Introduction
Acute ischemic stroke has high rates of mortality
and morbidity and is a major cause of mortality globally (1). Ischemic stroke accounts for almost
80% of all types of stroke, usually caused by a thrombotic or
embolic occlusion of the middle cerebral artery or its branches
(2). This occlusion triggers brain
injury via a complex series of pathophysiological processes,
resulting in neuronal death or neuronal apoptosis and subsequent
development of neurological disorders. There is an urgent
requirement for the development of neuroprotective therapies for
acute cerebral ischemia, which salvage the ischemic penumbra. This
strategy is reflected by the increasing use of acute
re-canalization therapies, including thrombolysis (3) and the mechanical removal of clots
(4). Various neuroprotective
compounds have been developed to treat ischemic damage (5); however, satisfactory drug treatments
in clinical practice are limited.
Allium sativum, commonly known as garlic, has
been widely utilized globally as a condiment. Previous studies have
suggested possible medicinal applications and health benefits of
garlic (6,7). In addition, garlic has been used for
a long time in Traditional Chinese Medicine in the treatment of
various diseases (8).
Allicin (diallyl thiosulfinate), known as one of the
most active components of garlic, is responsible for the typical
smell and numerous beneficial functions of garlic (9). Previous studies have demonstrated
that allicin possesses a wide spectrum of pharmacological effects,
including anti-inflammatory, anti-fungal, anti-oxidant and
anti-tumoral activities (10,11).
Allicin has also been reported to exhibit protective effects on the
brain. It has been suggested that allicin mitigates traumatic brain
injury (12). However, whether
allicin protects the brain from ischemic injury remains to be
elucidated. Therefore, the present study aimed to elucidate the
effect of allicin and its underlying mechanisms of action in a rat
model middle cerebral artery occlusion (MCAO).
Materials and methods
Reagents
Allicin (purity, >98%) was purchased from the
National Institute for the Control of Pharmaceutical and Biological
Products (Beijing, China). A tumor necrosis factor (TNF)-α ELISA
kit was purchased from Beyotime Institute of Biotechnology
(Shanghai, China). A myeloperoxidase (MPO) assay kit was purchased
from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2,3,5-triphenyltet-razolium chloride (TTC) was purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Animals
A total of 60 adult male Sprague-Dawley rats
(250–300 g; 6–8 weeks-old) were purchased from the Experimental
Animal Center of Harbin Medical University (Harbin, China). All
animals were maintained under a 12-h light/dark regime with ad
libitum access to food and water. The rats were housed at 22°C
with 50% relative humidity. The animals were maintained in
accordance with the Guide for the Care and Use of Laboratory
Animals published by the United States' National Institutes of
Health (publication no. 85–23, revised 1996; Bethesda, MD, USA).
The protocol was approved by the Committee of Experimental Animals
of Harbin Medical University (Harbin, China).
Preparation of MCAO model
Rats were randomly assigned to three groups:
Sham-surgery group (n=20), MCAO group (n=20) and MCAO + allicin
group (n=20). The rats were anesthetized intraperitoneally (i.p.)
with chloral hydrate (350 mg/kg; Beyotime Institute of
Biotechnology). Subsequently, the rat was fixed in a supine
position and a midline skin incision was made to expose the right
common carotid artery, the internal carotid artery (ICA) and the
external carotid artery (ECA). The MCAO was performed as described
previously (13). Briefly, a 4-0
nylon monofilament (Sunbio Biotech, Beijing, China) with a
head-rounded tip was inserted into the ECA and gently advanced into
the ICA for ~20 mm to obstruct the blood flow of the right middle
cerebral artery. After a 90-minute occlusion, the filament was
withdrawn, allowing for the reperfusion for 24 h. The rectal
temperature of the rat was maintained at 37°C±0.5°C using a heating
pad. Rats in the sham group were subjected to the same procedure,
with the exception of the MCAO. Allicin was administered at a dose
of 50 mg/kg i.p. 3 h after reperfusion daily for five consecutive
days. Allicin was dissolved in 2% dimethyl sulfoxide (DMSO;
Sigma-Aldrich). Rats in the sham and MCAO groups were injected with
an equal volume of DMSO.
