserts in the 0.1% crystal violet solution for 30 min. The
inserts were then rinsed in water and allowed to air dry. Each
invasion experiment was performed in triplicate.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was used to quantify messenger (m) RNA
levels. KG-1a and K562 cells were treated with 1 µg/ml doxycycline
(Sigma-Aldrich) or 100 ng/ml anti-β1-integrin antibody
(anti-β1-integrin-Ab) at 37°C in a CO2 incubator for 24
h. A total of 1×106 cells were placed in 1.5 ml tubes
which were cooled on ice. Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen Life Technologies, Foster
City, CA, USA) according to the manufacturer's instructions. A
total of 4 µl RNA was used to perform RT using an ABI 7500
Real-Time-PCR system (Applied Biosystems, Foster City, CA, USA)
with the SYBR Green master mix (Applied Biosystems) and primers
(Da′an Gene Co., Guangzhou, China). The sequences of the primers
used for qPCR analysis were as follows: human MMP-2 forward, 5′-GGC
CCCACA GGA GGA GAA-3′ and reverse, 5′-GGT GCT GGC TGA GTA GAT
CCA-3′; human MMP-9 forward, 5′-AGA TGC GTG GAG AGT CGA AATC-3′ and
reverse, 5′-GTC TCG GGC AGG GAC AGTT-3′; human FAK forward, 5′-AGC
AAG AAG AGC GCA TGAGG-3′ and reverse, 5′-GGG CGG TGC TTC ATC
AGA-3′; human β-actin forward, 5′-GCA TGG GTC AGA AGG ATT CCT-3′
and reverse, 5′-TCG TCC CAG TTG GTG ACGAT-3′. The reaction
conditions were as follows: 93°C for 3 min, followed by 40 cycles
of 93°C for 30 sec and 55°C for 45 sec. β-actin was used as a
reference to obtain the relative fold change for targets using the
comparative Ct method (17). All
samples were analyzed in triplicate.
Western blot analysis
In one treatment set, KG1a and K562 cells were
treated with doxycycline at 0.1 or 1 µg/ml for 1, 3, 6 and
12 h. In another treatment set, KG1a and K562 cells were treated
with 1 µg/ml doxycycline or 100 ng/ml anti-β1-integrin-Ab
for 24 h at 37°C in a CO2 incubator. Total protein was
extracted from control leukemic cells and treated leukemic cells.
Protein samples (40 µg) were subjected to 10% SDS-PAGE
(Dingguo Biotech Corp) and transferred onto polyvinylidene
difluoride membranes (Merck Millipore). Membranes were then blocked
with 5% skimmed milk in Tris-buffered saline with Tween 20 and
reacted with the following primary antibodies: Anti-FAK (rabbit
polyclonal; cat. no. 3283; 1:1,000 dilution), anti-p-FAK
(Tyr576/577; rabbit polyclonal; cat. no. 3281; 1:1,000 dilution),
anti-p-FAK (Tyr925; rabbit polyclonal; cat. no. 3284; 1:1,000
dilution), anti-MMP2 (rabbit polyclonal; cat. no. 4022; 1:1,000
dilution) and anti-MMP9 (rabbit polyclonal; cat. no. 2270; 1:1,000
dilution), which were purchased from Cell Signaling Technology
(Danvers, MA, USA) as well as anti-p-FAK (Tyr397; rabbit
monoclonal; cat. no. 44-625G; 1:1,000 dilution; Invitrogen Life
Technologies). Following washing with PBS three times, membranes
were incubated with goat anti-rabbit IgG, horseradish
peroxidase-conjugated secondary antibody (Cell Signaling
Technology, Danvers, MA, USA; cat. no. 7074; 1:1,000 dilution).
Bands were visualized using enhanced chemiluminescence (SuperSignal
West Pico chemiluminescent substrate; Pierce Biotechnology, Inc.,
Rockford, IL, USA). GAPDH (rabbit monoclonal; cat. no. 5174;
1:1,000 dilution; Cell Signaling Technology) was used as internal
control and was detected on the same membrane.
Statistical analysis
Values are expressed as the mean ± standard
deviation, unless otherwise stated. Statistical significance was
evaluated using one-way analysis of variance tests with SPSS 11.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference between
values.
