Introduction
In 2006, Takahashi and Yamanaka (1) reported the direct reprogramming of
murine embryonic fibroblasts (MEFs) into pluripotent stem cells,
following the introduction of four transcription factors
(octamer-binding transcription factor 4, sex-determining region Y
box-2, Kruppel-like factor 4 and cMyc). Subsequently, a number of
laboratories have derived induced pluripotent stem cells from
somatic cells. The identification of a method with which to
reprogram induced pluripotent stem (iPS) cells from somatic cells
(2) may facilitate advances in
regenerative medicine. In contrast to embryonic stem cells, iPS
cells may be administered without raising ethical or alloimmune
concerns. iPS cells have exhibited the potential to differentiate
into a number of cell lineages, including CD34+
progenitor cells (3),
cardiomyocytes (4) and endothelial
cells (ECs) (5). They have the
capacity for unlimited growth and self-renewal, and are able to
differentiate into all types of mature tissue cells. In recent
years, accumulating evidence has indicated that iPS cells may
differentiate into ECs in vitro or in vivo (6,7).
ECs line the blood vessels of the circulatory
system, and form the barrier between the circulating blood and the
rest of the vessel wall. The endothelium is a dynamic and
heterogeneous organ with secretory, metabolic, synthetic and
immunological functions. Impaired EC function may result in
hypertension, thrombosis, inflammation and atherosclerosis
(8). Repair and regeneration of
vascular cells, in particular of ECs, has been a research focus for
a number of years. However, in the context of human disease, the
use of adult progenitor or vascular stem cells has certain
limitations, such as the identification and availability of
appropriate, and effective cell-types (9). Recently, the ability to derive ECs
from pluripotent stem cells has extended the scope of regenerative
medicine (10,11).
MiRNAs, an emerging class of highly conserved,
non-coding small RNAs, regulate gene expression at the
post-transcriptional level by inhibiting the translation of protein
from mRNA or by promoting the degradation of mRNA. MiRNAs are
involved in a number of core biological processes (12–15),
including cardiogenesis, hematopoietic lineage differentiation and
oncogenesis. Certain specific miRNAs that regulate endothelial cell
functions and angiogenesis, have been described (16,17).
For example, Let7-f, miR-27b and miR-130a have been identified as
proangiogenic miRNAs. Others, such as miR-221 and miR-222, have
been shown to inhibit in vitro endothelial cell migration,
proliferation and angiogenesis (16).
SirT1 is a member of the a NAD+-dependent
class III group of histone deacetylases and has been reported to be
involved in a variety of biological systems and cellular functions
(18). Recent data also suggests
that SirT1 is a critical mediator in the regulation of various
development genes during stem cell differentiation (19) and is important role in different
cellular differentiations, including endothelial progenitor cells
(20), hematopoietic cells
(21) and osteoblasts (22,23).
Several previous studies have demonstrated the regulation of miRNAs
in a myriad of vascular biological events. However, how they
control EC fate commitment and the mechanisms involved in these
differentiation processes remain to be elucidated. Therefore, the
present study aimed to determine the possible roles of miRNAs in
the differentiation processes in iPS cells.
Materials and methods
Materials
Cell culture media, serum and cell culture
supplements were obtained from the American Type Culture Collection
(ATCC; Manassas, VA, USA), Millipore, Invitrogen Life Technologies
(Carlsbad, CA, USA) and PAA. Antibodies against rabbit
anti-VE-cadherin (1:1,000; cat. no. Ab33168), and rabbit anti-SM22
(1:1,000; cat. no. ab14106) were obtained from Abcam. An antibody
against mouse anti-smooth muscle actin (SMA; 1:1,000; cat. no.
A5228) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Antibodies against rabbit anti-Sirt1 (1:1,000; cat. no. sc-15404),
goat anti-CD31 (1:500′ cat. no. sc-1506) and rabbit anti-GAPDH
(1:1,000; cat. no. sc-25778) were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The secondary antibodies for
western blotting were obtained from Dakocytomation and Abcam.
iPS cell culture and differentiation
Mouse iPS cells were generated in our laboratory, as
previously described (24). Mouse
iPS cells were cultured on gelatin-coated flasks (PBS containing
0.04% of gelatin from bovine skin; Sigma-Aldrich) in Dulbecco's
modified Eagle's medium (DMEM; ATCC) supplemented with 10% fetal
bovine serum, 100 IU/ml penicillin and 100 µg/ml streptomycin
(Invitrogen Life Technologies); 10 ng/ml recombinant human leukemia
inhibitory factor (Millipore); and 0.1 mM/0.05mM 2-mercaptoethanol
(Invitrogen Life Technologies) in a humidified incubator,
supplemented with 5% CO2. Cells were passaged every 2
days at a ratio of 1:6. Differentiation of iPS cells was induced by
seeding the cells on type IV mouse collagen (5 µg/ml)-coated dishes
in differentiation medium (DM) that contained α-MEM supplemented
with 10% FBS (Invitrogen Life Technologies), 0.05 mm
2-mercap-toethanol, 100 units/ml penicillin, and 100 µg/ml
streptomycin in the presence of 50 ng/ml vascular endothelial
growth factor (VEGF) for the time points indicated.
miR-199a transient transfection
Manipulation of miR-199a levels in iPS cells that
had differentiated with or without VEGF for 4 days, and cultured to
60–70% confluence, was performed using Pre-199b, or the
non-targeting control (Pre-ctrl; Ambion AB). Inhibition of miR-199a
was performed using the locked nucleic acid (LNA) inhibitor of
miR-199a, LNA-199b, or a negative control (LNA-ctrl; Exiqon). All
transfections were performed using Lipofectamine™ RNAiMAX
(Invitrogen Life Technologies), at a final concentration of 50 nM,
according to the manufacturer's instructions. In order to detect
miRNA, total RNA was isolated using the miRVana miRNA isolation kit
(Ambion), according to the manufacturer's instructions. Total and
miRNA-specific cDNA was generated using the TaqMan®
MicroRNA Reverse Transcription kit (Ambion), and miRVana
quantitative RT-PCR primer sets for miRNAs (Ambion). The polymerase
chain reaction (PCR) reaction was directly monitored using the ABI
PRISM 7000 Sequence Detection System (Applied Biosystems, CA).
Briefly, cDNA was produced from enriched miRNA using the TaqMan
MicroRNA RT kit. U6 RNA was used as an endogenous control.
