Introduction
Ovarian cancer is one of the most common solid
tumors amongst women, with high mortality rates and few treatment
options (1,2). Despite significant advances in
ovarian cancer detection and research towards reducing recurrence
rates, the five-year survival rate for patients with ovarian cancer
has remained relatively stable for >20 years (1,2). The
main obstacle for ovarian cancer therapy is disease recurrence,
which is characterized by metastasis (3). For these reasons, the development of
effective therapeutic strategies for ovarian cancer metastasis is
urgently required.
The deregulation of oncogenes and tumor suppressors
is closely associated with ovarian cancer development and
progression (4). MicroRNAs
(miRNAs), 18–25 nucleotide non-coding RNAs, bind to the
3′-untranslated region (UTR) of their target messenger RNAs
(mRNAs), which results in the inhibition of translation or directly
induces mRNA degradation (5). The
deregulation of miRNA expression has been implicated in the
development and progression of ovarian cancer (6). Of these deregulated miRNAs, miR-148a
functions as a tumor suppressor in various types of cancer,
including gastric, colorectal and non-small cell lung cancer
(7–9). In addition, the expression of
miR-148a was found to be reduced in ovarian cancer tissues and cell
lines, and overexpression of miR-148a markedly inhibited cell
proliferation in ovarian cancer cells, suggesting that the
involvement of miR-148a in the carcinogenesis of ovarian cancer may
occur via deregulation of cell proliferation (10). However, whether miR-148a has
effects on ovarian cancer cell migration and/or invasion has
remained to be elucidated, and the specific targets of miR-148a in
ovarian cancer have not previously been studied.
Sphingosine-1-phosphate (S1P) has been found to have
a critical role in the regulation of growth, metastasis and drug
resistance in human malignancies, via binding to S1P receptors
(S1PRs) and activating the downstream signaling pathways (11). S1PR1 is a member of the S1PRs, and
S1P exerts pro-survival and drug resistant effects on cancer cells
through binding to S1PR1 (12).
However, the association between S1PR1 and miR-148a in ovarian
cancer has remained to be determined.
In the present study, the expression levels of
miR-148a in ovarian cancer tissues and cell lines, as well as in
normal ovarian tissues and ovarian epithelial cells were evaluated.
In addition, the effects of miR-148a on the migration and invasion
of ovarian cancer cells, as well as the underlying molecular
mechanisms were investigated.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), TRIzol,
fetal bovine serum (FBS), miRNA Reverse Transcription kit and
Lipofectamine® 2000 were purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). An miRNA Q-PCR Detection kit was
purchased from GeneCopoeia, Inc. (Rockville, MD, USA). Mouse
anti-S1PR1 monoclonal antibody (cat. no. ab72806), mouse anti-GAPDH
monoclonal antibody (cat. no. ab8245) and rabbit anti-mouse
immunoglobulin G secondary antibody (cat. no. ab175743) were
purchased from Abcam (Cambridge, UK). An enhanced chemiluminescence
(ECL) kit was purchased from Pierce Biotechnology, Inc. (Rockford,
IL, USA). A Quick-Change Site-Directed Mutagenesis kit was
purchased from Stratagene (Agilent Technologies, Inc., Santa Clara,
CA, USA). The psiCHECK™2 vector was purchased from Promega Corp.
(Madison, WI, USA).
Tissue specimen collection
The study protocols were approved by the Ethics
Committee of Xinxiang Medical University (Xinxiang, China).
Informed consent was obtained from all patients with ovarian cancer
recruited for the present study. Twenty ovarian cancer tissues, as
well as their matched normal adjacent tissues, were collected at
the Department of Gynecology and Obstetrics, The First Affiliated
Hospital of Xinxiang Medical University (Weihui, China). Following
surgical resection, the tissues were immediately snap-frozen in
liquid nitrogen until use.
Cell culture
Human ovarian cancer cell lines, SKOV3, OVCAR and
A2780, and normal ovarian epithelial cell line HUM-CELL-0088 were
purchased from Nlunbio (Changsha, China). Cells were cultured in
DMEM supplemented with 10% FBS at 37°C in a humidified incubator
containing 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the tissues and cells
using TRIzol reagent, according to the manufacturer's instructions.
An miRNA Reverse Transcription kit was used to convert RNA (1 µg)
into cDNA, according to the manufacturer's instructions.
