Introduction
Ulcerative colitis (UC) is a common inflammatory
intestinal disease, which belongs to a group of conditions known as
inflammatory bowel diseases (IBD) (1). The pathogenesis of UC is believed to
result from interactions between various genetic, immune and
environmental factors (2). UC is
associated with intestinal characteristics and symptoms, including
weight loss, bloody diarrhea, fever and shortening of the colon
(3). General features of tissue
damage in UC include ulceration of the mucosa, blunting and loss of
crypts, as well as infiltration of inflammatory cells (4). These features frequently result in
epithelial dysplasia and DNA damage with microsatellite
instability, which may result in cancer progression (5). Involvement of the entire colon for
>10 years has been shown to predispose patients to colon cancer
(5). In addition, it is well known
that chronic inflammation of the colon contributes to colon
carcinogenesis (6). To prevent the
progression of cancer in patients with UC, an improved
understanding of the pathogenesis of UC at the molecular and
cellular level is required. UC is usually closely associated with
intervals of acute exacerbation, and corticosteroid administration
is effective for clinical remission (7). However, corticosteroids often have
severe side effects, including hormonal disturbance, peptic ulcers,
liver dysfunction and psychological issues (8). These adverse effects occasionally
lead to discontinuation of corticosteroid treatment, which results
in acute UC exacerbation (9).
Therefore, alternative treatments for UC are required in order to
help patients with UC avoid these clinical problems.
Recent studies have demonstrated that
pro-inflammatory cytokines are associated with the initiation of
colonic inflammation (10).
Furthermore, it has been reported that mucosa from patients with UC
exhibits increased expression levels of interleukin (IL)-6
(11,12). Mueller (13) previously detected high expression
of tumor necrosis factor (TNF)-α in the blood, colonic tissue and
stool samples of patients with UC (13). Therefore, in the field of UC
treatment, there is a strong interest in developing agents that are
able to block the generation or action of inflammatory
cytokines.
Cyclooxygenase (COX) is a prostaglandin synthetase
enzyme that is involved in the metabolism of arachidonic acid.
There are two isoenzymes of COX: COX-1 and COX-2. COX-1 is
expressed constitutively in the majority of tissues, particularly
in the gastrointestinal tract, and is responsible for the
production of prostaglandins that are associated with
gastrointestinal integrity. Although COX-2 expression is low in the
normal state, they are rapidly upregulated in response to various
stimuli, including cytokines, growth factors, hormones and
carcinogens. COX-2 is also responsible for producing prostaglandins
associated with the mediation of inflammation (14). COX-2 expression has been shown to
be increased in the mucosa of patients with UC (15,16).
Nuclear factor (NF)-κB is one of the most important
transcription factors, which is responsible for the induction of
genes that mediate innate and adaptive immunity. The role of NF-κB
in UC has been investigated by numerous studies (17,18).
Ma et al (19) demonstrated
that increases in intestinal epithelial tight-junction permeability
require the activation of NF-κB. In addition, increased DNA binding
activity of NF-κB, which is associated with high levels of IL-1 and
IL-6, is observed in patients with UC (20). These studies suggested that the
activation of NF-κB has a critical role in the initiation of
intestinal inflammation in UC. Therefore, inhibiting the activation
of NF-κB has been suggested as an effective anti-inflammatory
strategy for the treatment of UC (21).
Traditional herbal medicine has been the subject of
increased interest for its potential in the treatment of
inflammation. Igongsan (IGS) is a Korean herbal medicine that
contains Ginseng Radix, Atractylodis Rhizoma Alba, Poria
Sclerotium, Glycyrrhizae Radix et Rhizoma and Citri Unshius
Pericarpium. IGS is widely used to treat digestive disorders,
including abdominal pain, dyspepsia and diarrhea. The use of IGS is
based on knowledge from Traditional Korean Medicine (22), with few scientific studies
available regarding the effects of IGS on intestinal inflammation
supporting its clinical applications.
In order to support the traditional use of IGS by
providing experimental evidence, the present study examined the
effects of IGS and its constituent, ergosterol, on dextran sulfate
sodium (DSS)-induced colitis. The aims of the present study were to
assay the effects of IGS and ergosterol on the clinical signs of
colitis, including weight loss, colon shortening, diarrhea and
obscure/gross bleeding, and to investigate the effects of IGS and
ergosterol on inflammatory-associated gene expression in
DSS-treated colon tissue.
Materials and methods
Reagents
DSS (mol wt, 36,000–50,000) was purchased from MP
Biomedicals North America (Solon, OH, USA). Mouse TNF-α affinity
purified polyclonal, goat IgG (cat no. AF-410-NA), mouse TNF-α
biotinylated affinity purified polyclonal, goat IgG (cat no. BF410)
and recombinant mouse TNF-α (cat no. 410-MT) were purchased from
R&D Systems, Inc. (Minneapolis, MN, USA). Purified rat
anti-mouse IL-6 (cat no. 55440), biotin rat anti-mouse IL-6 (cat
no. 554402) and recombinant mouse IL-6 (cat no. 554582) were
purchased from BD Biosciences (San Diego, CA, USA). Avidin
peroxidase (cat no. A3151), ergosterol (cat no. E6510),
sulfasalazine (cat no. S0883) and 2,2′-azinobis
[3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt (ABTS; cat
no. A9941) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Hydrogen peroxide (cat no. 23150-0350) was purchased from Junsei
Chemical Co., Ltd (Tokyo, Japan). COX-2 (cat no. sc-1745),
NF-κB/p65 (cat no. sc-7151) and histone H1 (cat no. sc-10806) were
purchased from from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). Peroxidase AffiniPure rabbit anti-goat IgG (H+L) (cat no.
