Introduction
Contact dermatitis (CD), one of the most common
types of skin disorder in developed countries, is an inflammatory
skin disease, which is induced by repeated skin contact with a low
molecular weight chemical, termed a hapten (1). The onset of CD is predominantly due
to the recruitment of chemical-specific CD8+ T cells
(2). In addition, CD4+
T helper (Th)1 and Th17 contribute to the extension of the
inflammatory reaction by releasing pro-inflammatory cytokines,
including IFN-γ and TNF-α, which activate cells within the skin
(2,3). In mouse models, repeated antigen
application results in an immediate-type response 30 min after
antigen exposure, demonstrating the involvement of mast cells in
the immediate-type response (4,5).
Mast cells are commonly found at sites of CD and are
involved in immediate-type allergic reactions and inflammation
(6). It is well established that
immediate-type allergic reactions are markedly reduced in the
absence of mast cells, demonstrated using mouse models of mast cell
deficiency (7). The degranulation
of mast cells induces the secretion of bio-active substances,
including histamine, cytokines and chemokines (8). Cytokines, particularly TNF-α, are
released in late-phase allergic reactions and inflammation via the
recruitment of inflammatory cells (9).
It has been reported that cross-talking between
oxidative stress and inflammation causes cutaneous damage in CD
(10). There is increasing
evidence that oxidative stress accentuates the immunological damage
in CD (11,12), and certain antioxidants have
anti-inflammatory and anti-allergic effects in irritant or allergic
CD (13,14). Previously, it was reported that
dietary carotenoids significantly inhibited ear swelling and
reduced the levels of TNF-α and histamine in dinitrofluorobenzene
(DNFB)-treated mice (15).
Astaxanthin (3,3′-dihydroxy-β,β-carotene-4,4′-dione;
AST) is a keto-carotenoid and a type of xanthophyll, which are
found in microalgae, fungi, complex plants, seafood, flamingos and
quail (16). AST has been
investigated for its potential medical applications as it has
antioxidative and antitumor properties (17,18).
AST is known to have anti-inflammatory activities in
endotoxin-induced uveitis (19)
and laser-induced choroidal neovascularization (20).
Based on these previous observations, the present
study evaluated the anti-allergic and anti-inflammatory effects of
AST by using mouse model of CD in vivo and the RBL-2H3 mast
cell-like leukemia cell line in vitro. It was hypothesized
that AST treatment may serve as a therapeutic approach in patients
with CD, as an alternative to the use of steroids.
Materials and methods
Chemicals and reagents
The reagents, 1-fluoro-2,4-dinitrofluorobenzene
(dinitrofluorobenzene; DNFB), dimethyl sulfoxide (DMSO),
dexamethasone (DEX), phorbol 12-myristate 13-acetate (PMA), calcium
ionophore A23187, nuclear fast red solution and fetal bovine serum
(FBS) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Alcian blue was purchased from Muto Pure Chemical (Tokyo, Japan).
Dulbecco's Modified Eagle's Medium (DMEM/high glucose), penicillin
and streptomycin were purchased from GE Healthcare (Logan, UT,
USA). PrestoBlue cell viability reagent was purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA). The Cytometric
Bead Array kit was purchased from BD Biosciences (Franklin Lakes,
NJ, USA). The histamine assay kit, including
-nitrophenyl-N-acetyl-β -D-glucosaminide was purchased from
Oxford Biomedical Research (Rochester Hills, MI, USA).
Preparation of AST
Haematococcus pluvialis CCAP-34/1F (Subitec
Gmbh, Stuttgart, Germany) were cultured in Bold's basal medium
(21,22), with additional nitrogen (1 g/l) for
efficient growth, with conditions of 25°C, 160 rpm (incubator
rotation), 10% inoculum, pH 7.0 and 60-70 µm ol
photon.m−2.s−1 with fluorescent lamps. In
order to induce the production of AST and the cyst cells from the
vegetative cells, the cell culture (optical density at 650 nm, 0.5)
were transferred to the medium without nitrogen and cultured at
23°C and 300 µmol photon.m−2.s−1 for
15 days. Upon confirming the production of AST, cultured cells
(~4.2×105 cells/ml) were collected by centrifugation at 8,000 x g,
4°C for 5 min, were crushed and mixed with 25 ml acetone in a
sonicator (Branson 250, Danbury, CT, USA) and subjected to repeated
cold extraction followed by fractionation. Subsequently,
concentration of the extract was performed under reduced pressure,
extracted with n-hexane, and then washed with water three times. To
remove the residual water, 1 g sodium sulfate anhydrous was used
for extraction of the AST. For the subsequent experiments, the AST
was dissolved in 0.01% DMSO.
Animals
Male balb/c mice (6-weeks-old; 38 mice used) were
purchased from Samtaco (Incheon, Korea). The mice were housed under
specific pathogen-free conditions with a 12 h light/dark cycle and
free access to standard rodent food and water. All animal
experiments were approved by the Animal Care and Use Committee of
Pusan National University (Yangsan, Republic of Korea) and
performed, according to institutional guidelines
(PNU-2010-00065).