Reverse transcription-PCR (RT-PCR)
RT-PCR and quantitative PCR (qPCR) were performed as
described previously (25). Total
RNA was extracted using the RNeasy Mini kit (Qiagen, Shanghai,
China), according to the manufacturer's instructions. Reverse
transcription for RNA (2 µg) was performed using an Improm-IITM RT
kit (Promega, Madison, WI, USA) with RNase inhibitor (Promega), and
Random primers (Promega). cDNA was analyzed by PCR using 20 ng cDNA
in a 50 µl reaction volume containing primers and Ex-Taq DNA
polymerase (Takara, Dalian, China). The PCR conditions were as
follows: 32 cycles of 94°C for 60 sec, 58°C for 60 sec and 72°C for
90 sec.
qPCR
Relative gene expression was determined by qPCR,
using 2 ng of cDNA (relative to RNA amount) for each sample with
the SYBR Green Master Mix in a 20-µl reaction. Ct values were
measured using an ABI Prism 7000 sequence detector (Applied
Biosystems, Foster City, CA, USA). 18S ribosomal RNA was used as
the endogenous control, against which the quantities of RNA in each
sample were normalized. For each sample, PCR was performed in
duplicate in a 96-well reaction plate (Eppendorf, twin. tec real
time PCR plates). The gene was considered undetectable beyond 35
cycles. The following primer sets were used: Forward:
5′-CCAGTAAGTGCGGGTCATAA-3′ and reverse: 5′-CCGAGGGCCTCACTAAACC-3′
for 18S, forward: 5′-AAGAAACCGCTGATCGGCA-3′ and reverse:
5′-TCGGAAGAATTGGCCTCTGTC-3′ for VE-cadherin, forward:
5′-CAAACAGAAACCCGTGGAGAT-3′ and reverse:
5′-ACCGTAATGGCTGTTGGCTTC-3′ for CD31, forward:
5′-CGGGCAATTTCCATTGGCTTCTCA-3′ and reverse:
5′-GGCCACACGAAGCTCGTTATAG-3′ for SMA and forward:
5′-GATATGGCAGCAGTGCAGAG-3′ and reverse: 5′-AGTTGGCTGTCTGTGAAGTC-3′
for SM22.
Immunoblotting
Cells were harvested and washed with cold PBS,
re-suspended in lysis buffer (25 mM Tris-Cl, pH 7.5; 120 mM NaCl; 1
mM EDTA, pH 8.0; and 0.5% Triton X100), supplemented with protease
inhibitors (Sigma-Aldrich) and lysed by ultra-sonication (twice,
for 6 sec each time; Bradson Sonifier 150; Bradson, St. Louis, MO,
USA) in order to obtain whole cell lysates. The protein
concentration was determined using the Biorad Protein Assay
Reagent. Whole lysate (50 µg) was applied to SDS-PAGE gels and
transferred to Hybond PVDF membranes (Amersham Pharmacia Biotech,
St. Albans, UK), followed by a standard western blotting procedure.
The bound primary antibodies were detected by the use of
horseradish peroxidase-conjugated secondary antibody and the ECL
detection system (GE Health). The band density was semiquantified
using ImageJ v 1.45S software (NIH, Bethseda, MA, USA).
Luciferase reporter assay
For the luciferase reporter assays, 3×104
iPS cells were seeded into collagen-coated wells in a 12-well plate
in differentiated media (DM). After 72 h, cells were transfected
with luciferase plasmids under the control of the Sirt1 3′UTR
Lenti-reporter-Luc Vector (ABM), in addition to the Pre-199b, 199b
inhibitor and controls. Briefly, 0.33 µg/well of the reporter
plasmids were cotransfected with the Pre-199b, 199b inhibitor and
controls (2 µl/well) using jetPRIME®
(Polyplus-transfection SA), according to the manufacturer's
instructions. pGL3-Luc Renilla (0.1 µg/well) was included in
all transfection assays as an internal control. Luciferase and
Renilla (Promega Corporation) activity assays were detected
at 48 h following transfection using a standard protocol (2). The relative luciferase unit (RLU) was
defined as the ratio of luciferase activity to Renilla
activity, with that of the control set as 1.0.
In vitro tube formation assay
iPS cells were differentiated in the absence of VEGF
for 4 days, and then transfected with Pre-199a or Pre-ctrl. In
vitro angiogenesis assays were conducted after 48 h, as
described previously (2,24). Cell suspensions, containing
4×104 transfected cells, were placed on top of the 50
µl/well Matrigel (10 mg/ml; BD Matrigel Basement Membrane Matrix,
A6661; BD Biosciences, Franklin Lakes, NJ, USA) in 8-well chamber
slides (BD Biosciences). Rearrangement of cells and the formation
of capillary-like structures were observed at 6–12 h.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean, and were analyzed using GraphPad Prism 5 software
(GraphPad Software, Inc., La Jolla, CA, USA) with two-tailed
Student's t-test for two groups or pairwise comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
MiR-199a is involved in EC
differentiation from iPS cells
The expression of miR-199a was found to be
upregulated during EC differentiation from iPS cells on days 0 to 8
(Fig. 1A). In order to examine the
effect of miR-199a on EC differentiation, a chemically synthesized
miR-199a inhibitor or Pre-199b (mimic) were administered. iPS cells
were forced to differentiate towards ECs for 4 days and were
efficiently transfected with either miR-199a precursor molecules
(pre-miR-199a) or an miR-199a inhibitor (anti-miR-199a), resulting
in significant miR-199a upregulation and repression, respectively
(Fig. 1B and C). Furthermore, the
miR-199a mimic increased the mRNA (Fig. 1D) and protein (Fig. 1E and F) expression of the
endothelial markers, CD144 and CD31. By contrast, the miR-199a
inhibitor decreased the expression of EC markers. These initial
observations suggest the involvement of miR-199a in EC
differentiation from iPS cells.