305-035-003), peroxidase-conjugated AffiniPure goat anti-rabbit IgG
(catalog no. 111-035-003) and peroxidase-conjugated AffiniPure goat
anti-mouse IgG (catalog no. 115-035-062) antibodies were purchased
from Jackson Immuno Research Laboratories, Inc. (West Grove, PA,
USA).
Animals
Female BALB/c mice (four weeks old; body weight,
16–18 g) were purchased from the Dae-Han Experimental Animal Center
(Eumsung, Korea). The mice (n=48) were maintained in the College of
Pharmacy, Wonkwang University (Iksan, Korea), and 5–10 mice were
housed per cage in a laminar air-flow room. The atmosphere was
maintained at a temperature of 22±1°C and relative humidity of
55±10% with a 12 h light/dark cycle throughout the study. Animal
experimental procedures were approved by the Animal Ethics
Committee of Wonkwang University (approval number WKU14–05).
Preparation of IGS
IGS is a herbal medicine composed of five herbs:
Ginseng Radix (root of Panax ginseng, Araliaceae),
Atractylodis Rhizoma Alba (rhizome of Atractylodes macro-
cephala, Compositae), Poria Sclerotium (sclerotium of Poria
cocos, Polyporaceae), Glycyrrhizae Radix et Rhizoma (root and
rhizome of Glycyrrhiza uralensis, Leguminosae) and Citri
Unshius Pericarpium (Peel of Citrus unshiu, Rutaceae). The
composition of IGS is shown in Table
I. The components of IGS used in the present study were
purchased from Daehak Oriental Drugstore (Iksan, Korea), and their
identity was confirmed by Professor Seung-Heon Hong (Department of
Oriental Pharmacy, Wonkwang University, Jeonbuk, Korea). The
extract was prepared by decocting the dried prescription with
boiling distilled water (100 g/l). The extraction was decocted for
3 h, and was then filtered and lyophilized prior to being
maintained at 4°C (yield, 7.26%). The samples were dissolved in
distilled water and then filtered through a 0.22-µm syringe
filter (Merck Millipore, Carrigtwohill, Ireland).
 | Table IComponents of Igongsan |
Table I
Components of Igongsan
| Constituent | Amoutn (g) |
|---|
| Ginseng Radix | 20 |
| Atractylodis
Rhizoma Alba | 20 |
| Poria
Sclerotium | 20 |
| Glycyrrhizae Radix
et Rhizoma | 20 |
| Citri Unshius
Pericarpium | 20 |
Induction of colitis by DSS
Colitis was induced in mice as described in a
previous study by our group (23).
The mice were orally administered DSS dissolved in drinking water,
which is a widely used experimental model of colitis. Briefly,
acute colitis was induced in the mice by supplementing their
drinking water with 5%(w/v) DSS for 7 days. The mice were checked
daily for weight loss, stool consistency and the presence of gross
bleeding. The mice were randomized into three groups (n=6): Mice
receiving oral administration of IGS (100 mg/kg/day) or Ergosterol
(20 mg/kg/day), mice treated with sulfasalazine (SFZ; 150
mg/kg/day) as a positive control, or mice treated with water as a
negative control. IGS and SFZ were orally administered once a day
for 7 days prior to DSS treatment. The mice were sacrificed by
cervical dislocation and assessed after 7 days of treatment with
DSS.
Disease activity index (DAI)
The activity of intestinal disease was assessed
through the following manifestations: Weight loss, diarrhea
accompanied with blood and mucus, and shortening of the colon
(3). In the present study, the DAI
was obtained from the score of three major clinical symptoms:
Weight loss, diarrhea and rectal bleeding, as described in a
previous study by our group (23),
based on a study by Murthy et al (24). Loss of body weight was calculated
as the difference between initial and final weight. Diarrhea was
defined by the absence of fecal pellet formation in the colon, and
the presence of continuous fluid fecal material in the colon. The
appearance of rectal bleeding was defined as diarrhea containing
visible blood and gross rectal bleeding, and was scored as
described for diar-rhea. The DAI was calculated using the following
formula: DAI = (weight loss score) + (diarrhea score) + (rectal
bleeding score). The clinical parameters used here are
comprehensive functional measures that are analogous to the
subjective clinical symptoms observed in human UC (25). This method of scoring has been
validated by repeated studies (24,26).
Cytokine assay
A cytokine assay was performed using a modified
ELISA as described previously (27). Tissue segments excised from distal
colon were prepared by homogenization and extraction by PRO-PREP
Protein Extraction solution (Intron Biotechnology, Inc., Seongnam,
Korea). Following protein quantification (DC™ Protein Assay kit;
Bio-Rad Laboratories, Inc., Hercules, CA, USA), equal protein
concentrations of the tissue samples underwent ELISA. The ELISA was
performed by coating 96-well plates with mouse monoclonal
antibodies targeting TNF-α and IL-6 overnight at 4°C. Assay plates
were sequentially exposed to biotinylated mouse TNF-α and IL-6 for
1 h, avidin peroxidase for 40 min and 2,2′-azinobis ABTS substrate
solution containing 30% H2O2. The plates were
read at a wavelength of 405 nm with the VersaMax™ ELISA Microplate
Reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
Prostaglandin E2
(PGE2) assay
The concentration of PGE2 in the colon
tissue was measured by ELISA using a PGE2 assay kit
(Stressgen Biotechnologies Corporation, San Diego, CA USA),
according to the manufacturer's instructions. Duplicate aliquots of
supernatant were measured for each sample.