Induction of CD and experimental
design
The mice were sensitized by applying 50 µl
DNFB (0.1%, v/v) in acetone:olive oil (AOO; 4:1) on the dorsum of
each ear for each animal for three consecutive days. At 4 days-post
sensitization, 30 µl DNFB (0.2%, v/v) in AOO was applied
onto the dorsum of each ear every 2 days. The AST was dissolved in
AOO and then filtered using a syringe filter (0.45 nm; Millipore,
Billerica, MA, USA), prior to dilution in acetone. The AST was
dissolved in olive oil and was then filtered using a syringe filter
(0.45 nm), prior to dilution in acetone. The AST solution at a
final concentration of either 0.1 or 1 mg/ml, was applied to the
dorsum of each ear every 2 days. All animals, with the exception of
the naïve group, were sensitized and challenged with DNFB. The
animals in the naïve group were sensitized with vehicle, with
subsequent AOO application (n=6). The control animals (CTL group)
were sensitized and challenged with DNFB, followed by AOO
application (n=8). The AST0-treated animals were sensitized and
challenged with DNFB, followed by the application of either 0.1
mg/ml AST (0.1 AST group; n=8) or 1 mg/ml AST (1 AST group)
solution (n=8). The DEX-treated animals (DEX group) were sensitized
and challenged with DNFB, followed by application of 2.5 mg/ml
dexamethasone, and were used as a positive control. Sensitization
applications were performed on days 1, 2 and 3, and challenge
applications were performed on days 7, 9, 11 and 13. The naïve
group was treated with AOO in the same manner. In the AST and DEX
groups, the AST or DEX were applied on days 8, 10, 12 and 14. All
animals were sacrificed by cervical dislocation on day 15. The
experimental design is shown in Fig.
1.
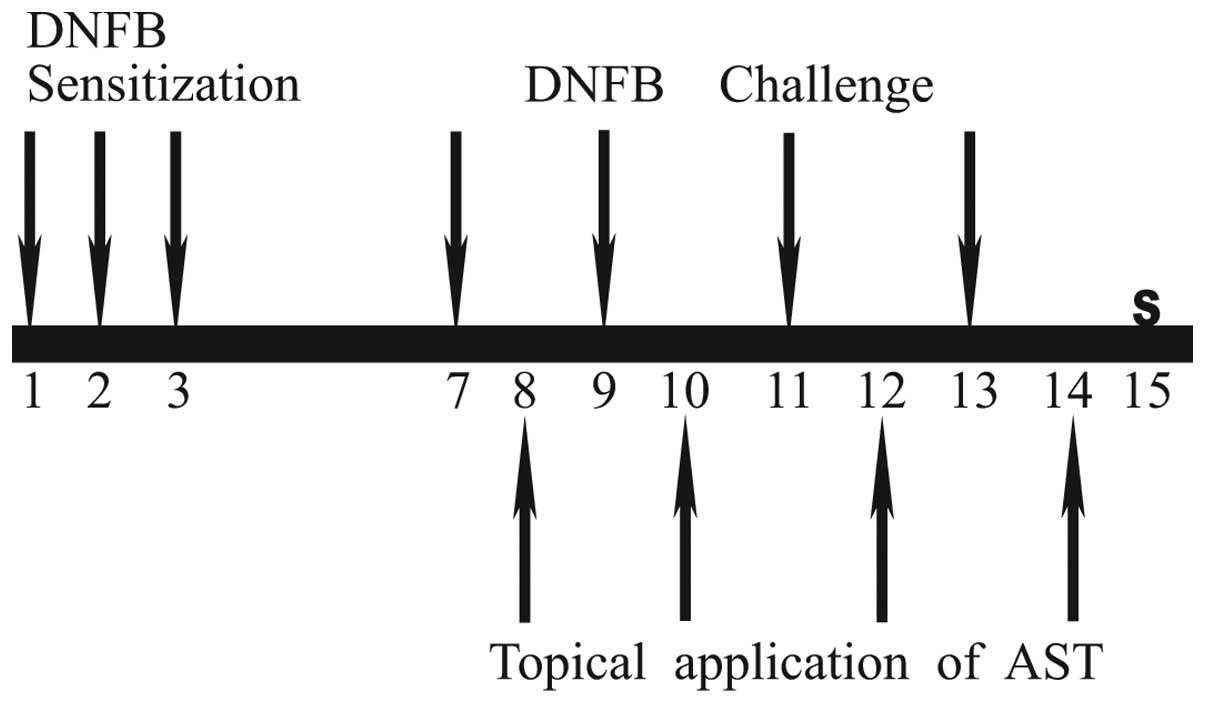 | Figure 1Experimental design for animal
investigations. The experimental groups, with the exception of the
naïve group, were sensitized by application of DNFB on days 1, 2
and 3. They were challenged on days 7, 9, 11 and 13. The naïve
group was treated with acetone:olive oil, in the same manner. In
the AST and DEX groups AST or DEX were applied on days 8, 10, 12
and 14. All animals were sacrificed (S) on day 15. DNFB,
dinitrofluorobenzene; AST, astaxanthinl; DEX, dexamethasone. |
Measurement of ear thicknesses and
weight
The mice were anesthetized with 30 mg/kg of zoletil
(Virbac, Carros, France), and the thicknesses of the right ear of
each mouse was measured using vernier calipers (Mitutoyo
Corporation, Tokyo, Japan) at the end of the experiment. The
weights of left ear pieces (5 mm in diameter), which were resected
by dermal punch (HB 925; Hebu Medical GmbH, Badstraße, G e r m a n
y) and were embedded in paraffin, were also measured.
