Introduction
Toll-like receptors (TLRs) are pattern recognition
receptors, which bind with pathogen-associated molecular patterns,
such as lipopolysaccharide (LPS), and damage-associated molecular
patterns (DAMPs), including S100A, high mobility group box 1
(HMGB1) and heat shock protein, which are released from necrotic
and apoptotic cells (1). TLR
signaling initiates divergent pathways, which exert either a
protumor response or an antitumor response (2). Activation of TLRs expressed on tumor
cell surfaces promotes tumor cell survival (3). TLR4 contributes to the immune
response to anticancer chemotherapy and radiotherapy (4). A recent study revealed that the
TLR4/NANOG oncogenic signaling pathway conferred cancer stem cell
chemoresistance in Hepatitis C virus (HCV)-associated
hepatocellular carcinoma (HCC) (5). Paradoxically, TLR agonists have also
been demonstrated to have potent anticancer effects. The TLR2/4
agonist, Bacillus Calmette-Guérin has been used successfully for
bladder cancer treatment (6). The
TLR3 agonists Ampligen and polyuridilyc acid have also been
developed for cancer treatment (7,8). In
addition, the TLR7/8 agonist imiquimod has received Food and Drug
Administration approval for the topical treatment of various
dermatological malignancies (9).
However, to the best of our knowledge, the systemic use of TLR7/8
agonists has not been investigated in a breast cancer model.
Angiogenesis is essential for tumor growth and
progression, therefore targeting angiogenesis is a promising
strategy for cancer therapy (10).
However, a number of studies have suggested that anti-angiogenic
therapy may induce immunosuppression and cancer stem cell
enrichment (11–13). Anti-angiogenic therapy with
vascular endothelial growth factor antibody and sunitinib may
recruit myeloid-derived suppressor cells (MDSCs) in tumor-bearing
mice (11,14), which may promote tumor angiogenesis
through matrix metallopeptidase 9 secretion and direct
differentiation into endothelial cells (15); however, eventually, resistance is
developed to anti-angiogenic therapy. Sunitinib is an
anti-angiogenesis agent and in the present study it was used in
combination with the TLR7/8 agonist to determine whether it
increases the efficacy of anti-angiogenesis therapies.
In the present study, the antitumoral effect of
TLR7/8 was investigated in a murine breast cancer model. The
synergistic antitumor effect of the TLR7/8 agonist, R848, plus
