Introduction
Atherosclerosis, a complex physiopathological
process, is initiated by the infiltration of low-density
lipoproteins (LDLs) into the subendothelial spaces, where they
accumulate and become modified, predominantly by oxidation
(1–3). Macrophages, which are derived from
monocytes in these areas, take up oxidized LDL (ox-LDL) through
scavenger receptor pathways, resulting in lipid droplet
accumulation and foam cell formation. Foam cells are known to be
important in the development and progression of atherosclerosis
through the production of various bioactive molecules and proteins,
including growth factors and cytokines, and adipose
differentiation-related protein or adipophilin (4,5).
Adipophilin is a 50 kDa protein isolated from differentiating
adipocytes. Adipohilin is encoded by a gene that is expressed in
atherosclerotic plaques rich in macrophage-derived foam cells,
which increases lipid storage (6,7).
This suggests that adipophilin may be a specific marker for lipid
accumulation in cells.
Previous studies have indicated that PKCα is
expressed by the formation of foam cells, and that
apolipoprotein-induced cholesterol efflux is reversed by a PKCα
inhibitor (8,9). This suggests that PKC is closely
associated with the accumulation of cholesterol esters.
Acyl-coenzyme A:cholesterol acyltransferase 1 (ACAT1) is the
predominant enzyme involved in the synthesis cholesterol ester in
cells, which maintains the intracellular metabolic balance of
cholesterol. The distribution of synthetic lipids is determined by
the status of the cells. ACAT1 is located in the endoplasmic
reticulum (10). When the
accumulation of intracellular lipids increases, the synthetic
lipids form lipid droplets, which are then stored in the cytoplasm
(11). Robenek H et al
generated foam cells from macrophages incubated with acetylated LDL
(12), and demonstrated that
adipophilin accumulates in the leaf of endoplasmic reticulum in the
outer face of the cytoplasm, which is close to the lipid droplet
surface (12). Therefore, the
subcellular localization of the two substances, adipophilin and
ACAT1, are close. Gao J et al observed that adipophilin can
promote cell uptake and binding of fatty acids, therefore, fatty
acids can be induced by the expression of adipophilin (13). As fatty acy1-CoA was used as the
substrate of ACAT1 in the cells, adipophilin and ACAT1 were also
demonstrated to be closely associated in function, and the data
revealed that adipophilin and ACAT1 are associated with the
accumulation of intracellular cholesterol ester.
The present study was performed to examine the
signaling pathway linking ox-LDL and the accumulation of
intracellular cholesterol ester, and the association between
adipophilin and ACAT1.
Materials and methods
Materials
RPMI-1640 medium, trypsin and fetal bovine serum
(FBS) was purchased from Hyclone (Logan, UT, USA). the ReverAidTM
First-Strand cDNA Synthesis kit and Lipofectamine 2000 and TRIzol
reagent were purchased from Invitrogen Life Technologies (Carlsbad,
CA, USA). The bicinchoninic acid (BCA) protein assay reagent was
purchased from Pierce Biotechnology, Inc., Rockford, IL, USA).
β-actin rabbit anti-human antibody (cat. no. ab199406), and
horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary
antibody (cat. no. 2109) were purchased from Abcam (Cambridge, UK).
Rabbit polyclonal anti-PKCα (sc-358943), anti-phosphorylated
(p)-PKCα (Thr638; cat. no. 135685), anti-adipophilin (cat. no.
32888) and anti-ACAT1 (cat. no. sc-69836) were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). All other reagents were
obtained at optimal grade, available from commercial sources.
Cell cultures
The RAW264.7 mouse macrophage-like cell line,
purchased from the Cell Bank at the Shanghai Institute of Cell
Biology of Chinese Academy of Sciences (Shanghai, China), was
incubated using RPMI-1640 medium containing 25 mmol/l HEPES buffer
and 10% fetal bovine serum at 37°C in 5% CO2. Safe
Packaging Line PA317 cells, were provided by the Department of
Biochemistry of Central South University (Hengyang, China), were
cultured in DMEM-high containing 25 mmol/l HEPES buffer and 10% FBS
at 37°C in 5% CO2. The cells were cultured without serum
for at least 6 h prior to initiation of the experiments.
Preparation of ox-LDL
The native LDL was obtained from Sigma-Aldrich (St.
Louis, MO, USA). LDL was oxidized with CuSO4 at 37°C for
18 h and transferred into ethylene diamine tetraacetic acid (EDTA;
200 µmol/l) in phosphate-buffered saline (PBS) for 24 h at
4°C to remove Cu2+. Subsequently, the product was
dialyzed in PBS for 24 h at 4°C to remove the EDTA. LDL oxidation
was confirmed using thiobarbituric acid reaction substances
(Shanghai XiTang Biotechnology Co., Ltd., Shanghai, China), with
malondialdehyde as the standard. The content of ox-LDL was
1.12±0.056, compared with 0.30±0.067 nmol/100 µg protein in
the native LDL preparation (P<0.01). The ox-LDL was then
sterilized by filtration and stored at 4°C, as previously described
(14).