Histopathological examination
Following measurement of the ear thickness and
weights, the ear tissues were resected and paraffin-embeded. The
sections were stained with hematoxylin and eosin (HE) for
histopathological observation, including the evaluation of immune
cell infiltration and spongiosis. Alcian blue staining was also
used to evaluate the distribution of mast cells and nuclear fast
red solution (Sigma-Alrich) was used for counter staining. The
stained tissues were observed using a light microscope.
Measurement of cytokine production
At the end of the experiment, the resected ear
tissues were lysed and homogenized with protein extraction solution
(Intron bio, Daejeon, Korea) using a bullet blender (Next advance,
Averill Park, N Y, USA), to obtain the tissue lysates. Subsequently
50 g of the lysates were used to measure the levels of IFN-γg and
TNF-α. The cytokine levels were measured using a cytometric bead
array kit (BD Biosciences). All experimental procedures were
performed according to the manufacturer's instructions.
Cell culture
The RBL-2H3 cells were purchased from the Korean
Cell Line Bank (Seoul, Korea) and grown in DMEM/high glucose,
supplemented with 10% FBS, 100 U/ml penicillin and 100 g/ml
streptomycin at 37°C in a humidified incubator under 5%
CO2.
Measurement of cell proliferation
The RBL-2H3 cells were plated at a density of
1×105 cells/well in a 96-well plate and were left to
reach ~70% confluence. AST (0-800 µg/ml) in complete DMEM
was added to each well and the wells were incubated for 2 h at
37°C. The effects of AST on cell viability were assessed using 10
µl PrestoBlue Cell Viability Reagent (cat. no. A13261;
Invitrogen Life Technologies) with 90 µl mixture (containing
the cells and medium) according to the manufacturer's instructions.
Cell viability was measured using a microplate reader (Infinite
M200; Tecan Group Ltd.).
Hexosaminidase release assay
The inhibitory effects of AST on the secretion of
β-hexosaminidase from the RBL-2H3 cells were measured using a
modification of the method previously described by Matsuda et
al (23). Briefly, the RBL-2H3
cells were plated at a density of 2×104 cells/well in a
96-well plate. The cells were incubated overnight for attachment,
and were treated with the indicated concentrations of AST for 1 h,
prior to stimulation with 50 nM PMA and 1 µM A23187 at 37°C
for 60 min. Following stimulation, 50 µl of each sample was
incubated with 50 µl 1 mM
p-nitrophenyl-N-acetyl-β-D-gl ucosaminide dissolved in 0.1 M
citrate buffer (pH 5.0) in a 96-well plate at 37°C for 1 h. The
reaction was terminated with 200 µl/well 0.1 M carbonate
buffer (pH 10.5). The absorbance at 405 nm was measured using a
microplate reader (Tecan 200 Pro, Tecan Group Ltd., Männedorf,
Switzerland). The percentage of inhibition of β-hexosaminidase
release was calculated using the following equation:
β-hexosaminidase release (%) = absorbance of supernatant /
(absorbance of supernatant + absorbance of pellet) x 100.
Histamine release assay
The RBL-2H3 cells were plated at a density of
2×104 cells/well in a 96-well plate. The cells were then
incubated overnight in a complete medium, followed by treatment for
1 h with the indicated concentrations of AST, prior to stimulation
with 50 nM PMA and 1 µM A23187 at 37°C for 30 min. The
histamine contents were measured using a histamine detection kit
(Oxford Biochemical Research), according to the manufacturer's
instructions.
Statistical analysis
All statistical comparisons were made using
Student's t-test. SigmaPlot version 11.0 (Systat software, Inc.,
San Jose, CA, USA) was used for statistical analysis. All data are
presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of AST on ear swelling induction
in CD mice
The repeated application of DNFB induced ear
swelling, which is a feature of CD. The effects of AST on ear
swelling were evaluated by measuring the ear thicknesses (Fig. 2A) and weights (Fig. 2B). In the CTL group, the ear
thickness and weights were increased significantly compared with
the naïve group. In the Dex group, the ear thickness and weights
were decreased markedly compared with the CTL group. However, when
different concentrations of AST were applied to the CD mice,
following repeated application of DNFB, the increases in the
thickness and weight of the mice were effectively reduced,
confirming that AST may decrease the inflammatory reactions.
Effects of AST on histopathological
changes of ear tissues in CD mice
In order to determine the histological changes of
the CD mice in response to AST, HE staining was used following DNFB
challenge and/or topical AST application to the mice. As shown in
Fig. 3, although no abnormal
changes were observed in the ear tissues from the naïve group
(Fig. 3A), the epidermises from
the tissues in the CTL group in Fig.