sunitinib was also assessed. The present study aimed to investigate
the potential use of a TLR7/8 agonist in cancer therapy.
Materials and methods
Cell culture and reagents
Murine 4T1 breast cancer cells were maintained in
RPMI-1640 (Gibco-BRL, Gaithersburg, MD, USA) supplemented with 10%
fetal bovine serum (Gibco Life Technologies, Grand Island, NY, USA)
and amikacin (100 µg/ml; Henan Topfond Pharmaceutical Co.,
Ltd., Henan, China) in a humidified atmosphere containing 5%
CO2 at 37°C. R848 was purchased from Sigma-Aldrich (St.
Louis, MO, USA) and sunitinib was purchased from Pfizer (New York,
NY, USA).
Tumor challenge and therapeutics
The study was approved by the Ethics Committee of
Sichuan University (Chengdu, China). BALB/c mice were purchased
from Beijing HFK Bioscience (Beijing, China), and were conditioned
under a specific pathogen free environment in the State Key
Laboratory of Biotherapy (Chengdu, China). Female BALB/c mice (age,
6–8 weeks) were inoculated subcutaneously with 1×106 4T1
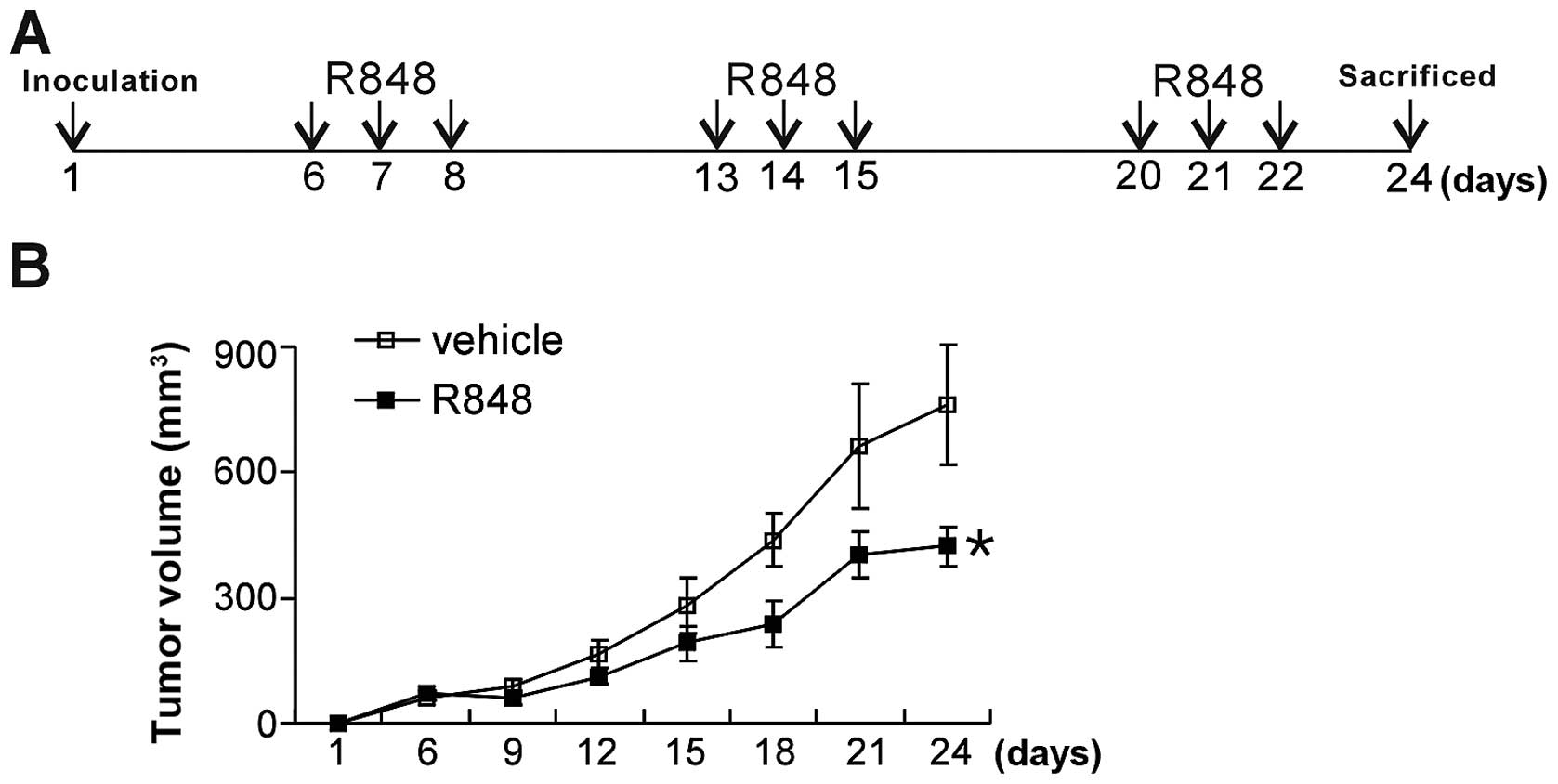
cells. At day 6 after inoculation, mice were treated with vehicle
(6% dimethyl sulfoxide + 94% normal saline) and R848 [3 mg/kg,
intraperitoneally (IP)] (n=5/group). For combination therapy, tumor
bearing mice were also treated with sunitinib [10 mg/kg, per
os (P.O.)], or sunitinib (10 mg/kg, P.O.) plus R848 (3 mg/kg,
IP) (n=5/group). The delivery strategy for R848 is described in
Fig. 1A. Briefly, R848 was
injected IP for three continuous days in a week for three weeks.
Sunitinib was gavaged daily for 16 days. Tumor diameters were
measured with a vennor caliper (Shanghai Taihai Measuring Tools
Co., Ltd., Shanghai, China) and tumor volumes were calculated by
the formula: 0.52 × a × b2, where a is the larger
diameter and b is the smaller diameter of the tumor mass.
Twenty-four days after inoculation, mice were sacrificed by
cervical dislocation.
Flow cytometry
At day 24 after tumor inoculation, blood from
tumor-bearing mice was collected. Red blood cells were lysed and
then mononuclear cells were stained using CD4-PerCP-Cy5.5 (rat
anti-mouse; monoclonal; 553052) and CD69-PE (armenian hamster
anti-mouse; monoclonal; 553237) antibodies (BD Pharmingen, San
Diego, CA, USA). For flow cytometric analysis, cells were acquired
using a FACSCalibur (342973) flow cytometer and then analyzed with
CellQuest software, version 6.0 (BD Biosciences, Mountain View, CA,
USA).