Neurological score
The neurological score was assessed by an observer
blinded to the animal groups after 24 h reperfusion according to
previously described methods (14). The neurological deficits were
evaluated on a five-point scale as follows: No neurological
deficits = 0; failure to fully extend left paw = 1; circling to the
left = 2; falling to the left = 3; no spontaneous walking and
depressed levels of consciousness = 4.
Evaluation of brain water content
At 24 h after MCAO, the brain water content was
detected according to a previously described method (15). The rats were sacrificed by deep
anesthesia with chloral hydrate (350 mg/kg, i.p.) and brains were
immediately removed and placed on a frozen plate. Tissue samples
were collected from infarct areas of ischemic rats and from the
corresponding areas in the sham-surgery rats. The samples were
weighed to determine the wet weight. Subsequently, samples were
dried in a desiccating oven at 110°C for 24 h and then weighed to
determine the dry weight. The brain water content was calculated
using the following formula: brain water content (%) = (wet weight
− dry weight) ×100%/wet weight.
Evaluation of infarct size
The animals were sacrificed 24 h after reperfusion
(n=6 in each group) by deep anesthesia with chloral hydrate (350
mg/kg, i.p.). The brains were rapidly removed and cut into five
coronal sections (2 mm). The sections were incubated in a solution
of 2% TTC at 37°C for 30 min. The infarct sizes were measured using
ImageJ software version 1.6 (National Institutes of Health).
Immunohistochemistry
At 24 h after reperfusion, the rats were
anesthetized and transcardially perfused with saline, followed by
4% paraformaldehyde (Beijing Solarbio Science & Technology Co.,
Ltd., Beijing, China) in phosphate-buffered saline. Subsequently,
the samples were dehydrated in a graded series of ethanol and
embedded in paraffin (Leica Biosystems, Wetzlar, Germany).
Following epitope retrieval at 120°C for 5–10 min using citrate
buffer (10 mM citric acid, 0.05% Tween 20; pH 6.0), the sections
were incubated with 3% H2O2 for 15 min and
then with 5% bovine serum albumin (Beyotime Institute of
Biotechnology) for 1 h. The sections were then incubated with
primary mouse monoclonal neuronal nuclei (NeuN) antibody (1:100;
cat. no. MAB377; EMD Millipore, Billerica, MD, USA) overnight at
4°C, followed by incubation with goat anti-mouse secondary antibody
(1:1,000; P044701; Dako, Carpinteria, CA, USA) for 30 min at 37°C.
The immunoreactivity was visualized using a Dako Envision
system-horseradish peroxidase kit (Dako). Finally, counterstaining
was performed using hematoxylin (Beyotime Institute of
Biotechnology). NeuN-positive staining in the cortex was identified
by the presence of deep brown staining of cells specifically
localized to the nucleus (TI-S; Nikon Corporation, Tokyo, Japan).
The images were analyzed using the Image-Pro plus 6.0 image
analysis system (Media Cybernetics, Inc., Rockville, MD, USA).
Western blot analysis
Total proteins of the cortex were extracted and
quantified according to the bicinchoninic acid protein assay kit
(Beyotime Institute of Biotechnology). The protein samples were
subjected to 8% SDS-PAGE (Beijing Solarbio Science & Technology
Co., Ltd.) followed by electrotransfer onto polyvinylidene
difluoride membranes (Millipore, Boston, MA, USA). Subsequently,
the membranes were blocked with 5% non-fat milk for 2 h at room
temperature. The membranes were then incubated overnight at 4°C
with the rabbit anti-cleaved caspase-3 polyclonal antibody
(1:1,000; cat. no. 9661; Cell Signaling Technology, Inc., Danvers,
MA, USA) and with rabbit anti-β-actin polyclonal antibody (1:3,000;
sc-7210; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), followed
by three washes with Tris-buffered saline with Tween-20 (TBST;
Beijing Solarbio Science & Technology Co., Ltd.). Subsequently,
the membranes were incubated with the secondary antibody (1:5,000;
Sc-2004; goat anti-rabbit immunoglobulin G-horseradish peroxidase;
Santa Cruz Biotechnology, Inc.) for 2 h at room temperature,
followed by three washes with TBST. Finally, the bands were
detected via chemiluminescence with the ECL Plus western blotting
detection kit (EMD Millipore). Blots were analyzed using the
Bio-Rad Imaging software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and Quantity One software package version 4.6.2 (Bio-Rad
Laboratories).