3B exhibited hyperplasia, severe edema and spongiosis. In
addition, a marked infiltration of mononuclear cells was observed
in the CTL group (Fig. 3B).
However, when the mice in the CTL group were treated with AST, a
reduction in the pathophysiological reactions of hyperplasia, edema
and spongiosis was observed in the ear tissues (Fig. 3C and D). The DEX group, as a
positive control, exhibited infiltration of immune cells (Fig. 3E). Therefore, AST may alleviate
inflammatory or allergic reaction in CD mice.
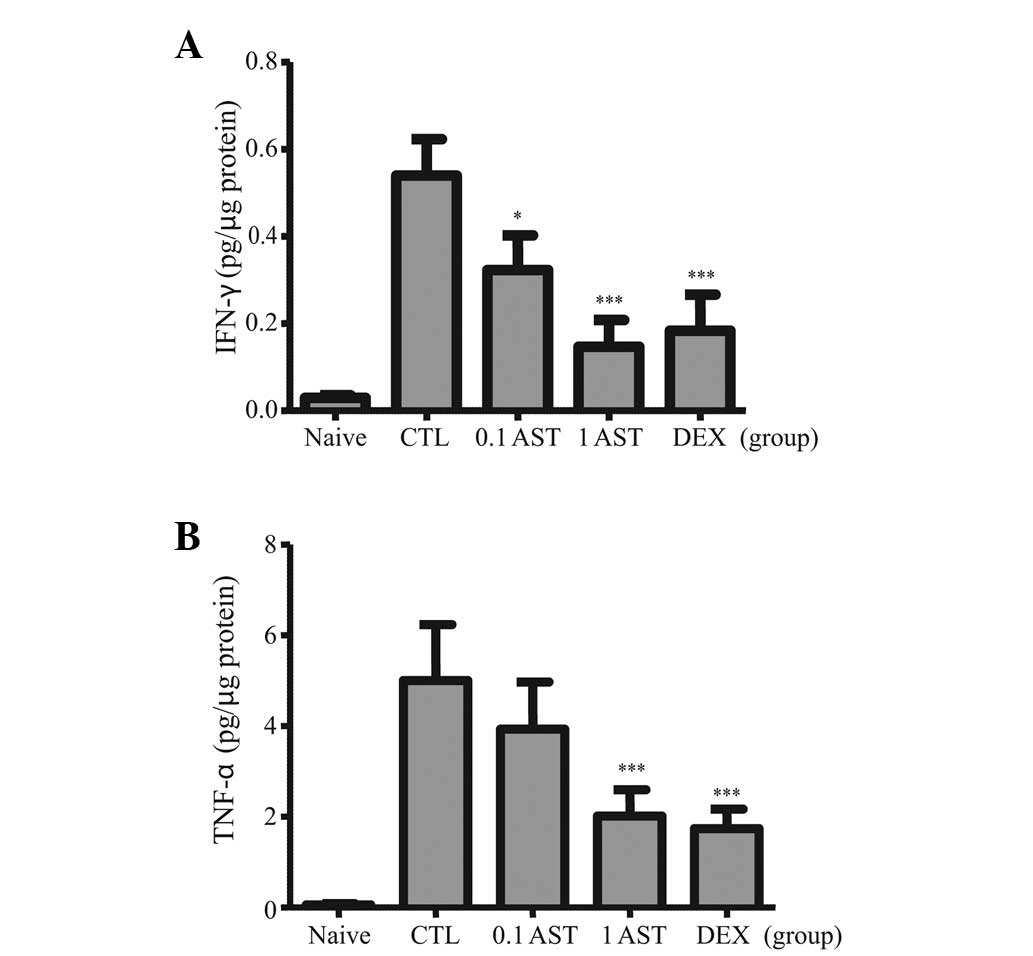
Effects of AST on the levels of IFN-γ and
TNF-α in CD mice
Pro-inflammatory cytokines, which induce
inflammatory reactions were assessed in CD mice, with or without
AST treatment. The repeated application of DNFB in the CTL groups
resulted in increased quantities of IFN-γ (Fig. 4A) and TNF-α (Fig. 4B) in the ear homogenates. However,
treatments with either 0.1 or 1 mg/ml AST significantly suppressed
the augmented level of IFN-γ in the CTL group. In addition,
treatment with 1 mg/ml AST significantly repressed the augmented
level of TNF-α (1 AST group in Fig.
4B, whereas, treatment with 0.1 mg/ml AST repressed the
augmented level of TNF-α, but without statistical significance (0.1
AST group in Fig. 4B). Notably, as
shown in Fig. 4, the levels of
IFN-γ and TNF-α in the 1 AST group exhibited a similar pattern as
the DEX group. Thus, these data suggested that AST negatively
regulated the IFN-γ and TNF-α pro-inflammatory cytokines in the CD
mice.