Immunohistochemistry and
immunofluorescence
Tumor tissues were paraffin-embedded and sectioned.
Tumor sections were stained with an antibody against HMGB1 (rabbit
monoclonal; ab79823; Abcam, Cambridge, UK), followed by
biotin-conjugated secondary antibody and streptavidin-horseradish
peroxidase complex. 3,3′-diaminobenzidine was used as the enzyme
substrate. For tumor vasculature assessment, frozen tumor sections
were stained with CD31-flurorescein isothiocyanate antibody (rat
monoclonal; 102405; BioLegend, San Diego, CA, USA). Images were
captured for each tumor by using a Leica DM2500 microscope (Leica
Microsystems GmbH, Wetzlar, Germany).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay
For apoptotic assessment, paraffin tumor sections
were subject to a TUNEL assay (Promega, Madison, WI, USA),
according to the manufacturer's instructions. Briefly, tumor
samples were deparaffinized and fixed with 4% methanol-free
paraformaldehyde for 10 min at room temperature. The samples were
washed with phosphate-buffered saline (PBS) twice for 5 min and
then permeabilized with 20 µg/ml proteinase K solution at
room temperature. Tissue sections were then fixed with 4%
methanol-free formaldehyde solution for another 5 min at room
temperature. The samples were washed with PBS and incubated with
equilibration buffer for 10 min at room temperature, then the
equilibration buffer was removed and reaction buffer containing
terminal deoxynucleotidyl trans-ferase enzyme, nucleotide mix and
equilibration buffer was added to the sections. The slides were
incubated at 37°C for 60 min inside the humidified chamber. Slides
were protected from direct light. The reaction was terminated by
immersing the samples in 2X saline-sodium citrate for 15 min at
room temperature. Samples were then washed thoroughly to remove
unincorporated fluorescein-dUTP. Immunofluorescence microscopy was
