Introduction
Colon cancer is one of the most prevalent types of
cancer in the United States and is the second most frequent cause
of cancer-associated mortality (1). In addition, the worldwide incidence
rates of colorectal cancer have been increasing steadily in
previous years. Although early-stage colorectal cancer can be
successfully treated by surgery, advanced-stage colon cancer
frequently recurs and becomes fatal, even in patients receiving
combination chemotherapy (2).
Chemotherapeutic agents, including cisplatin, are routinely used in
the treatment of advanced-stage colon cancer; however, they provide
only minimal survival benefits as a result of several factors: Drug
resistance, side effects and toxicity (3,4).
Previously, the development of cancer chemoprevention protocols
using natural or synthetic agents, which prevent or suppress the
progression to invasive cancer, have been recognized as a field
with enormous potential to reduce the cancer burden (5). Therefore, there is an urgent
requirement for novel chemopreventive agents with minimal or no
side effects and toxicities. In previous years, bioactive compounds
derived from natural sources have become the focus of a substantial
amount of attention from researchers seeking to develop
chemopreventive agents, primarily due to the potential
cancer-preventive and/or therapeutic activities of several of these
compounds at non-toxic levels. However, continued research into the
mechanism of action of such compounds is required.
Fucoidan is a sulfated polysaccharide located in the
cell wall matrix of brown seaweeds, including Ascophyllum
nodosum, Cladosiphon okamuranus, Ecklonia kurome, Fucus evanescens,
Fucus vesiculosus, Hizikia fusiforme, Laminaria angustata and
Undaria pinnatifida (6–8).
Structurally, fucoidan is a heparin-like molecule with a
substantial percentage of l-fucose and sulfated ester
groups, as well as small quantities of d-xylose, d-galactose, d-mammose and glucuronic acid
(9). Among the several analogues
of fucoidan, the predominant form is isolated from Undaria
pinnatifida and is described as a sulfated galactofucan
(10). Fucoidan has various
biological activities, including anti-cancer (11), anti-inflammatory, anti-angiogenic
(12), anti-coagulant (13) and anti-human immunodeficiency virus
(14) activities. In previous
in vivo studies performed using xenograft models, fucoidan
was reported to suppress the growth of Ehrlich ascites carcinoma
(15,16) and Lewis lung adenocarcinoma
(17), and was also demonstrated
to inhibit the metastasis of Lewis lung adenocarcinoma (17) and 13762 MAT rat mammary
adenocarcinoma (18). These
findings demonstrated that fucoidan inhibits the growth of human
non-small-cell bronchopulmonary carcinoma (NSCLS-N6) cells
(19) and human lymphoma HS-Sultan
cells (11), and also inhibits the
invasion of HT1080 human fibrosarcoma cells and the angiogenic
activity of HeLa human uterine carcinoma cells (20).
Fucoidan cannot be hydrolyzed by digestive enzymes
in the human small intestine (21)
and therefore, the consumption of this compound can result in an
increase in the concentration of luminal fucoidan within the large
intestine. Thus, fucoidan may prove to be an excellent candidate
for the prevention of colon carcinogenesis, provided that it exerts
cancer-preventive effects in the colon. However, its inhibitory
mechanism on colon cancer proliferation and metastasis remains to
be elucidated. In the present study, the effect of fucoidan on the
migration and proliferation of HT-29 human colon cancer cells, and
its underlying anti-cancer mechanisms of action were
investigated.
Materials and methods
Preparation of fucoidan
Fuciodan extract from the seaweed Fucus
vesiculous was obtained from Sigma-Aldrich (St. Louis, MO,
USA). Fucoidan powder was dissolved in phosphate-buffered saline
(Gibco Life Technologies, Carlsbad, CA, USA), sterilized by
filtration through a 0.45-μm pore filter (Sartorius Biotech
GmbH, Göttingen, Germany) and stored as fucoidan extract (20 mg/ml)
at 4°C until use.
