Introduction
Autoimmune hepatitis (AIH) is a type of chronic,
autoimmune inflammation of the liver, which is characterized by
histological interface hepatitis, hypergammaglobulinemia and the
production of autoantibodies (1).
During AIH, the self-tolerance, also termed homeostatic processes,
is defective, resulting in dysfunction of T-lymphocytes, including
over-activated CD4 and CD8 T-cells, which mediate autoimmune liver
injury (2). Previous evidence has
indicated that immune regulatory cells, including regulatory
T-cells (3) and natural killer T
cells (4) are critical for the
maintenance of immune tolerance in AIH. Another subset of
regulatory cells, termed myeloid-derived suppressor cells (MDSCs)
have identified to possess a marked ability to suppress T-cell
responses, therefore, representing an attractive candidate for
immune intervention to reconstitute self-tolerance in autoimmune
conditions (5).
MDSCs are a heterogeneous population of immature
myeloid cells, derived from dendritic cell (DC) progenitors,
macrophages and granulocytes in cancer, inflammation and autoimmune
disease, which have been defined as CD11b (Mac-1) and Gr-1 for
granulocytic MDSCs and CD11b, Gr-1, CD115 and F4/80 for monocytic
MDSCs (6). These exist in an
activated state, which is characterized by the increased production
of reactive oxygen species (ROS), reactive nitrogen species (RNS)
and arginase-1. They are potent suppressors of T-cell functions,
using arginase-1 and inducible nitric oxide synthase (iNOS)
(7). In humans, the identification
of MDSCs is indistinct due to the absence of specific markers
therefore, their phenotype is established, according to their
functional definition (8).
Generation and accumulation of MDSCs in peripheral blood and
regional tissues in response to tumor, infection or trauma is
associated with multiple growth factors and cytokines, including
granulocyte macrophage colony-stimulating factor, vascular
endothelial growth factor, interleukin (IL)-1β, IL-6, stem cell
factor, cyclooxygenase-2 (prostaglandin-endoperoxide synthase 2),
interferon (IFN)-γ, IL-4, IL-13 and transforming growth factor
(TGF)-β, which induce MDSCs generation and promoted MDSCs
activation (9).
In autoimmune diseases, including inflammatory bowel
disease, experimental autoimmune encephalomyelitis and experimental
autoimmune uveoretinitis, the frequency of human MDSCs with
suppressive function are increased in peripheral blood from
patients and rodents, which suggests a novel MDSC-mediated immune
regulatory pathway in autoimmune diseases (10). The characterization of MDSCs during
AIH remains to be elucidated. The present study investigated the
frequency and function of MDSCs in AIH, and further analyzed the
clinical relevance of MDSC frequency with disease status in
patients with AIH.
Subjects and methods
Subjects and healthy donors
A total of 48 peripheral blood and three liver
samples were obtained from patients with AIH between October 2009
and October 2010 at The Third Affiliated Hospital of Anhui Medical
University (Hefei, China). AIH was diagnosed, according to the
International Autoimmune Hepatitis Group diagnostic scoring system
in 1999 (total score >17) (11). A number of the patients were
diagnosed, according to pathology (Table I). None of the patients received
treatment with glucocorticoids or immunosuppres-sants, and 15
patients with AIH were treated with Prednisone for 6 months.
Prednisone alone was used to achieve a clinical remission, at an
initial dose of 40 mg/day for 4 weeks, followed by 10 mg/day for
maintenance. In addition, 24 peripheral blood samples and two liver
tissue samples were obtained from healthy individuals, as a normal
control. Tissue samples were obtained by liver biopsy. No
statistically significant differences were observed between the two
groups in age or gender ratio (P>0.05). The present study was
approved by the Ethics Committee of The Third Affiliated Hospital
of Anhui Medical University, and written informed consent was
obtained from each subject. Table
I shows the clinical characteristics of the patients with AIH,
who were involved in the present study.
 | Table IClinical characteristics of the
patients and healthy subjects recruited. |
Table I
Clinical characteristics of the
patients and healthy subjects recruited.
| Group | AIH (n=48) | LC (n=15) | No-LC (n=33) | HC |
|---|
| Age (years) | 45.6±8.9 | 52.3±6.5 | 41.8±5.7 | 41.2±3.6 |
| Gender M/F | 15/33 | 6/9 | 9/24 | 7/17 |
| IAH scoring | >17 | >17 | >17 | ND |
| ALT (U/l) | 228.81±275.99 | 169.67±130.34 | 255.69±279.82 | ND |
| AST (U/l) | 284.93±198.56 | 219.33±279.89 | 314.76±272.06 | ND |
| CHE (U/l) |
5267.91±2038.02 |
4473.09±1669.718 |
5629.185±1988.06 | ND |
| TBIL
(µmol/l) | 59.00±74.79 | 48.62±85.12 | 63.72±84.07 | ND |
| DBIL
(µmol/l) | 32.59±47.08 | 29.26±19.11 | 34.10±53.96 | ND |
| IgG (g/l) | 32.53±6.61 | 33.52±6.81 | 32.08±6.58 | ND |
| PA (%) | 82.03±21.52 | 77.53±19.09 | 84.07±23.01 | ND |
| MDSCs (%) | 1.76±1.22 | 0.70±0.42 | 2.23±1.16 | 0.91 |
Antibodies
Fluorescein isothiocyanate (FITC)-conjugated Lin1
(cat. no. 340546; 20 µl), mouse anti-human leukocyte antigen
(HLA)-DR, peridin chlorophyl protein (PerCP)-conjugated mouse
anti-HLA-DR (cat. no. 552764; 20 µl), mouse anti-CD8 (cat.