performed on a fluorescence microscope (Leica DM2500).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical comparisons between two groups were measured
with Student's t-test. Statistical comparisons among four groups
were measured using a one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
TLR7/8 agonist exhibits antitumoral
effects
To assess the antitumoral effect of a TLR7/8
agonist, tumor-bearing mice were treated with R848 (3 mg/kg). The
delivery strategy of R848 is described in Fig. 1A. R848 significantly retarded tumor
growth compared with the vehicle group (Fig. 1B), suggesting that TLR7/8 agonist
R848 has a robust antitumoral effect in the breast cancer
model.
TLR7/8 agonist reduces tumor vasculature
and induces apoptosis
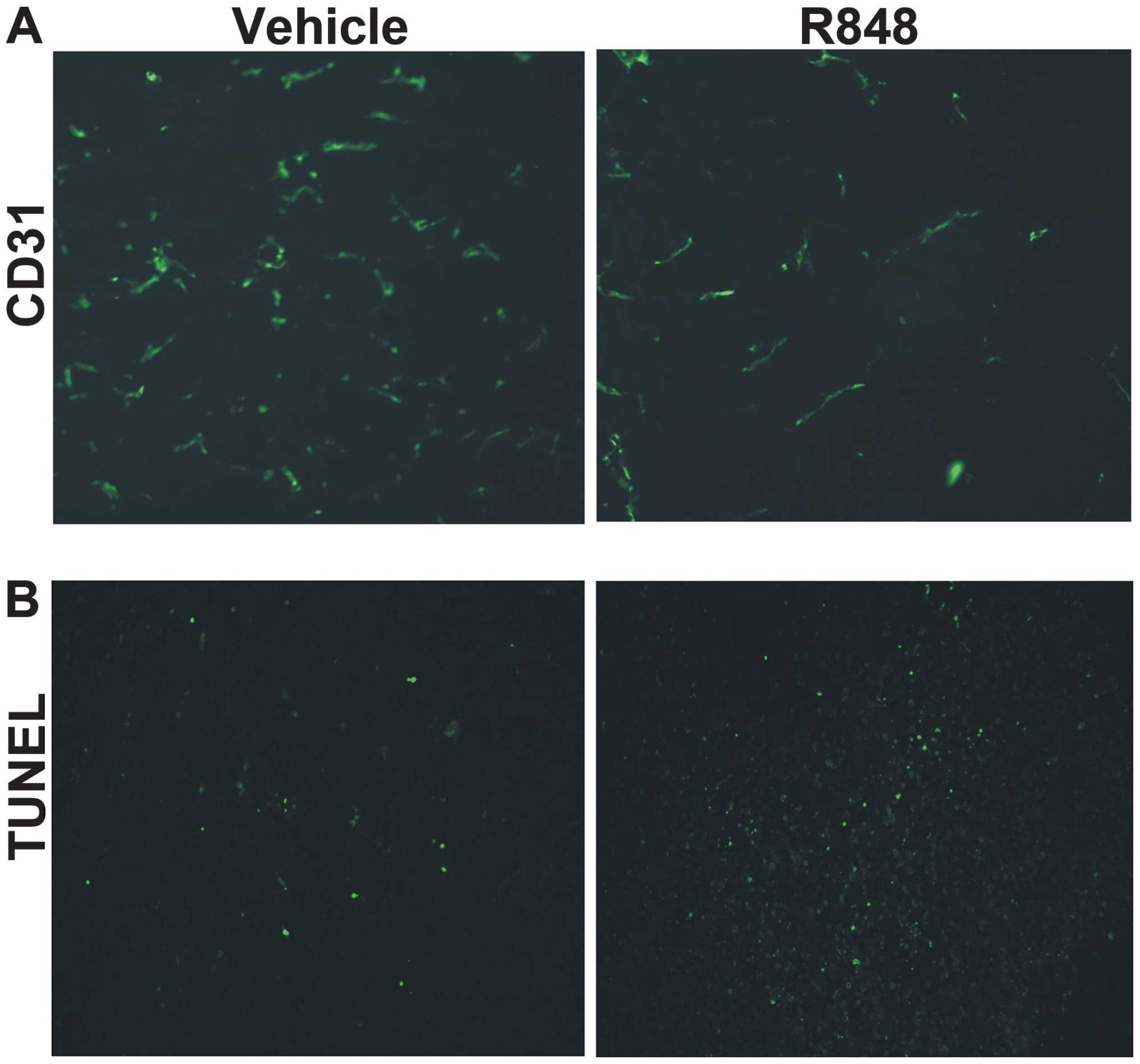
In order to observe the vasculature in the tumor
microenvironment, tumor sections were stained for CD31 expression.
Tumor vessel density was markedly decreased in the R848-treated
group, while tumors in the control group exhibited clear CD31
staining (Fig. 2A). A TUNEL assay
was performed to detect apoptosis following treatment. Compared
with the control group, there was a greater increase in tumor cell
death in the mice treated with R848 (Fig. 2B). The present data suggests that
TLR7/8 agonist therapy may reduce the density of tumor vasculature
and induce tumor cell apoptosis.
TLR7/8 agonist upregulates HMGB1 and
activates CD4+ T cells
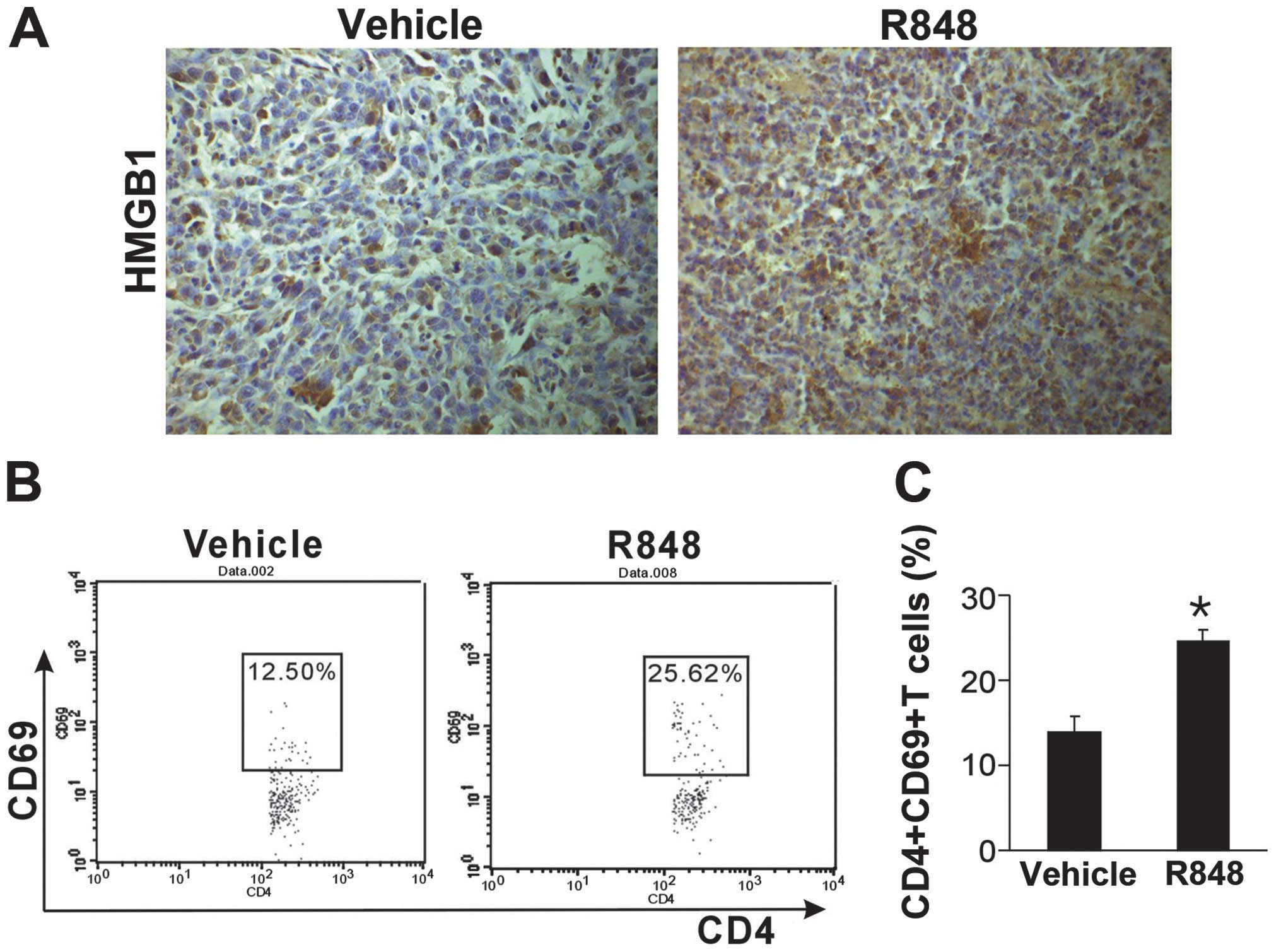
HMGB1 has been observed to be an endogenous danger
signal to alert the immune system. To investigate whether HMGB1 is
involved in the antitumoral effect of R848, tumor sections were
stained with an antibody against HMGB1. Compared with the control
group, TLR7/8 activation with R848 exhibited higher HMGB1
expression (Fig. 3A). It was
subsequently determined whether CD4+ T cells were