Western blot analysis
The western blot analyses were performed as
described previously (23).
Briefly, the proteins were extracted from the distal colon tissue
samples. The proteins were then separated using the Gradi-Gel 2
Gradient PAGE analysis kit (Elpis Biotech, Inc., DaeJeon, Korea)
7.5% gel for SDS-PAGE prior to being transferred onto
nitrocellulose membranes (GE Healthcare Bio-Sciences, Piscataway,
NJ, USA). The membranes were then incubated with primary and
secondary antibodies sequentially, and the protein bands were
visualized using an Enhanced Chemiluminesence Detection system (GE
Healthcare Bio-Sciences), according to the manufacturer's
instructions. Relative protein levels were evaluated using GAPDH or
histone as the reference protein in each condition. The band
intensities were measured using ImageJ software, version 1.48
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean of at least three experiments. The data were examined by
one-way analysis of variance with Tukey's post hoc test. PASW
Statistics software, version 18.0.0 (SPSS, Inc., Chicago, IL, USA)
was used to conduct the statistical analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
IGS alleviates clinical symptoms of
DSS-induced colitis
It has previously been reported that DSS-induced
colitis in mice has a similar phenotype to human acute and chronic
UC (28). In the present study,
the inhibitory effects of IGS on the intestines of mice with
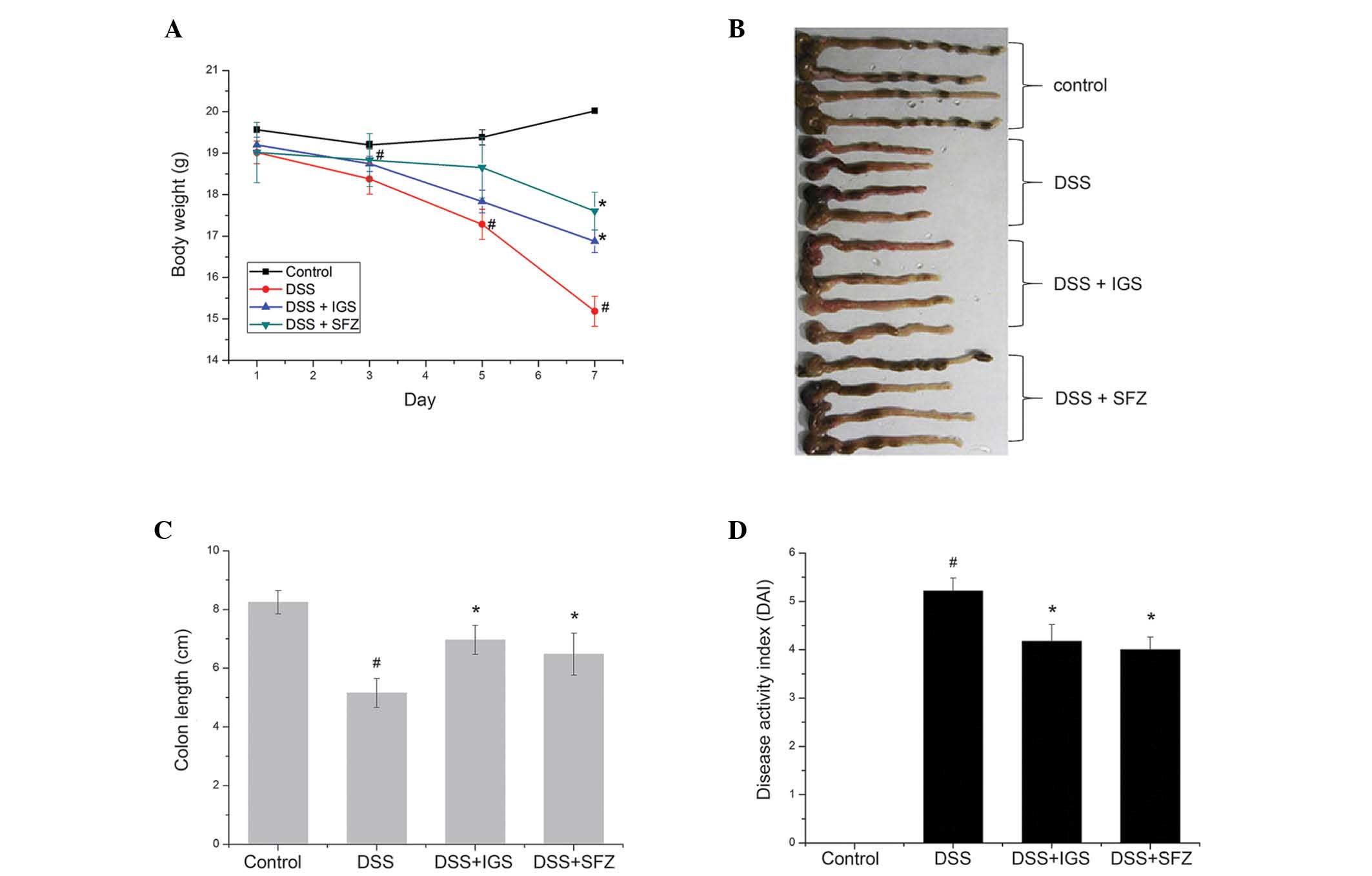
DSS-induced experimental colitis were evaluated (Fig. 1). The physiological symptoms of
colitis (weight loss, colon shortening, diarrhea and obscure/gross
bleeding) were observed following 7 days of 5% DSS treatment, and
the DAIs of the mice were calculated. Mice treated with DSS
exhibited significant weight loss (15.19±0.37 g; Fig. 1A) and colon shortening (5.20±0.45
cm) (Fig. 1B and C), as compared
with the control group (20.05±0.14 g and 8.23±0.34 cm,
respectively; P<0.05). The percentage of body weight loss and
colon shortening was ~26.01 and 42%, respectively. Mice treated
with IGS had a significant attenuation of body weight loss
(16.88±0.28 g) and colon shortening (6.23±0.50 cm) as compared with
that in the mice with DSS-induced colitis (P<0.05) (Fig. 1A–C). In addition, the DAI was
markedly decreased in the mice treated with IGS (4.25±0.33) as
compared with that in the mice treated with DSS alone (5.22±0.25;
P<0.05) (Fig. 1D). SFZ is a
drug widely used to treat colitis, which was used as the positive
control in the present study; results for the SFZ group were
similar to those of the IGS group within the error range.