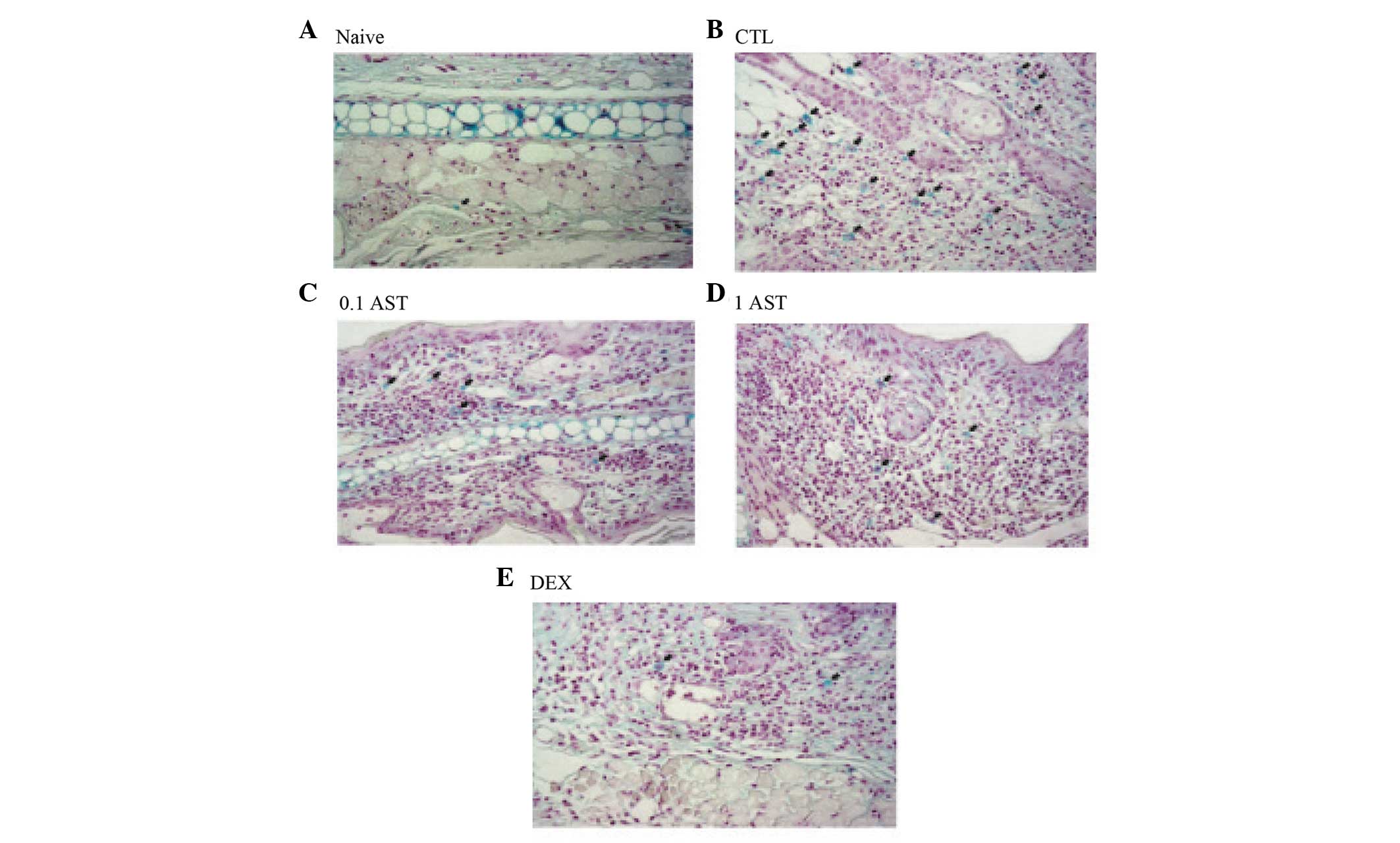
Effects of AST on the density of mast
cells in CD mice
Since mast cells are found at sites of CD (6), the present study evaluated them in CD
mice (Fig. 5). In the naïve group,
few mast cells were observed (Fig.
5A). In the CTL group, a marked increase in the number of mast
cells against DNFB (filled arrow in Fig. 5B) was observed in the mice.
Treatments with 0.1 and 1 mg/ml AST to the mice sensitized to DNFB
decreased the density of mast cells compared with the CTL group
(Fig. 5C and D). Few mast cells
were observed in the DEX group (Fig.
5E). Therefore, AST decreased the density of mast cells in the
CD mice, indicating that AST exhibited anti-allergic or
inflammatory activities.