activated following R848 treatment. CD4+CD69+
T cells in the blood were evaluated using flow cytometry. It was
identified that there was an increased quantity of activated
CD4+CD69+ T cells in the peripheral blood
following R848 therapy (Fig. 3B).
The present results suggest that TLR7/8 activation induces HMGB1
release and activates T cells.
Synergistic antitumoral effects of R848
and sunitinib
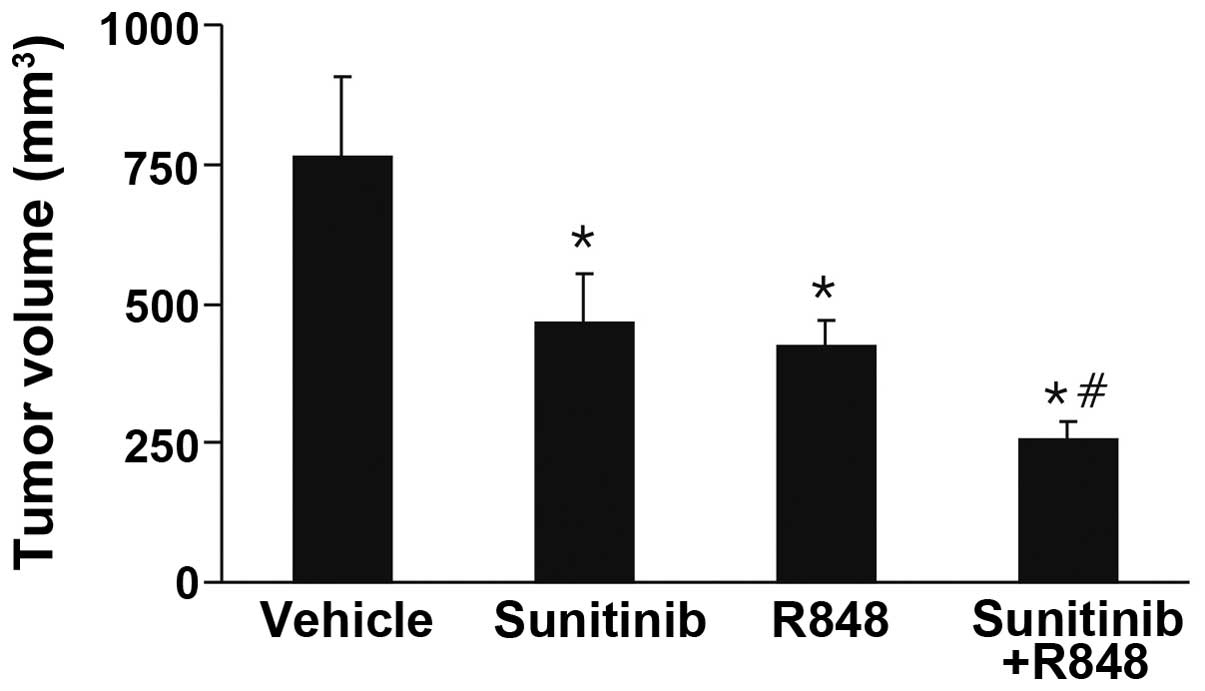
A recent study demonstrated that a systemic TLR7/8
agonist may enhance the effect of radiotherapy (16). To determine whether a systemic
TLR7/8 agonist enhanced the antitumoral effect of anti-angiogenic
therapy, 4T1 tumor-bearing mice were treated with R848 and
sunitinib. As expected, a synergistic effect between R848 and
sunitinib was observed. The combinational treatment robustly
retarded tumor growth, compared with vehicle and monotherapy groups
(Fig. 4). These data suggested
that systemic delivery of sunitinib and R848 has a synergistic
antitumoral effect.
Discussion
TLRs have been observed to be expressed on the tumor
surface (3). DAMP activation of
TLRs expressed on tumor cell surfaces initiates numerous signaling
cascades, which mediate angiogenesis and tumor survival and
progression (17). The
pluripotency marker NANOG was recently found to be the direct
target of TLR4. The activated TLR4/NANOG oncogenic signaling
pathway is associated with tumor stem cell chemoresistance in
HCV-associated HCC (5). In
addition, by inducing immunosuppressive cytokines, TLR4 signaling
also promotes immune escape of cancer cells (18). Paradoxically, TLR agonists may also
exert marked antitumoral effects. Several approaches employ TLR
activation in immune responses to enhance tumor surveillance and
cytotoxicity (2). In the present
study, it was identified that TLR7/8 activation with R848 may