IGS inhibits increases in TNF-α and IL-6
levels in DSS-induced colitis
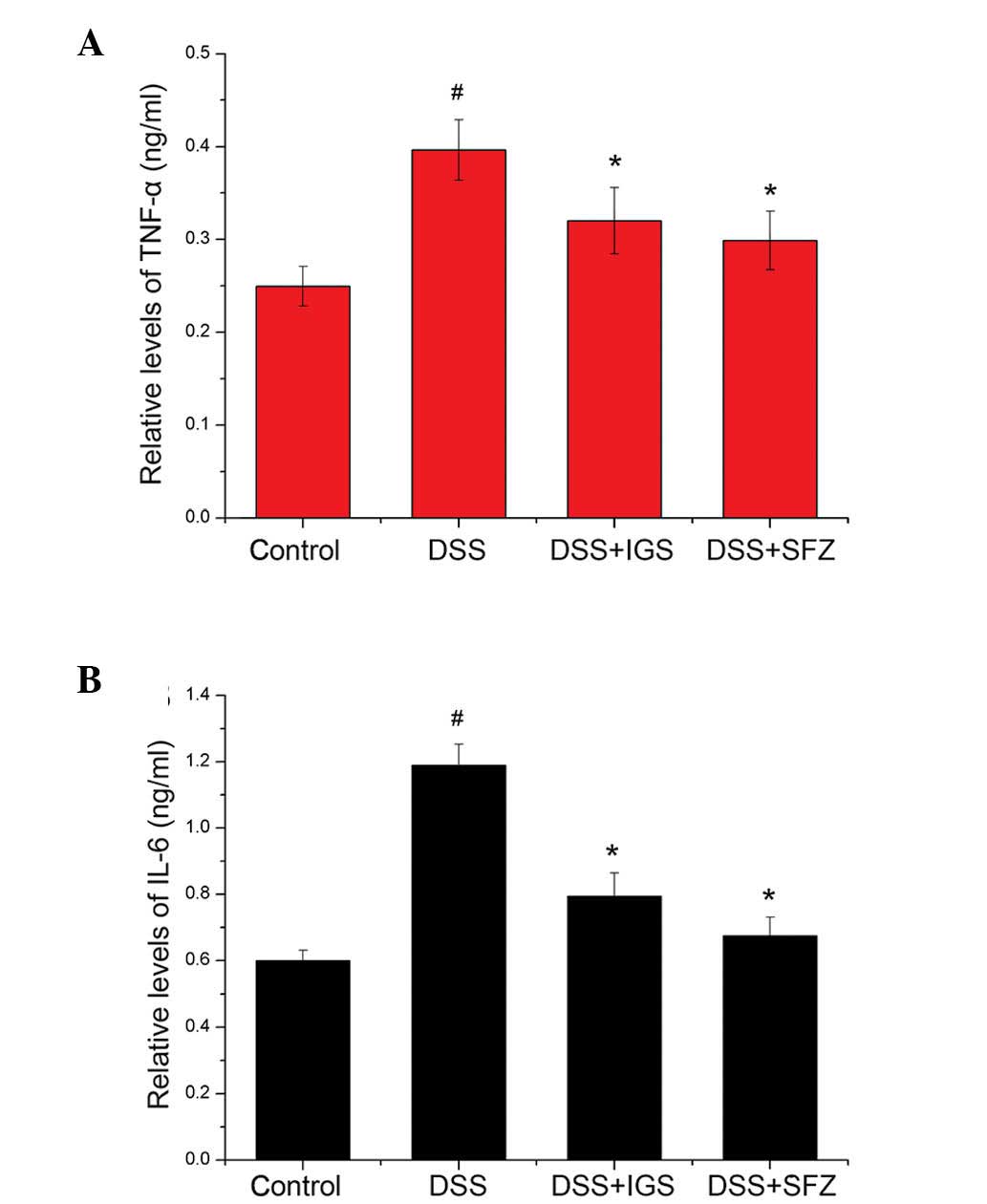
The effects of IGS on TNF-α and IL-6 levels in colon
tissue from mice with experimental colitis were determined. At the
end of the experiment, the mouse colons were homogenized and
examined using ELISA. As shown in Fig.