Cell cultures
The human HT-29 colon cancer cell line was obtained
from the American Type Culture Collection (Manassas, VA, USA). The
cells were maintained in Dulbecco's modified Eagle's medium (4.5
g/l glucose), supplemented with 10% fetal calf serum, l-glutamine and antibiotics
(Biological Industries, Beit Haemek, Israel) at 37°C with 5%
CO2 in a humidified incubator.
Cell viability assay
Exponentially growing colon cancer cells in 96-well
plates (5,000 cells/well) were sub-confluently incubated with
fucoidan (0, 50, 100 and 200 μg/ml) for various durations
(24 and 48 h). Cell viability was determined using a modified
version of the MTT assay (Promega, Madison, WI, USA), which was
based on the conversion of the tetrazolium salt of MTT to the
formazan product by mitochondrial dehydrogenase. A total of 10
μl MTT solution was added to each well and incubated for 4 h
at 37°C. The color was extracted with dimethyl sulfoxide
(Sigma-Aldrich) at 37°C for 20 min. The formazan product was
quantified by measuring the absorbance of the reaction at 570 nm
using a microplate reader (Infinite F50; Tecan, Männedorf,
Switzerland).
Wound-healing migration assay
The HT-29 cells were seeded onto six-well plates
(25×104 cells/well) and grown to 90% confluence in 2 ml
growth medium. The cell monolayers were damaged using a 2 mm-wide
tip to generate a line-shaped wound. The cells were subsequently
treated with fucoidan (0, 50, 100 and 200 μg/ml) for 48 h at
37°C. The cells were allowed to migrate and images were captured by
an inverted microscope (IX71; Olympus, Tokyo, Japan).
Tumor sphere culture
To generate tumor spheres, the colon cancer cells
(200 cells/ml) were seeded into siliconized spinner flasks (Bellco,
Vineland, NJ, USA), followed by agitation at 70 rpm for three days.
Spinner flasks were siliconized by the application of Sigma coat
(Sigma-Aldrich), followed by drying for a minimum of 24 h. The
cells were cultured with growth medium. Spheroids formed at day
three and images of the spheres were captured by an inverted
microscope (IX71; Olympus).
Western blot analysis
The total protein was extracted using
radioimmunoprecipitation lysis buffer (Thermo Fisher Scientific,
Waltham, MA, USA). The cell lysates were separated via SDS-PAGE
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) and were
transferred onto a polyvinylidene fluoride membrane (Millipore,
Billerica, MA, USA). The membranes were blocked with 5% non-fat
milk (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and were
subsequently incubated with the appropriate primary antibodies:
Mouse monoclonal cyclin D1 (1:1,000; cat. no. sc-20044; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), mouse monoclonal cyclin E
(1:1,000; cat. no. sc-377100; Santa Cruz Biotechnology, Inc.),
rabbit polyclonal CDK2 (1:1,000; cat. no. sc-748; Santa Cruz
Biotechnology, Inc.), mouse monoclonal CDK4 (1:1,000; cat. no.
sc-56277; Santa Cruz Biotechnology, Inc.), mouse monoclonal matrix
metalloproteinase 2 (MMP 2; 1:1,000; cat. no. sc-13594; Santa Cruz
Biotechnology, Inc.), rabbit monoclonal p-Akt (1:1,000–1:2,000;
cat. no. OMA1-03061; Pierce Biotechnology, Inc., Rockford, IL,
USA), rabbit poly-clonal p-mammalian target of rapamycin (mTOR;
1:1,000; cat. no. sc-101738;Santa Cruz Biotechnology, Inc.), mouse
monoclonal p-p70s6k (1:1,000; cat. no. sc-8416; Santa Cruz
Biotechnology, Inc.), rabbit cleaved caspase-3 (1:1,000; cat. no.