Measurement of serum TNF-α
At 24 h after the MCAO, blood samples (1 ml) were
harvested from the femoral vein. Following centrifugation at 1,000
× g for 15 min, the supernatant was collected and stored at −80°C.
Serum TNF-α was assayed using a TNF-α ELISA kit according to the
manufacturer's instructions.
Evaluation of MPO
Following reperfusion, brain tissue from the
penumbral cortex was immediately harvested and stored at −80°C. The
brain tissue was homogenized in ice-cold phosphate-buffered saline.
Subsequently, the homogenate was centrifuged at 1,000 × g for 15
min. MPO was detected using an MPO kit according to manufacturer's
instructions (Nanjing Jiancheng Bioengineering Institute).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical analyses were performed using the SPSS 16.0
software package (SPSS, Inc., Chicago, IL, USA). Data analysis was
performed by one-way analysis of variance, followed by a least
significant difference-t-test for inter-group comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Allicin ameliorates neurological deficits
following MCAO
As shown in Fig. 1,
the rats in the sham group did not exhibit any neurological
deficits, while the MCAO rats exhibited severe neurological
deficits according the neurological scoring system. Allicin
treatment markedly ameliorated the neurological deficits compared
with those in the MCAO group (P<0.05).
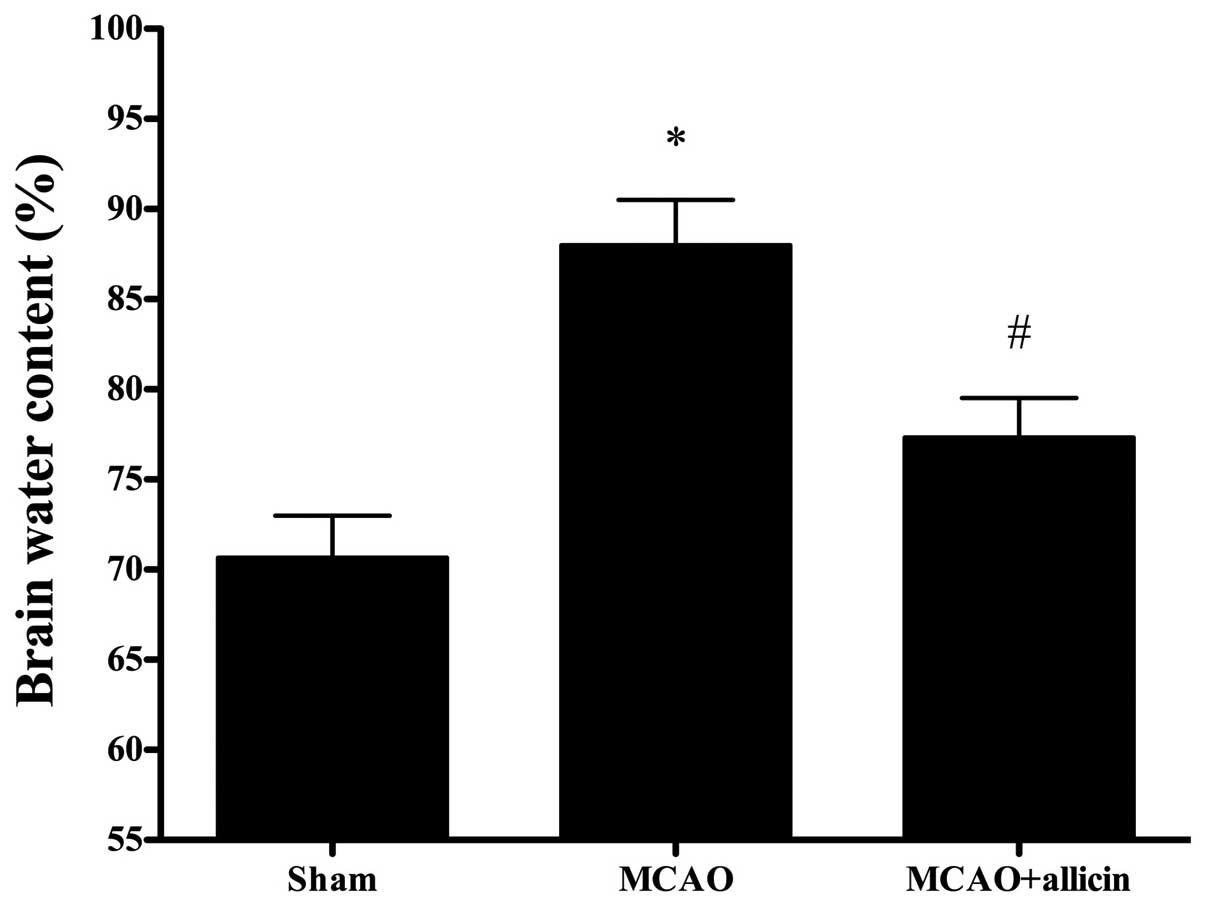
Allicin decreases brain edema following
MCAO
At 24 h after MCAO, the brain water content of the
MCAO rats was markedly higher than that of the sham-surgery rats.
Treatment with allicin significantly decreased brain edema compared
with those in the MCAO group (P<0.05; Fig. 2).
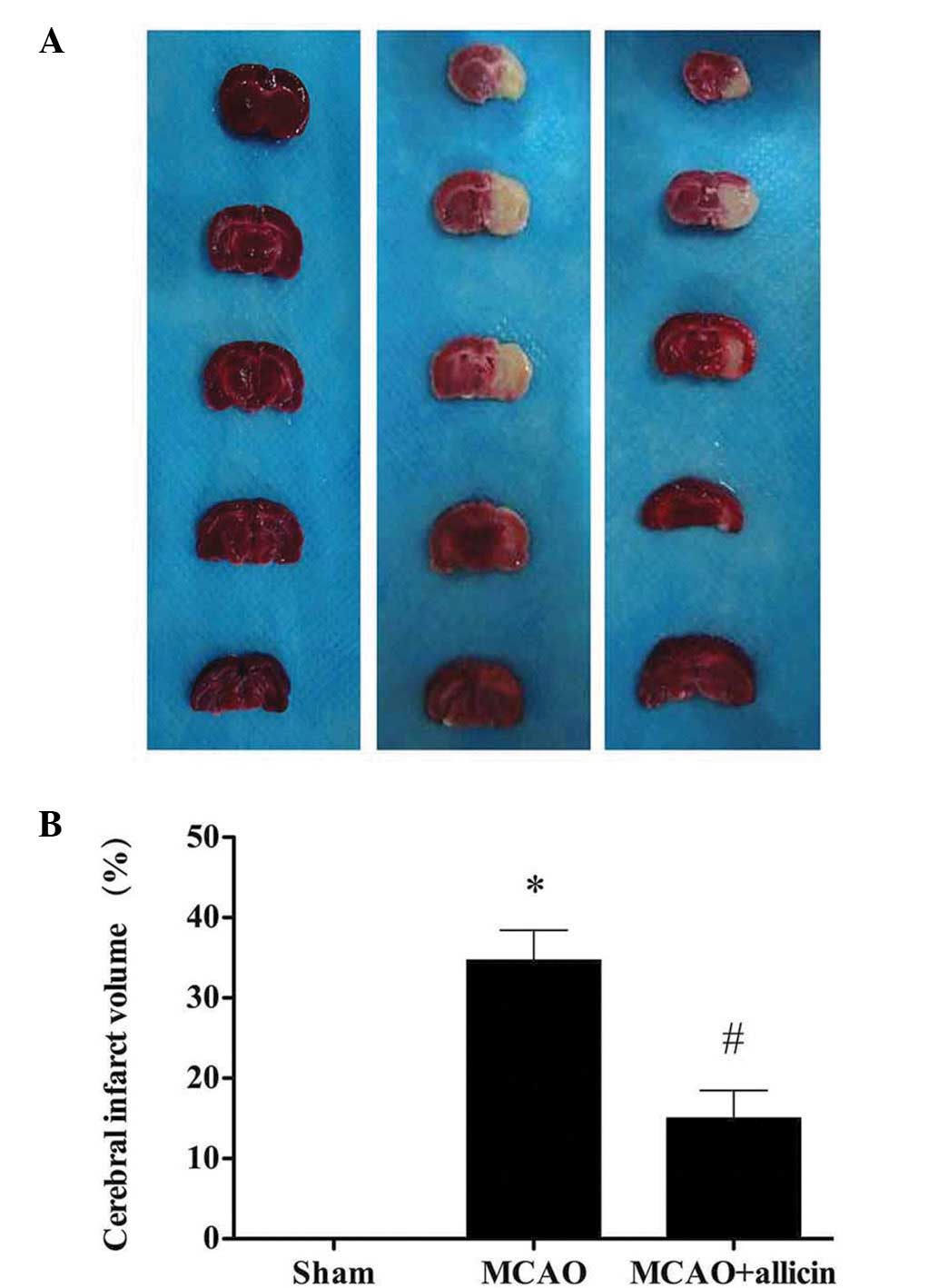
Allicin reduces the brain infarct volume
following MCAO
The rats in the sham group did not exhibit any