2A and B, the levels of TNF-α and IL-6 were significantly
increased in the colon tissue of DSS-treated mice (0.4±0.03 and
1.2±0.06 ng/ml) as compared with those in the control mice
(0.25±0.02 and 0.6±0.04 ng/ml; P<0.05). However, administration
of IGS reduced the DSS-induced increase in TNF-α and IL-6 levels
(0.32±0.04 and 0.8±0.07 ng/ml, respectively; P<0.05). The rate
of inhibition of TNF-α and IL-6 by IGS was 21.71 and 33.34%,
respectively. In comparison, the rate of inhibition of TNF-α and
IL-6 by SFZ was 25.01 and 36.11%, respectively.
IGS inhibits increases in COX-2
expression and PGE2 levels in DSS-induced colitis
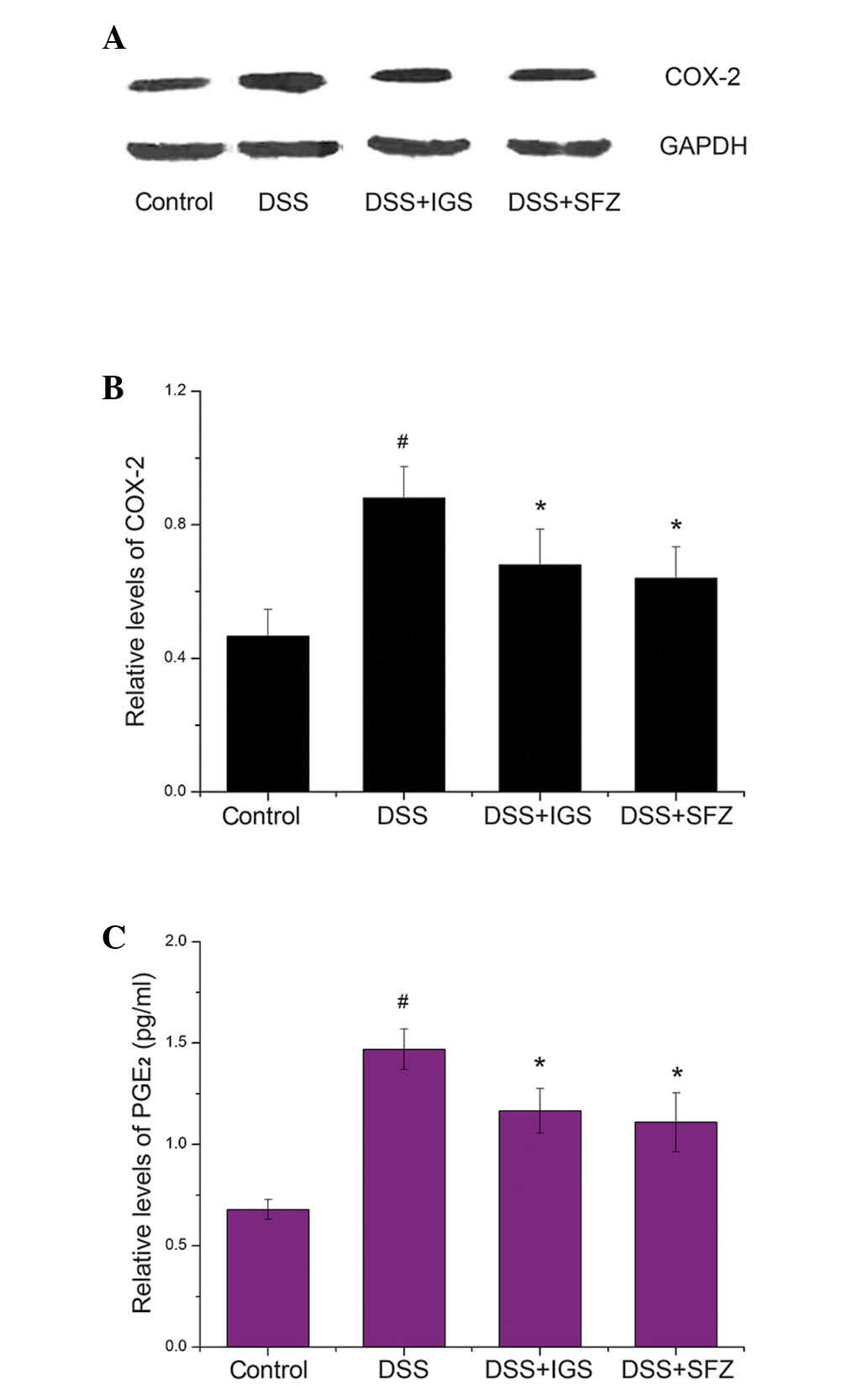
The effects of IGS on the protein expression levels
of COX-2 were determined by western blot analysis. The expression
levels of COX-2 were significantly increased in colon tissue of
mice treated with DSS (0.88±0.10) as compared with those in the
control mice (0.46±0.08; P<0.05). However, treatment with IGS
reduced the expression levels of COX-2 in the DSS-treated mice
(0.66±0.11; P<0.05) (Fig. 3A and
B).