no. 561967; dilution, 0.2 mg/ml), allophycocyanin (APC)-conjugated
mouse anti-CD11b (cat. no. 553312; dilution, 0.2 mg/ml), anti-CD3
and mouse anti-CD4 (cat. no. 340443; dilution, 6 µg/ml) and
mouse phycoerthrin (PE)-conjugated anti-CD33 (cat. no. 555450; 20
µl) antibodies were purchased from Becton Dickinson (San
Diego, CA, USA). Mouse anti-CD3 (cat. no. SAB4700040; dilution, 1
mg/ml) and mouse anti-CD28 (cat. no. SAB4700739; dilution, 1 mg/ml)
monoclonal antibodies were purchased from Sigma-Aldrich (St. Louis,
MO, USA).
Flow cytometric analysis
Cell surface staining was performed, according to
established methods (12). A total
of 1–3×105 cells/condition were incubated for 30 min at
4°C with different combinations of the following antibodies:
(FITC)-Lin1 (20 µl/test), (PerCP)-HLA-DR (20
µl/test), (APC)-CD11b (10 µl/test) and (PE)-CD33 (20
µl/test). Following two washes with 1 ml phosphate buffered
saline (PBS), the cells were resuspended in 100 µl 1%
paraformalde-hyde (Beijing Ding Guo Chang Sheng Biotechnology Co.,
Ltd., Beijing, China) and analyzed using a FACSCalibur (Becton
Dickinson). The data were analyzed using FlowJo v.5.7.2 software
(Tree Star, Ashland, Oregon, USA).
Cell isolation and sorting
Peripheral blood mononuclear cells (PBMCs) were
isolated using Ficoll-Hypaque density gradient centrifugation
(IAE-1) at 2,700 x g for 20 min at 22°C from heparinized blood. The
Lin1-/low HLA-DR- CD33+ CD11b+ MDSCs were
isolated from the PBMCs using Lin1 and HLA-DR negative selection
(Miltenyi Biotech, Bergisch Gladbach, Germany), followed by
analysis using a BD FACS Aria (Becton Dickinson). The purity of the
MDSCs was >85%. The CD3+ T cells were separated from the PBMCs
via CD3-positive selection using a MidiMACS separator unit,
according to the manufacturer's instructions (Miltenyi Biotech,).
The purity of the CD3+ T cells was >96%.
Proliferation assay and the detection of
cytokines
Carboxylfluorescein succinimidyl ester (CFSE)
labeling was used to detect the proliferation of CD3+ T
cells. Briefly, 1.3×106 CD3+ T cells were co-cultured
with MDSCs at ratios of 1:0, 1:1, 3:1 and 10:1 in RPMI-1640 medium,
supplemented with 10% fetal bovine serum (FBS; Gibco Life
Technologies, Carlsbad, CA, USA) and stimulated with anti-CD3 and
CD28 monoclonal antibodies (Sigma-Aldrich). All CD3+ T cells were
seeded into a 96-well plate (Costar, Lowell, MA, USA) in the
presence or absence of MDSCs, as mentioned above. The cells were
harvested following culturing for 5 days and stained with
anti-CD4-APC and anti-CD8-PerCp. As the CFSE signal was diluted
with each cell division, cells exhibiting low fluorescence
intensity of CFSE were considered to have proliferated. IFN-γ in
the supernatant was measured using an ELISA kit (RapidBio
Laboratory, Calabasas, CA, USA), according to the manufacturer's
instructions.
Apoptosis assay
The CD3+ T cells were co-cultured with MDSCs at the
ratios mentioned above and treated with anti-CD3 and CD28
monoclonal antibodies. Following incubation for 48 h, the cells
were collected and stained with annexin-V-FITC, 7-amino-actinomycin
D (7-AAD) (eBioscience, San Diego, CA, USA), anti-CD4-APC and
anti-CD8-PerCp to analyze the apoptosis of the CD4 and CD8
cells.
Expression of iNOS and determination of
NO
To confirm the suppression of MDSCs, iNOS were first
detected on the surface of MDSCs from the patients and healthy
subjects using anti-iNOS (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA). The NO content of the supernatant was subsequently
determined using an ELISA kit (Rapidbio Lab, CA).
Immunohistochemistry was also performed to detect the expression
levels of iNOS in the liver tissues and MDSCs. The expression of
iNOS was detected using a rabbit-anti-iNOS antibody (cat. no.
ab92765; Abcam, Cambridge, UK) in lymphocyte markers on the liver
tissue and PBMCs were detected using an immunohistochemical kit
(Zhongshan Golden Bridge Co., Beijing, China).
Statistical analysis
All statistical analyses were performed using SPSS
13.0 software for Windows (SPSS, Inc., Chicago, IL, USA).
Comparisons between different groups were analyzed using a
Mann-Whitney U test. A Wilcoxon matched-pairs signed ranks test was
used to compare the data from the same individuals. Correlation
analysis was performed using the Spearman rank correlation test.
All data are presented as the mean ± standard deviation. P<0.05
was considered to indicate a statistically significant
difference.