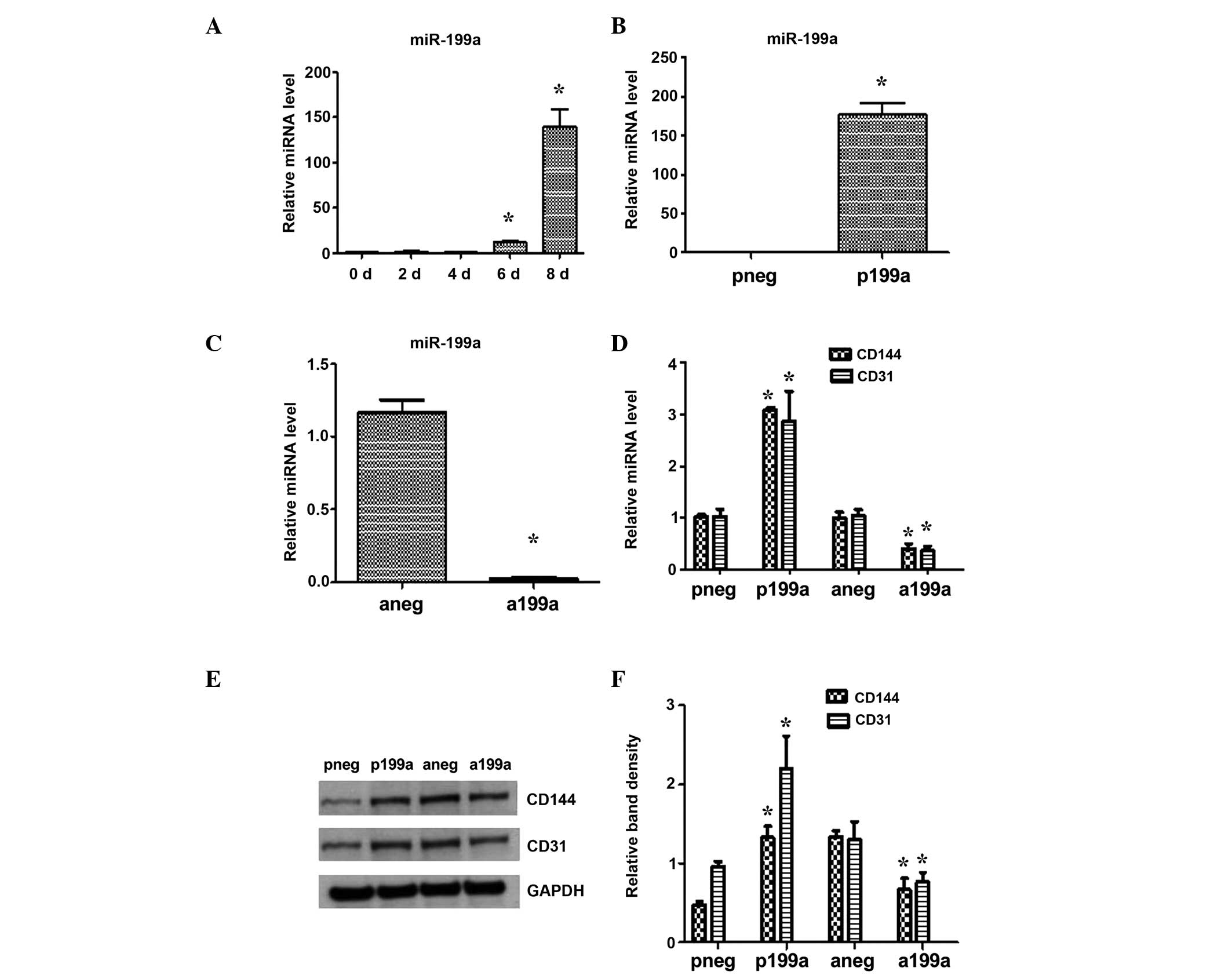 | Figure 1Effect of miR-199a in EC
differentiation from iPS cells. Mouse iPS cells were seeded on
Collagen IV-coated dishes and cultured in differentiated media
supplemented with 50 ng/ml VEGF. (A) The expression of miR-199a was
found to be upregulated during EC differentiation, as demonstrated
by RT-qPCR. (B) iPS cells were forced to differentiate towards ECs
for 4 days and then efficiently transfected with either miR-199a
precursor molecules (pre- miR-199a) or an miR-199a inhibitor
(anti-miR-199a), which resulted in significant miR-199a
upregulation and repression, respectively. (C) On day 7, the cells
were harvested and further RT-qPCR analysis demonstrated that
miR-199a increased the mRNA (D) and protein (E) and (F) expression
of the endothelial markers, CD144 and CD31. Data are presented as
the mean ± SEM. n=3. *P<0.05. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; SEM, standard
error of the mean; miRNA, microRNA; EC, endothelial cell; iPS
cells, induced pluripotent stem cells; VEGF, vascular endothelial
growth factor; pneg, mimic negative control; P199a, miR-199b mimic;
Aneg, inhibitor negative control; A199b, miR-199b inhibitor. |
MiR-199a acts as a phenotypic switch in
vascular cell differentiation
Subsequent experiments demonstrated that miR-199a
concomitantly suppressed the mRNA and protein expression of the
smooth muscle cells (SMC) markers, SMA and SM22, when iPS cells
were induced to differentiate towards vascular progenitor cells, by
seeding the cells on Collagen IV and culturing them in DM in the
absence of VEGF. By contrast, the miR-199a inhibitor favored the
induction of the SMC markers (Fig. 2A
and B). These results indicate an association between miR-199a
and SMC differentiation, and it is hypothesized that miR-199a may
act as a phenotypic switch during vascular cell
differentiation.