COX-2 catalyzes the biosynthesis of PGE2;
therefore, the present study determined whether IGS exerted an
effect on the levels of PGE2 in colon tissue. As shown
in Fig. 3C, PGE2 levels
were enhanced in response to DSS (1.46±0.10 pg/ml); however, this
increase was significantly inhibited by IGS (1.16±0.10 pg/ml;
P<0.05). The rate of inhibition of PGE2 production by
IGS (100 mg/kg/day) was 32.22%.
IGS suppresses the activation of NF-κB
p65 in DSS-induced colitis
Activation of NF-κB p65 is involved in colitis
(18); therefore, inhibition of
NF-κB activation has been suggested as an anti-inflammatory
therapeutic strategy for colitis. The present study investigated
whether IGS regulated the activation of NF-κB p65 in colitis. The
activation of NF-κB p65 was increased in the colon tissue of the
DSS group (1.70±0.09) as compared with that in the control group
(0.83±0.08; P<0.05). However, oral IGS administration
significantly suppressed the DSS-induced activation of NF-κB p65 in
colon tissue (1.23±0.10; P<0.05) (Fig. 4).
Ergosterol alleviates clinical symptoms
of DSS-induced colitis
Ergosterol is a component of IGS. The present study
evaluated the inhibitory effects of ergosterol on DSS-induced
colitis. As shown in Figs. 5A–D,
ergosterol significantly inhibited DSS-induced weight loss, colon
shortening and DAI in the mice (DSS vs. DSS + Ergo: 16.08±0.49 g
vs. 17.45±0.58 g; 5.40±0.23 cm vs. 6.32±0.16 cm; and 2.25±0.57 vs.
1.75±0.32) (P<0.05). In addition, it was observed that
ergosterol attenuated DSS-induced activation of NF-κB p65 in colon
tissue from mice with experimental colitis (DSS vs. DSS + Ergo,
1.32±0.10 vs. 1.07±0.11; P<0.05) (Fig. 5E and F).
Discussion
Traditional herbal medicines have been used
effectively for centuries, based on traditional knowledge; however,
the pharmacological mechanisms of action of these herbal
prescriptions have yet to be fully elucidated. The present study
aimed to provide experimental evidence regarding the use of IGS as
a potential therapeutic drug for patients with UC. The present
study demonstrated that IGS is able to inhibit inflammation and
colon injury provoked by DSS treatment. The results indicated that
there is an important molecular mechanism by which IGS ameliorates
intestinal inflammation.
UC is a typical inflammatory disease of the
intestine, which belongs to a group of conditions known as IBD.
However, despite numerous years of extensive research implicating
immune dysfunction, genetic susceptibility and bacterial flora
within the intestinal environment as possible factors associated
with the development of the disease (29), its pathogenesis has remained to be
elucidated. The signs and symptoms of UC include abdominal pain,
weight loss and diarrhea accompanied with bleeding (30–32).
Therapies for UC include glucocorticosteroids and SFZ (33,34);
however, these treatments often have severe adverse effects.
Therefore, the requirement for anti-inflammatory agents with fewer
side effects is growing. Recently, traditional herbal medicine has
received increased interest regarding the treatment of such
disorders. Previous studies by our group have demonstrated that
various herbal medicines have anti-colitis effects in mice
(23,35). The present study investigated the
effects of IGS on UC, using the DSS-induced mouse model of colitis.
Treatment with IGS reduced DSS-induced weight loss and colon
shortening. In addition, the DAI, which is a scale determined by
measuring the levels of three major clinical symptoms (weight loss,
diarrhea and rectal bleeding), was markedly inhibited in the group
treated with IGS. The protective effect of IGS on colon shortening
and DAI was similar to the protective effect of SFZ. These results
therefore suggested that IGS may effectively inhibit the symptoms
of DSS-induced colitis.
Pro-inflammatory cytokines are closely associated
with the initiation of the inflammatory response in colitis. It has
previously been shown that TNF-α is strongly associated with the
pathogenesis and progression of intestinal inflammatory disorders
(36). It has also been reported
that levels of IL-6 are markedly elevated in patients with UC, and
that IL-6 has an integral role in its pathogenesis (37). Therefore, studies on novel
biological therapies for UC have focused on blocking components of
the inflammatory cascade, such as cytokines. COX-2 is also closely
associated with numerous pathological processes, including UC. DSS
elicits COX-2 expression to regulate the production of
prostaglandins, which induce gastrointestinal fluid secretion in
the inflammatory pathway (38).
The present study demonstrated that the levels of TNF-α, IL-6 and
COX-2 were increased in the colon tissue of DSS-treated mice as
compared with those in the control mice, and treatment with IGS
reduced these levels. In addition, the inhibitory effect of IGS on
the increases in TNF-α, IL-6 and COX-2 levels in DSS-treated colon
tissue was similar to the effect of SFZ. These results indicated
that the anti-inflammatory effects of IGS may be attributed to the
regulation of inflammatory mediators in DSS-induced colitis.
NF-κB is an important transcription factor,
particularly for the activation of numerous inflammatory mediators,
cytokines including IL-1β and IL-6, and COX-2. NF-κB p65 has been
reported to be a critically important factor in chronic
inflammatory diseases (39).
Therefore, NF-κB is considered an ideal target for a molecular
therapy of UC, and extensive efforts have been made to develop
novel treatments targeting NF-κB (17,40,41).
In the present study, it was observed that the activation of NF-κB
p65 was significantly increased in the colon tissue of DSS-treated
mice as compared with that in the control group, and this increase
was attenuated by treatment with IGS. These results indicated that
the mechanism of action of IGS is a novel mechanism involving the
regulation of NF-κB p65 activation in DSS-induced colitis.