infarct area in the brain, while in the MCAO group, a clear
infarction was observed. Following treatment with allicin, the
infarct volume in the MCAO + allicin group was significantly lower
compared with that in the MCAO group (P<0.05; Fig. 3).
Allicin rescues neuronal survival
following MCAO
Compared with the sham group, the number of
NeuN-positive neurons was significantly decreased in the MCAO
group. The decrease of NeuN-positive neurons was attenuated by
allicin treatment (P<0.05; Fig.
4).
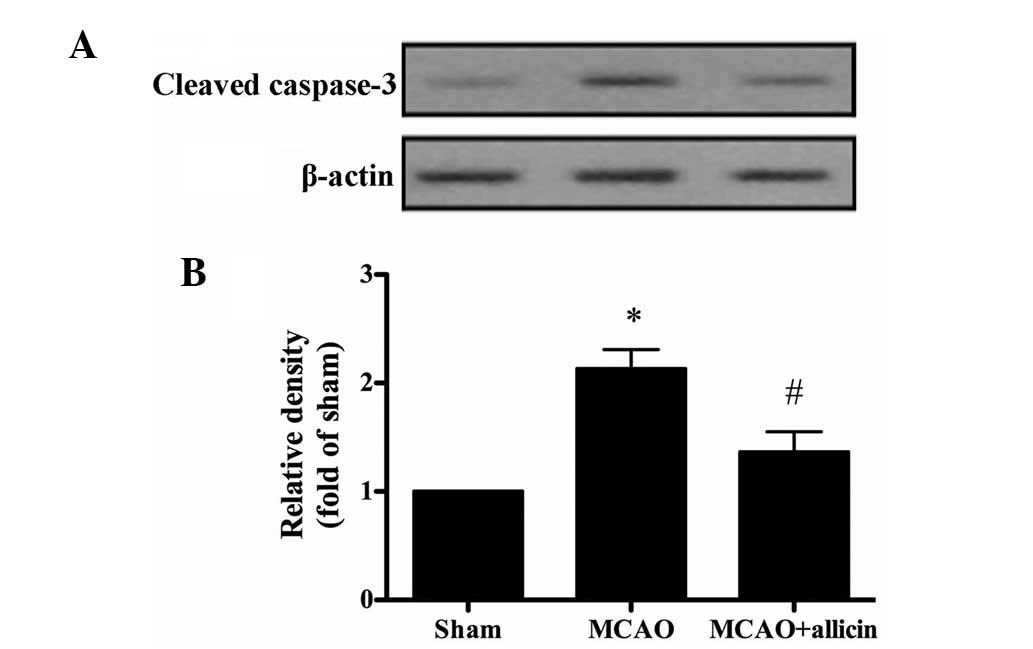
Allicin inhibits cerebral
ischemia-induced neuronal apoptosis
To identify whether the neuroprotective effect of
allicin was associated with its anti-apoptotic activity, the levels
of cleaved caspase-3 were assessed. Compared with those in the sham
group, the levels of cleaved caspase-3 were significantly increased
in the MCAO group. Of note, allicin markedly decreased the levels
of cleaved caspase-3 (P<0.05; Fig.