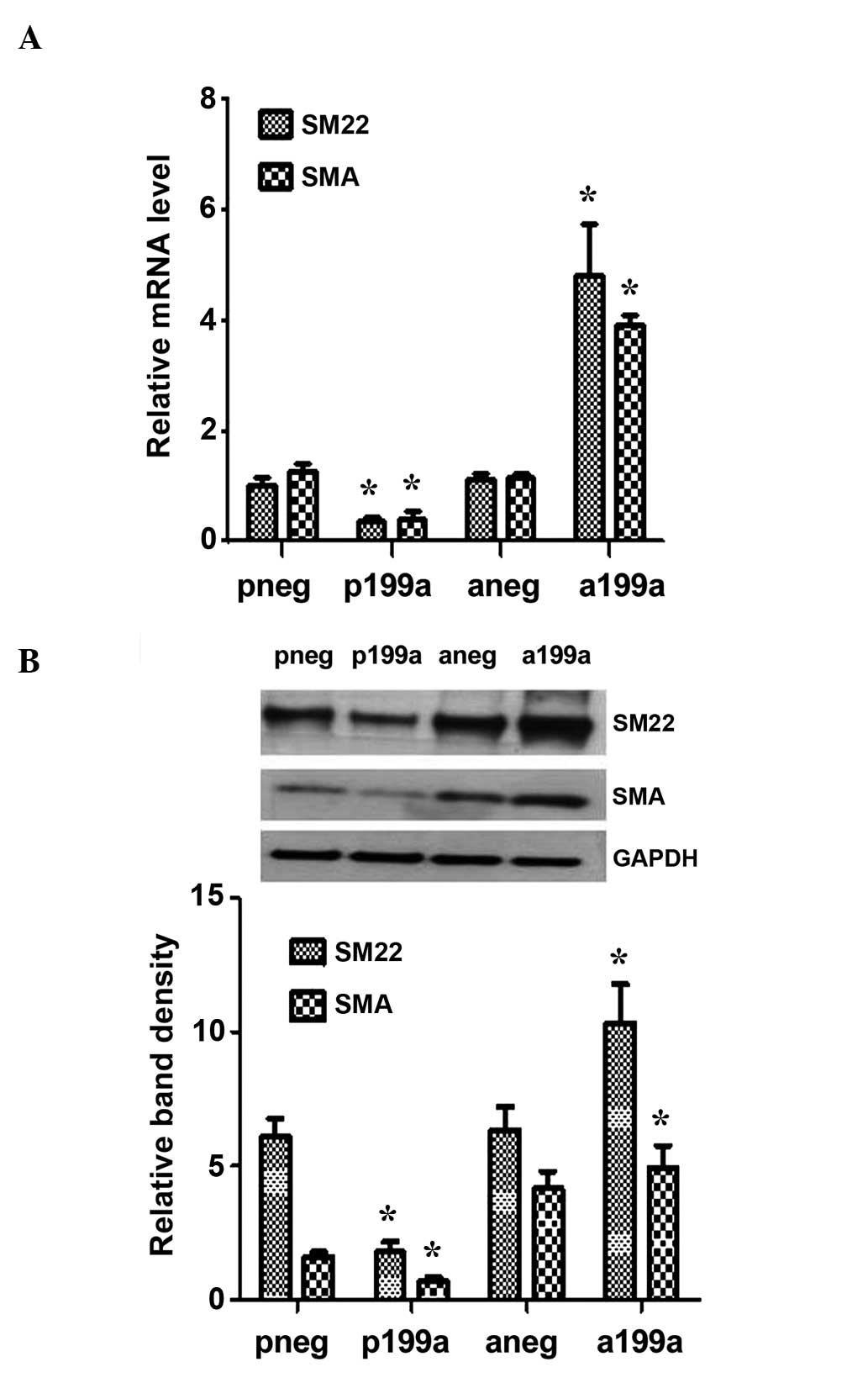 | Figure 2miR-199a may act as a phenotypic
switch in vascular cell differentiation. iPS cells were seeded on
collagen IV-coated dishes, and cultured with differentiated media
in the absence of VEGF for 4 days. They were then forced to
differentiate towards vascular progenitor cells. (A) Overexpression
of miR-199a suppressed the mRNA (A) and protein (B) expression of
the SMC markers, SMA and SM22, as demonstrated by reverse
transcription-quantitative polymerase chain reaction and western
blotting, respectively. The miR-199a inhibitor favored the
induction of the SMC markers. Data are presented as the mean ± SEM.
n=3. *P<0.05. miRNA, microRNA; iPS cell, induced
pluripotent stem cell; VEGF, vascular endothelial growth factor;
SMC, smooth muscle cell; pneg, mimic negative control; P199a,
miR-199b mimic; Aneg, inhibitor negative control; A199b, miR-199b
inhibitor. |
MiR-199a induces angiogenesis in
vitro
In order to evaluate the role of miR-199a in
angiogenesis, the levels of miR-199a were modulated either by
overexpression using an miR-199a mimic, or by knockdown using an
miR-199a inhibitor. In brief, mouse iPS cells were seeded on
collagen IV-coated plates and cultured in DM in the absence of
VEGF. The miR-199a mimic or control were then introduced to the
cells by transfection. After 4 days, the cells were subjected to
Matrigel assays plugs in vitro. The results demonstrated
that in the presence of the miR-199a mimic, the differentiated
cells had formed vascular structures within a few hours, in
comparison with the control cells, in which where few or no defined
vascular structures were observed (Fig. 3A and B). Further experiments were
performed in the presence of the miR-199a inhibitor. These cells
were then subjected to Matrigel plugs assays in vitro. The
results demonstrated that the formation of vascular structures was
suppressed in cells treated with the miR-199a inhibitor, in
comparison with the control cells.