Results
Effect of doxycycline and
anti-β1-integrin on leukemic cell migration
The inhibitory effects of doxycycline and
anti-β1-integrin-Ab blocking treatment on the invasion capacity of
KG1a and K562 cells were investigated using Matrigel®
matrix-coated Transwell® chamber assays. As shown in
Table I, doxycycline and
anti-β1-integrin-Ab were demonstrated to significantly decrease the
number of migrated KG1a (P<0.001) and K562 (P<0.001) cells
compared with that of the control group. This therefore indicated a
marked reduction in the invasion capacity of these cells.
 | Table IEffects of doxycycline and anti-β1
integrin-Ab on the invasiveness of KG1a and K562 human leukemia
cell lines. |
Table I
Effects of doxycycline and anti-β1
integrin-Ab on the invasiveness of KG1a and K562 human leukemia
cell lines.
| Group | No. of migrated
leukemia cells
|
|---|
| KG1a | K562 |
|---|
| Control | 25.00±3.91 | 24.25±4.57 |
| Doxycycline | 11.75±4.43a | 7.00±2.16a |
| Anti-β1
integrin-Ab | 5.00±1.00a | 3.50±1.29a |
Transcription of FAK and gelatinases in
leukemic cells following doxycycline or anti-β1-integrin-Ab
treatment
RT-qPCR was conducted in order to further understand
the influence of doxycycline or anti-β1-integrin-Ab treatment on
the expression of FAK and gelatinases in the KG1a and K562 leukemic
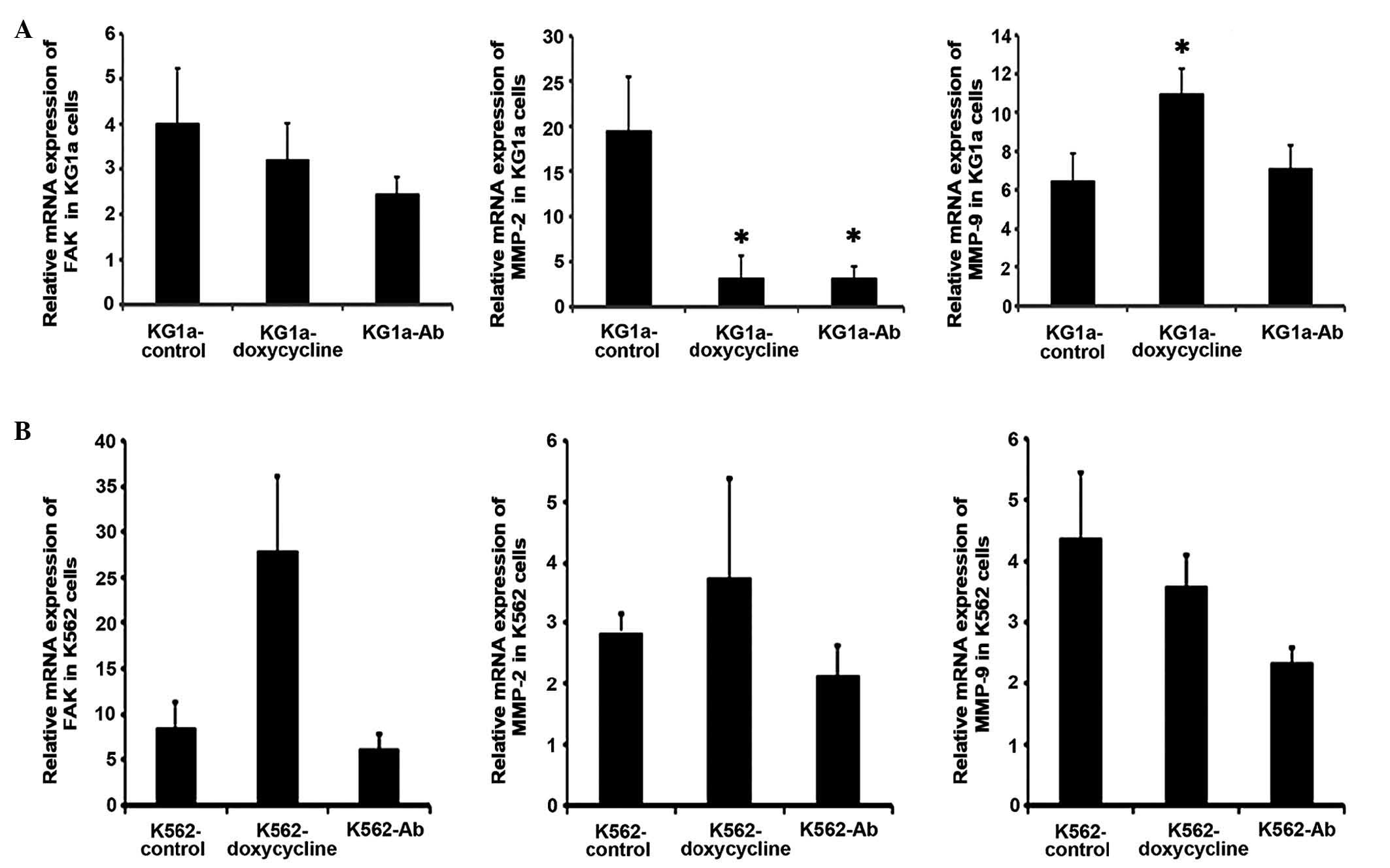
cell lines (Fig. 1A and B).
As shown in Fig.