Therefore, it may be hypothesized that IGS exerts its
anti-inflammatory effect in UC by regulating the activation of
NF-κB.
IGS is composed of five herbs, each of which have
been reported to have various effects; in particular, ginsenoside
Rb1, Rd and Re of Ginseng Radix (42–44),
hesperidin of Unshius Pericarpium (45), and glycyrrhizic acid of
Glycyrrhizae Radix et Rhizoma (46) have been shown to exhibit
anti-colitis effects. Ergosterol is a compound present in Poria
Sclerotium. It has previously been reported that ergosterol may be
beneficial in the treatment of the allergic response by decreasing
immu-noglobulin E in mucosal-type mast cells (47). However, there is currently no
information available regarding the effects of ergosterol on
colitis. Therefore, an aim of the present study was to evaluate the
possible modulating effects of ergosterol on colitis. The results
of the present study demonstrated that ergosterol was able to
attenuate clinical symptoms of DSS-induced colitis in mice.
In conclusion, the present study demonstrated that
treatment with IGS significantly reduced the clinical symptoms and
the levels of inflammatory mediators in a DSS-induced murine model
of colitis. These results suggested that IGS may be a useful
therapeutic candidate for colitis. However, further studies are
required to reveal the precise mechanisms underlying the effects of
IGS in intestinal inflammatory disorders.
Acknowledgments
The present study was supported by the National
Research Foundation of Korea grant funded by the Korean government
(MSIP) (grant nos. 2011-0010435, 2013R1A2A2A03006068, 2012-0007669
and 2011-0030130).
References
|
1
|
Hyams JS: Inflammatory bowel disease.
Pediatr Rev. 21:291–295. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Danese S, Sans M and Fiocchi C:
Inflammatory bowel disease: The role of environmental factors.
Autoimmun Rev. 3:394–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hendrickson BA, Gokhale R and Cho JH:
Clinical aspects and pathophysiology of inflammatory bowel disease.
Clin Microbiol Rev. 15:79–94. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lichtenstein GR and Rutgeerts P:
Importance of mucosal healing in ulcerative colitis. Inflamm Bowel
Dis. 16:338–346. 2010. View Article : Google Scholar
|
|
5
|
Ullman T, Croog V, Harpaz N, Sachar D and
Itzkowitz S: Progression of flat low-grade dysplasia to advanced
neoplasia in patients with ulcerative colitis. Gastroenterology.
125:1311–1319. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eaden JA, Abrams KR and Mayberry JF: The
risk of colorectal cancer in ulcerative colitis: A meta-analysis.
Gut. 48:526–535. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Domènech E: Inflammatory bowel disease:
Current therapeutic options. Digestion. 73(Suppl 1): 67–76. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buchman AL: Side effects of corticosteroid
therapy. J Clin Gastroenterol. 33:289–294. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Farrell RJ and Kelleher D: Glucocorticoid
resistance in inflammatory bowel disease. J Endocrinol.
178:339–346. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, de Haar C, Chen M, et al:
Disease-related expression of the IL6/STAT3/SOCS3 signalling
pathway in ulcerative colitis and ulcerative colitis-related
carcinogenesis. Gut. 59:227–235. 2010. View Article : Google Scholar
|
|
11
|
Papadakis KA and Targan SR: Role of
cytokines in the pathogenesis of inflammatory bowel disease. Annu
Rev Med. 51:289–298. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ogata H and Hibi T: Cytokine and
anti-cytokine therapies for inflammatory bowel disease. Curr Pharm
Des. 9:1107–1113. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mueller C: Tumour necrosis factor in mouse
models of chronic intestinal inflammation. Immunology. 105:1–8.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vane JR, Bakhle YS and Botting RM:
Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 38:97–120.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roberts PJ, Morgan K, Miller R, Hunter JO
and Middleton SJ: Neuronal COX-2 expression in human myenteric
plexus in active inflammatory bowel disease. Gut. 48:468–472. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Agoff SN, Brentnall TA, Crispin DA, et al:
The role of cyclooxygenase 2 in ulcerative colitis-associated
neoplasia. Am J Pathol. 157:737–745. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bantel H, Berg C, Vieth M, Stolte M, Kruis
W and Schulze-Osthoff K: Mesalazine inhibits activation of
transcription factor NF-kappaB in inflamed mucosa of patients with
ulcerative colitis. Am J Gastroenterol. 95:3452–3457. 2000.
|
|
18
|
Andresen L, Jørgensen VL, Perner A, Hansen
A, Eugen-Olsen J and Rask-Madsen J: Activation of nuclear factor
kappaB in colonic mucosa from patients with collagenous and
ulcerative colitis. Gut. 54:503–509. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma TY, Iwamoto GK, Hoa NT, et al:
TNF-alpha-induced increase in intestinal epithelial tight junction
permeability requires NF-kappa B activation. Am J Physiol
Gastrointest Liver Physiol. 286:G367–G376. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Atreya I, Atreya R and Neurath MF:
NF-kappaB in inflammatory bowel disease. J Intern Med. 263:591–596.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jobin C and Sartor RB: The I kappa
B/NF-kappa B system: A key determinant of mucosalinflammation and
protection. Am J Physiol Cell Physiol. 278:C451–462.