Transfection of small interfering
(si)RNA
siRNA targeting PKCα, adipophilin and ACAT1 was
purchased from Santa Cruz Biotechnology, Inc. A control siRNA,
specific for red fluorescent protein (CCACTACCTGAGCACCCAG) was used
as a negative control. The cells (4×105/well in 6-well
plates) were cultured without antibiotics or serum at 37°C for 6 h
at 50% confluence. Different concentrations of RNA interference
reagent (A; siRNA; 100 nmol/l per well of a 12-well plate) and RNA
transfection reagent (B; Lipofectamine 2000; 2 µl/well of a
12-well plate) were diluted. Prior to transfection, the
interference reagent was mixed with the transfection reagent and
incubated for 30 min. The cells were then washed three times with
PBS at room temperature then cultured in 30% serum Dulbecco's
modified Eagle's medium.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent,
according to the manufacturer's instructions. The RNA (1 µg)
was reverse transcribed into cDNA using a Taq Man Reverse
Transcription Reagent kit (Applied Biosystems Life Technologies,
Foster City CA, USA), of which 2 µg was used in an Real Time
qPCR Detection system (StepOne™ Real-Time PCR systems; Applied
Biosystems Life Technologies) for the evaluation of the relative
mRNA levels of apo (a). GAPDH served as the control. The primer
sequences and amplification-specific gene products were as follows:
GAPDH, sense 5′-TGCCATCAACGACCCCTT CA-3′ and antisense
5′-TGACCTTGCCCACAGCCTTG-3′; adipophilin, sense
5′-TGCCATCAACGACCCCTT-3′ and anti-sense 5′-ACAGTGGGACTCATCGGTGTC-3;
and ACAT1, sense 5′-TGCCTGAGCTACTTCTACCCA-3′and antisense
5′-CACGTAACGACAAGTCCAGGT-3′. The PCR cycling conditions were as
follows: GAPDH, denaturation at 94°C for 5 min, 35 cycles of 94°C
for 30 sec, 60°C for 30 sec and 72°C for 45 sec, and 72°C for 5
min; adipophilin, denaturation at 94°C for 5 min, 35 cycles of 94°C
for 1 min, 55°C for 1 min and 72°C for 30 sec, and 72°C for 5 min;
ACAT1, denaturation at 94°C for 5 min, 35 cycles of 95°C for 30
sec, 60°C for 30 sec and 72°C for 40 sec, and 72°C for 5 min. The
quantitative results for ACAT1 and adipophilin were normalized to
the levels of GAPDH mRNA. The resulting PCR products were separated
on a 1.2% agarose gel and stained with ethidium bromide. The cycle
threshold (Ct) value of each sample was calculated from the
threshold cycles, using the software embedded in the RT-qPCR
machine (SDS 2.3), wherein the relative expression of adipophilin
and ACAT mRNA were normalized to the levels of GAPDH. Relative
expression levels were determined by normalization (15).
Western blot analysis
The cells (2×106/well in 6-well plates)
were immediately lysed in ice-cold lysis buffer containing 50 mM
tris, 150 mM sodium chloride, 2 mM EDTA, 1 mM phenylmethyl
sulfonylfluoride (94:6), 1% NP-40, 1% sodium deoxycholate, 0.1%
SDS, 50 mM sodium fluoride, 1 mM sodium orthovanadate, 15 mM sodium
pyrophosphate, and 10 mM β-glycerophosphate. Following lysis, the
samples were centrifuged at 10,000 rpm for 10 min at 48°C, and the
supernatants were collected. The protein concentration was
determined using Coomassie brilliant blue (Biotechnology Co., Ltd.,
Suzhou, China). The proteins were separated by 10% SDS-PAGE and
electrophoretically transferred onto a polyvinylidene difluoride
membrane (EMD Millipore, Billerica, MA, USA). The membranes were
blocked at 4°C for 4–6 h with tris-buffered saline containing Tween
20 (TBS-T). The membrane was then immunoblotted with the following
antibodies: Anti-adipophilin (1:500), anti-ACAT1 (1:500), anti-PKCα
(1:500), anti-phospho-PKCα and anti-β-actin (1:1,000) for 3 h at
room temperature. After washing three times with TBS-T, the
membranes were incubated at room temperature for 1 h with
HRP-conjugated goat anti-rabbit or anti-mouse secondary antibodies
(1:3,000). The membranes were washed with TBS-T and immunoreactive
proteins were visualized using an Enhanced Chemilluminence (ECL)
Plus kit (Shanghai XiTang Biotechnology Co., Ltd.), and were
exposed to X-ray film. The immunoreactive bands were quantified
using AlphaImager 2200 software (Beijing Representative Office of
Natural Gene, Ltd., Beijing, China), and the data represent the
protein variation following treatment.