1A, in KG1a cells, the levels of FAK mRNA were not
significantly altered by doxycycline or anti-β1-integrin-Ab
treatment compared with those of untreated control cells. By
contrast, MMP-2 mRNA expression was significantly decreased
following treatment with doxycycline or anti-β1-integrin-Ab
compared with the control group (P<0.05). Furthermore, MMP-9
mRNA expression was significantly increased by doxycycline
(P<0.05), whereas anti-β1-integrin-Ab treatment exhibited no
significant effect. As shown in Fig.
1B, no significant changes were detected in the levels of FAK,
MMP-2 and MMP-9 mRNA in K562 cells following treatment with
doxycycline or anti-β1-integrin-Ab.
Effects of different concentrations of
doxycycline on FAK protein expression and phosphorylation
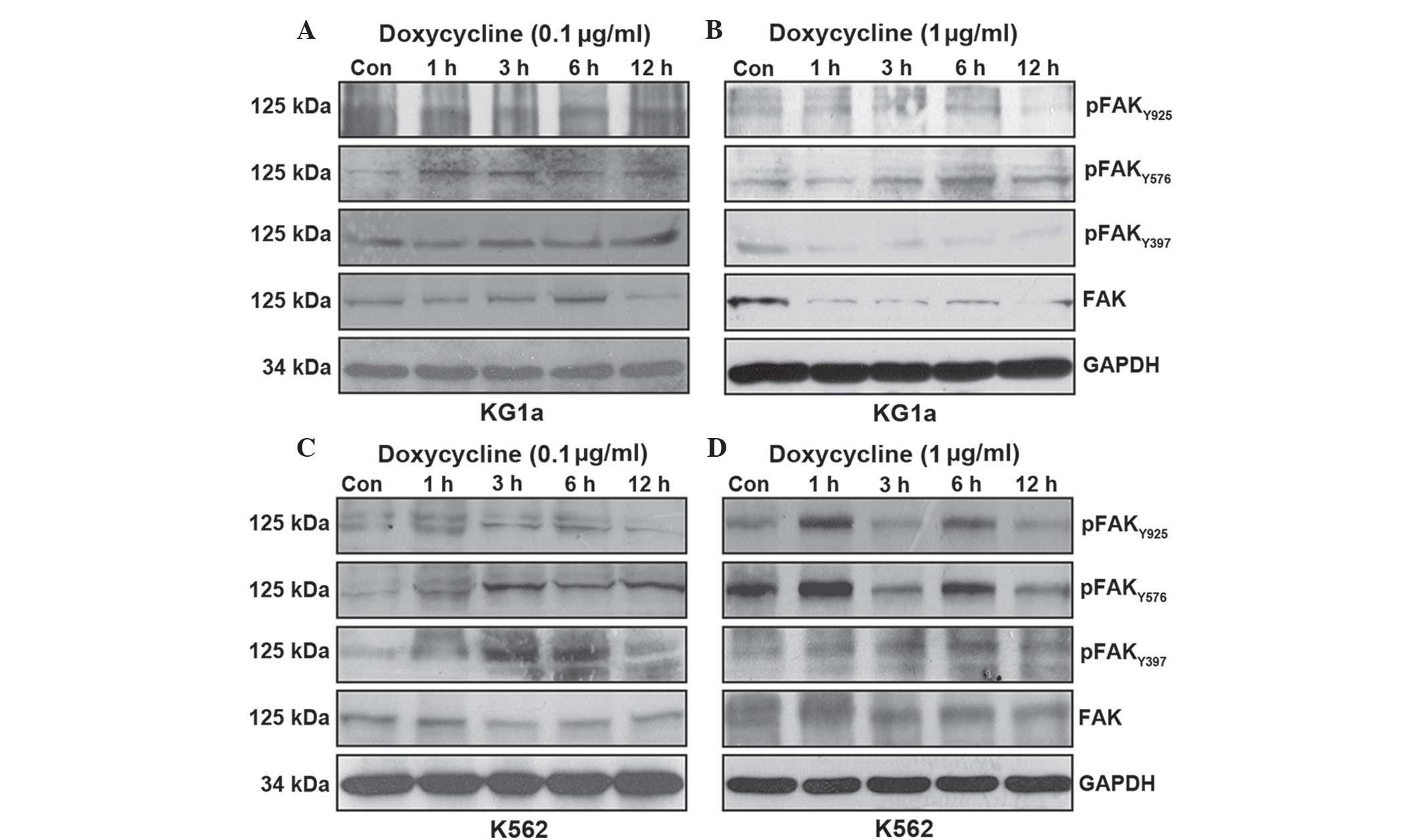
In order to investigate the mechanism by which
doxycycline inhibited leukemic cell migration, FAK protein
expression and phosphorylation were evaluated using western blot
analysis. KG1a and K562 cells were treated with doxycycline at 0.1
or 1 µg/ml for 1, 3, 6 and 12 h. FAK is known to undergo
adhesion-dependent phos-phorylation on six tyrosine residues: 397,
407, 576, 577, 861 and 925 (12);
however, since neither the Tyr-407 or Tyr-861 site has been
reported to function in mediating interactions with effecter
molecules, the functional significance of these sites remains
uncertain (18). Therefore,
Tyr397, Tyr576/577 and Tyr 925 were analyzed in the present
study.