retard tumor growth and induce increased levels of tumor cell
apoptosis. Recent studies have demonstrated that the topical TLR7
agonist imiquimod can induce therapeutic effects in breast cancer
metastasis to the skin (19). In
addition, TLR agonists have been demonstrated to have adjuvant
effects in cancer therapy. Topical imiquimod had been observed to
inhibit tumor growth and act synergistically with radiotherapy in a
mouse model of cutaneous breast cancer (20). Systemic administration of the
TLR7/8 agonist R848 in combination with radiation was demonstrated
to enhance antitumoral immune responses in lymphoma-bearing mice
(16). The present study revealed
that the systemic administration of the TLR7/8 agonist R848 was
effective for the treatment of a murine model of breast cancer,
which may result from reduced vasculature and increased apoptosis.
It was identified that R848 increased the levels of activated T
cells in the blood, while the clarification of the exact T cells
type requires further investigation.
Angiogenesis is tightly regulated during embryonic
development and wound healing. In addition, dysregulated
angiogenesis is common in malignant diseases (21). Targeting angiogenesis represents a
promising strategy for cancer therapy (10). However, several factors have been
suggested to be resistant to anti-angiogenic therapy, such as
elevated fibroblast growth factor (22), and recruitment of MDSCs (11) and T helper 17 cells (23). Thus, the development of novel
strategies is required to overcome the drawbacks of current
anti-angiogenic therapy. In the present study, the synergistic
antitumoral effect of the anti-angiogenic agent sunitinib and
TLR7/8 agonist R848 was observed in a murine breast cancer
model.
HMGB1 is a chromatin-binding nuclear protein with
215 amino acid residues, which is involved in numerous inflammatory
and malignant diseases (24).