Oil red O staining
Lipid accumulation was measured in the RAW264.7
cells through the staining of neutral fats and cholesterol esters
using Oil Red O. The cells were seeded into 6-well plates with
slides at a density of 4×105 cells/cm2 and
cultured for 24 h. Subsequently, the cells were rinsed with PBS
three times and fixed with 50% isopropyl alcohol for 1 min at room
temperature. The cells were then incubated with fresh filtered oil
red O solution (60% saturated oil red O/40% deionized water) for 10
min. For analysis, the slides were then counterstained with
hematoxylin and mounted in glycerol/gelatin solution. Images of the
cells were captured using a light microscope (XSZ-2105; Olympus
Corporation, Tokyo, Japan). The intracellular lipid droplets were
stained red and the nuclei were stained blue (14).
High-performance liquid chromatography
(HPLC) assays
HPLC analysis was performed, as described previously
(14). Briefly, the cells were
washed three times with PBS. The appropriate volume (1 ml) of 0.5%
NaCl was added to between ~50 and 200 mg cellular proteins per ml.
The cells (2×106/well in 6-well plates) were sonicated
using an ultrasonic processor (JY92-IIN/JY92-IIDN; Shanghai Selon
Scientific Instrument Co., Ltd., Shanghai, China) for 2 min. The
protein concentration in the cell solution was measured using a BCA
kit. A 0.1 ml aliquot of the cell solution (containing 5–20
µg protein) was used to measure free cholesterol, and a
different aliquot was used to measure total cholesterol. Free
cholesterol was dissolved in isopropanol (1 mg cholesterol/ml) and
stored at −20°C as a stock solution. A cholesterol standard
calibration solution ranging between 0 and 40 mg cholesterol/ml was
obtained by diluting the cholesterol stock solution in the same
cell lysis buffer. Subsequently, 0.1 ml each sample (cholesterol
standard calibration solutions or cell solutions) was supplemented
with 10 ml reaction mixture, containing 500 mM MgCl2,
500 mM Trise HCl (pH 7.4), 10 mM dithiothreitol and 5% NaCl. A
total of 0.4 units cholesterol oxidase in 10 ml 0.5% NaCl was added
to each tube for free cholesterol determination, while 0.4 units
cholesterol oxidase and 0.4 units cholesterol esterase were added
to each tube for total cholesterol measurement. The total reaction
solution in each tube was incubated at 37°C for 30 min, and 100 ml
methanol:ethanol mixture (1:1) was then added to terminate the
reaction. Each solution was maintained at cold temperature for 30
min to enable protein precipitation and then centrifuged at 272 × g
for 10 min at 15°C. Subsequently, 10 ml supernatant was transferred
onto a chromatograph system (PerkinElmer, Inc., Waltham, MA, USA),
which consisted of a PerkinElmer Series 200 vacuum degasser, pump,
PerkinElmerSeries 600 LINK, PerkinElmer series 200 UV/vis detector,
and a Discovery C-18 HLPC column (Supelco, Inc., Bellefonte, PA,
USA). The column was eluted using an
isopropanol:n-heptane:acetonitrile mixture (35:13:52) at a flow
rate of 1 ml/min for 8 min. The absorbance at 216 nm was monitored.
Data were analyzed using Total Chrom software (PerkinElmer,
Inc.).
Statistical analysis
All results are expressed as the mean ± standard
deviation from three independent experiments, and data were
analyzed using one-way analysis of variance and Student's t-test,
using SPSS 13.0 software(SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Effect of ox-LDL on the expression levels
of PKCα, adipophilin and ACAT in RAW264.7 cells
In the present study, the effects of ox-LDL on the
expression levels of PKCα, p-PKCα, adipo-philin and ACAT1 in
RAW264.7 cells were first examined. The RAW264.7 cells were
maintained in fresh serum-free medium for 6 h to obtain
synchronisation of growth, following which the medium was replaced
with fresh serum-free medium containing different concentrations of
ox-LDL (50 mg/l) and incubated with the cells for 24 h (14). The results of RT-qPCR and western
blotting (Fig. 1) indicated that
ox-LDL induced the expression of adipophilin and ACAT1. Ox-LDL is
expected to increase the expression and phosphorylation of PKC in
THP1 macrophages. The results of the present study demonstrated
that ox-LDL did not increase the expression of PKCα in the RAW264.7
cells, but ox-LDL increased the phosphorylation of PKCα and the
accumulation of intracellular lipid droplets.