The effects of doxycycline on FAK protein expression
and phosphorylation were not consistent in the leukemic cells.
Exposure of KG1a cells to 0.1 µg/ml doxycycline decreased
only the total protein expression of FAK and this effect was only
apparent following 12 h of treatment (Fig. 2A). Of note, following treatment of
KG1a cells with 1 µg/ml doxycycline, decreased total FAK
protein expression and Tyr397 phosphorylation were observed at 1 h
post treatment; in addition, Tyr925 phosphorylation was inhibited
following 12 h of treatment (Fig.
2B). Doxycycline treatment had no significant effects on
Tyr576/566 phosphorylation at either dose (Fig. 2A and B).
By contrast, exposure of K562 cells to 0.1
µg/ml doxycycline revealed a time-dependent decrease in
Tyr925 phosphorylation (Fig. 2C).
However, an increased concentration (1 µg/ml) of doxycycline
exhibited no effect on total FAK expression and Tyr397
phosphorylation, although downregulation of Tyr576/577 and Tyr925
phosphorylation occurred following 12 h of treatment with 1
µg/ml doxycycline (Fig.
2D).
Expression of FAK, pFAK, gelatinases
MMP-2 and MMP-9 following doxycycline or anti-β1-integrin-Ab
treatment
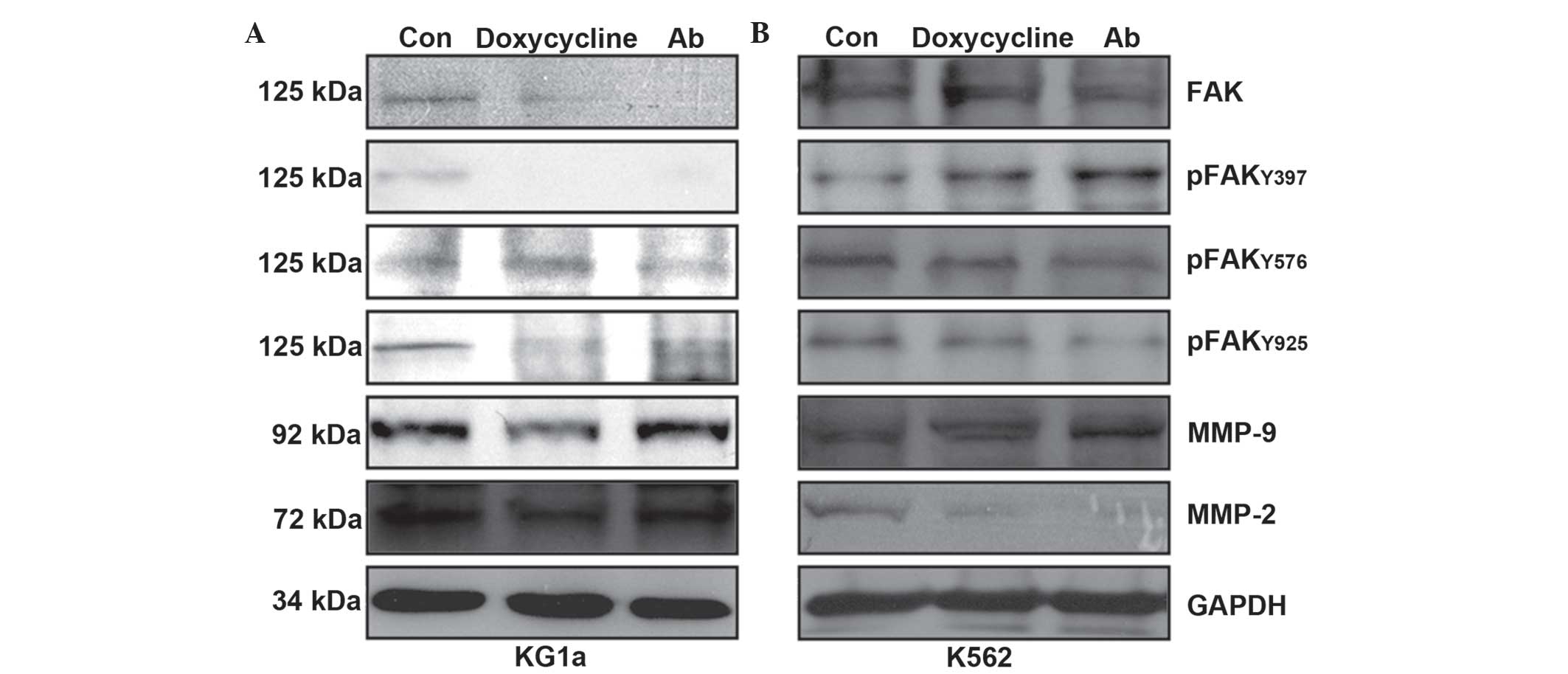
In order to investigate whether signaling downstream
of the FAK pathway was involved in the doxycycline- or
anti-β1-integrin-Ab-mediated downregulation of gelatinases, KG1a
and K562 cells were treated with doxycycline (1 µg/ml) or
anti-β1-integrin-Ab (100 ng/ml) for 24 h. As shown in Fig. 3A, the expression of MMP-2, FAK,
Tyr397-p-FAK and Tyr925-p-FAK were potently decreased by
doxycycline and anti-β1-integrin-Ab treatment in KG1a cells. The
anti-β1-integrin-Ab also inhibited the expression of MMP-9 in KG1a
cells. As shown in Fig. 3B, both
doxycycline and anti-β1-integrin-Ab inhibited MMP-2,
Tyr576/577-p-FAK and Tyr925-p-FAK in K562 cells, while FAK,
Tyr397-p-FAK and MMP-9 were not impacted. However, exposure of K562
cells to identical conditions decreased only MMP-9 protein
expression following doxycycline treatment (Fig. 3B).