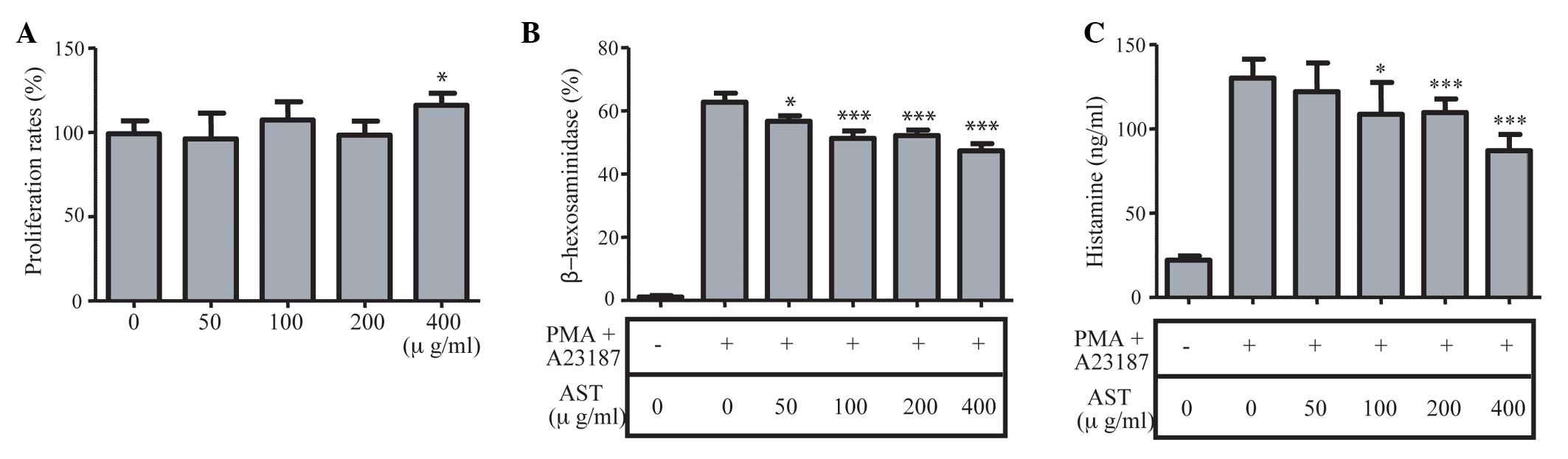
Effects of AST on the degranulation of
RBL-2H3 cells
Since the degranulation of mast cells causes
allergic or inflammatory reactions (9), the present study examined the
secretion of β-hexosaminidase and histamine in the RBL-2H3 cells,
following stimulation of the cells by PMA and ionophore A23187 to
induce inflammation in vitro. As the combination of PMA and
A23187 activate cell proliferation to cause inflammation, the
present study examined the role of AST in the proliferation of
cells. As shown in Fig. 6A,
although treatment with 400 g/ml AST marginally elevated the
proliferation rates of the RBL-2H3 cells, AST did not affect cell
proliferation (Fig. 6A). The
levels of β-hexosaminidase were measured, as shown in Fig. 6B. Pretreatment with >50 g/ml AST
reduced the levels of β-hexosaminidase in a dose-dependent manner
(Fig. 6B). Similarly, pretreatment
with>100 µg/ml AST also reduced the levels of histamine
(Fig. 6C). Therefore, the
degranulation of mast cells released β-hexosaminidase and histamine
from the RBL-2H3 cells, and AST had an inhibitory role in their
production in vitro.
Discussion
Physiological stress, air pollution, exposure to
chemicals or exposure to ultraviolet (UV) light can enhances the
productions of free radicals and highly reactive forms of oxygen
(24). Oxidative damage has been
closely linked to skin diseases, including CD. Reactive oxygen
species are involved in the pathogenesis of allergic reactions in
the skin and trigger the induction and maintenance of cutaneous
inflammation (24).
As AST is a potent antioxidant, it has important
applications in the nutraceutical, cosmetics and food industries.
It exhibits marked antioxidant activity, suggesting its potential
in targeting a number of health conditions (25). AST has several essential biological
functions, including antioxidative action against lipid
peroxidation, protection against damage by UV light and reduction
of the immune response (26).
The present study demonstrated anti-allergic and
anti-inflammatory actions of AST in vitro and in
vivo, respectively. The topical application of AST was found to
suppress allergic reactions in a mouse model of DNFB-induced
allergic CD. The increases in ear thickness and weight were
prevented by the application of AST (Fig. 2). Inflammatory hyperplasia, edema
and spongiosis are recognized as the microscopic hallmark of
inflammatory skin disease, including allergic contact and nummular
dermatitis (27). In the present
study, the topical application of AST effectively reduced
inflammatory hyperplasia, epidermal spongosis, edema and
mononuclear cell infiltration (Fig.
3). These results suggested that epidermal spongiosis, edema
and inflammatory cell infiltration resulted in enlargement of ear
thickness and weight, and that AST prevented these inflammatory
reactions effectively in DNFB-induced CD.
Repeated application of DNFB induces T helper (Th)1
skewing inflammation in the skin (28). IFN-γ, the hallmark of the Th 1
skewing reaction of T cell, is responsible for the increased
production of various cytokines and chemokines in the skin,
including interleukin-1, TNF-α, granulocyte macrophage
colony-stimulating factor and macrophage inflammatory protein
(MIP)-2, which results in marked infiltration of leukocytes
(29). Skin contact with a
specific allergen induces the release of TNF-α, another Th1
cytokine, during the sensitization phase (1). Furthermore, TNF-α exerts a
stimulatory effect on skin cells, resulting in the recruitment of
leukocytes during the elicitation of a contact hypersensitivity
response (30). In the present
study, AST treatment effectively reduced the production levels of
IFN-γ and TNF-α in the inflammatory tissues (Fig. 4). These data suggested AST as an
anti-inflammatory agent against the Th 1 skewing reaction,
resulting in a reduction of inflammatory reactions, including
hyperplasia and spongiosis and immune cell infiltration.