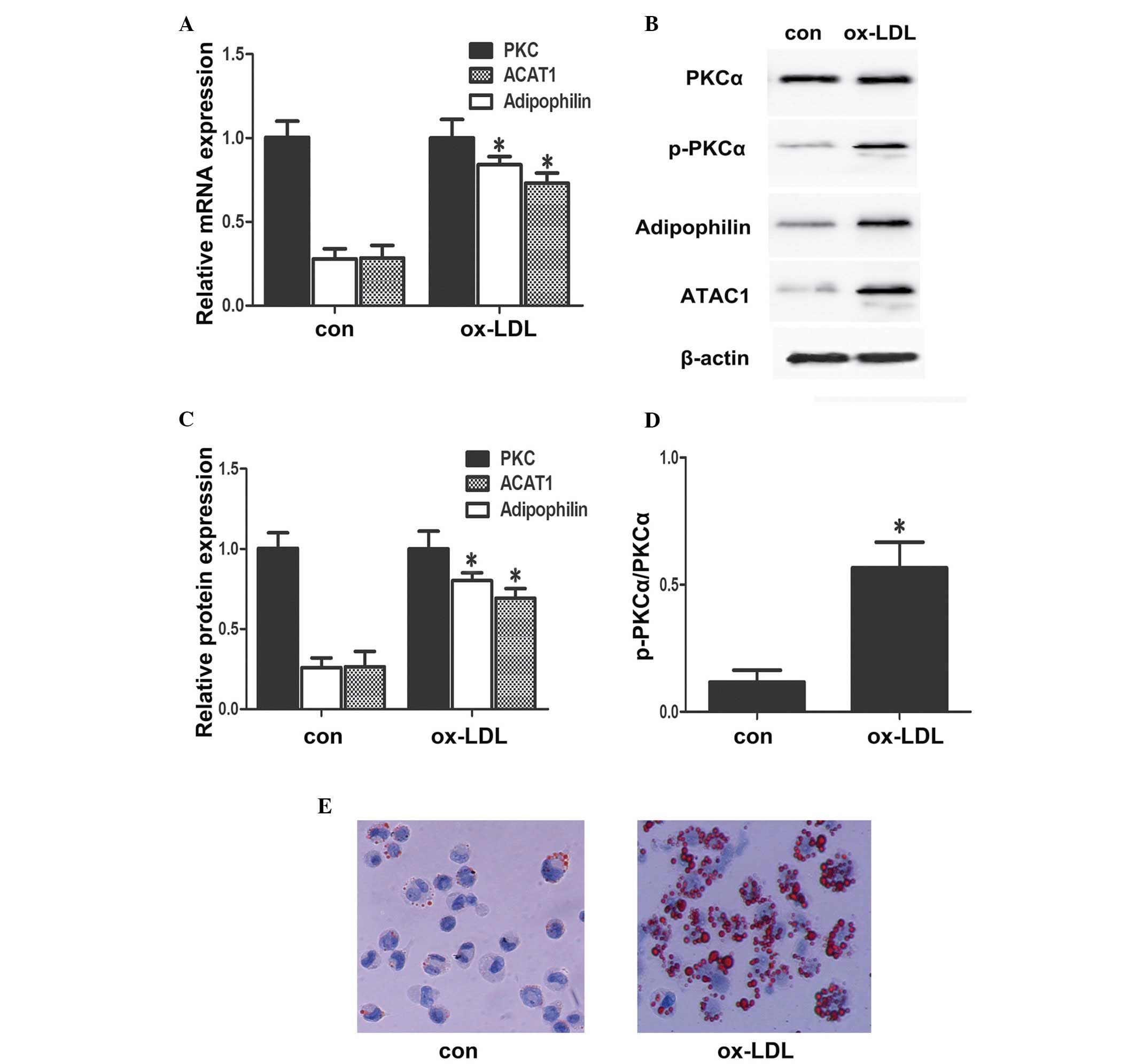 | Figure 1Ox-LDL increases the phosphorylation
of PKCα, and the expression levels of adipophilin and ACAT1, and
increases lipid droplet accumulation. (A) RAW264.7 cells were
incubated with either 50 mg/ml Ox-LDL for 24 h or with 10% BSA for
24 h. The mRNA expression levels of PKCα, adipophilin and ACAT1
were analyzed using reverse transcription-quantitative polymerase
chain reaction and normalized to GAPDH transcripts. (B–D)
Expression levels of PKCα, p-PKCα, edipophilin and ACAT1 in whole
cell lysates from RAW264.7 cells treated for 24 h with 50 mg/ml
Ox-LDL, analyzed using western blot analysis and densitometry. (E)
RAW264.7 cells were incubated with either 50 mg/ml Ox-LDL for 24 h
or 10% BSA for 24 h. The cells were then stained with oil red O.