Results
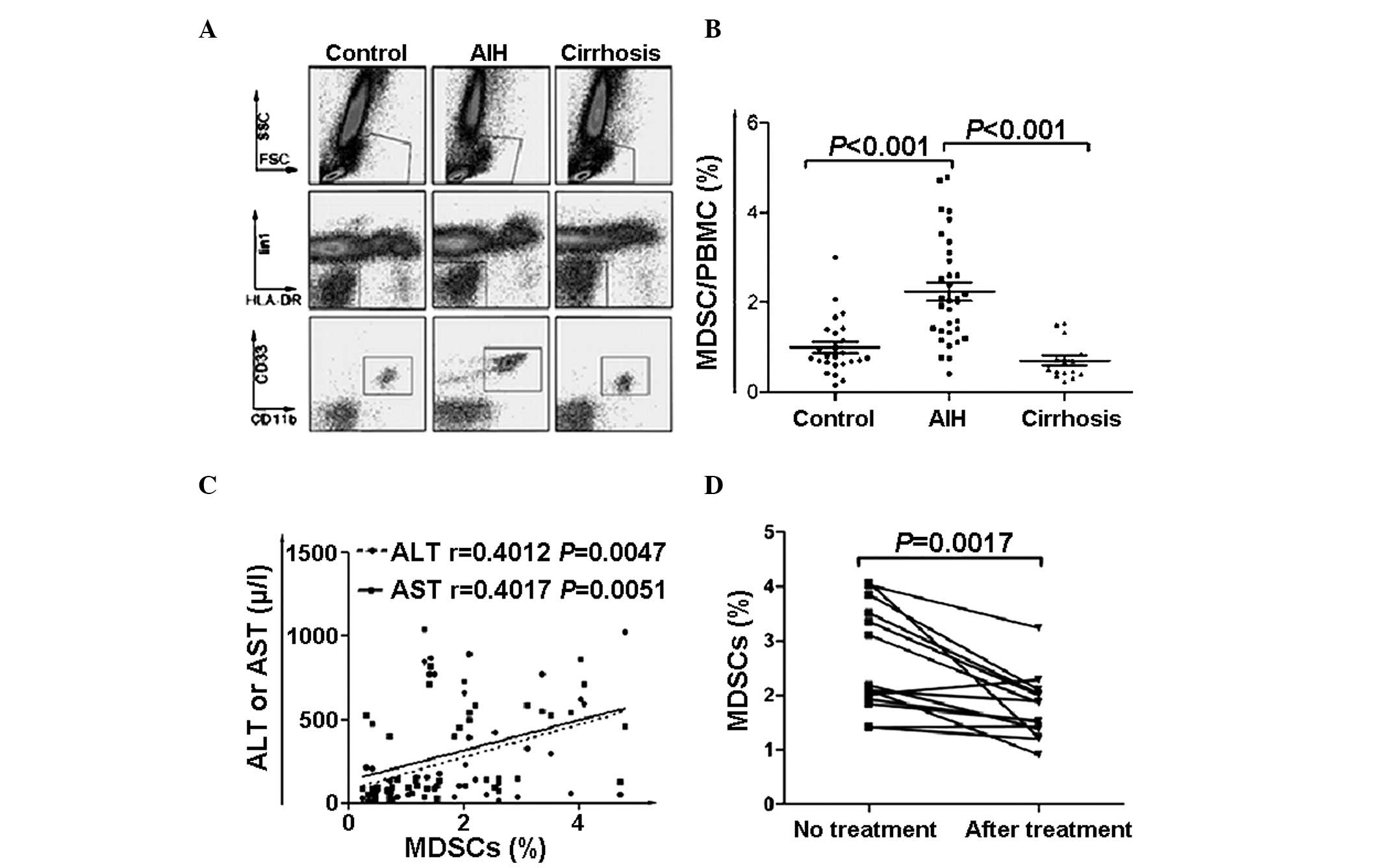
Increased frequency of MDSCs
(Lin1−/low HLA-DR− CD33+
CD11b+) in peripheral blood from patients with AIH
Due to the heterogeneity of MDSCs in humans, mature
lymphocytes and HLA-DR+ groups were excluded from the
present study, with CD33+ was reserved as a myeloid
marker and CD11b as its functional marker. Accordingly, MDSCs were
defined as the population of CD33+ CD11b+
Lin1− HLA-DR− (Fig. 1A) cells. The MDSCs frequency in
PBMCs was determined in samples from patients diagnosed with AIH
without cirrhosis, AIH with cirrhosis, and healthy subjects. There
was a significantly higher frequency of circulating MDSCs in the
AIH patients without cirrhosis compared with the AIH patients with
cirrhosis and the healthy subjects (P=0.012). No significant
difference was observed between the healthy subjects and the AIH
patients with cirrhosis (Fig. 1B;
PControl:AIH<0.001,
PAIH:Cirrhosis<0.001).
Frequency of MDSCs in the peripheral
blood correlates with clinical features
A significant positive correlation was observed
between the MDSC frequency and the levels of ALT/AST (Fig. 1C), which suggested that the
frequency of MDSCs was associated with liver inflammation. However,
other clinical indicators, including total bilirubin, direct
bilirubin, cholinesterase, alkaline phosphatase, immuno-globulin G
and prealbumin, revealed no correlation with the frequency of MDSCs
(P>0.05). In order to determine the effect of hormones on the
frequency of MDSCs, 15 patients receiving hormone therapy were
included. After 6 months of treatment, the majority of these
patients were in remission, accompanied with a reduced frequency of
MDSCs (Fig. 1D; P=0.0017).