Mast cells are important in adaptive and innate
immunity, and their functions in skin immunity have been reported
(31). Several studies have
demonstrated an increase in the density of mast cells at
inflammatory sites, and they are noted to undergo degranulation to
produce small-molecule chemical mediators, including histamine and
β-hexosaminidase (8,32). Mast cells affect the functions of
keratinocytes and fibroblasts through the release of TNF-α or
histamine, which act on keratinocytes and promotes their production
of adhesion molecules, proinflammatory cytokines, chemokines and
growth factors (33). Mast cells
and their mediators, particularly histamine, induce the activation
and proliferation of fibroblasts (33,34).
In addition, mast cells recruit neutrophils during T cell-mediated
delayed-type hypersensitivity reactions through TNF-α and MIP-2
(35). In the present study, AST
effectively reduced the density of mast cells and mononuclear cells
(Figs. 3 and 5). In addition, treatment with AST
significantly inhibited the release of histamine and
β-hexosaminidase from the RBL-2H3 cells in vitro (Fig. 6). These data suggested that AST may
contribute to alleviate ear swelling and hyperplasia due to its
suppression of inflammatory reactions, including the infiltration
of neutrophils, activation of keratinoctyes and proliferation of
fibroblasts.
Taken together, the present study demonstrated the
anti-inflammatory and anti-allergic actions of AST, in terms of
histopathologic change, mediator release and cytokine production.
These results suggested that AST has similar effects to the
dexamethasone in restricting the Th1 skewing reaction, and that AST
may be used to treat patients with CD, particularly in patients
exhibiting side effects caused by steroid treatment.
Acknowledgments
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea,
funded by the Ministry of Education (grant no. 2011-0024355); a
grant from the New Growth Engine Industry Division, Busan
Metropolitan City (grant no. 2010-2012; and the Aquaculture
Industry Division funded grant by the National Fisheries Research
and Development Institute, Gangneung, Korea (grant no.
RP-14-AQ-62). Dr Seong-A Ju was additionally supported by the
Priority Research Center Program (2009-0094050) through the
National Research Foundation of Korea.
References
|
1
|
Saint-Mezard P, Rosieres A, Krasteva M, et
al: Allergic contact dermatitis. Eur J Dermatol. 14:284–295.
2004.PubMed/NCBI
|
|
2
|
Cavani A and De Luca A: Allergic contact
dermatitis: novel mechanisms and therapeutic perspectives. Curr
Drug Metab. 11:228–233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kapsenberg ML, Wierenga EA, Stiekema FE,
Tiggelman AM and Bos JD: Th1 lymphokine production profiles of
nickel-specific CD4+ T-lymphocyte clones from nickel contact
allergic and non-allergic individuals. J Invest Dermatol. 98:59–63.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimada Y, Hasegawa M, Kaburagi Y, et al:
Lselectin or ICAM-1 deficiency reduces an immediate-type
hypersensitivity response by preventing mast cell recruitment in
repeated elicitation of contact hypersensitivity. J Immunol.
170:4325–4334. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Natsuaki M, Yano N, Yamaya K and Kitano Y:
Immediate contact hypersensitivity induced by repeated hapten
challenge in mice. Contact Dermatitis. 43:267–272. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Askenase P, Van Loveren H, Kraeuter-Kops
S, et al: Defective elicitation of delayed-type hypersensitivity in
W/Wv and SI/SId mast cell-deficient mice. J Immunol. 131:2687–2694.
1983.PubMed/NCBI
|
|
7
|
Dudeck A, Dudeck J, Scholten J, et al:
Mast cells are key promoters of contact allergy that mediate the
adjuvant effects of haptens. Immunity. 34:973–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Theoharides TC, Alysandratos KD, Angelidou
A, et al: Mast cells and inflammation. Biochim Biophys Acta.
1822:21–33. 2012. View Article : Google Scholar :
|
|
9
|
Broide DH: Molecular and cellular
mechanisms of allergic disease. J Allergy Clin Immunol.
108:S65–S71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fuchs J, Zollner TM, Kaufmann R and Podda
M: Redox-modulated pathways in inflammatory skin diseases. Free
Radic Biol Med. 30:337–353. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sharkey P, Eedy DJ, Burrows D, McCaigue MD
and Bell AL: A possible role for superoxide production in the
pathogenesis of contact dermatitis. Acta Derm Venereol. 71:156–159.
1991.PubMed/NCBI
|
|
12
|
Finnen MJ, Lawrence CM and Shuster S:
Inhibition of dithranol inflammation by free radical scavengers.