Intracellular lipid droplets are stained red and nuclei are stained
blue (magnification, ×100). Ox-LDL, oxidized low density
lipoprotein; con, control; PKC, protein kinase C; p-,
phosphorylated; ATAC1, adipophilin and acyl-coenzymeA:cholesterol
acyltransferse 1. |
Effect of the accumulation of cholesterol
esters and expression of adipophilin and ACAT1 on PKCα
inhibition
PKCα is expressed and activated by foam cell
formation, as previously reported (8). Consistent with PKCα, adipophilin and
ACAT1 are also expressed significantly during foam cell formation
(15,16). Therefore, the present study
investigated the involvement of PKCα signals in Ox-LDL-induced
expression of adipophilin and ACAT1 in RAW264.7 cells. To confirm
whether the expression of adipophilin and ACAT1 were due to PKCα
signaling, the RAW264.7 cells were pre-incubated with either PKCα
siRNA or scramble siRNA (NA) for 24 h followed by 50 mg/ml Ox-LDL
for 24 h. As shown in Fig. 2,
following treatment with PKCα siRNA for 24 h, the expression levels
of adipophilin and ACAT1 were decreased significantly. The increase
in intracellular lipid droplets and cholesterol esters was also
inhibited by PKCα siRNA (Fig. 3;
Table I). These data demonstrated
that ox-LDL induced the accumulation of cholesterol esters and the
expression levels of adipophilin and ACAT1 via PKCα signaling.
 | Figure 2Ox-LDL-induced expression of
adipophilin and ACAT1 by PKCα. (A) RAW264.7 cells were
pre-incubated with PKCα siRNA or NA for 24 h followed by 50 mg/ml
ox-LDL for 24 h. mRNA levels of adipophilin and ACAT1 were
determined using reverse transcription-quantitative polymerase
chain reaction and normalized to GAPDH. (B-D) RAW264.7 cells were
pre-incubated with 10 µM PKCα siRNA for 24 h followed by 50
mg/ml ox-LDL for 24 h. Cell proteins were separated by sodium
dodecyl sulfate polyacrylamide gel electrophoresis and
immunoblotted with polyclonal anti-adipophlin and anti-ACAT1
antibodies. All data are expressed as the mean ± standard deviation
of three independent experiments, each performed in triplicate.
*P<0.05, vs. ox-LDL group; #P<0.05, vs.
ox-LDL+NA. Ox-LDL, oxidized low density lipoprotein; siRNA, small
interfering RNA; NA, scramble siRNA; con, control; PKC, protein
kinase C; p-, phosphorylated; ATAC1, adipophilin and
acyl-coenzymeA:cholesterol acyltransferse 1. |
 | Table IEffect of PKCα siRNA on the
intracellular lipid content of the RAW264.7 cells. |
Table I
Effect of PKCα siRNA on the
intracellular lipid content of the RAW264.7 cells.
| Group | TC (mg/g
protein) | FC (mg/g
protein) | CE (mg/g
protein) | CE/TC (%) |
|---|
| Control | 162±29 | 136±21 | 26±20 | 16.1±2.6 |
| Ox-LDL | 253±30 | 167±22 | 86±21 | 33.9±1.3 |
| Ox-LDL+NA | 233±27 | 161±24 | 72±27 | 30.9±1.5 |
| Ox-LDL+siRNA | 182±25a,b | 143±27a,b | 39±19a,b | 21.4±1.2a,b |
Effect of the expression of ACAT1 and
accumulation of cholesterol esters on adipophilin inhibition in
RAW264.7 cells
Forcheron et al demonstrated that adipophilin
and ACAT1 are expressed in atherosclerotic plaques and, compared
with the surrounding tissue of the plaques, are increased
significantly (17). This
indicates that adipophilin is closely associated with ACAT1. In
order to examine the association between adipophilin and ACAT1, the
present study determined the expression level of ox-LDL-induced
ACAT1 using RT-qPCR and western blot analysis, and the accumulation
of intracellular lipids was detected using oil red O staining
following pre-incubation of the RAW264.7 cells with adipophilin
siRNA or scramble siRNA for 24 h. The results, as shown in Figs. 4 and 5 and Table
II, demonstrated that inhibited adipophilin partly attenuated
the ox-LDL induced expression of ACAT1, and decreased the
accumulation of intracellular lipid droplets and cholesterol
esters. A previous study indicated that cholesterol from
internalized lipoproteins is converted to cholesteryl esters by
ACAT1 (18). In the present study,
ACAT1 siRNA was transfected into RAW264.7 cells prior to ox-LDL
treatment, and the results (Table
III) revealed that the accumulation of cholesteryl esters was
inhibited by ACAT1 siRNA. Therefore, these data suggested that
ox-LDL increased the accumulation of cholesterol esters, at least
in part, through the PKCα-adipophilin-ACAT1 pathway.
 | Table IIEffect of adipophilin siRNA on the
intracellular lipid content of the RAW264.7 cells. |
Table II
Effect of adipophilin siRNA on the
intracellular lipid content of the RAW264.7 cells.