Discussion
Tetracycline is a polyketide, which is produced by
the Streptomyces genus of Actinobacteria and has been used
as a broad-spectrum antibiotic for decades (19). Tetracycline functions as a protein
synthesis inhibitor by binding to the 16S ribosomal RNA portion of
the 30S ribosomal subunit and preventing amino-acyl transfer RNA
from binding to the ribosome (20). Doxycycline, a member of the
tetracycline group of antibiotics, has been reported to have a
variety of antitumor effects in vitro (21), including impairment of
mitochondrial protein synthesis (22,23),
proliferation arrest in the G1 phase of the cell cycle
(24) and induction of apoptosis
via caspase-3 activation (8). The
present study confirmed that doxycycline (1 µg/ml) exerted
inhibitory effects on the proliferation of leukemia cells, with no
significant cytotoxic effects detected using cell counting kit-8
assays in vitro (data not shown).
Studies have demonstrated that doxycycline exhibited
direct weak cytotoxic and indirect inhibitory effects on tumor cell
proliferation, angiogenesis, metastasis and migration through
multiple targets (11,25,26).
However, the molecular mechanism of the antitumor effects of
doxycycline remains to be fully elucidated. It was speculated that
the interaction between tumor cells and ECM may be a critical stage
in this process, leading to a series of consequential biological
actions that control important tumor cell phenotypes (27,28).
The FAK gene is ubiquitously expressed and encodes a
non-receptor tyrosine kinase that localizes to focal adhesions on
the cell membrane (29). FAK is a
crucial signaling component activated by numerous stimuli,
including growth factor receptors (epidermal and vascular
endothelial growth factor receptors) and integrins, in order to
regulate proliferation, survival and motility in normal cells as
well as tumor cells (18). Breast
cancer models have been employed to evaluate the role of FAK in
regulating tumorigenic and metastatic properties (30). In addition, a study in human and
mouse melanoma cell lines indicated that doxycycline inhibited
adhesion and migration through downregulating the FAK signaling
pathway (11). Furthermore, FAK
signaling has been critically implicated in the generation of
gelatinases and subsequent tumor invasion (31). However, it remained to be
elucidated whether doxycycline exerts these effects on leukemia
cells.
Acute leukemia is a hematopoietic malignancy that is
widely circulated from its onset and may be regarded as a prototype
of metastatic cancer (13). A
previous study demonstrated that expression of FAK in leukemia was
associated with enhanced blast migration and poor prognosis
(16). Expression of gelatinases
was also reported to have an essential role in the invasive
capacity of AML and chronic myeloid leukemia, with emerging
evidence suggesting that expression of these molecules may be
mediated through the FAK/phosphoinositide 3-kinase
(PI-3K)/extracellular signal-regulated kinase (ERK) signaling
pathways (16,32,33).
The present study investigated the effects of
doxycycline on the invasiveness of two myelogenous leukemia cell
lines, KG1a and K562, as well as examined the role of the FAK
signaling pathway and its influence on gelatinases in these
effects. FAK is known to typically activate the migration of
leukemic cells through the formation of integrin-dependent focal
adhesions; in addition, β1-integrin (CD29) has been reported to be
expressed by the KG1a and K562 cell lines (34,35).