Released passively by dying and necrotic cells or actively induced
by cytokines, HMGB1 is a typical DAMP, which alerts the immune
system and behaves as an endogenous immune adjuvant (4). HMGB1 has a crucial role in the
activation and functional maturation of dendritic cells (DCs)
(4,25), which initiate the adaptive immune
response. HMGB1-activated DCs have been observed to induce the
clonal expansion and Th1 polarization of alloreactive T cells
(26). In addition, DC-conditioned
medium containing HMGB1 may polarize naive CD4+ T cells
toward a Th1 phenotype (25). In
the present study, an increased number of activated CD4+
T cells were observed in the blood following R848 therapy. In the
primary tumor sites, the TLR7/8 agonist treatment increased HMGB1
expression, which may induce an effective antitumor immune
response.
Although the present results demonstrated that
TLR7/8 agonist alone or as an adjuvant can retard tumor growth,
other studies have suggested that TLR7 is associated with
carcinogenesis and immunosuppression (27,28).
A novel TLR7 agonist GpC-oligodeoxynucleotide can induce
indoleamine-pyrrole 2,3-dioxygenase expression in human DCs,
allowing those cells to assist forkhead box p3+regulatory T cell
generation (27). Through driving
stromal inflammation, TLR7 ligation markedly accelerates tumor
progression. Mice lacking TLR7 within their inflammatory cells are
protected from pancreatic carcinogenesis (28). In addition, the role of HMGB1 in
cancer is complex. Elevated expression of HMGB1 occurs in a number
of different types of tumor (29)
and is associated with tumor development (30), metastasis (31) and angiogenesis (32). HMGB1-mediated autophagy through
controlling the formation of Beclin 1-PI3KC3 complex contributes to
chemotherapy resistance in osteosarcoma (33). Thus, the molecular mechanism of
action should be further investigated in future studies.
In conclusion, it was demonstrated that R848, a
TLR7/8 agonist, can suppress tumor growth in breast cancer. In
addition, the combination of TLR7/8 agonist and sunitinib had a
synergistic antitumoral effect. Therefore, TLR7/8 agonists may be a
potential adjuvant for future anti-angiogenic therapy.
Acknowledgments
The present study was supported by the National
Natural Science Funds (grant no. 81272523).
References
|
1
|
Bianchi ME: DAMPs, PAMPs and alarmins: all
we need to know about danger. J Leuk Biol. 81:1–5. 2007. View Article : Google Scholar
|
|
2
|
Ridnour LA, Cheng RY, Switzer CH, et al:
Toll-like receptors in the tumor microenvironment-poor prognosis or
new therapeutic opportunity. Clin Cancer Res. 19:1340–1346. 2013.
View Article : Google Scholar
|
|
3
|
Huang B, Zhao J, Li H, et al: Toll-like
receptors on tumor cells facilitate evasion of immune surveillance.
Cancer Res. 65:5009–5014. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Apetoh L, Ghiringhelli F, Tesniere A, et
al: Toll-like receptor 4-dependent contribution of the immune
system to anticancer chemotherapy and radiotherapy. Nat Med.
13:1050–1059. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen CL, Tsukamoto H, Liu JC, et al:
Reciprocal regulation by TLR4 and TGF-β in tumor-initiating
stem-like cells. J Clin Invest. 123:2832–2849. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoo KH, Lim TJ and Chang SG: Monthly
intravesical bacillus Calmette-Guerin maintenance therapy for
non-muscle-invasive bladder cancer: 10-year experience in a single
institute. Exp Ther Med. 3:221–225. 2012.PubMed/NCBI
|
|
7
|
Salaun B, Zitvogel L, Asselin-Paturel C,
et al: TLR3 as a biomarker for the therapeutic efficacy of
double-stranded RNA in breast cancer. Cancer Res. 71:1607–1614.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jasani B, Navabi H and Adams M: Ampligen:
a potential toll-like 3 receptor adjuvant for immunotherapy of
cancer. Vaccine. 27:3401–3404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schon MP and Schon M: TLR7 and TLR8 as
targets in cancer therapy. Oncogene. 27:190–199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weis SM and Cheresh DA: Tumor
angiogenesis: molecular pathways and therapeutic targets. Nat Med.