| Group | TC (mg/g
protein) | FC (mg/g
protein) | CE (mg/g
protein) | CE/TC (%) |
|---|
| Con | 165±24 | 133±25 | 32±23 | 19.3±1.6 |
| Ox-LDL | 267±28 | 169±24 | 98±22 | 36.7±1.4 |
| Ox-LDL+NA | 243±27 | 166±24 | 77±27 | 31.6±1.7 |
| Ox-LDL+siRNA | 178±18a,b | 130±21a,b | 30±20a,b | 22.4±1.2a,b |
 | Table IIIEffect of ACAT1 siRNA on the
intracellular lipid content of the RAW264.7 cells. |
Table III
Effect of ACAT1 siRNA on the
intracellular lipid content of the RAW264.7 cells.
| Group | TC (mg/g
protein) | FC (mg/g
protein) | CE (mg/g
protein) | CE/TC (%) |
|---|
| Con | 168±24 | 136±26 | 32±21 | 19.3±1.6 |
| Ox-LDL | 266±28 | 171±24 | 95±22 | 35.7±1.4 |
| Ox-LDL+NA | 231±27 | 160±24 | 71±27 | 30.7±1.7 |
| Ox-LDL+siRNA | 171±19a,b | 145±23a,b | 26±21a,b | 15.2±1.1a,b |
Discussion
Macrophages contribute to the formation of arterial
lesions by accumulating excessive amounts of lipids, predominantly
cholesterol esters, through the accumulation of cholesterol esters
by a variety of mechanisms, including ACAT1, the enzyme responsible
for the esterification of intracellular free cholesterol. Thus,
elimination of accumulated cholesterol esters from macrophage foam
cells represents a promising therapeutic approach in the prevention
of atherosclerotic lesions (19).
Adipophilin is a 50 kDa protein, which is
upregulated in atherosclerotic plaques and is associated with the
development of atherosclerosis. Lipid storage is facilitated by the
production of large quantities of lipid vesicle coating proteins,
including adipophilin and perilipin (6,7),
thus preventing lipid efflux from macrophages. A previous study
suggested that ox-LDL increases the expression of adipophilin in
THP1 macrophage-derived foam cells, and inhibition of adipophilin
reduces the formation of lipid droplets and foam cells. These
observations suggested that adipophilin is involved in lipid
storage and is relevant to atherosclerosis. Our previous study also
indicated that ox-LDL induces the expression of adipophilin
(14). In the present study, the
results demonstrated that ox-LDL increased the expression of
adipophilin. Furthermore, previous studies demonstrated that the
knockdown of adipo-philin by siRNA attenuated the ox-LDL-induced
expression of adipophilin and accumulation of lipid droplets
(20–22). These results demonstrated that
adipophilin was involved in the accumulation of lipid droplets and
promoted the formation of foam cells.
ACAT1 is a 56 kDa protein present in the brain,
liver, adrenal glands and macrophages, and is important in the
formation of macrophage-derived foam cells in atherosclerotic
lesions by catalyzing the formation of cholesteryl esters (19,23).
Inhibition of the expression of ACAT1 has reduced the incidence of
atherosclerosis (24). Yang et
al reported that ox-LDL increases the expression of ACAT1 in
macrophages and causes macrophages to become foamy in appearance,
which is a hallmark appearance of early atherosclerotic lesions
(25). In advanced lesions, in
addition to cholesteryl esters, free, unesterified, cholesterol
also accumulates in macrophages. In the present study, RAW264.7
cells were preincubated with ACAT1 siRNA or scramble siRNA for 24
h, followed by treatment with 50 mg/ml Ox-LDL for 24 h. The results
demonstrated that the ox-LDL-induced accumulation of intracellular
lipid droplet and cholesterol esters were suppressed by ACAT1
siRNA. This finding was consistent with that of Naomi et al.
In addition, previous studies have reported that adipophilin and
ACAT1 are present in atherosclerotic plaques simultaneously
(26) and, compared with the
tissue surrounding the plaque, adipophilin and ACAT1 exhibited
increased levels of expression in atherosclerotic plaques (26). Therefore, the present study
hypothesized that there is a molecular interaction between
adipophilin and ACAT1 and, to investigate whether ACAT1 was the
downstream factor of adipophilin, the RAW264.7 cells were
transfected with adipophilin siRNA prior to ox-LDL. The results
indicated that ACAT1 siRNA partly attenuated the ox-LDL-induced
expression of ACAT1 and decreased accumulation of intracellular
lipid droplets and cholesterol esters. This demonstrated that
ox-LDL induced the accumulation of intracellular lipid droplets and
cholesterol esters, at least in part, via the adipophilin-ACAT1
pathway, and ACAT1 may be a downstream factor of adipophilin.