9664; Cell Signaling Technology, Danvers, MA, USA) and mouse
monoclonal β-actin (1:3,000; cat. no. sc-47778; Santa Cruz
Biotechnology, Inc.) in Tris-buffered saline in 0.1% Tween-20
(TBST) and incubated overnight at 4°C. The membranes were
subsequently washed three times with TBST, incubated at 4°C
overnight with goat anti-mouse (1:10,000; cat. no. sc-2005; Santa
Cruz Biotechnology, Inc.) and goat anti-rabbit (1:10,000; cat. no.
sc-2004; Santa Cruz Biotechnology, Inc.) secondary antibodies. The
bands were visualized by enhanced chemiluminescence reagents
(Amersham Biosciences, Uppsala, Sweden). Quantification of band
intensity was performed using TINA 2.0 (Raytest, Straubenhardt,
Germany) and normalized against the intensity of β-actin.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. All experiments were analyzed by Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference. All data were analyzed using Sigma plot 8.0 software
(Systat Software Inc., San Jose, CA, USA).
Results
Fucoidan inhibits the proliferation of
human HT-29 colon cancer cells
The effects of various fucoidan concentrations (50,
100, 200 μg/ml) on the growth of HT-29 cells were initially
assessed by measuring the viable cell numbers via the MTT assay.
Fucoidan reduced the number of viable HT-29 cells in a dose- and
time-dependent manner (Fig. 1A).
In addition, the present study assessed whether fucoidan affects
the expression levels of the cell cycle regulatory proteins, cyclin
D1, cyclin E, CDK2 and CDK4. These proteins were demonstrated to be
maximally decreased following 48 h of treatment with 200
μg/ml fucoidan (Fig. 1B and
C). Collectively, these data demonstrated that fucoidan may
suppress the proliferation of colon cancer cells.
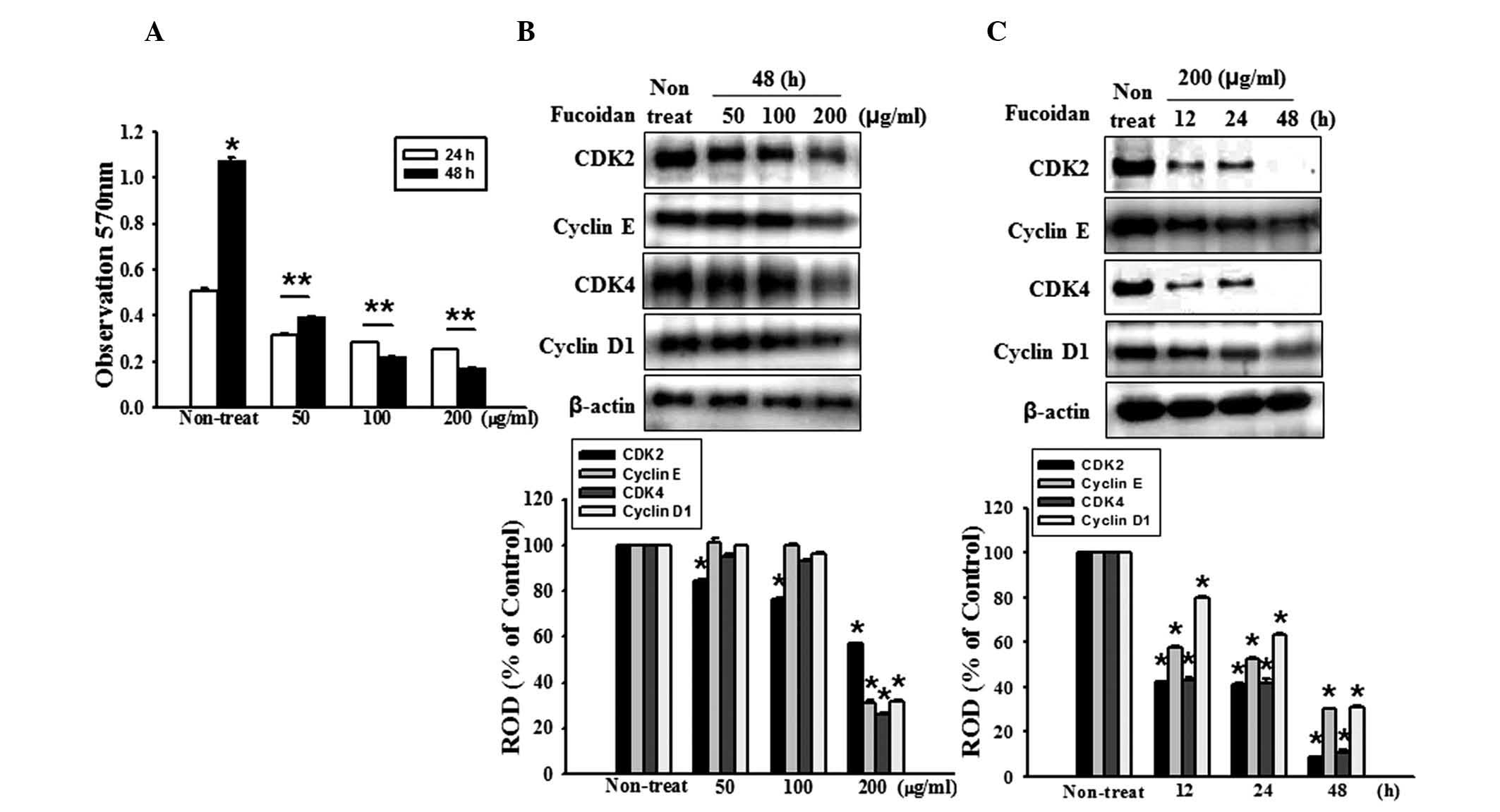 | Figure 1Fucoidan inhibits the proliferation of
HT-29 cells. (A) HT-29 cell proliferation was measured using an MTT
assay. Values are expressed as the mean ± standard error of the
mean from three independent experiments (*P<0.05,
**P<0.01, vs. 48 h fucoidan treatment). (B) HT-29
cells were incubated for 48 h with various concentrations of
fucoidan (0, 50, 100 and 200 μg/ml) and the expression
levels of CDK2, cyclin E, CDK4 and cyclin D1 were assessed by
western blotting. (C) HT-29 cells were treated with fucoidan for
different durations (0–48 h). The expression levels of CDK 2,
cyclin E, CDK4 and cyclin D1 were assessed by western blotting.