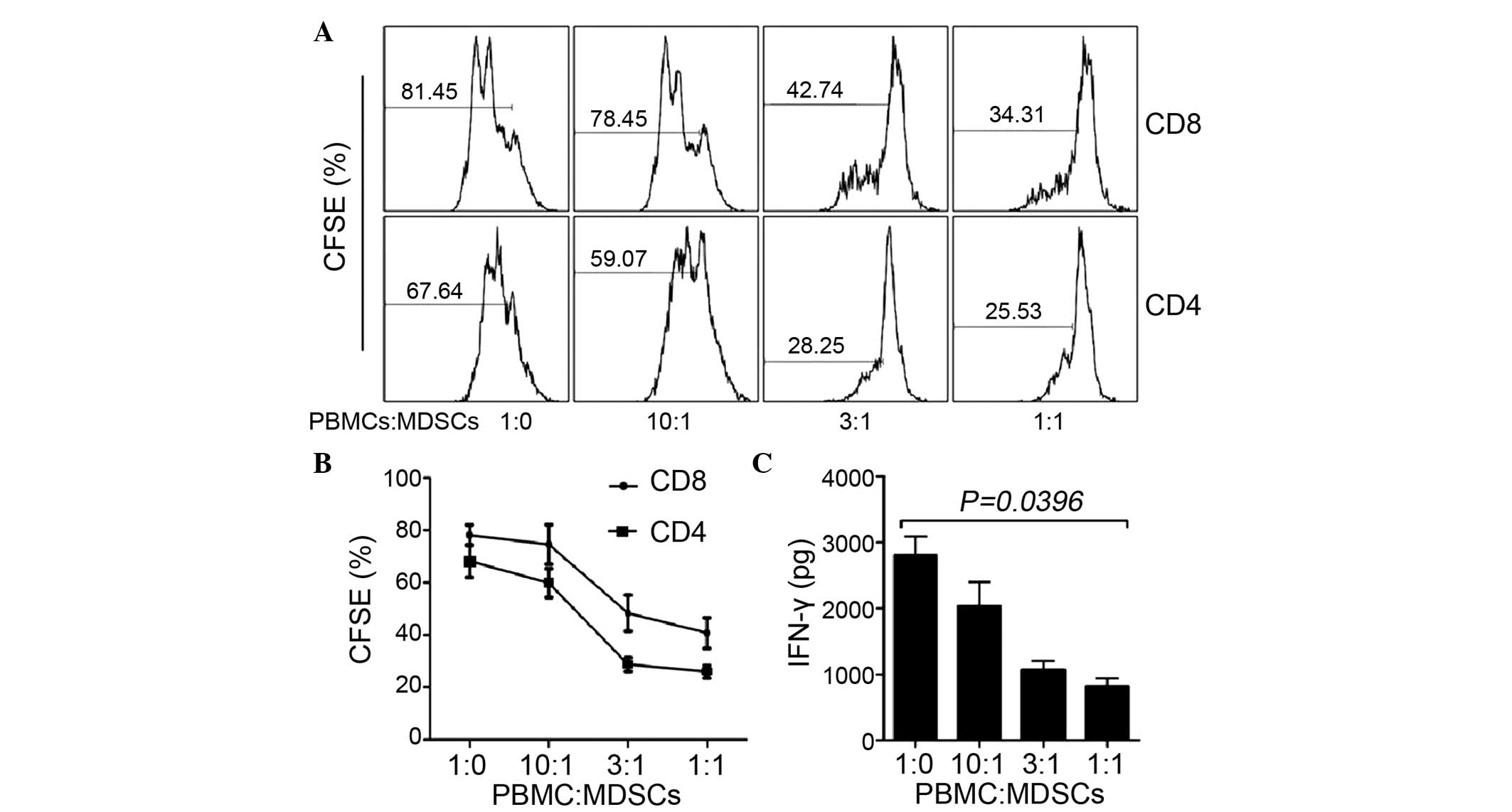
MDSCs inhibit the proliferation of T
cells in vitro
To investigate the effects of MDSC on T cells, MDSCs
were sorted and subsequently co-cultured with CFSE-labeled
CD3+ T cells at different ratios. Following culture for
5 days, the proliferation of the CD4 and CD8 T cells were detected
using flow cytometry. As the cell density of the MDSCs increased,
the proliferation of CD4 and CD8 T cells was inhibited (Fig. 2A and B). In addition, the present
study examined the capacity of MDSCs to inhibit cytokine secretion.
An ELISA was performed to detect IFN-γ in the culture supernatant.
The results demonstrated that the concentration of IFN-γ decreased
with the increase in MDSCs (Fig.
2C; PALT=0.047, PAST=0.051), which
suggested that the MDSCs inhibited cytokine secretion in a
dose-dependent manner.