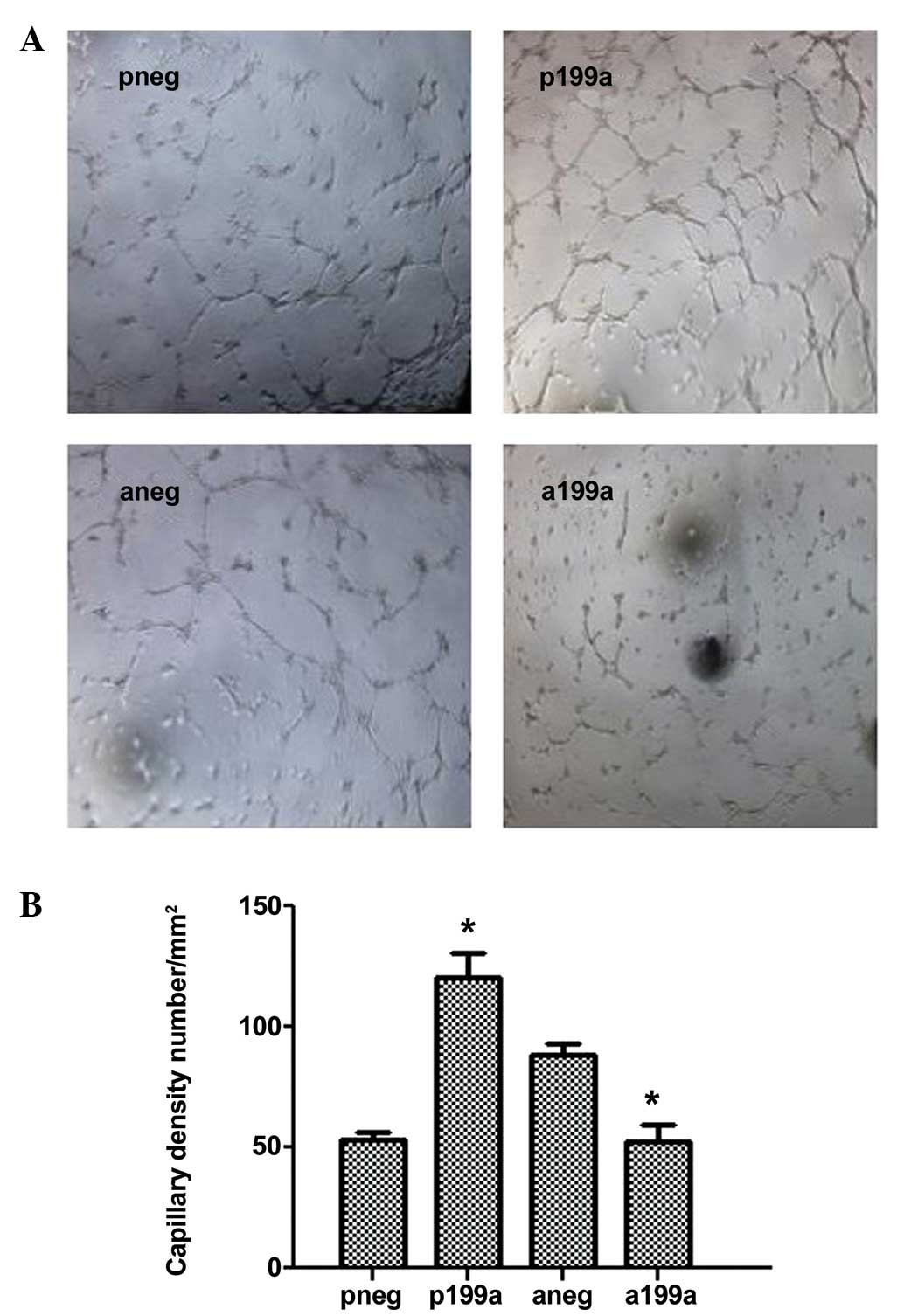 | Figure 3miR-199a induced angiogenesis in
vitro. iPS cells were seeded on collagen IV-coated plates and
cultured in differentiated media in the absence of VEGF for 4 days.
Then the miR-199a mimic or control were introduced to the cells by
transfection. After 2 days, the cells were subjected to Matrigel
assays plugs in vitro. (A) Upper panel: miR-199a formed
vascular structures within a 4–6 h in the in vitro Matrigel
assays, compared with the control where less defined vascular
structures were observed. Lower panel: Additional experiments were
performed, using the miR-199a inhibitor. The miR-199a inhibitor
suppressed the formation of vascular structures in the in
vitro Matrigel plugs assays, compared with the control cells.
(B) Quantification of results in (A). Data are presented as the
mean ± SEM. n=3. *P<0.05. miRNA, microRNA; iPS cell,
induced pluripotent stem cell; VEGF, vascular endothe-lial growth
factor; pneg, mimic negative control; P199a, miR-199b mimic; Aneg,
inhibitor negative control; A199b, miR-199b inhibitor. |
MiR-199a targets Sirt1
In order to further investigate the mechanisms
underlying the regulation of EC differentiation by miR-199a,
potential mRNA targets of miR-199a were scrutinized. Sirt1 emerged
as one of the primary targets of miR-199a in several computational
algorithmic databases, including Targetscan (www.targetscan.org), pictar (www.pictar.
mdc-berlin.de) iand miRanda (www.microrna.org), and two highly conserved binding
sites for miR-199a have also previously been identified within the
Sirt1 3′UTR (26). The results of
the present study supported this hypothesis, as the Sirt1 gene and
protein expression was significantly downregulated or upregulated
following overexpression or inhibition of miR-199a in the
differentiating iPS cells (Fig. 4A and
B), respectively. This indicated that Sirt1 is regulated,
directly or indirectly, by miR-199a. In order to investigate this
possibility, the 3′UTR of Sirt1, which contained the binding sites
for miR-199a, was cloned into a luciferase reporter. The results
from the miRNA reporter assay demonstrated that the activity of
luciferase from the construct, containing the Sirt1 3′UTR, was
significantly downregulated following miR-199a overexpression
(Fig. 4C). This data has provides
clear evidence that Sirt1 is a target of miR-199a.