Therefore, it was hypothesized that treatment with a blocking
anti-β1-integrin-Ab may inhibit migration of leukemic cells at the
levels of transcription, translation and phosphorylation. In the
present study, KG1a and K562 cells were treated with 100 ng/ml
anti-β1-integrin-Ab for 24 h. As expected, the anti-β1-integrin-Ab
potently decreased migration of the leukemic cells in
Matrigel® invasion assays. In addition, although mRNA
levels of MMP-2 were significantly decreased in KG1a cells, MMP-9
mRNA levels were unchanged following treatment with
anti-β1-integrin-Ab; these results were comparable to the effects
of doxycycline. However, mRNA levels of MMP-2, MMP-9 and FAK
remained stable in K562 cells following doxycycline or
anti-β1-integrin-Ab. Furthermore, at the protein level, the
expression levels of FAK and MMP-2 as well as the phosphorylation
of Tyr397 and Tyr925 were potently decreased by anti-β1-integrin-Ab
treatment of KG1a cells. These results were comparable to the
effects of doxycycline in KG1a. In K562 cells, anti-β1-integrin-Ab
treatment inhibited the expression of MMP-2 and phosphorylation of
Tyr576 and Tyr925.
Cell migration is essential to tumor invasion and
metastasis; therefore, the present study focused on the capacity of
doxycycline to attenuate the migration of leukemic cells through
inhibiting the FAK signaling pathway. FAK activation and
degradation of the ECM have important roles in cell migration
(36); therefore, it was
hypothesized that doxycycline-mediated reduction of FAK and
gelatinases may lead to decreased cell invasiveness. This
hypothesis was tested in the present study using
Matrigel® invasion assays, which demonstrated that
exposure of KG1a and K562 leukemic cells to doxycycline decreased
their invasive capacity. mRNA levels of FAK and the gelatinases
(MMP-2 and MMP-9) were almost unchanged in K562 cells following
doxycycline treatment; however, identical treatment of KG1a cells
resulted in significantly decreased MMP-2 mRNA levels, while those
of MMP-9 were increased. These data indicated that doxycycline
exhibited no significant effect on FAK transcription in leukemic
cells and demonstrated that the different leukemic cell lines had
various sensitivities to doxycycline at the level of gelatinase
transcription. These observations suggested that, paradoxically,
transcription of FAK and gelatinases may not be essential for
leukemic cell migration.
Phosphorylation, in particular tyrosine
phosphorylation, is important for kinase activity and represents
another mode of FAK regulation. FAK contains several tyrosine
residues, including Tyr397, 407, 576, 577, 861 and 925, which are
able to be phosphorylated (12).
Tyr397 autophosphorylation is a key event in FAK activation, which
results in the generation of a Src-homology-2 (SH2) binding site
for Src (37). Following upstream
activation of Src, the Tyr576/577 site of FAK is phosphorylated,
which promotes maximal FAK catalytic activation (18). Src was also reported to
phosphorylate the downstream effector FAK at Y407, 861 and 925.
Phosphorylation of FAK by Src regulates its kinase activity and
localization as well as its cellular motility and invasion
(38). Of note, phosphorylation of
the FAK C-terminal Tyr925 has been associated with Src-induced
focal contact dynamics. In addition, phosphorylation of Tyr925 was
reported to induce the generation of growth factor receptor-bound
protein 2 (GRB2) binding site for GRB2, which activated the
Rasmitogen-activated protein kinase signaling cascade to promote
focal contact turnover and contribute to cancer cell migration
(39).
There is evidence to suggest that FAK-mediated
signaling via Ras-related C3 botulinum toxin substrate 1 and Jun
N-terminal kinases as well as FAK/PI-3K/ERK signaling may
contribute to the expression of gelatinases and FAK-enhanced
motility (40). Activation of MMPs
is known to promote matrix proteolysis, leading to the
extracellular release of integrin-matrix contacts, thereby
facilitating focal contact remodeling and cell migration (41).
The present study demonstrated that the invasion
ability of KG1a and K562 cells was inhibited by doxycycline;
however, these results also indicated the existence of two
different mechanisms underlying this effect in these leukemic cell
lines. In KG1a cells, decreased expression of FAK following
doxycycline treatment resulted in the inhibition of Tyr397 and
Tyr925 phosphorylation, which subsequently led to downregulation of
gelatinase expression, attenuation of focal contact turnover and
blockade of ECM degradation. In K562 cells, doxycycline had no
effect on expression of FAK and phosphorylation of Tyr397; however,
doxycycline was able to downregulate Tyr576/577 and Tyr925
phosphorylation, ultimately leading to decreased focal contact
remodeling and expression of MMP-2.
In the present study, analogous results to those of
doxycy-cline were observed following treatment of KG1a and K562
cells with anti-β1-integrin-Ab, which attenuated migration in these
leukemic cells through inhibiting the expression and
phosphorylation of FAK. These results suggested that doxycycline
exerted its antimigratory effects through the FAK signaling
pathway. In addition, a previous study demonstrated that
dysfunction of β1-integrin and FAK in K562 cells lead to abnormal
adhesive characteristics (42). It
was therefore proposed that disruption of aberrant
β1-integrin-mediated signaling by doxycycline restored normal
adhesion mechanisms, which further indicated that the integrin-FAK
signaling pathway may be a potential target of doxycycline.