2000.PubMed/NCBI
|
|
22
|
Kim SJ, Shin HJ, Lee BJ, et al: The
antiinflammatory mechanism of Igongsan in mouse peritoneal
macrophages via suppression of NF-κB/Caspase-1 activation.
Phytother Res. 28:736–744. 2014. View
Article : Google Scholar
|
|
23
|
Kim SJ, Kim YG, Kim DS, et al: Oldenlandia
diffusa ameliorates dextran sulphate sodium-induced colitis through
inhibition of NF-κB activation. Am J Chin Med. 39:957–969. 2011.
View Article : Google Scholar
|
|
24
|
Murthy SN, Cooper HS, Shim H, Shah RS,
Ibrahim SA and Sedergran DJ: Treatment of dextran sulfate
sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis
Sci. 38:1722–1734. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|
|
26
|
Porter SN, Howarth GS and Butler RN: An
orally administered growth factor extract derived from bovine whey
suppresses breath ethane in colitic rats. Scand J Gastroenterol.
33:967–974. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim MS, Lim WK, Cha JG, et al: The
activation of PI 3-K and PKC zeta in PMA-induced differentiation of
HL-60 cells. Cancer Lett. 171:79–85. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okayasu I, Hatakeyama S, Yamada M, Ohkusa
T, Inagaki Y and Nakaya R: A novel method in the induction of
reliable experimental acute and chronic ulcerative colitis in mice.
Gastroenterology. 98:694–702. 1990.PubMed/NCBI
|
|
29
|
Fiocchi C: Inflammatory bowel disease:
Evolutionary concepts in biology, epidemiology, mechanisms and
therapy. Curr Opin Gastroenterol. 29:347–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ardizzone S and Bianchi Porro G: Biologic
therapy for inflammatory bowel disease. Drugs. 65:2253–2286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rufo PA and Bousvaros A: Current therapy
of inflammatory bowel disease in children. Paediatr Drugs.
8:279–302. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sato K, Ohkura S, Kitahara Y, et al:
Involvement of CPI-17 downregulation in the dysmotility of the
colon from dextran sodium sulphate-induced experimental colitis in
a mouse model. Neurogastroenterol Motil. 19:504–514. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sandborn WJ and Targan SR: Biologic
therapy of inflammatory bowel disease. Gastroenterology.
122:1592–1608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ishiguro K, Ando T, Maeda O, et al:
Paeonol attenuates TNBS-induced colitis by inhibiting NF-kappaB and
STAT1 transactivation. Toxicol Appl Pharmacol. 217:35–42. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim SJ, Kim KW, Kim DS, et al: The
protective effect of Cassia obtusifolia on DSS-induced colitis. Am
J Chin Med. 39:565–577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Raddatz D, Bockemühl M and Ramadori G:
Quantitative measurement of cytokine mRNA in inflammatory bowel
disease: Relation to clinical and endoscopic activity and outcome.
Eur J Gastroenterol Hepatol. 17:547–557. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zheng P, Niu FL, Liu WZ, Shi Y and Lu LG:
Anti-inflammatory mechanism of oxymatrine in dextran sulfate
sodium-induced colitis of rats. World J Gastroenterol.
11:4912–4915. 2005.PubMed/NCBI
|
|
38
|
Beubler E, Schuligoi R, Chopra AK, Ribardo
DA and Peskar BA: Cholera toxin induces prostaglandin synthesis via
post-transcriptional activation of cyclooxygenase-2 in the rat
jejunum. J Pharmacol Exp Ther. 297:940–945. 2001.PubMed/NCBI
|
|
39
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bak YK, Lampe JW and Sung MK: Effects of
dietary supplementation of glucosamine sulfate on intestinal
inflammation in a mouse model of experimental colitis. J
Gastroenterol Hepatol. 29:957–963. 2014. View Article : Google Scholar
|
|
41
|
Song YA, Park YL, Kim KY, et al: Black tea
extract prevents lipo-polysaccharide-induced NF-κB signaling and
attenuates dextran sulfate sodium-induced experimental colitis. BMC
Complement Altern Med. 11:912011. View Article : Google Scholar
|
|
42
|
Joh EH, Lee IA, Jung IH and Kim DH:
Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1
activation - the key step of inflammation. Biochem Pharmacol.
82:278–286. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang XL, Guo TK, Wang YH, et al:
Ginsenoside Rd attenuates the inflammatory response via modulating
p38 and JNK signaling pathways in rats with TNBS-induced relapsing
colitis. Int Immunopharmacol. 12:408–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee IA, Hyam SR, Jang SE, Han MJ and Kim
DH: Ginsenoside Re ameliorates inflammation by inhibiting the
binding of lipopoly-saccharide to TLR4 on macrophages. J Agric Food
Chem. 60:9595–9602. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu L, Yang ZL, Li P and Zhou YQ:
Modulating effect of Hesperidin on experimental murine colitis
induced by dextran sulfate sodium. Phytomedicine. 16:989–995. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu Y, Xiang J, Liu M, Wang S, Lee RJ and
Ding H: Protective effects of glycyrrhizic acid by rectal treatment
on a TNBS-induced rat colitis model. J Pharm Pharmacol. 63:439–446.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kageyama-Yahara N, Wang P, Wang X, et al:
The inhibitory effect of ergosterol, a bioactive constituent of a
traditional Japanese herbal medicine saireito on the activity of
mucosal-type mast cells. Biol Pharm Bull. 33:142–145. 2010.
View Article : Google Scholar : PubMed/NCBI
|