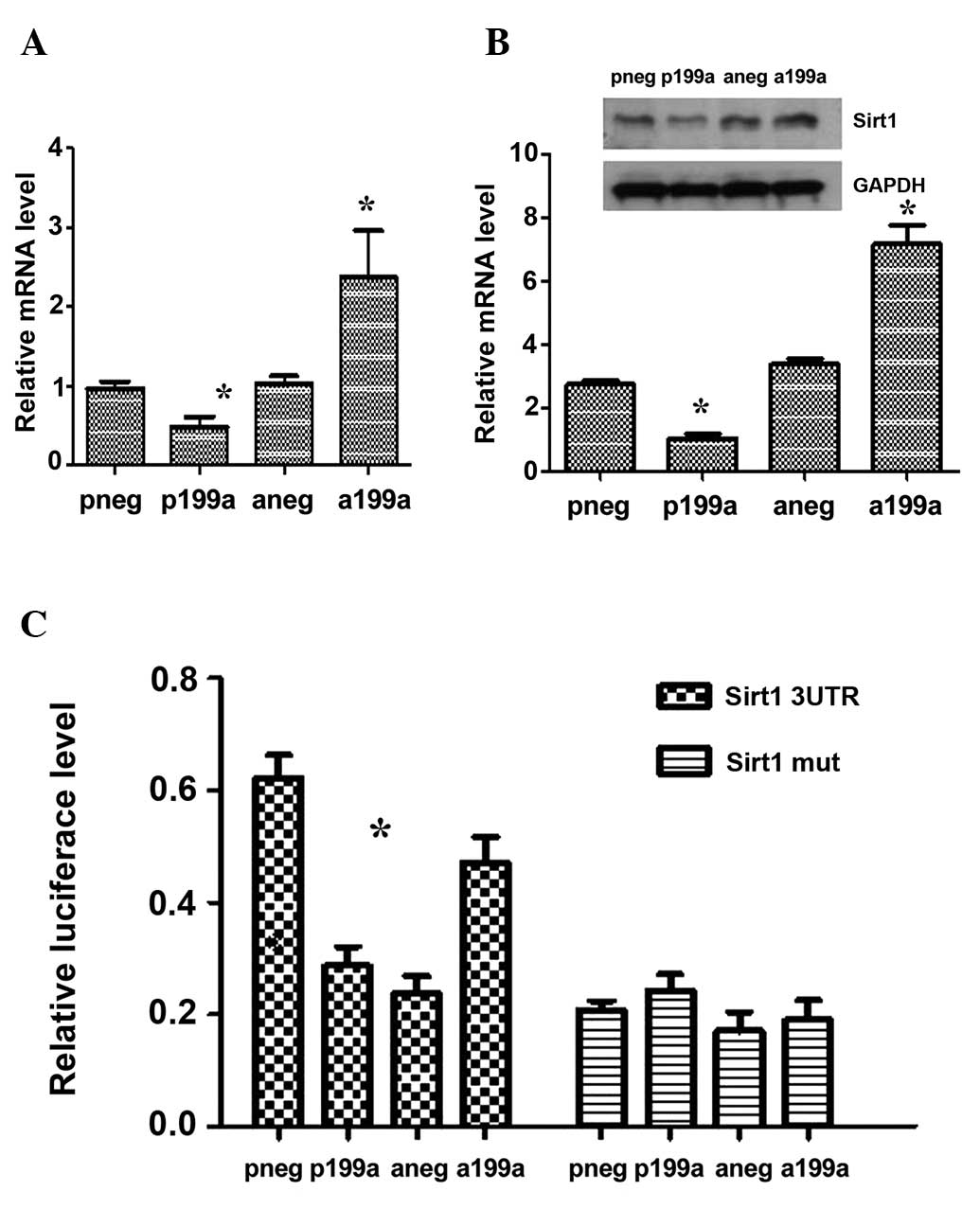 | Figure 4miR-199a targeted Sirt1. miR-199a
suppressed the mRNA (A) and protein (B) expression of Sirt1 during
EC differentiation derived from iPS cells. The miR-199a inhibitor
induced the expression of Sirt1. (C) iPS cells were forced to
differentiate towards ECs for 4 days. Cotransfection of miR-199a or
inhibition with the luciferase plasmid of the 3′UTR of Sirt1 was
then performed. After 48 h, the cells were subjected to luciferase
analysis, which demonstrated that the 3′UTR of Sirt1 is a direct
target for miR-199a. Data are presented as the mean ± SEM. n=3.
*P<0.05. miRNA, microRNA; Sirt1, sirtuin 1; EC,
endothelial cell; iPS cell, induced pluripotent stem cell; UTR,
untranslated region; pneg, mimic negative control; P199a, miR-199b
mimic; Aneg, inhibitor negative control; A199b, miR-199b
inhibitor. |
Discussion
iPS-derived ECs may be used to treat damaged
vessels, to avoid restenosis and to develop tissue-engineered
vascular grafts, which are significant challenges for regenerative
medicine (27). To date, studies
have demonstrated the regulation by miRNAs of a myriad of vascular
biological events (28). By
studying the non-coding RNAs and their regulatory signalling, it is
possible to improve understanding of the molecular mechanisms that
govern cell fate specification. The present study, aimed to
elucidate the molecular mechanisms underlying the differentiation
of iPS cells into ECs, and to identify miRNAs and their respective
targets involved in this process, in order to aid the development
of novel strategies for the treatment of vascular diseases, such as
atherosclerosis; for the enhancement of neovascularization
following ischemia; and for the prevention of atherosclerotic
inflammation.
miR-199a is well-conserved in different species
(29), and has been identified by
diverse high-throughput screenings in a number of animal models of
disease. The functions of miR-199a vary between systems. For
example, miR-199a is involved in cardiomyocyte protection by rapid
downregulation under hypoxic conditions and prompts hypoxia
inducible factor 1α (HIF1a) expression (30). miR-199a has also been shown to be
involved in stem cell differentiation (29), with differences in the expression
of miR-199a detected prior to and following stem cell
differentiation. For example, following the differentiation of
human embryonic stem cells into pancreatic islet-like cells,
miR-199a expression was found to be upregulated (31). Studies in ovarian cancer stem cells
have shown that the two subtypes of epithelial ovarian cancer stem
cells, exhibit distinct expression patterns of miR-199a (32). However, there is insufficient
evidence to demonstrate how miR-199a controls commitment to EC
differentiation or the mechanisms involved in these processes. To
the best of our knowledge, the current study reports for the first
time, that miR-199a is involved in EC differentiation from iPS
cells. The expression of miR-199a was shown to be upregulated
during EC differentiation, and an abundant expression of miR-199a
was detected in the later stages of this process. Notably, miR-199a
was demonstrated to target Sirt1. miR-199a has already been
described as a potential regulator of Sirt1, in two independent
studies. In the first, miR-199a was shown to promote migration and
tube formation of human cytomegalovirus-infected endothelial cells
via the downregulation of Sirt1 and eNOS (33). The second study found that
downregulation of miR-199a repressed HIF1a and Sirt1 expression,
and reinforced hypoxia preconditioning in cardiac myocytes
(34). In accordance with these
previous findings, the present study provides clear evidence that
Sirt1 is an mRNA target of miR-199a and that miR-199a negatively
regulates this target gene during EC differentiation from iPS
cells. This hypothesis is supported by several lines of
experimentation. Firstly, Sirt1 gene and protein expression levels
were negatively regulated by miR-199a, as demonstrated in the
experiments involving miR-199a overexpression and inhibition.
Secondly, overexpression of miR-199a significantly upregulated
Sirt1 3′UTR activity, while this upregulation was abrogated when
the miR-199a binding sites were mutated.
Notably, in the present study, miR-199a exhibited
the potential to induce angiogenesis, using Matrigel plug assays.
In previous studies, ECs derived from pluripotent stem cells were
incorporated into the microvasculature of ischemic tissue, thereby
enhancing perfusion and improving function (35,36).
Currently, the potential of iPS cells to differentiate towards
therapeutic cells is based on directed empiricism, while they are
entirely dependent on combinations of growth factors, media and
matrices to favor differentiation into the desired lineage. With
regard to vascular regeneration, it is important to gain an
improved understanding of epigenetic alterations, transcriptional
activity and microRNA patterns associated with the differentiation
processes, in order to efficiently generate therapeutic cells.