In conclusion, the results of the present study
demonstrated that doxycycline exerted a potent activity against the
migration of leukemic cells in vitro. In addition to
downregulation of FAK and its phosphorylation, the inhibition of
downstream gelati-nases was suggested to be involved in
doxycycline-mediated inhibition of migration. These results
therefore indicated the potential of doxycycline to attenuate the
migration of leukemic cells through inhibiting the FAK signaling
pathway.
Acknowledgments
The present study was supported by a grant from the
Medical & Health Technology Fund of Guangzhou City (grant no.
201102A212004).
References
|
1
|
Chiang AC and Massagué J: Molecular basis
of metastasis. New Engl J Med. 359:2814–2823. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedl P and Alexander S: Cancer invasion
and the microenvironment: Plasticity and reciprocity. Cell.
147:992–1009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagase H and Woessner JF Jr: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
McLean GW, Carragher NO, Avizienyte E,
Evans J, Brunton VG and Frame MC: The role of focal-adhesion kinase
in cancer - a new therapeutic opportunity. Nat Rev Cancer.
5:505–515. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rolain JM, Boulos A, Mallet MN and Raoult
D: Correlation between ratio of serum doxycycline concentration to
MIC and rapid decline of antibody levels during treatment of Q
fever endocarditis. Antimicrob Agents Chemother. 49:2673–2676.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fife RS, Sledge GW Jr, Sissons S and
Zerler B: Effects of tetracyclines on angiogenesis in vitro. Cancer
Lett. 153:75–78. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwasaki H, Inoue H, Mitsuke Y, Badran A,
Ikegaya S and Ueda T: Doxycycline induces apoptosis by way of
caspase-3 activation with inhibition of matrix metalloproteinase in
human T-lymphoblastic leukemia CCRF-CEM cells. J Lab Clin Med.
140:382–386. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fife RS, Sledge GW Jr, Roth BJ and Proctor
C: Effects of doxycycline on human prostate cancer cells in vitro.
Cancer Lett. 127:37–41. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tu G, Xu W, Huang H and Li S: Progress in
the development of matrix metalloproteinase inhibitors. Curr Med
Chem. 15:1388–1395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun T, Zhao N, Ni CS, et al: Doxycycline
inhibits the adhesion and migration of melanoma cells by inhibiting
the expression and phosphorylation of focal adhesion kinase (FAK).
Cancer Lett. 285:141–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mitra SK, Hanson DA and Schlaepfer DD:
Focal adhesion kinase: in command and control of cell motility. Nat
Rev Mol Cell Biol. 6:56–68. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Freireich EJ: Acute leukemia. A prototype
of disseminated cancer. Cancer. 53:2026–2033. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Janowska-Wieczorek A, Marquez LA,
Matsuzaki A, et al: Expression of matrix metalloproteinases (MMP-2
and -9) and tissue inhibitors of metalloproteinases (TIMP-1 and -2)
in acute myelogenous leukaemia blasts: comparison with normal bone
marrow cells. Br J Haematol. 105:402–411. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Recher C, Ysebaert L, Beyne-Rauzy O, et
al: Expression of focal adhesion kinase in acute myeloid leukemia
is associated with enhanced blast migration, increased cellularity
and poor prognosis. Cancer Res. 64:3191–3197. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berken A, Abel J and Unfried K:
beta1-integrin mediates asbestos-induced phosphorylation of AKT and
ERK1/2 in a rat pleural mesothelial cell line. Oncogene.
22:8524–8528. 2003.PubMed/NCBI
|
|
17
|
Ramakers C, Ruijter JM, Deprez RH and
Moorman AF: Assumption-free analysis of quantitative real-time
polymerase chain reaction (PCR) data. Neurosci Lett. 339:62–66.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hanks SK, Ryzhova L, Shin NY and Brabek J:
Focal adhesion kinase signaling activities and their implications
in the control of cell survival and motility. Front Biosci.
8:d982–996. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Adimora AA: Treatment of uncomplicated
genital Chlamydia trachomatis infections in adults. Clin Infect
Dis. 35(Suppl 2): S183–S186. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Olson MW, Ruzin A, Feyfant E, Rush TS III,
O'Connell J and Bradford PA: Functional, biophysical and structural
bases for antibacterial activity of tigecycline. Antimicrob Agents
Chemother. 50:2156–2166. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fife RS and Sledge GW Jr: Effects of
doxycycline on in vitro growth, migration and gelatinase activity
of breast carcinoma cells. J Lab Clin Med. 125:407–411.