Subsequently, PCR was performed using a miRNA Q-PCR Detection kit
on an ABI 7500 Thermocycler (Applied Biosystems Life Technologies,
Carlsbad, CA, USA), according to the manufacturer's instructions.
The PCR conditions were as follows: 50°C for 2 min, followed by 40
cycles at 95°C for 15 sec and 60°C for 1 min. The U6 gene was used
as an internal reference. The relative expression was analyzed
using the 2−ΔΔCt method.
Western blot analysis
The tissues or cells were solubilized in cold
radioimmunoprecipitation assay lysis buffer (Sigma-Aldrich, St.
Louis, MO, USA). Proteins were subsequently separated with 12%
SDS-PAGE, transferred to a polyvinylidene difluoride (PVDF)
membrane (Life Technologies, Carlsbad, CA, USA) and incubated with
Tris-buffered saline with 5% Tween-20 (Sigma-Aldrich) containing 5%
milk at room temperature for 3 h. The PVDF membrane was then
incubated with mouse anti-S1PR1 antibody (1:100) and mouse
anti-GAPDH antibody (1:50), respectively, at room temperature for 3
h. Following washing three times with phosphate-buffered saline
Tween-20, the PVDF membrane was incubated with rabbit anti-mouse
secondary antibody (1:5,000) at room temperature for 1 h. An ECL
kit was used to perform chemiluminescent detection, and the
relative protein expression levels were analyzed with Image-Pro
Plus software version 6.0 (Media Cybernetics, Inc., Rockville, MD,
USA). GAPDH was used as an internal reference.
Transfection
Transfection was performed using Lipofectamine 2000,
according to the manufacturer's instructions. For miR-148a
functional analysis, SKOV3 cells were transfected with scrambled
miRNA as a negative control, miR-148a mimic or miR-148a inhibitor
(all from Invitrogen Life Technologies), respectively. For S1PR1
functional analysis, SKOV3 cells were transfected with
S1PR1-specific short interfering (si)RNA or S1PR1 plasmid (all from
GenePharma, Co., Ltd, Shanghai, China), respectively.
Dual luciferase reporter assay
In accordance with the manufacturer's instructions,
a Quick Change Site-Directed Mutagenesis kit was used to generate a
mutant 3′-UTR of S1PR1. The wild-type or mutant 3′-UTR of S1PR1 was
then inserted into the psiCHECK™2 vector by restriction enzyme
digestion using XhoI and EcoRI (New England Biolabs,
Ipswich, MA, USA). The plasmid was then ligated using T4 DNA ligase
(New England Biolabs). SKOV3 cells were cultured to ~70%
confluence, and were subsequently transfected with
psiCHECK™2-S1PR1-3′-UTR or psiCHECK™2-mutant S1PR1-3′-UTR vector,
with or without 100 nM miR-148a mimic, respectively. Following 48 h
of transfection, luciferase activity was determined using an LD400
luminometer (Beckman Coulter, Brea, CA, USA). Renilla
luciferase activity was normalized to firefly luciferase
activity.
Migration assay
A Corning-Costar 3494 Transwell (Corning Life
Sciences, Oneonta, NY, USA) was used to analyze SKOV3 cell
migration. Briefly, a cell suspension (5×105 cells/ml)
was prepared in serum-free DMEM. For each group, DMEM supplemented
with 10% FBS was added to the lower chamber, and the cell
suspension was added into the upper chamber. Following incubation
for 24 h, cells that had not migrated through the membrane were
removed. Cells that had migrated through the membrane were stained
with 0.1% crystal violet, rinsed in water and air-dried. Six fields
were randomly selected under the microscope (TS100; Nikon Corp.,
Tokyo, Japan), and the number of stained cells within these fields
was counted.
Invasion assay
In order to evaluate cell invasion, 24-well
Transwell chambers (Bioscience Research Reagents; Merck Millipore,
Temecula, CA, USA) containing a layer of matrix gel were used. A
cell suspension (5×105 cells/ml) was prepared in
serum-free media. The cell suspension was added into the upper
Transwell chamber, and DMEM with 10% FBS was added into the lower
chamber. Following incubation for 24 h, the non-invading cells and
the matrix gel on the interior of the inserts were removed. Cells
on lower surface were stained with 0.1% crystal violet. Six
microscopic fields were randomly selected and the number of stained
cells was determined under a microscope (Nikon Corp.).