Refined experimental protocols are required to reliably guide iPS
cells to a vascular lineage (37,38).
The bioengineered vessels derived from these vascular cells, and
their incorporation of into bypass grafts or stents, are
anticipated future directions in stem cell research
(39–41).
In addition, inhibition of miR-199a resulted in the
robust induction of SMC marker expression. In 2007, Ferreira et
al (42) reported that
vascular progenitor cells isolated from human embryonic stem cells,
gave rise to EC- and SMC-like cells, and formed vascular networks
in vivo, providing strong evidences that ECs and SMCs are
derived from a common progenitor. Therefore, it is possible that a
molecular switch may regulate vascular cell differentiation, and
may maintain the balance of these two cell lineages during
development, disease-repair and cell differentiation. Therefore,
the present findings may suggest a unique role miR-199a as a
vascular cell phenotypic switch.
In conclusion, the present study has demonstrated a
novel role for miR-199a in the differentiation of ECs from iPS
cells, and has provided strong evidence that miR-199a negatively
regulates its target gene, Sirt1, during EC differentiation.
Furthermore, the current data support the hypothesis that miR-199a
inhibits the differentiation of iPS cells into SMCs, which
indicates that miR-199a may act as a potential regulator of the
phenotypic switch during vascular cell differentiation. Finally,
the induction of angiogenesis by miR-199a, was evaluated using
Matrigel plug assays. These findings significantly increase the
current understanding of the molecular mechanisms underlying EC
differentiation, and may benefit future research into regenerative
medicine and aid the development of the clinical application of
this therapy.
Acknowledgments
This study was supported by the Zhejiang Provincial
Natural Science Foundation of China (grant no. LQ14H020001) and the
National Natural Science Foundation of China (grant no.
81373161/H1006).
References
|
1
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Margariti A, Winkler B, Karamariti E,
Zampetaki A, Tsai TN, Baban D, Ragoussis J, Huang Y, Han JD, Zeng
L, et al: Direct reprogramming of fibroblasts into endothelial
cells capable of angiogenesis and reendothelialization in
tissue-engineered vessels. Proc Natl Acad Sci USA. 109:13793–13798.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park SW, Jun Koh Y, Jeon J, Cho YH, Jang
MJ, Kang Y, Kim MJ, Choi C, Sook Cho Y, et al: Efficient
differentiation of human pluripotent stem cells into functional
CD34+ progenitor cells by combined modulation of the
MEK/ERK and BMP4 signaling pathways. Blood. 116:5762–5772. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dambrot C, Passier R, Atsma D and Mummery
CL: Cardiomyocyte differentiation of pluripotent stem cells and
their use as cardiac disease models. Biochem J. 434:25–35. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Z, Hu S, Ghosh Z, Han Z and Wu JC:
Functional characterization and expression profiling of human
induced pluripotent stem cell-and embryonic stem cell- derived
endothelial cells. Stem Cells Dev. 20:1701–1710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rufaihah AJ, Huang NF, Kim J, Herold J,
Volz KS, Park TS, Lee JC and Zambidis ET: Reijo-Pera R and Cooke
JP: Human induced pluripotent stem cell-derived endothelial cells
exhibit functional heterogeneity. Am J Transl Res. 5:21–35.
2013.
|
|
7
|
Wong WT, Huang NF, Botham CM, Sayed N and
Cooke JP: Endothelial cells derived from nuclear reprogramming.
Circ Res. 111:1363–1375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Otsuka F, Finn AV, Yazdani SK, Nakano M,
Kolodgie FD and Virmani R: The importance of the endothelium in
athero-thrombosis and coronary stenting. Nat Rev Cardiol.
9:439–453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Collado M, Blasco MA and Serrano M:
Cellular senescence in cancer and aging. Cell. 130:223–233. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen
H, Dean DB and Zhang C: MicroRNAs are aberrantly expressed in
hypertrophic heart: Do they play a role in cardiac hypertrophy. Am
J Pathol. 170:1831–1840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sayed D, Hong C, Chen IY, Lypowy J and
Abdellatif M: MicroRNAs play an essential role in the development
of cardiac hypertrophy. Circ Res. 100:416–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen T, Huang Z, Wang L, Wang Y, Wu F,
Meng S and Wang C: MicroRNA- 125a- 5p partly regulates the
inflammatory response, lipid uptake, and ORP9 expression in oxLDL-
stimulated monocyte/macrophages. Cardiovasc Res. 83:131–139. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen T, Li Z, Jing T, Zhu W, Ge J, Zheng
X, Pan X, Yan H and Zhu J: MicroRNA- 146a regulates the maturation
process and pro- inflammatory cytokine secretion by targeting CD40L
in oxLDL- stimulated dendritic cells. FEBS Lett. 585:567–573. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen T, Li Z, Tu J, Zhu W, Ge J, Zheng X,
Yang L, Pan X, Yan H and Zhu J: MicroRNA- 29a regulates pro-
inflammatory cytokine secretion and scavenger receptor expression
by targeting LPL in oxLDL- stimulated dendritic cells. FEBS Lett.
585:657–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen T, Yan H, Li Z, Jing T, Zhu W, Ge J,
Zheng X, Pan X and Zhu J: MicroRNA- 155 regulates lipid uptake,
adhesion/chemokine marker secretion and SCG2 expression in
oxLDL-stimulated dendritic cells/macrophages. Int J Cardiol.
147:446–447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuehbacher A, Urbich C, Zeiher AM and
Dimmeler S: Role of Dicer and Drosha for endothelial microRNA
expression and angiogenesis. Circ Res. 101:59–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Urbich C, Kuehbacher A and Dimmeler S:
Role of microRNAs in vascular diseases, inflammation, and
angiogenesis. Cardiovasc Res. 79:581–588. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Y, Duan W, Li Y, Jin Z, Yan J, Yu S
and Yi D: Novel Role of Silent Information Regulator 1 in
Myocardial Ischemia. Circulation. 128:2232–2240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Calvanese V, Lara E, Suárez-Alvarez B, et
al: Sirtuin 1 regulation of developmental genes during
differentiation of stem cells. Proc Natl Acad Sci USA.