1995.PubMed/NCBI
|
|
22
|
Pilkington GJ, Parker K and Murray SA:
Approaches to mitochondrially mediated cancer therapy. Semin Cancer
Biol. 18:226–235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kroon AM, Dontje BH, Holtrop M and Van den
Bogert C: The mitochondrial genetic system as a target for
chemotherapy: tetracyclines as cytostatics. Cancer Lett. 25:33–40.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van den Bogert C, van Kernebeek G, de Leij
L and Kroon AM: Inhibition of mitochondrial protein synthesis leads
to proliferation arrest in the G1-phase of the cell cycle. Cancer
Lett. 32:41–51. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun B, Zhang S, Zhang D, et al:
Doxycycline influences microcirculation patterns in B16 melanoma.
Exp Biol Med (Maywood). 232:1300–1307. 2007. View Article : Google Scholar
|
|
26
|
Saikali Z and Singh G: Doxycycline and
other tetracyclines in the treatment of bone metastasis.
Anti-cancer Drugs. 14:773–778. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gilmore AP, Owens TW, Foster FM and
Lindsay J: How adhesion signals reach a mitochondrial
conclusion-ECM regulation of apoptosis. Curr Opin Cell Biol.
21:654–661. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sieg DJ, Hauck CR, Ilic D, et al: FAK
integrates growth-factor and integrin signals to promote cell
migration. Nat Cell Biol. 2:249–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Golubovskaya VM and Cance W: Focal
adhesion kinase and p53 signal transduction pathways in cancer.
Front Biosci. 15:901–912. 2010. View
Article : Google Scholar
|
|
30
|
Costa P, Scales TM, Ivaska J and Parsons
M: Integrin-specific control of focal adhesion kinase and RhoA
regulates membrane protrusion and invasion. PLoS One. 8:e746592013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Duivenvoorden WC, Vukmirović-Popović S,
Kalina M, Seidlitz E and Singh G: Effect of zoledronic acid on the
doxycycline-induced decrease in tumour burden in a bone metastasis
model of human breast cancer. Br J Cancer. 96:1526–1531. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mon NN, Ito S, Senga T and Hamaguchi M:
FAK signaling in neoplastic disorders: a linkage between
inflammation and cancer. Ann N Y Acad Sci. 1086:199–212. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dutta A, Sen T and Chatterjee A: Culture
of K562 human myeloid leukemia cells in presence of fibronectin
expresses and secretes MMP-9 in serum-free culture medium. Int J
Clin Exp Pathol. 3:288–302. 2010.PubMed/NCBI
|
|
34
|
Wang C, Chen Z, Li Z and Cen J: The
essential roles of matrix metalloproteinase-2, membrane type 1
metalloproteinase and tissue inhibitor of metalloproteinase-2 in
the invasive capacity of acute monocytic leukemia SHI-1 cells. Leuk
Res. 34:1083–1090. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liesveld JL, Winslow JM, Frediani KE, Ryan
DH and Abboud CN: Expression of integrins and examination of their
adhesive function in normal and leukemic hematopoietic cells.
Blood. 81:112–121. 1993.PubMed/NCBI
|
|
36
|
Guo-Bao W, Xiao-Qin C, Qi-Rong G, Jie L,
Gui-Nan L and Yue L: Arsenic Trioxide overcomes cell
adhesion-mediated drug resistance through down-regulating the
expression of beta (1)-integrin in K562 chronic myelogenous
leukemia cell line. Leuk Lymphoma. 51:1090–1097. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheng SY, Sun G, Schlaepfer DD and Pallen
CJ: Grb2 promotes integrin-induced focal adhesion kinase (FAK)
autophos-phorylation and directs the phosphorylation of protein
tyrosine phosphatase alpha by the Src-FAK kinase complex. Mol Cell
Biol. 34:348–361. 2014. View Article : Google Scholar :
|
|
38
|
Brunton VG, Avizienyte E, Fincham VJ, et
al: Identification of Src-specific phosphorylation site on focal
adhesion kinase: dissection of the role of Src SH2 and catalytic
functions and their consequences for tumor cell behavior. Cancer
Res. 65:1335–1342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Katz BZ, Romer L, Miyamoto S, et al:
Targeting membrane-localized focal adhesion kinase to focal
adhesions: roles of tyrosine phosphorylation and SRC family
kinases. J Biol Chem. 278:29115–29120. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li S, Butler P, Wang Y, et al: The role of
the dynamics of focal adhesion kinase in the mechanotaxis of
endothelial cells. Natl Acad Sci USA. 99:3546–3551. 2002.
View Article : Google Scholar
|
|
41
|
Brinckerhoff CE and Matrisian LM: Matrix
metalloproteinases: a tail of a frog that became a prince. Nat Rev
Mol Cell Biol. 3:207–214. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lundell BI, McCarthy JB, Kovach NL and
Verfaillie CM: Activation-dependent alpha5beta1 integrin-mediated
adhesion to fibronectin decreases proliferation of chronic
myelogenous leukemia progenitors and K562 cells. Blood.
87:2450–2458. 1996.PubMed/NCBI
|