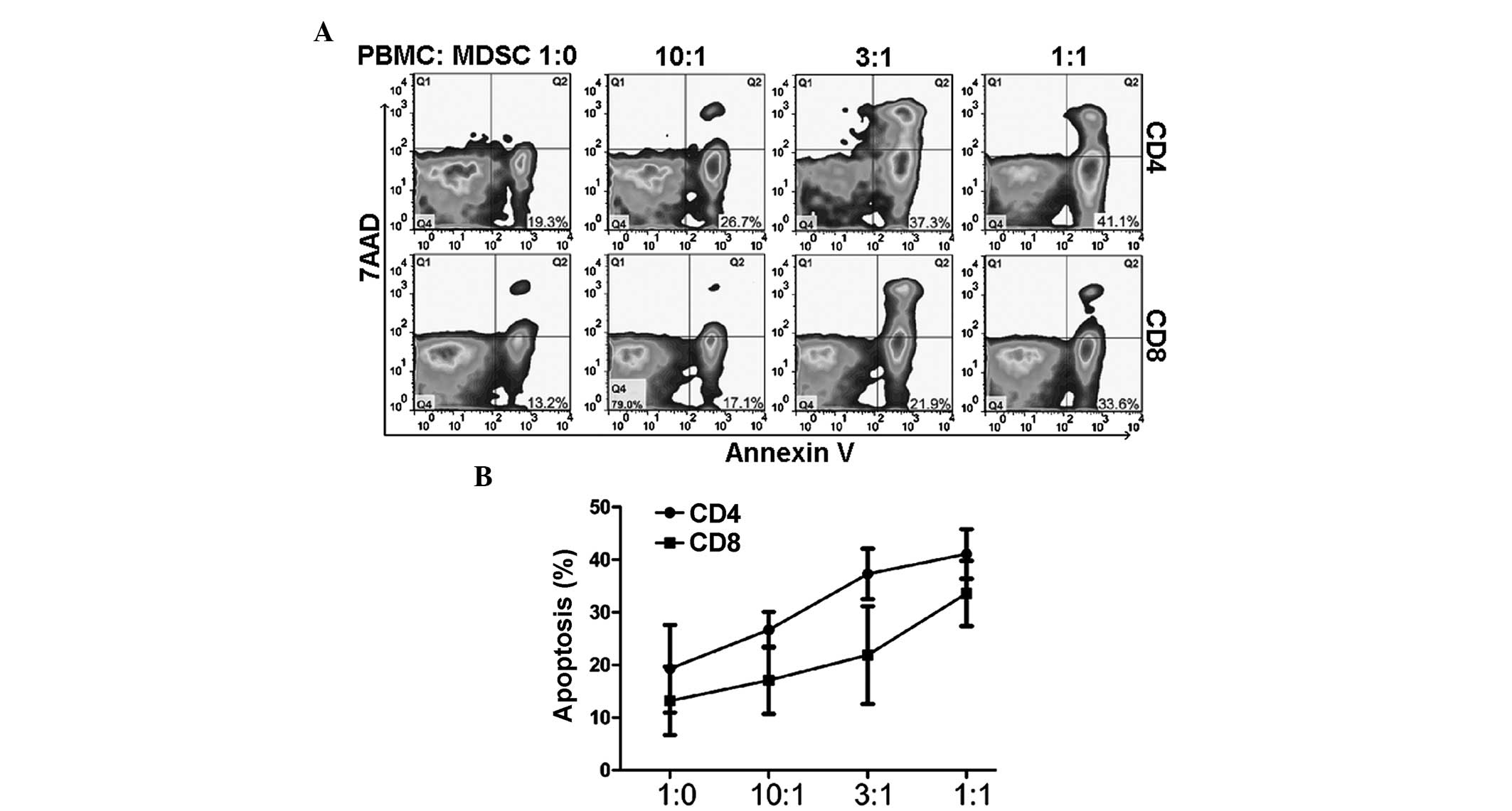
MDSCs promote T cell apoptosis in
vitro
Apoptosis detection was performed using annexin V
and 7AAD staining. (Fig. 3). The
sorted MDSCs were cocultured with PBMC, and the apoptosis of CD4
and CD8 T cells was detected. The results revealed that MDSCs
promoted CD4+ and CD8+ T cell apoptosis in a
dose-dependent manner (Fig. 3;
P=0.018).