Statistical analysis
Values are expressed as the mean ± standard
deviation of three independent experiments. Statistical analysis of
differences was performed by one-way analysis of variance using
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
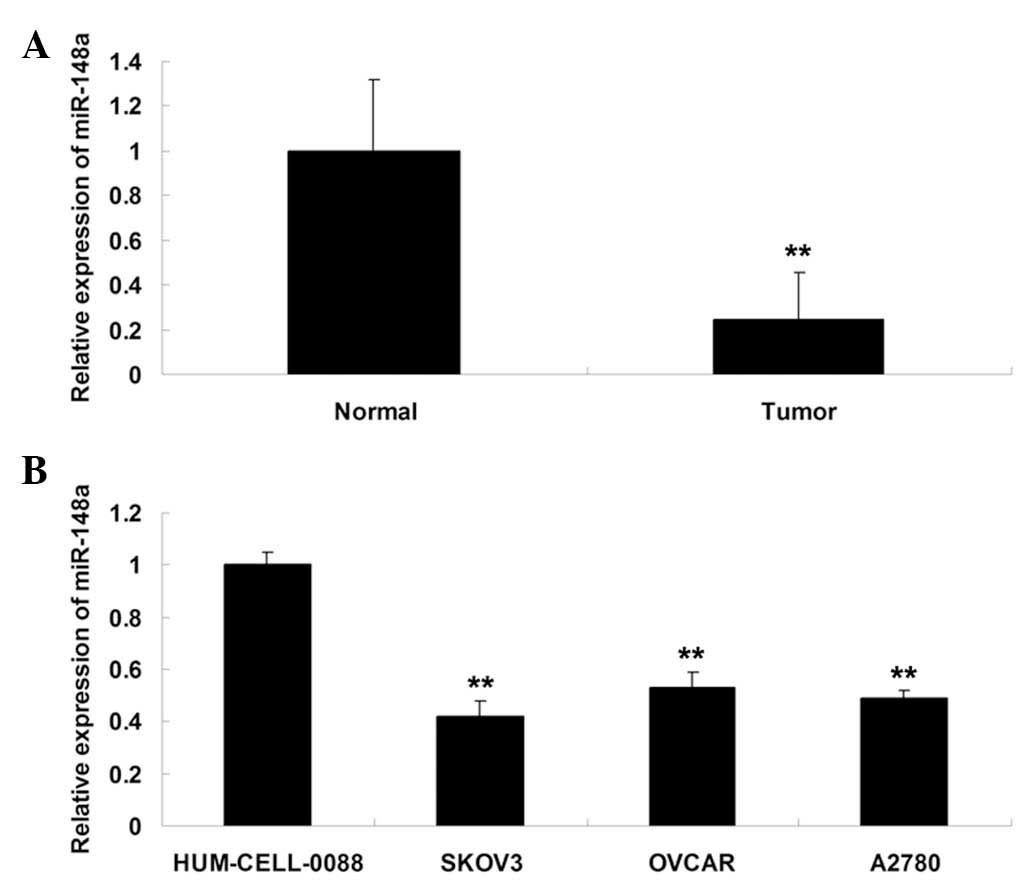
miR-148a is downregulated in ovarian
cancer tissues and cell lines
RT-qPCR was performed in order to determine the
expression levels of miR-148a in twenty ovarian cancer tissue
specimens and their matched normal adjacent tissues, three ovarian
cancer cell lines and one normal ovarian epithelial cell line. As
shown in Fig. 1A, miR-148a
expression in ovarian cancer tissues was significantly
downregulated, compared with that of their matched normal adjacent
tissues. In addition, miR-148a expression was downregulated in the
three ovarian cancer cell lines, SKOV3, OVCAR and A2780, compared
with that of the HUM-CELL-0088 normal ovarian epithelial cell line
(Fig. 1B). SKOV3 cells were chosen
for subsequent experiments as they showed the most significant
decrease in miR-148a expression levels among the three ovarian
cancer cell lines.