Values are expressed as the mean ± sandard error of the mean of
four independent experiments for each condition, as determined from
densitometry against to β-actin (*P<0.05, vs.
non-treated cells). ROD, relative optical density. |
Fucoidan inhibits the expression of MMP-2
and the migration of HT-29 cells
The degradation of the extracellular matrix (ECM) is
crucial for cellular migration and invasion, indicating the
inevitable involvement of matrix-degrading proteinases. Therefore,
the present study examined the effect of fucoidan on the expression
of MMP-2, a key molecule involved in ECM degradation, by western
blotting. The expression of MMP-2 was gradually reduced in response
to an increase in fucoidan concentration (Fig. 2A). Cell migration is a measure of
the metastatic potential of cancer cells; therefore, the influence
of fucoidan on cell migration was investigated using a
wound-healing assay. The HT-29 cells treated with fucoidan
demonstrated a reduction in cell migration (Fig. 2B). Collectively, these data
demonstrated that fucoidan suppressed the migratory properties of
human colon cancer cells.
Fucoidan suppresses the signaling of
PI3K
The above findings have demonstrated that fucoidan
significantly inhibits the proliferation and migration of HT-29
cells, and also reduced the expression of MMP-2 in HT-29 cells.
However, the signaling mechanisms responsible for fucoidan on
proliferation and migration remain to be elucidated. PI3K/Akt has
been suggested as a key pathway involved in the regulation of
proliferation and migration. The present study revealed that
fucoidan markedly inhibited the phospholylation of PI3K and its
downstream target, Akt, in a dose- and time-dependent manner
(Fig. 3A and B). Based on these
results, fucoidan potently inhibited the proliferation and
migration of human colon cancer cells, possibly by suppressing the
PI3K/Akt pathway.
Fucoidan suppresses mTOR signaling
Based on the above finding that fucoidan suppresses
the PI3K-Akt pathway in human HT-29 colon cancer cells, whether
fucoidan modulates mTOR and its downstream signaling molecules was
investigated. As shown in Fig. 4A and
B, fucoidan inhibited the phospholylation of mTOR in a dose-
and time-dependent manner. In addition, the phosphorylation of
p70S6K, an immediate downstream target of mTOR and an indicator of
mTOR activity, was also significantly suppressed (Fig. 4C and D).