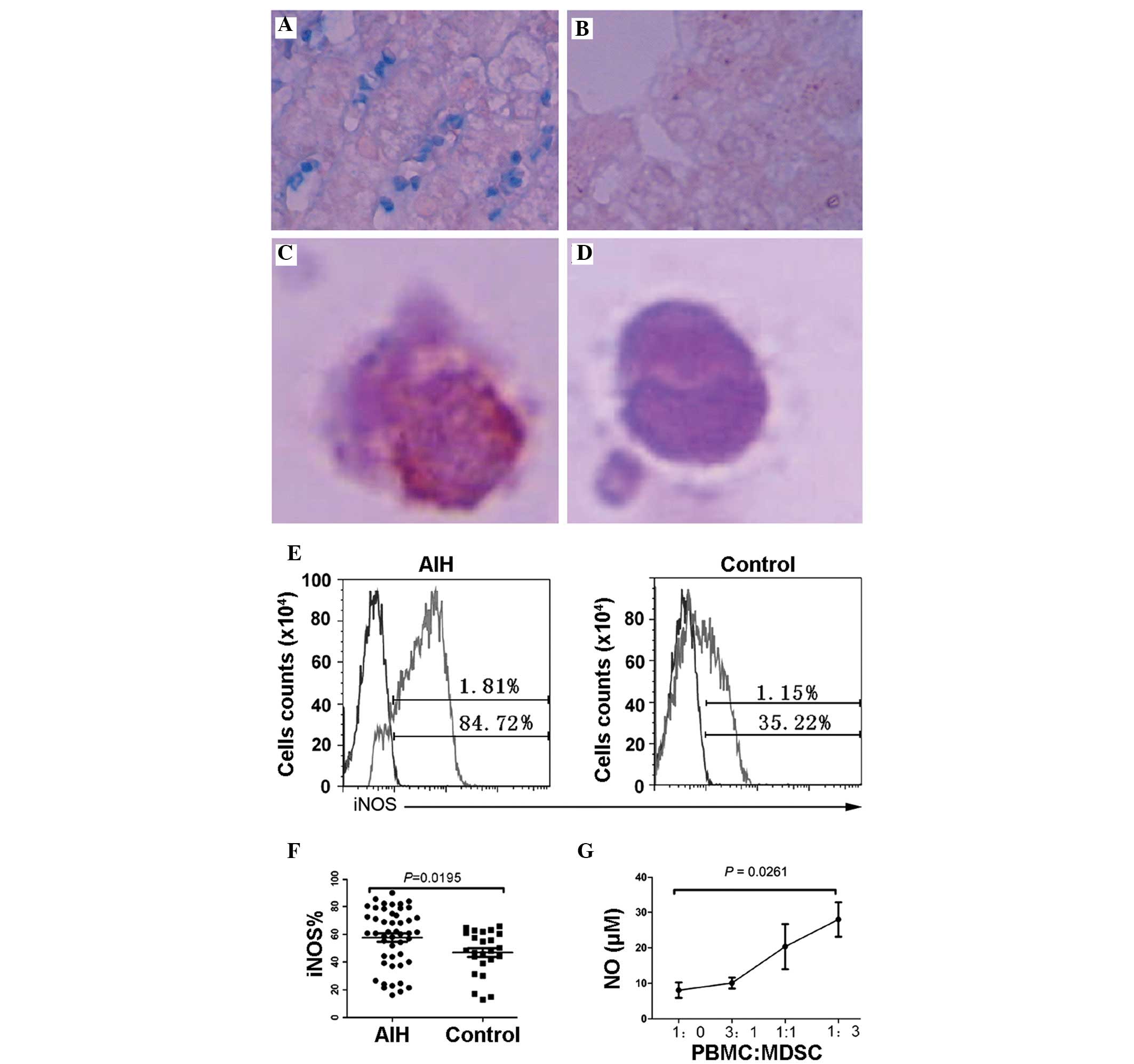
Expression of iNOS in MDSCs
The present study subsequently examined the in
situ expression of iNOS by lymphocytes in the liver. The
expression of iNOS in the lymphocytes of the AIH liver tissue was
significantly higher, compared with the healthy liver tissue
(Fig. 4A and B). In addition,
MDSCs from the peripheral blood were sorted and stained with iNOS
monoclonal antibody, which demonstrated that the expression level
of iNOS in the MDSCs was significantly higher than in the other
PBMCs. (Fig. 4C and D). The PBMCs
from 48 patients and 24 healthy individuals were stained and
analyzed. The expression of iNOS in the circulating MDSCs from
patients with AIH was significantly higher than in the healthy
individuals (Fig. 4E and F;
P=0.0195). Correspondingly, the concentrations of NO in the
supernatant, detected using ELISA, were also associated with the
frequency of MDSCs (Fig. 4G;
P=0.0261).
Discussion
MDSCs are essential in regulating immune responses
during autoimmune diseases. The present study demonstrated for the
first time, to the best of our knowledge, the frequency and
function of MDSCs from patients with AIH. The results demonstrated
that increased frequencies of peripheral blood MDSCs were
associated with liver injury. Hormone therapy reduced the frequency
of peripheral blood MDSCs in patients with AIH. MDSCs from patients
with AIH suppressed the proliferation and cytokine secretion of T
cells. In addition, MDSC promoted the apoptosis of T cells in a
dose-dependent manner in vitro. Notably, higher expression
levels of iNOS in the MDSCs were observed in the in situ
liver tissues from patients with AIH, compared with the healthy
subjects. Additionally, peripheral blood MDSCs expressed iNOS and
produced NO.
As precursors of macrophages, granulocytes and
dendritic cells, MDSCs are heterogeneous, immature and plastic. In
humans, MDSCs remains to be fully characterized owing to the lack
of specific markers (13).
Generally, human MDSCs are identified as
CD14−CD11b+ cells, or populations of cells
expressing the myeloid-associated marker, CD33, but not the MHC
Class II molecules, HLA-DR, mature myeloid or lymphoid cell markers
(14). In addition, a group of
CD15+ MDSCs have been identified in the peripheral blood
of patients with cancer (15). A
previous study characterized a novel population of MDSCs, which
were CD14+ and HLA-DR−/low. They are
significantly increased in the peripheral blood and tumor tissues
of patients with hepatocellular carcinoma, exhibit arginase
activity and suppress autologous T cell proliferation (16). The present study used
Lin1−/low HLA-DR-CD33+ CD11b+ as
markers of human peripheral blood MDSCs, and investigated the
frequency of MDSCs in the peripheral blood of patients with AIH.
The results demonstrated an increased frequency of peripheral blood
MDSCs, which reduced following hormone treatment, suggesting that
MDSCs are essential during the progression and treatment of
AIH.