S1PR1 expression is upregulated in
ovarian cancer tissues and cell lines
A western blotting assay was performed to examine
the protein expression of S1PR1 in the aforementioned tissues and
cells. As shown in Fig. 2A, the
protein levels of S1PR1 were markedly increased in ovarian cancer
tissues, compared with those in their matched normal adjacent
tissues. Concurrently, S1PR1 expression was also upregulated in
ovarian cancer cells compared with that of normal ovarian
epithelial cells (Fig. 2B).
miR-148a negatively regulates the protein
expression of S1PR1 in SKOV3 cells
As miR-148a was downregulated while S1PR1 was
upregulated in ovarian cancer cells and tissues, the association
between these two factors was further examined in SKOV3 ovarian
cancer cells. Following transfection of SKOV3 cells with scrambled
miRNA, miR-148a mimic or miR-148a inhibitor, respectively, the
expression levels of miR-148a in each group were determined, and
the results indicated that the transfection efficiency was
satisfactory (Fig. 3A). The
protein expression levels of S1PR1 in each group were also
determined, and the results indicated that S1PR1 was significantly
downregulated following overexpression of miR-148a, but was
upregulated following inhibition of miR-148a in SKOV3 cells
(Fig. 3B), suggesting that
miR-148a may negatively regulate the protein expression of S1PR1 in
SKOV3 ovarian cancer cells.
miR-148a inhibits SKOV3 cell migration
and invasion via targeting S1PR1
The roles of miR-148a and S1PR1 in the regulation of
cell migration and invasion in SKOV3 cells were further evaluated.
The results revealed that overexpression of miR-148a or inhibition
of S1PR1 suppressed SKOV3 cell migration and invasion (Fig. 4). However, overexpression of S1PR1
reversed the inhibitory effect of miR-148a overexpression on SKOV3
cell migration and invasion. These data suggested that miR-148a
suppressed SKOV3 cell migration and invasion, at least in part, via
inhibition of S1PR1.
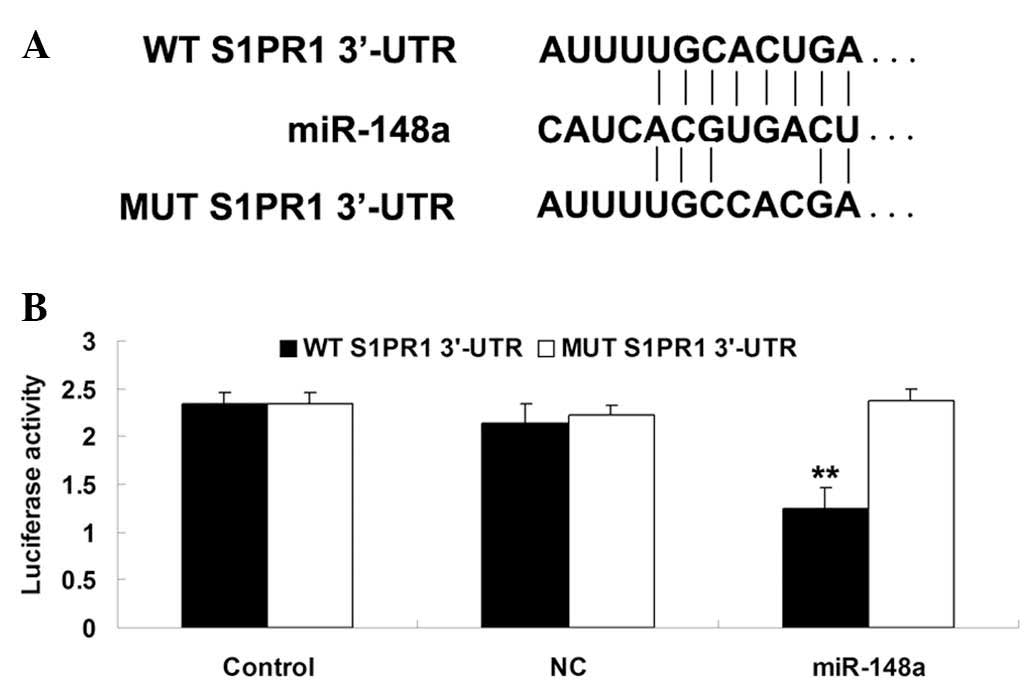
S1PR1 is a novel target of miR-148a in
SKOV3 cells
In order to investigate whether S1PR1 was a target
of miR-148a, wild-type and mutant S1PR1 3′-UTR were generated
(Fig. 5A). Subsequently, a
luciferase reporter assay was performed in SKOV3 cells, and
luciferase activity was demonstrated to be significantly
downregulated in SKOV3 cells co-transfected with the wild-type
3′UTR of S1PR1 and miR-148a mimic (Fig. 5B). However, luciferase activity
remained unchanged in the SKOV3 cells co-transfected with mutant
S1PR1 3′UTR and miR-148a mimic. These data indicated that S1PR1 may
be a direct target of miR-148a in SKOV3 cells.