Fucoidan increases the activation of
caspases
Caspases are central effectors of apoptosis. To
examine the mechanism of fucoidan-induced apoptosis, western
blotting was used to detect anti-cleaved caspase-3, which detects
the cleaved forms of the enzymes to determine whether or not
fucoidan activated caspases. Treatment with fucoidan increased the
expression of cleaved caspase-3 in a dose- and time-dependent
manner (Fig. 5A and B).
Fucoidan inhibits cancer sphere
formation
In order to generate spheroid cells, HT-29 cells
were enzymatically dissociated and inoculated on ultra-low
attachment culture plates in serum-free medium. As is often the
case for HT-29 cells, the majority survived and generated floating
spherical colonies following 3–5 days in culture (Fig. 6A). To investigate the
cancer-sphere-formation capacity, the HT-29 cells were treated with
fucoidan during sphere formation. The results demonstrated that
sphere formation by the HT-29 cells was inhibited by fucoidan in a
time-dependent manner (Fig. 6B).
At 200 μg/ml fucoidan, the efficiency of cancer sphere
formation was reduced.
Discussion
Given the high mortality rate as a result of colon
cancer and the significant morbidity, apparent toxicity and poor
response rates of current chemotherapeutic regimens, there has been
a big push to identify novel therapeutic modalities with fewer
toxicity profiles. The PI3K/Akt/mTOR signaling axis is critical in
the proliferation, resistance to apoptosis, angiogenesis and
metastasis, and is central to the development and maintenance of
colorectal cancer cells (21–23).
Accordingly, inhibition of the activity and expression of the Akt
pathway may lead to the development of effective therapy in
patients with colon cancer. Although numerous studies have made
great efforts to develop potential anti-cancer agents inhibiting
the Akt pathway, the majority of clinical trials remain to be
successfully completed (24).
In search of efficacious tumor suppressors, a wide
variety of natural products and the pharmacologically active
components of plants are promising candidates. These natural
products have several advantages over synthetic chemicals with
regards to side effects and pharmacophore diversity. Certain
products from various natural resources have previously been
characterized as potential therapeutics with anti-cancer activity
(22). Among them, fucoidan has
been demonstrated to possess anti-proliferative and cytotoxic
effects on MCF-7 breast cancer cells; however, not on human mammary
epithelial cells (23).
Previously, fucoidan was demonstrated to inhibit metastasis by
suppressing MMP-2/29 and reducing the expression and secretion of a
vascular endothelial growth factor (14). However, the underlying mechanism of
its inhibition of cancer cell proliferation and metastasis remains
to be elucidated. The present study partly identified, for the
first time, to the best of our knowledge, the underlying molecular
mechanism of the anti-proliferative and anti-metastatic effects of
fucoidan.
Cell viability was assessed using an MTT assay in
the absence or presence of various concentrations of fucoidan.
Fucoidan inhibited cell growth, the expression of MMP-2 and cell
migration capacity at 200 μg/ml. Therefore, the
anti-proliferative and anti-migratory effects of fucoidan observed
in the present study were dependent on its cancer-preventive
effects.
The Akt signaling pathway is known to regulate the
development and progression of various types of tumor (24–26).
There is an accumulating number of studies demonstrating the
importance of the Akt signaling pathway in the inhibition of cell
growth (24–27). Previous studies have revealed a
decreased activation of Akt in growth-retarded tumor cells
(24–27). Several previous studies have also
demonstrated that targeting of the PI3K/Akt signaling pathway with
anti-sense small interfering (si)RNA or small molecule inhibitors
results in the downregulation of tumor invasion and tumorigenesis
in malignant cancer cells (28,29).