It has been reported that the immunosuppressive
activities of MDSCs require direct cell-cell contact, which is
through cell-surface receptors and soluble mediators, and the
suppressive activity of MDSCs is associated with the metabolism of
arginine and iNOS (17). Another
important factor contributing to the suppressive activity of MDSCs
is ROS (18). Increased production
of ROS has emerged as one of the predominant characteristics of
MDSCs from tumor-bearing mice and patients with cancer (19). NO, produced by iNOS, is also
involved in the suppressive activity of MDSCs (20). A previous study also demonstrated
that peroxynitrite is a crucial mediator of the suppression of
T-cell function by MDSCs (21).
Notably, a novel population of MDSCs, described as CD14+
DR−/low, have been demonstrated to promote the de
novo development of forkhead box P3+ regulatory T cells in
vivo. In addition, MDSCs secrete immunosuppressive cytokines,
including TGF-β and IL-10, to inhibit T cell function (22). The present study demonstrated
higher expression levels of iNOS in the peripheral blood MDSCs and
also in in situ liver tissues from patients with AIH. In
vitro experiments revealed that MDSCs suppressed the function
of T cells by inhibiting proliferation and the cytokine secretion
of T cells, and by promoting apoptosis of the T cells.
MDSC expansion is associated with inflammation and
autoimmunity. In experimental autoimmune encephalomyelitis, a mouse
model of multiple sclerosis, the number of MDSCs has been observed
to increase in the spleen and blood (23). A significant increase in the number
of MDSCs has also been detected in experimental autoimmune
uveoretinitis and in inflammatory bowel diseases (24). In mouse models of systemic lupus
erythematosus, alopecia areata and type 1 diabetes, MDSCs
demonstrate increased frequency and immune suppression (25). The present study revealed for the
first time, to the best of our knowledge, that the frequency of
peripheral blood MDSCs in patients with AIH was increased, which
was consistent with the mouse models of autoimmune disease.
Previous studies have demonstrated that activated
CD4+ and CD8+ T cells induce autoimmune liver injury through
antibody-dependent cell-mediated cytotoxicity and the cytotoxic T
lymphocyte (CTL) killing effect. Th1 cells cytokines, including
IFN-γ, IFN-α, IL-1 and IL-2, together with T helper 2 cell
cytokines, including IL-4, IL-6, IL-10, IL13, IL-17 and TGF-β, are
also induced during the pathology of liver injury (26). The activation, recruitment and
amplification of MDSCs is closely associated with the stimulation
of inflammatory cytokines (27),
among which IFN-γ, IL-6, IL-10, IL-13 and TGF-β stimulate MDSCs
recruitment and amplification, and IFN-γ, IL-4, IL-13, IL-17 and
TGF-β induce MDSC activation (28). Therefore, the present study
hypothesized that MDSCs present a compensatory mechanism against
inflammation. The present study demonstrated that the level of
MDSCs were higher during the severe inflammatory reaction stage,
and the increase in MDSCs was positively correlated with ALT and
AST, which suggested that MDSCs correlated with the level of liver
inflammation. By contrast, during the cirrhosis period of AIH, the
frequency of MDSCs declined. This weakened suppression of MDSCs
exacerbated the liver injury.
MDSC are involved in the immune response, not only
in inflammation associated with cancer, but also in autoimmune
disease, including AIH. The present study investigated the
frequency and function of peripheral blood MDSCs from patients with
AIH. The increased frequency of circulating MDSCs in patients with
AIH was associated with liver injury, and effective hormone
treatment reduced the frequency of MDSCs. In addition, higher
expression levels of iNOS were observed in the in situ liver
tissues of patients with AIH. This characterization of MDSCs
improves current understanding of the pathogenesis of AIH and may
provide a possible method of immune intervention.
Acknowledgments
This study was supported by the Hefei Science and
Technology Development Special Fund (no. 201154-19-2) and the Hefei
Science and Technology Program (no. 2013183-29).
References
|
1
|
Manns MP and Vogel A: Autoimmune
hepatitis, from mechanisms to therapy. Hepatology. 43(Suppl 2):
132–144. 2006. View Article : Google Scholar
|
|
2
|
Longhi MS, Ma Y, Mieli Vergani G and
Vergani D: Aetiopathogenesis of autoimmune hepatitis. J autoimmun.
34:7–14. 2010. View Article : Google Scholar
|
|
3
|
Longhi MS, Meda F, Wang P, Samyn M,
Mieli-Vergani G, Vergani D and Ma Y: Expansion and de novo
generation of potentially therapeutic regulatory T cells in
patients with autoimmune hepatitis. Hepatology. 47:581–591. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kawamura H, Aswad F, Minagawa M,
Govindarajan S and Dennert G: P2X7 receptors regulate NKT cells in
autoimmune hepatitis. J Immunol. 176:2152–2160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peranzoni E, Zilio S, Marigo I, Dolcetti
L, Zanovello P, Mandruzzato S and Bronte V: Myeloid-derived
suppressor cell heterogeneity and subset definition. Curr Opin
Immunol. 22:238–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Solito S, Falisi E, Diaz-Montero CM, Doni
A, et al: A human promyelocytic-like population is responsible for
the immune suppression mediated by myeloid-derived suppressor
cells. Blood. 118:2254–2265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haile LA, von Wasielewski R,
Gamrekelashvili J, Krüger C, Bachmann O, Westendorf AM, et al:
Myeloid-derived suppressor cells in inflammatory bowel disease: a
new immunoregulatory pathway. Gastroenterology. 135:871–881.