5).
Allicin attenuates increases in serum
levels of TNF-α following MCAO
A cerebral ichemia/reperfusion (I/R) injury causes
the production of TNF-α (16).
Thus, the levels of serum TNF-α were assessed. Fig. 6 shows that, compared with the MCAO
group, allicin significantly decreased the serum levels of TNF-α
(P<0.05).
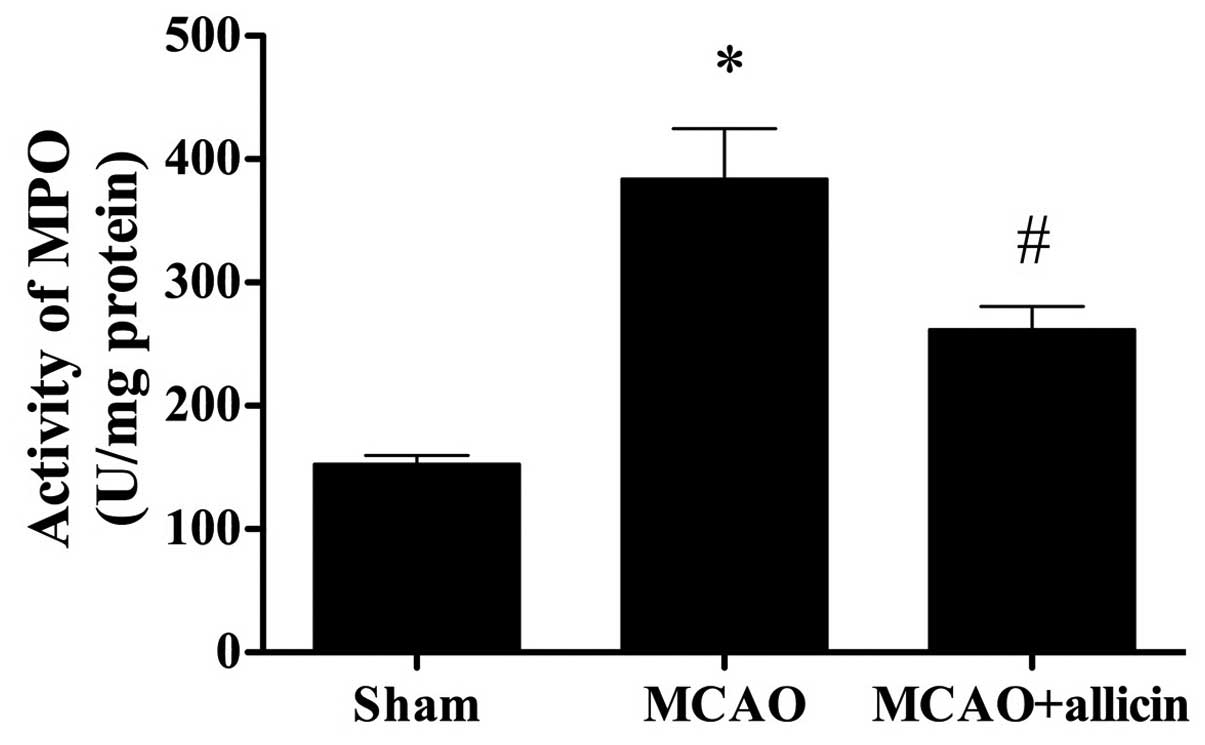
Allicin attenuates increases in MPO
activity following MCAO
As indicated in Fig.