17:1359–1370. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shojaei F, Wu X, Malik AK, et al: Tumor
refractoriness to anti-VEGF treatment is mediated by CD11b+ Gr1+
myeloid cells. Nat Biotechnol. 25:911–920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shojaei F, Wu X, Qu X, et al:
G-CSF-initiated myeloid cell mobilization and angiogenesis mediate
tumor refractoriness to anti-VEGF therapy in mouse models. Proc
Natl Acad Sci USA. 106:6742–6747. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Conley SJ, Gheordunescu E, Kakarala P, et
al: Antiangiogenic agents increase breast cancer stem cells via the
generation of tumor hypoxia. Proc Natl Acad Sci USA. 109:2784–2789.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Finke J, Ko J, Rini B, et al: MDSC as a
mechanism of tumor escape from sunitinib mediated anti-angiogenic
therapy. Int Immunopharmacol. 11:856–861. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang L, DeBusk LM, Fukuda K, et al:
Expansion of myeloid immune suppressor Gr+ CD11b+ cells in
tumor-bearing host directly promotes tumor angiogenesis. Cancer
Cell. 6:409–421. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dovedi SJ, Melis MH, Wilkinson RW, et al:
Systemic delivery of a TLR7 agonist in combination with radiation
primes durable antitumor immune responses in mouse models of
lymphoma. Blood. 121:251–259. 2013. View Article : Google Scholar
|
|
17
|
Sato Y, Goto Y, Narita N and Hoon DS:
Cancer cells expressing toll-like receptors and the tumor
microenvironment. Cancer Microenviron. 2(Suppl 1): 205–214. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He W, Liu Q, Wang L, et al: TLR4 signaling
promotes immune escape of human lung cancer cells by inducing
immunosuppressive cytokines and apoptosis resistance. Mol Immunol.
44:2850–2859. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Adams S, Kozhaya L, Martiniuk F, et al:
Topical TLR7 agonist imiquimod can induce immune-mediated rejection
of skin metastases in patients with breast cancer. Clin Cancer Res.
18:6748–6757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dewan MZ, Vanpouille-Box C, Kawashima N,
et al: Synergy of topical toll-like receptor 7 agonist with
radiation and low-dose cyclophosphamide in a mouse model of
cutaneous breast cancer. Clin Cancer Res. 18:6668–6678. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Potente M, Gerhardt H and Carmeliet P:
Basic and therapeutic aspects of angiogenesis. Cell. 146:873–887.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Casanovas O, Hicklin DJ, Bergers G, et al:
Drug resistance by evasion of antiangiogenic targeting of VEGF
signaling in late-stage pancreatic islet tumors. Cancer Cell.
8:299–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chung AS, Wu X, Zhuang G, et al: An
interleukin-17-mediated paracrine network promotes tumor resistance
to anti-angiogenic therapy. Nat Med. 19:1114–1123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Urbonaviciute V, Furnrohr BG, Meister S,
et al: Induction of inflammatory and immune responses by
HMGB1-nucleosome complexes: implications for the pathogenesis of
SLE. J Exp Med. 205:3007–3018. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dumitriu IE, Baruah P, Valentinis B, et
al: Release of high mobility group box 1 by dendritic cells
controls T cell activation via the receptor for advanced glycation
end products. J Immunol. 174:7506–7515. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Messmer D, Yang H, Telusma G, et al: High
mobility group box protein 1: an endogenous signal for dendritic
cell maturation and Th1 polarization. J Immunol. 173:307–313. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Volpi C, Fallarino F, Bianchi R, et al: A
GpC-rich oligonucleotide acts on plasmacytoid dendritic cells to
promote immune suppression. J Immunol. 189:2283–2289. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ochi A, Graffeo CS, Zambirinis CP, et al:
Toll-like receptor 7 regulates pancreatic carcinogenesis in mice
and humans. J Clin Invest. 122:4118–4129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ellerman JE, Brown CK, de Vera M, et al:
Masquerader: high mobility group box-1 and cancer. Clin Cancer Res.
13:2836–2848. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mittal D, Saccheri F, Vénéreau E, et al:
TLR4-mediated skin carcinogenesis is dependent on immune and
radioresistant cells. EMBO J. 29:2242–2252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sims GP, Rowe DC, Rietdijk ST, et al:
HMGB1 and RAGE in Inflammation and Cancer. Annu Rev Immunol.
28:367–388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mitola S, Belleri M, Urbinati C, et al:
Cutting Edge: Extracellular high mobility group box-1 protein is a
proangiogenic cytokine. J Immunol. 176:12–15. 2006. View Article : Google Scholar
|
|
33
|
Huang J, Ni J, Liu K, et al: HMGB1
promotes drug resistance in osteosarcoma. Cancer Res. 72:230–238.
2012. View Article : Google Scholar
|