881e1–e5. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goh C, Narayanan S and Hahn YS:
Myeloid-derived suppressor cells: the dark knight or the joker in
viral infections? Immunol Rev. 255:210–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ugel S, Delpozzo F, Desantis G, Papalini
F, Simonato F, Sonda N, et al: Therapeutic targeting of
myeloid-derived suppressor cells. Curr Opin Pharmacol. 9:470–481.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Manns MP, Czaja AJ, Gorham JD, Krawitt EL,
Mieli-Vergani G, Vergani D and Vierling JM: Diagnosis and
management of autoimmune hepatitis. Hepatology. 51:2193–2213. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Monu NR and Frey AB: Myeloid-derived
suppressor cells and anti-tumor T cells: A complex relationship.
Immunol Invest. 41:595–613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Highfill SL, Rodriguez PC, Zhou Q, Goetz
CA, et al: Bone marrow myeloid-derived suppressor cells (MDSCs)
inhibit graft-versus-host disease (GVHD) via an
arginase-1-dependent mechanism that is up-regulated by
interleukin-13. Blood. 116:5738–5747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lechner MG, Megiel C, Russell SM, Bingham
B, Arger N, Woo T and Epstein AL: Functional characterization of
human Cd33+ and Cd11b+ myeloid-derived suppressor cell subsets
induced from peripheral blood mononuclear cells co-cultured with a
diverse set of human tumor cell lines. J Transl Med. 9:902011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goedegebuure P, Mitchem JB, Porembka MR,
Tan MC, Belt BA, Wang-Gillam A, et al: Myeloid-derived suppressor
cells: general characteristics and relevance to clinical management
of pancreatic cancer. Curr Cancer Drug Targets. 11:734–751. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hoechst B, Ormandy LA, Ballmaier M, Lehner
F, Krüger C, Manns MP, et al: A new population of myeloid-derived
suppressor cells in hepatocellular carcinoma patients induces
CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 135:234–243. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu CY, Wang YM, Wang CL, Feng PH, Ko HW,
Liu YH, et al: Population alterations of L-arginase- and inducible
nitric oxide synthase-expressed CD11b+/CD14 (-)/CD15+/CD33+
myeloid-derived suppressor cells and CD8+ T lymphocytes in patients
with advanced-stage non-small cell lung cancer. J Cancer Res Clin
Oncol. 136:35–45. 2010. View Article : Google Scholar
|
|
18
|
Jayaraman P, Parikh F, Lopez-Rivera E,
Hailemichael Y, Clark A, Ma G, et al: Tumor-expressed inducible
nitric oxide synthase controls induction of functional
myeloid-derived suppressor cells through modulation of vascular
endothelial growth factor release. J Immunol. 188:5365–5376. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Raber PL, Thevenot P, Sierra R, et al:
Subpopulations of myeloid-derived suppressor cells impair T cell
responses through independent nitric oxide-related pathways. Int J
Cancer. 134:2853–2864. 2014. View Article : Google Scholar :
|
|
20
|
Jia W, Jackson-Cook C and Graf MR:
Tumor-infiltrating, myeloid-derived suppressor cells inhibit T cell
activity by nitric oxide production in an intracranial rat
glioma+vaccination model. J Neuroimmunol. 223:20–30. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu T, Ramakrishnan R, Altiok S, Youn JI,
Cheng P, Celis E, et al: Tumor-infiltrating myeloid cells induce
tumor cell resistance to cytotoxic T cells in mice. J Clin Invest.
121:4015–4029. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lechner MG, Liebertz DJ and Epstein AL:
Characterization of cytokine-induced myeloid-derived suppressor
cells from normal human peripheral blood mononuclear cells. J
Immunol. 185:2273–2284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ioannou M, Alissafi T, Lazaridis I, Deraos
G, Matsoukas J, Gravanis A, et al: Crucial role of granulocytic
myeloid-derived suppressor cells in the regulation of central
nervous system autoimmune disease. J Immunol. 188:1136–1146. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kerr EC, Raveney BJ, Copland DA, Dick AD
and Nicholson LB: Analysis of retinal cellular infiltrate in
experimental autoimmune uveoretinitis reveals multiple regulatory
cell populations. J Autoimmun. 31:354–361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin B, Ma G, Yen CY, Zhou Z, Wang GX,
Divino CM, et al: Myeloid-derived suppressor cells prevent type 1
diabetes in murine models. J Immunol. 185:5828–5834. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carambia A and Herkel J: CD4 T cells in
hepatic immune tolerance. J Autoimmun. 34:23–28. 2010. View Article : Google Scholar
|
|
27
|
Ribechini E, Greifenberg V, Sandwick S and
Lutz MB: Subsets, expansion and activation of myeloid-derived
suppressor cells. Med Microbiol Immunol. 199:273–281. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He D, Li H, Yusuf N, Elmets CA, Li J,
Mountz JD and Xu H: IL-17 promotes tumor development through the
induction of tumor promoting microenvironments at tumor sites and
myeloid-derived suppressor cells. J Immunol. 184:2281–2288. 2010.
View Article : Google Scholar : PubMed/NCBI
|