7, the MPO activity in the sham group was at a normal level,
while that in the MCAO group was markedly elevated (P<0.05). Of
note, treatment with allicin significantly lowered cerebral MPO
activity (P<0.05).
Discussion
In the present study, it was demonstrated that
allicin exerted a neuroprotective effect against cerebral I/R
injury. Treatment with allicin significantly ameliorated
neurological deficits, decreased the cerebral infarct area, as well
as attenuated neuronal death, apoptosis and inflammation.
Therefore, the results of the present study suggested that allicin
may be of therapeutic value in the treatment of ischemic
stroke.
In the signaling pathways involved in apoptosis,
ischemia induces the translocation of B-cell lymphoma 2-associated
X protein into the outer membrane of the mitochondria, leading to
the release of cytochrome C from the mitochondria to the cytoplasm.
This process causes the activation of pro-caspase-3 into active
caspase-3 (cleaved caspase-3), a critical executioner of apoptosis
(17), triggering apoptosis
(18,19).
Apoptosis, induced by cerebral I/R injury, is one of
the major causes of cell death in the ischemic penumbra (20). The infarct size is determined by
the number of apoptotic neurons in the penumbra (21). Therefore, inhibiting apoptosis in
the ischemic penumbra may be a promising therapeutic target for
mitigating cerebral infarct size following cerebral I/R injury. In
the present study, the number of NeuN-positive cells in the MCAO
group was significantly decreased, while it was markedly increased
following allicin treatment. In addition, cleaved caspase-3 levels
were increased in rats subjected to MCAO, which was attenuated by
allicin treatment. Therefore, the results of the present study
demonstrated that allicin has anti-apoptotic effects.
In addition, inflammation is critical in brain
injury and infarction induced by cerebral ischemia. During
inflammation, inflammatory cytokines and cells are of great
significance. Inflammation-associated cytokines include pro- and
anti-inflammatory cytokines according to their ability to activate
or inhibit inflammation (22).
Important pro-inflammatory cytokines, including TNF-α, interleukin
(IL)-1β and IL-6, are responsible for the initiation of
inflammatory reactions and inducing the expression of other
cytokines following I/R injury (23). In the present study, allicin
markedly reduced TNF-α and MPO activity, indicating that allicin
protects the brain from ischemia via an anti-inflammatory
pathway.
In conclusion, the present study indicated that
allicin has a protective effect against cerebral I/R injury in
rats, which may be ascribed to its anti-inflammatory and
anti-apoptotic properties. The findings of the present study
provided further insight into the mechanism by which allicin exerts
its neuroprotective effects and paved a way for the development of
a novel therapeutic target in the clinical treatment of cerebral
ischemic stroke.
References
|
1
|
Saito T, Nito C, Ueda M, et al: Continuous
oral administration of atorvastatin ameliorates brain damage after
transient focal ischemia in rats. Life Sci. 94:106–114. 2014.
View Article : Google Scholar
|
|
2
|
Durukan A and Tatlisumak T: Acute ischemic
stroke: Overview of major experimental rodent models,
pathophysiology, and therapy of focal cerebral ischemia. Pharmacol
Biochem Behav. 87:179–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hacke W, Kaste M, Bluhmki E, et al ECASS
Investigators: Thrombolysis with alteplase 3 to 4.5 hours after
acute ischemic stroke. N Engl J Med. 359:1317–1329. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith WS, Sung G, Saver J, et al Multi
MERCI Investigators: Mechanical thrombectomy for acute ischemic
stroke: Final results of the Multi MERCI trial. Stroke.