The results of the present study demonstrated that fucoidan
inhibited the phosphorylation of PI3K/Akt in a time- and
dose-dependent manner. PI3K and Akt are well-known upstream
regulators of the mTOR signaling pathway in mammalian cells. As
evidence of this hypothesis, siRNA-mediated gene silencing of PI3K
and Akt inhibited the activation of p70S6K1, a downstream target of
mTOR, and subsequently led to the suppression of migration,
invasion and proliferation (30).
In the present study, fucoidan significantly decreased the
phosphorylation of mTOR and p70S6K1. In addition, since previous
studies indicated that spheroid cells were more resistant to
chemotherapeutic drugs (31–34),
the present study assessed the sphere formation capacity of HT-29
cells during treatment with fucoidan. The results revealed that
fucoidan inhibited sphere formation in a time-dependent manner.
Fucoidan concentrations of 200 μg/ml caused significant
inhibition of sphere formation in HT-29 cells.
In conclusion, the present study demonstrated for
the first time, to the best of our knowledge, that fucoidan
inhibited cell growth, migration and sphere formation by
suppressing the PI3K/Akt/mTOR pathway and reducing the expression
of MMP-2 in human HT-29 colon cancer cells. In vivo and
clinical investigations are required for the development of
fucoidan as a novel therapeutic agent and alternative remedy in
patients with cancer. By any measure, fucoidan may be a promising
candidate as an efficacious anti-cancer reagent with minimal side
effects in normal cells.
Acknowledgments
This study was supported by a National Research
Foundation grant funded by the Korean government (no. 2011-0009610)
and a grant from the Korean Health Technology R&D Project,
Ministry of Health and Welfare, Republic of Korea (grant no.
HI14C2253).
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chung KY and Saltz LB: Adjuvant therapy of
colon cancer: current status and future directions. Cancer J.
13:192–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rabik CA and Dolan ME: Molecular
mechanisms of resistance and toxicity associated with platinating
agents. Cancer Treat Rev. 33:9–23. 2007. View Article : Google Scholar :
|
|
4
|
Macdonald JS and Astrow AB: Adjuvant
therapy of colon cancer. Semin Oncol. 28:30–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mann JR, Backlund MG and DuBois RN:
Mechanisms of disease: Inflammatory mediators and cancer
prevention. Nat Clin Pract Oncol. 2:202–210. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li B, Lu F, Wei X and Zhao R: Fucoidan:
structure and bioactivity. Molecules. 13:1671–1695. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamasaki-Miyamoto Y, Yamasaki M, Tachibana
H and Yamada K: Fucoidan induces apoptosis through activation of
caspase-8 on human breast cancer mcf-7 cells. J Agric Food Chem.
57:8677–8682. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bilan MI, Grachev AA, Ustuzhanina NE,
Shashkov AS, Nifantiev NE and Usov AI: Structure of a fucoidan from
the brown seaweed Fucus evanescens C.Ag. Carbohydr Res.
337:719–730. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gideon TP and Rengasamy R: Toxicological
evaluation of fucoidan from Cladosiphon okamuranus. J Med Food.
11:638–642. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JB, Hayashi K, Hashimoto M, Nakano T
and Hayashi T: Novel antiviral fucoidan from sporophyll of Undaria
pinnatifida (Mekabu). Chem Pharm Bull (Tokyo). 52:1091–1094. 2004.
View Article : Google Scholar
|
|
11
|
Aisa Y, Miyakawa Y, Nakazato T, et al:
Fucoidan induces apoptosis of human HS-sultan cells accompanied by
activation of caspase-3 and down-regulation of ERK pathways. Am J
Hematol. 78:7–14. 2005. View Article : Google Scholar
|
|
12
|
Koyanagi S, Tanigawa N, Nakagawa H, Soeda
S and Shimeno H: Oversulfation of fucoidan enhances its
anti-angiogenic and antitumor activities. Biochem Pharmacol.
65:173–179. 2003. View Article : Google Scholar
|
|
13
|
Dürig J, Bruhn T, Zurborn KH, Gutensohn K,
Bruhn HD and Béress L: Anticoagulant fucoidan fractions from Fucus
vesiculosus induce platelet activation in vitro. Thromb Res.