Discussion
The results of the present study indicated that
miR-148a expression was markedly downregulated in ovarian cancer
tissues and cell lines, compared with that of the normal adjacent
tissues and ovarian epithelial cells, respectively. S1PR1 was
further identified as a direct target of miR-148a, and the protein
expression of S1PR1 was significantly upregulated in ovarian cancer
tissues and cell lines. In addition, it was demonstrated that S1PR1
expression was negatively regulated by miR-148a. Investigation into
the underlying molecular mechanisms revealed that the inhibitory
effect of miR-148a on ovarian cancer cell migration and invasion
was via direct targeting of S1PR1.
miRNAs are able to negatively regulate gene
expression via inhibition of mRNA translation or induction of mRNA
degradation. The deregulation of miRNA expression is a key
molecular mechanism by which the expression levels of oncogenes or
tumor suppressors are mediated in cancer cells (13). Zhou et al (10) examined the expression levels of
miR-148a in 78 patients with epithelial ovarian cancer, 17 normal
ovarian epithelium tissues and two ovarian cancer cell lines. The
results revealed that the expression of miR-148a was markedly
reduced in ovarian cancer tissues and cell lines (10), consistent with the results of the
present study. Furthermore, miR-148a mimics were transfected into
ovarian cancer cell lines, and the results demonstrated that the
upregulation of miR-148a inhibited ovarian cancer cell
proliferation (10). Accordingly,
miR-148a may represent a novel biomarker for early detection or
therapeutic targets of ovarian cancer. However, to the best of our
knowledge, the specific role of miR-148a in the regulation of cell
migration and invasion in ovarian cancer cells, as well as the
underlying molecular mechanisms, has not previously been
reported.
The effects of miR-148a on cell migration and
invasion in other types of cancer have been demonstrated. For
example, Zheng et al (14)
revealed that the overexpression of miR-148a inhibited gastric
cancer cell migration and invasion in vitro and lung
metastasis formation in vivo. Another study reported that
miR-148a overexpression inhibited cell migration and invasion in
prostate cancer cells (15). In
the present study, it was demonstrated that restoration of miR-148a
expression significantly suppressed cell migration and invasion in
ovarian cancer SKOV3 cells. Based on the above findings, it was
hypothesized that the inhibitory effects of miR-148a on cancer cell
migration and invasion may be universal.
The underlying molecular mechanisms by which
miR-148a mediated ovarian cancer cell migration and invasion were
further investigated, and the results indicated that S1PR1 was
involved in the miR-148a-mediated inhibition of cell migration and
invasion in ovarian cancer SKOV3 cells. S1P has been found to be
aberrantly expressed in patients with ovarian cancer, and is
involved in the regulation of key cellular processes that
contribute to the development and progression of ovarian cancer
(16,17). In addition, agents that block the
S1P/S1PRs signaling pathway were reported to inhibit ovarian cancer
cell growth or induce apoptosis (18). Furthermore, the S1P/S1PRs signaling
pathway has been found to be involved in the regulation of ovarian
cancer invasion potential (19).
Wang et al (17) revealed
that physiological concentrations of S1P promoted the migration and
invasion of ovarian cancer cells but inhibited the migration of
human ovarian epithelial cells. In addition to the effects of S1P
in SKOV3 cells, S1P was also found to induce the invasion of OVCAR3
ovarian cancer cells, an effect which was inhibited by VPC 23019,
an antagonist of S1PR1 (20). The
results of the present study indicated that the expression of S1PR1
was markedly increased in ovarian cancer tissues and cell lines,
compared with that of normal ovarian tissues and epithelial cells.
Another study indicated that S1PR1 was expressed in the hen and
human ovary, as well as in ovarian tumors (21). Furthermore, S1PR1 was identified as
a novel target of miR-148a, and its expression was negatively
regulated by miR-148a in ovarian cancer cells. These findings
further confirmed the association between miR-148a and S1PR1 in
ovarian cancer cells.
In conclusion, the results of the present study
revealed that miR-148a had an inhibitory effect on migration and
invasion in ovarian cancer cells, at least in part, via direct
inhibition of S1PR1, a novel target of miR-148a. Therefore,
miR-148a may potentially be used as a molecular agent for the
prevention and treatment of invasion and metastasis in ovarian
cancer, while S1PR1 may present a promising target for clinical
applications.