PKCα is a member of the serine/threonine family of
kinases. Previous studies have indicated that ox-LDL-induced PKCα
activation depends on O2•− (27) and that the expression of PKCα is
high in atherosclerotic plaque (28). The present study also demonstrated
that the overexpression and activation of PKCα was induced by
ox-LDL. These findings suggest that PKCα signaling may be involved
in the ox-LDL-induced accumulation of cholesterol esters and
expression of adipophilin and ACAT1. PKCα activation stimulates
PPARγ, and the expression of adipophilin and ACAT1 are regulated by
PPARγ (29,30). Thus, the present study hypothesized
that ox-LDL-induced expression of adipophilin and ACAT1 may occur
through PKCα signaling. To explain this hypothesis, the involvement
of alternative signaling pathways were examined. The RAW264.7 cells
were pre-incubated with PKCα siRNA or scramble siRNA for 24 h and
then treated with ox-LDL. The results revealed that the expression
levels of adipophilin and ACAT1 were inhibited, and the
accumulation of intracellular lipid droplets and cholesterol esters
were reversed, suggesting that ox-LDL-induced expression of
adipophilin and ACAT1 depend on PKCα signaling.
In conclusion, the present study demonstrated that
ox-LDL induced the upregulation of the expression levels of
adipophilin and ACAT1, and increased the accumulation of cellular
cholesterol esters by PKCα signaling. In addition, ox-LDL increased
the accumulation of cellular cholesterol esters via
PKCα-adipophilin-ACAT1 and PKCα-ACAT1 signaling. These findings
provide evidence for the role of ox-LDL in promoting the
progression of atherosclerosis.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (grant. no. 30971268), the
Scientific Research Foundation for Returned Overseas Chinese
Scholars from Ministry of Education (grant. no. 2008)890) and the
Science and Technology Innovatice Research Team in Higher Education
Institutions (Hunan, China).
References
|
1
|
Paim LR, Schreiber R, Matos-Souza JR,
Silva AA, Campos LF, et al: Oxidized low-density lipoprotein,
matrix-metalloproteinase-8 and carotid atherosclerosis in spinal
cord injured subjects. Atherosclerosis. 231:341–345. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pirillo A, Norata GD and Catapano AL:
LOX-1, OxLDL and atherosclerosis. Mediators Inflamm.
2013:1527862013. View Article : Google Scholar
|
|
3
|
Yang H, Mohamed AS and Zhou SH: Oxidized
low density lipoprotein, stem cells and atherosclerosis. Lipids
Health Dis. 11:2–9. 2012. View Article : Google Scholar
|
|
4
|
Straub BK, Gyoengyoesi B, Koenig M,
Hashani M, Pawella LM, et al: Adipophilin/perilipin-2 as a lipid
droplet-specific marker for metabolically active cells and diseases
associated with metabolic dysregulation. Histopathology.
62:617–631. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Buers I, Hofnagel O, Ruebel A, Severs NJ
and Robenek H: Lipid droplet associated proteins: an emerging role
in atherogenesis. Histol Histopathol. 26:631–642. 2011.PubMed/NCBI
|
|
6
|
Song Y, Li H, Zhang LY, Ye Q and Li Q:
Expression profile of ADRP in macrophages and foam cells. Xi Bao Yu
Fen Zi Mian Yi Xue Za Zhi. 25:309–311. 2009.In Chinese. PubMed/NCBI
|
|
7
|
Nuotio K, Isoviita PM, Saksi J, Ijäs P,
Pitkäniemi J, Sonninen R, et al: Adipophilin expression is
increased in symptomatic carotid atherosclerosis: correlation with
red blood cells and cholesterol crystals. Stroke. 38:1791–1798.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Via DP, Pons L, Dennison DK, et al:
Induction of acetyl' LDL receptor activity by phorbol ester in
human monocyte cell line THP-1. J Lipid Res. 30:1515–1524.
1989.PubMed/NCBI
|
|
9
|
Slater SJ, Seiz JL, Cook AC, Stagliano BA
and Buzas CJ: Inhibition of protein kinase C by resveratrol.
Biochim Biophys Acta. 1637:59–69. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Freeman NE, Rusinol AE, Linton M, Hachey
DL, Fazio S, Sinensky MS and Thewke D: Acyl-coenzyme A: Cholesterol
acyltransferase promotes oxidized LDL/oxysterol-induced apoptosis
in macrophages. J Lipid Res. 46:1933–1943. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang Y, Yang X, Ma L, Cai X, Wang L, Yang
C, et al: Homocysteine-mediated cholesterol efflux via ABCA1 and
ACAT1 DNA methylation in THP-1 monocyte-derived foam cells. Acta
Biochim Biophys Sin (Shanghai). 45:220–228. 2013. View Article : Google Scholar
|
|
12
|
Robenek H, Robenek MJ and Troyer D: PAT
family proteins pervade lipid droplet cores. J Lipid Res.