107:13736–13741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng BB, Yan ZQ, Yao QP, et al:
Association of SIRT1 expression with shear stress induced
endothelial progenitor cell differentiation. J Cell Biochem.
113:3663–3671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ou X, Chae HD, Wang RH, et al: SIRT1
deficiency compromises mouse embryonic stem cell hematopoietic
differentiation, and embryonic and adult hematopoiesis in the
mouse. Blood. 117:440–450. 2011. View Article : Google Scholar :
|
|
22
|
Shakibaei M, Shayan P, Busch F, et al:
Resveratrol mediated modulation of Sirt-1/Runx2 promotes osteogenic
differentiation of mesenchymal stem cells: potential role of Runx2
deacetylation. PLoS One. 7:e357122012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Srivastava S, Bedi U and Roy P:
Synergistic actions of insulin-sensitive and Sirt1-mediated
pathways in the differentiation of mouse embryonic stem cells to
osteoblast. Mol Cell Endocrinol. 361:153–164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Di Bernardini E, Campagnolo P, Margariti
A, Zampetaki A, Karamariti E, Hu Y and Xu Q: Endothelial lineage
differentiation from induced pluripotent stem cells is regulated by
microRNA- 21 and transforming growth factor β2 (TGF-β2) pathways. J
Biol Chem. 289:3383–3393. 2014. View Article : Google Scholar :
|
|
25
|
Margariti A, Zampetaki A, Xiao Q, Zhou B,
Karamariti E, Martin D, Yin X, Mayr M, Li H, Zhang Z, et al:
Histone deacetylase 7 controls endothelial cell growth through
modulation of beta- catenin. Circ Res. 106:1202–1211. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu X, Zhang L, Wen G, Zhao H, Luong LA,
Chen Q, Huang Y, Zhu J, Ye S, Xu Q, Wang W and Xiao Q: Upregulated
sirtuin 1 by miRNA-34a is required for smooth muscle cell
differentiation from pluripotent stem cells. Cell Death Differ.
19:2014.
|
|
27
|
Hibino N, Duncan DR, Nalbandian A, Yi T,
Qyang Y, Shinoka T and Breuer CK: Evaluation of the use of an
induced puripotent stem cell sheet for the construction of tissue-
engineered vascular grafts. J Thorac Cardiovasc Surg. 143:696–703.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cai X, Hagedorn CH and Cullen BR: Human
microRNAs are processed from capped, polyadenylated transcripts
that can also function as mRNAs. RNA. 10:1957–1966. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gu S and Chan WY: Flexible and versatile
as a chameleon -sophisticated functions of microRNA- 199a. Int J
Mol Sci. 13:8449–8466. 2012. View Article : Google Scholar
|
|
30
|
Joshi HP, Subramanian IV, Schnettler EK,
Ghosh G, Rupaimoole R, Evans C, Saluja M, Jing Y, Cristina I, Roy
S, et al: Dynamin 2 along with microRNA- 199a reciprocally regulate
hypoxia- inducible factors and ovarian cancer metastasis. Proc Natl
Acad Sci USA. 111:5331–5336. 2014. View Article : Google Scholar
|
|
31
|
Chen BZ, Yu SL, Singh S, Kao LP, Tsai ZY,
Yang PC and Chen BH: Shoei-Lung Li S: Identification of microRNAs
expressed highly in pancreatic islet- like cell clusters
differentiated from human embryonic stem cells. Cell Biol Int.
35:29–37. 2011. View Article : Google Scholar
|
|
32
|
Yin G, Chen R, Alvero AB, Fu HH, Holmberg
J, Glackin C, Rutherford T and Mor G: TWISTing stemness,
inflammation and proliferation of epithelial ovarian cancer cells
through MIR199A2/214. Oncogene. 29:3545–3553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang S, Liu L, Wang R, Tuo H, Guo Y, Yi
L, Wang J and Wang D: MiR- 199a- 5p promotes migration and tube
formation of human cytomegalovirus- infected endothelial cells
through downregulation of SIRT1 and eNOS. Arch Virol.
158:2443–2452. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rane S, He M, Sayed D, Vashistha H,
Malhotra A, Sadoshima J, Vatner DE, Vatner SF and Abdellatif M:
Downregulation of miR- 199a derepresses hypoxia- inducible factor-
1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in
cardiac myocytes. Circ Res. 104:879–886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Z, Wu JC, Sheikh AY, Kraft D, Cao F,
Xie X, Patel M, Gambhir SS, Robbins RC, et al: Differentiation,
survival, and function of embryonic stem cell derived endothelial
cells for ischemic heart disease. Circulation. 116(Suppl 11):
I46–I54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamahara K, Sone M, Itoh H, Yamashita JK,
Yurugi-Kobayashi T, Homma K, Chao TH, Miyashita K, Park K, Oyamada
N, et al: Augmentation of neovascularization [corrected] in
hindlimb ischemia by combined transplantation of human embryonic
stem cells-derived endothelial and mural cells. PloS One.
3:e16662008. View Article : Google Scholar
|
|
37
|
Mauritz C, Schwanke K, Reppel M, Neef S,
Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler
J, et al: Generation of functional murine cardiac myocytes from
induced pluripotent stem cells. Circulation. 118:507–517. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nishikawa S, Goldstein RA and Nierras CR:
The promise of human induced pluripotent stem cells for research
and therapy. Nat Rev Mol Cell Biol. 9:725–729. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Asahara T and Kawamoto A: Endothelial
progenitor cells for postnatal vasculogenesis. Am J Physiol Cell
Physiol. 287:C572–C579. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao R and Daley GQ: From fibroblasts to
iPS cells: Induced pluripotency by defined factors. J Cell Biochem.
105:949–955. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang L, Zhou J, Lu Q, Wei Y and Hu S: A
novel small- diameter vascular graft: In vivo behavior of
biodegradable three- layered tubular scaffolds. Biotechnol Bioeng.
99:1007–1015. 2008. View Article : Google Scholar
|
|
42
|
Ferreira LS, Gerecht S, Shieh HF, Watson
N, Rupnick MA and Dallabrida SM: Vunjak-Novakovic G and Langer R:
Vascular progenitor cells isolated from human embryonic stem cells
give rise to endothelial and smooth muscle like cells and form
vascular networks in vivo. Circ Res. 101:286–294. 2007. View Article : Google Scholar : PubMed/NCBI
|


