39:1205–1212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katsura K, Suda S, Abe A, Kanamaru T, Toda
Y and Katayama Y: Brain protection therapy in acute cerebral
infarction. J Nippon Med Sch. 79:104–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Asdaq SM: Antioxidant and hypolipidemic
potential of aged garlic extract and its constituent, s-allyl
cysteine, in rats. Evid Based Complement Alternat Med.
2015:3285452015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arreola R, Quintero-Fabián S, López-Roa
RI, Flores-Gutiérrez EO, Reyes-Grajeda JP, Carrera-Quintanar L and
Ortuño-Sahagún D: Immunomodulation and anti-inflammatory effects of
garlic compounds. J Immunol Res. 2015:4016302015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ginter E and Simko V: Garlic (Allium
sativum L.) and cardiovascular diseases. Bratisl Lek Listy.
111:452–456. 2010.PubMed/NCBI
|
|
9
|
Lawson LD and Gardner CD: Composition,
stability, and bioavailability of garlic products used in a
clinical trial. J Agric Food Chem. 53:6254–6261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hunter R, Caira M and Stellenboom N:
Thiolsulfinate allicin from garlic: Inspiration for a new
antimicrobial agent. Ann NY Acad Sci. 1056:234–241. 2005.
View Article : Google Scholar
|
|
11
|
Chan JY, Tsui HT, Chung IY, Chan RY, Kwan
YW and Chan SW: Allicin protects rat cardiomyoblasts (H9c2 cells)
from hydrogen peroxide-induced oxidative injury through inhibiting
the generation of intracellular reactive oxygen species. Int J Food
Sci Nutr. 65:868–873. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou YF, Li WT, Han HC, et al: Allicin
protects rat cortical neurons against mechanical trauma injury by
regulating nitric oxide synthase pathways. Brain Res Bull.
100:14–21. 2014. View Article : Google Scholar
|
|
13
|
Ye R, Yang Q, Kong X, et al: Ginsenoside
Rd attenuates early oxidative damage and sequential inflammatory
response after transient focal ischemia in rats. Neurochem Int.
58:391–398. 2011. View Article : Google Scholar
|
|
14
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hatashita S, Hoff JT and Salamat SM:
Ischemic brain edema and the osmotic gradient between blood and
brain. J Cereb Blood Flow Metab. 8:552–559. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saad MA, Abdelsalam RM, Kenawy SA and
Attia AS: Montelukast, a cysteinyl leukotriene receptor-1
antagonist protects against hippocampal injury induced by transient
global cerebral ischemia and reperfusion in rats. Neurochem Res.
40:139–150. 2015. View Article : Google Scholar
|
|
17
|
Fernandes-Alnemri T, Litwack G and Alnemri
ES: CPP32, a novel human apoptotic protein with homology to
Caenorhabditis elegans cell death protein Ced-3 and mammalian
interleukin-1 beta-converting enzyme. J Biol Chem. 269:30761–30764.
1994.PubMed/NCBI
|
|
18
|
Lee Y and Gustafsson AB: Role of apoptosis
in cardiovascular disease. Apoptosis. 14:536–548. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Crow MT, Mani K, Nam YJ and Kitsis RN: The
mitochondrial death pathway and cardiac myocyte apoptosis. Circ
Res. 95:957–970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mattson MP, Duan W, Pedersen WA and
Culmsee C: Neurodegenerative disorders and ischemic brain diseases.
Apoptosis. 6:69–81. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Broughton BR, Reutens DC and Sobey CG:
Apoptotic mechanisms after cerebral ischemia. Stroke. 40:e331–e339.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen T, Liu W, Chao X, et al: Salvianolic
acid B attenuates brain damage and inflammation after traumatic
brain injury in mice. Brain Res Bull. 84:163–168. 2011. View Article : Google Scholar
|
|
23
|
Yasuda Y, Shimoda T, Uno K, et al:
Temporal and sequential changes of glial cells and cytokine
expression during neuronal degeneration after transient global
ischemia in rats. J Neuroinflammation. 8:702011. View Article : Google Scholar : PubMed/NCBI
|