46:1331–1338. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao J, Ye H and Serrero G: Stimulation of
adipose differentiation related protein (ADRP) expression in
adipocyte precursors by long-chain fatty acids. J Cell Physiol.
182:297–302. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Q, Dai Z, Liu Z, Liu X, Tang C, Wang
Z, et al: Oxidized low-density lipoprotein activates adipophilin
through ERK1/2 signal pathway in RAW264.7 cells. Acta Biochim
Biophys Sin (Shanghai). 42:635–645. 2010. View Article : Google Scholar
|
|
15
|
Yuan ZH, Liu XH, Wang ZQ, Jia W, Niu XL,
Huang AF, et al: Adipophilin accumulates cellular cholesteryl ester
by PKC and ACAT1 in THP-1 macrophages. Chinese J Pathophysiol.
24:833–842. 2008.In Chinese.
|
|
16
|
Qiao YC, Huang AF, Tian GP, Liu XH and
Yuan ZH: Adipophilin facilitate Acyl-coenzyme A: cholesterol
acyltransferase 1 expression in RAW264.7 cells. Chin J
Arterioscler. 17:643–647. 2009.In Chinese.
|
|
17
|
Forcheron F, Legedz L, Chinetti G, Feugier
P, Letexier D, Bricca G, et al: Genes of cholesterol metabolism in
human atheroma: overexpression of perilipin and genes promoting
cholesterol storage and repression of ABCA1 expression.
Arterioscler Thromb Vasc Biol. 25:1711–1717. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thomas JP, Geiger PG, Maiorino M, Ursini F
and Girotti AW: Enzymatic reduction of phospholipid and cholesterol
hydroper-oxides in artificial bilayers and lipoproteins. Biochim
Biophys Acta. 1045:252–260. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang TY, Li BL, Chang CC and Urano Y:
Acyl-coenzyme A: cholesterol acyltransferases. Am J Physiol
Endocrinol Metab. 297:E1–E9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Larigauderie G, Furman C, Jaye M, Lasselin
C, Copin C, Fruchart JC, et al: Adipophilin enhances lipid
accumulation and prevents lipid efflux from THP-1 macrophages:
Potential role in atherogenesis. Arterioscler Thromb Vasc Biol.
24:504–510. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Imamura M, Inoguchi J, Ikuyama S,
Taniguchi S, Kobayashi K, Nakashima N and Nawata H: ADRP stimulates
lipid accumulation and lipid droplet formation in murine
fibroblasts. Am J Physiol Endocrinol Metab. 283:E775–E783. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan ZH, Yang YZ, Yin WD, et al: Induced
expression of adipo-philin with high cholesterol diet in rabbit
atherosclerotic lesions and reduced lipid accumulation with
adipophilin antisense in mouse macrophages. Prog Biochem Biophys.
30:549–554. 2003.
|
|
23
|
Kitayama K, Tanimoto T, Koga T, Terasaka
N, Fujioka T, Inaba T, et al: Importance of acyl-coenzyme
A:cholesterol acyl-transferase 1/2 dual inhibition for
anti-atherosclerotic potency of pactimibe. Eur J Pharmacol.
540:121–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rong JX, Blachford C, Feig JE, Bander I,
Mayne J, Kusunoki J, et al: ACAT inhibition reduces the progression
of preexisting, advanced atherosclerotic mouse lesions without
plaque or systemic toxicity. Arterioscler Thromb Vasc Biol.
33:4–12. 2013. View Article : Google Scholar :
|
|
25
|
Yang L, Yang JB, Chen J, Yu GY, Zhou P,
Lei L, et al: Enhancement of human ACAT1 gene expression to promote
the macrophage-derived foam cell formation by dexamethasone. Cell
Res. 14:315–323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Daugherty A, Rateri DL and Lu H: As
macrophages indulge, atherosclerotic lesions bulge. Circ Res.
102:1445–1447. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kruth HS, Huang W, Ishii I and Zhang WY:
Macrophage foam cell formation with native low density lipoprotein.
J Biol Chem. 277:34573–34580. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Karunakaran D, Kockx M, Owen DM, Burnett
JR, Jessup W and Kritharides L: Protein kinase C controls vesicular
transport and secretion of apolipoprotein E from primary human
macrophages. J Biol Chem. 288:5186–5197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
von Knethen A, Soller M, Tzieply N,
Weigert A and Johann AM: PPARgamma1 attenuates cytosol to membrane
translocation of PKCalpha to desensitize monocytes/macrophages. J
Cell Biol. 176:681–694. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kang J, Cheng B and Jiang L: PPARγ signal
transduction pathway in the foam cell formation induced by
visfatin. Sheng Li Xue Bao. 62:427–432. 2010.In Chinese. PubMed/NCBI
|



