References
|
1
|
Miles GD, Seiler M, Rodriguez L, Rajagopal
G and Bhanot G: Identifying microRNA/mRNA dysregulations in ovarian
cancer. BMC Res Notes. 5:1642012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ozols RF: Treatment goals in ovarian
cancer. Int J Gynecol Cancer. 15(Suppl 1): S3–S11. 2005. View Article : Google Scholar
|
|
3
|
Kleppe M, Amkreutz LC, Van Gorp T, Slangen
BF, Kruse AJ and Kruitwagen RF: Lymph-node metastasis in stage I
and II sex cord stromal and malignant germ cell tumours of the
ovary: A systematic review. Gynecol Oncol. 133:124–127. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Titone R, Morani F, Follo C, Vidoni C,
Mezzanzanica D and Isidoro C: Epigenetic control of autophagy by
microRNAs in ovarian cancer. Biomed Res Int. 2014:3435422014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Imbar T and Eisenberg I: Regulatory role
of microRNAs in ovarian function. Fertil Steril. 101:1524–1530.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Llauradó M, Majem B, Altadill T, et al:
MicroRNAs as prognostic markers in ovarian cancer. Mol Cell
Endocrinol. 390:73–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xia J, Guo X, Yan J and Deng K: The role
of miR-148a in gastric cancer. J Cancer Res Clin Oncol.
140:1451–1456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takahashi M, Cuatrecasas M, Balaguer F, et
al: The clinical significance of MiR-148a as a predictive biomarker
in patients with advanced colorectal cancer. PLoS One.
7:e466842012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Song Y, Wang Y, Luo J and Yu W:
MicroRNA-148a suppresses epithelial-to-mesenchymal transition by
targeting ROCK1 in non-small cell lung cancer cells. Mol Cell
Biochem. 380:277–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou X, Zhao F, Wang ZN, et al: Altered
expression of miR-152 and miR-148a in ovarian cancer is related to
cell proliferation. Oncol Rep. 27:447–454. 2012.
|
|
11
|
Tabasinezhad M, Samadi N, Ghanbari P, et
al: Sphingosin 1-phosphate contributes in tumor progression. J
Cancer Res Ther. 9:556–563. 2013. View Article : Google Scholar
|
|
12
|
Selvam SP and Ogretmen B: Sphingosine
kinase/sphingosine 1-phosphate signaling in cancer therapeutics and
drug resistance. Handb Exp Pharmacol. 216:3–27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin H, Dai T, Xiong H, et al: Unregulated
miR-96 induces cell proliferation in human breast cancer by
downregulating transcriptional factor FOXO3a. PloS one.
5:e157972010. View Article : Google Scholar
|
|
14
|
Zheng B, Liang L, Wang C, et al:
MicroRNA-148a suppresses tumor cell invasion and metastasis by
downregulating ROCK1 in gastric cancer. Clin Cancer Res.
17:7574–7583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujita Y, Kojima K, Ohhashi R, et al:
MiR-148a attenuates paclitaxel resistance of hormone-refractory,
drug-resistant prostate cancer PC3 cells by regulating MSK1
expression. J Biol Chem. 285:19076–19084. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hong G, Baudhuin LM and Xu Y:
Sphingosine-1-phosphate modulates growth and adhesion of ovarian
cancer cells. FEBS Lett. 460:513–518. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang D, Zhao Z, Caperell-Grant A, et al:
S1P differentially regulates migration of human ovarian cancer and
human ovarian surface epithelial cells. Mol Cancer Ther.
7:1993–2002. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dai L, Xia P and Di W: Sphingosine
1-phosphate: A potential molecular target for ovarian cancer
therapy? Cancer Invest. 32:71–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smicun Y, Reierstad S, Wang FQ, Lee C and
Fishman DA: S1P regulation of ovarian carcinoma invasiveness.
Gynecol Oncol. 103:952–959. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park KS, Kim MK, Lee HY, et al: S1P
stimulates chemotactic migration and invasion in OVCAR3 ovarian
cancer cells. Biochem Biophys Res Commun. 356:239–244. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bradaric MJ, Barua A, Penumatsa K, et al:
Sphingosine-1 phosphate receptor (S1p1), a critical receptor
controlling human lymphocyte trafficking, is expressed in hen and
human ovaries and ovarian tumors. J Ovarian Res. 4:42011.
View Article : Google Scholar : PubMed/NCBI
|