Lancet. 217:1129–1130. 1984. View Article : Google Scholar
|
|
13
|
Senaldi G, Pointaire P, Piguet PF and Grau
GE: Protective effect of N-acetylcysteine in hapten-induced
irritant and contact hypersensitivity reactions. J Invest Dermatol.
102:934–937. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sur R, Nigam A, Grote D, Liebel F and
Southall MD: Avenanthramides, polyphenols from oats, exhibit
anti-inflammatory and anti-itch activity. Arch Dermatol Res.
300:569–574. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakai S, Sugawara T and Hirata T:
Inhibitory effect of dietary carotenoids on
dinitrofluorobenzene-induced contact hypersensitivity in mice.
Biosci Biotechnol Biochem. 75:1013–1015. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fassett RG and Coombes JS: Astaxanthin: a
potential therapeutic agent in cardiovascular disease. Mar Drugs.
9:447–465. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Campoio TR, Oliveira FA and Otton R:
Oxidative stress in human lymphocytes treated with fatty acid
mixture: role of carotenoid astaxanthin. Toxicol In Vitro.
25:1448–1456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yasui Y, Hosokawa M, Mikami N, Miyashita K
and Tanaka T: Dietary astaxanthin inhibits colitis and
colitis-associated colon carcinogenesis in mice via modulation of
the inflammatory cytokines. Chem Biol Interact. 193:79–87. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suzuki Y, Ohgami K, Shiratori K, et al:
Suppressive effects of astaxanthin against rat endotoxin-induced
uveitis by inhibiting the NF-kappaB signaling pathway. Exp Eye Res.
82:275–281. 2006. View Article : Google Scholar
|
|
20
|
Izumi-Nagai K, Nagai N, Ohgami K, et al:
Inhibition of choroidal neovascularization with an
anti-inflammatory carotenoid astaxanthin. Invest Ophthalmol Vis
Sci. 49:1679–1685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Starr RC and Zeikus JA: UTEX - The culture
collection of algae at the University of Texas at Austin. J Phycol.
29(Suppl): 1–106. 1993. View Article : Google Scholar
|
|
22
|
Kwak TW, Cha JY, Lee CW, Kim YM, Yoo BH,
Kim SG, Kim JM, Park SH and An WG: Anti-inflammatory and
antioxidant effect of astaxanthin derived from microalgae. J Life
Sci. 21:1377–1384. 2011.In Korean. View Article : Google Scholar
|
|
23
|
Matsuda H, Morikawa T, Managi H and
Yoshikawa M: Antiallergic principles from Alpinia galanga:
Structural requirements of phenylpropanoids for inhibition of
degranulation and release of TNF-alpha and IL-4 in RBL-2H3 cells.
Bioorg Med Chem Lett. 13:3197–3202. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bickers DR and Athar M: Oxidative stress
in the pathogenesis of skin disease. J Invest Dermatol.
126:2565–2575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guerin M, Huntley ME and Olaizola M:
Haematococcus astaxanthin: applications for human health and
nutrition. Trends Biotechnol. 21:210–216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lorenz RT and Cysewski GR: Commercial
potential for Haematococcus microalgae as a natural source of
astaxanthin. Trends Biotechnol. 18:160–167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Machado-Pinto J, McCalmont T and Golitz L:
Eosinophilic and neutrophilic spongiosis: clues to the diagnosis of
immunobullous diseases and other inflammatory disorders. Semin
Cutan Med Surg. 15:308–316. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dearman RJ, Basketter DA and Kimber I:
Characterization of chemical allergens as a function of divergent
cytokine secretion profiles induced in mice. Toxicol Appl
Pharmacol. 138:308–316. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kobayashi Y: The role of chemokines in
neutrophil biology. Front Biosci. 13:2400–2407. 2008. View Article : Google Scholar
|
|
30
|
Grabbe S and Schwarz T: Immunoregulatory
mechanisms involved in elicitation of allergic contact
hypersensitivity. Immunol Today. 19:37–44. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gould HJ, Sutton BJ, Beavil AJ, et al: The
biology of IgE and the basis of allergic disease. Annu Rev Immunol.
21:579–628. 2003. View Article : Google Scholar
|
|
32
|
Navi D, Saegusa J and Liu FT: Mast cells
and immunological skin diseases. Clin Rev Allergy Immunol.
33:144–155. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kanda N and Watanabe S: Histamine enhances
the production of granulocyte-macrophage colony-stimulating factor
via protein kinase Calpha and extracellular signal-regulated kinase
in human keratinocytes. J Invest Dermatol. 122:863–872. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jordana M, Befus A, Newhouse M,
Bienenstock J and Gauldie J: Effect of histamine on proliferation
of normal human adult lung fibroblasts. Thorax. 43:552–558. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Biedermann T, Kneilling M, Mailhammer R,
et al: Mast cells control neutrophil recruitment during T
cell-mediated delayed-type hypersensitivity reactions through tumor
necrosis factor and macrophage inflammatory protein 2. J Exp Med.
192:1441–1452. 2000. View Article : Google Scholar : PubMed/NCBI
|