85:479–491. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McClure MO, Moore JP, Blanc DF, et al:
Investigations into the mechanism by which sulfated polysaccharides
inhibit HIV infection in vitro. AIDS Res Hum Retroviruses. 8:19–26.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Itoh H, Noda H, Amano H, Zhuaug C, Mizuno
T and Ito H: Antitumor activity and immunological properties of
marine algal polysaccharides, especially fucoidan, prepared from
sargassum thunbergii of phaeophyceae. Anticancer Res. 13:2045–2052.
1993.PubMed/NCBI
|
|
16
|
Zhuang C, Itoh H, Mizuno T and Ito H:
Antitumor active fucoidan from the brown seaweed, umitoranoo
(Sargassum thunbergii). Biosci Biotechnol Biochem. 59:563–567.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alekseyenko TV, Zhanayeva SY, Venediktova
AA, et al: Antitumor and antimetastatic activity of fucoidan, a
sulfated polysaccharide isolated from the Okhotsk Sea Fucus
evanescens brown alga. Bull Exp Biol Med. 143:730–732. 2007.In
English, Russian. View Article : Google Scholar
|
|
18
|
Coombe DR, Parish CR, Ramshaw IA and
Snowden JM: Analysis of the inhibition of tumour metastasis by
sulphated polysaccharides. Int J Cancer. 39:82–88. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Riou D, Colliec-Jouault S, Pinczon du Sel
D, et al: Antitumor and antiproliferative effects of a fucan
extracted from ascophyllum nodosum against a non-small-cell
bronchopulmonary carcinoma line. Anticancer Res. 16:1213–1218.
1996.PubMed/NCBI
|
|
20
|
Ye J, Li Y, Teruya K, et al:
Enzyme-digested fucoidan extracts derived from seaweed mozuku of
cladosiphon novae-caledoniae kylin inhibit invasion and
angiogenesis of tumor cells. Cytotechnology. 47:117–126. 2005.
View Article : Google Scholar
|
|
21
|
Bilan MI, Grachev AA, Ustuzhanina NE,
Shashkov AS, Nifantiev NE and Usov AI: A highly regular fraction of
a fucoidan from the brown seaweed Fucus distichus L. Carbohydr Res.
339:511–517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
WCRF/AICR: Policy and Action for cancer
prevention. Food, nutrition and physical activity: A global
perspective. World cancer research fund and American Institute for
Cancer Research; 2009
|
|
23
|
Rajamanickam S and Agarwal R: Natural
products and colon cancer: current status and future prospects.
Drug Dev Res. 69:460–471. 2008. View Article : Google Scholar
|
|
24
|
Hill MM and Hemmings BA: Inhibition of
protein kinase B/Akt. implications for cancer therapy. Pharmacol
Ther. 93:243–251. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ye M, Hu D, Tu L, et al: Involvement of
PI3K/Akt signaling pathway in hepatocyte growth factor-induced
migration of uveal melanoma cells. Invest Ophthalmol Vis Sci.
49:497–504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Faivre S, Kroemer G and Raymond E: Current
development of mTOR inhibitors as anticancer agents. Nat Rev Drug
Discov. 5:671–688. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burleson KM, Boente MP, Pambuccian SE and
Skubitz AP: Disaggregation and invasion of ovarian carcinoma
ascites spheroids. J Transl Med. 4:62006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
L'Espérance S, Bachvarova M, Tetu B,
Mes-Masson AM and Bachvarov D: Global gene expression analysis of
early response to chemotherapy treatment in ovarian cancer
spheroids. BMC Genomics. 9:992008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shield K, Riley C, Quinn MA, Rice GE,
Ackland ML and Ahmed N: Alpha2beta1 integrin affects metastatic
potential of ovarian carcinoma spheroids by supporting
disaggregation and proteolysis. J Carcinog. 6:112007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zietarska M, Maugard CM, Filali-Mouhim A,
et al: Molecular description of a 3D in vitro model for the study
of epithelial ovarian cancer (EOC). Mol Carcinog. 46:872–885. 2007.
View Article : Google Scholar : PubMed/NCBI
|




















